Abstract
Patterning is a process by which ordered arrangements of cells and tissue structure are attained. The term derived from developmental biology is also useful for the study of colonic carcinogenesis, in which the patterning of neoplastic tubules is necessary for properties of growth, invasion, and metastasis. Interestingly the nuclear expression and transcriptional activity of β-catenin, a major oncoprotein in colonic carcinogenesis, is decisive for the first patterning of a tubule in embryogenesis, which creates the primitive gut and is called the gastrulation. Thus, basic patternings of embryogenesis and carcinogenesis might be linked. To test this hypothesis we compared morphological patterns and immunohistochemical β-catenin stainings in colonic adenomas and adenocarcinomas with the gastrulation steps. Two analogies were found: 1) the patterning of invasion with reconstruction in adenocarcinomas corresponded to the epithelio-mesenchymal transition, ingression, and rearrangement of cells during the first phase of gastrulation; and 2) the patterning of tubular branching in adenomas and adenocarcinomas resembled the endodermal invagination during the second phase. The intratumorous distribution and intensity of nuclear β-catenin expression was significantly correlated with the two patternings, similar to the findings in gastrulation. The results indicate microenvironmental regulations of nuclear β-catenin expression and a return of neoplastic cells to embryonic transcriptional susceptibilities during colonic carcinogenesis.
The properties of neoplastic growth and invasion are acquired by a series of well-defined mutations during the colonic adenoma-carcinoma sequence. Loss of function mutations of the APC gene open the gate to this sequence in ∼80% of the sporadic tumors. 1 They lead to an increase of the cytoplasmatic free pool of β-catenin, which is a component of the WNT/wingless pathway. 2,3 In an E-cadherin-bound, cell membrane-associated fraction, β-catenin participates in cell-cell adhesion. The oncogenic potential of β-catenin is derived from its nuclear pool that associates with members of the TCF (T cell factor) family of transcription factors. The resulting transcription complex was shown to activate the genes of c-myc and cyclin D1 associated with proliferation. 4,5
Later, the matrix metalloproteinase-7, an important molecule for tissue remodeling, 6,7 and the mesenchymal marker protein fibronectin 8 were found to be target genes of the β-catenin transcriptional activity. Moreover, a spatial predominance of nuclear β-catenin expression was found at the invasion front. 9 Most recent data indicate that heterogeneous intensities and spatial distributions of nuclear β-catenin expression do not correlate with the rate of proliferation, but with tumor size 10 and the grade of dysplasia 11 in adenomas as well as with the invasiveness in adenocarcinomas. 9
These observations prompted us to evaluate the spatial patterning and nuclear expression of β-catenin in colonic carcinogenesis. Patterning or pattern formation is defined as a spatiotemporal process by which ordered arrangements of cells and tissue structure are attained. The term is mostly applied to embryonic cells and the compartimentation and morphogenesis in developmental biology, but it is useful for neoplastic cells and the neomorphogenesis in tumor biology, too. Thus growth and invasiveness in colonic neoplasms can be considered as a patterning of neoplastic tubules. Growth will be achieved by a new branching and extension of tubules in addition to cellular proliferation. Invasion requires an ingression of tumor cells combined with a reconstruction of tubules, which is also necessary in the satellite foci and metastases. Many studies of colon neoplasms dealt with the proliferation and differentiation, 12,13 whereas the patterning was rarely investigated and is poorly understood. 14,15
β-catenin is decisively involved in the first patterning of a tubule in embryogenesis, which creates the primitive gut (archenteron) and is called the gastrulation. 16,17 During this process the WNT signal pathway is activated, causing nuclear expressions of β-catenin similar to colon carcinoma. 16-22 The program of gastrulation is highly conserved from echinodermata to mammals and its simplest appearance is well documented for the sea urchin. Thus, nuclear β-catenin could link a basic phylogenetic process of patterning from embryogenesis to carcinogenesis. To test this hypothesis we analyzed the patterning in colonic adenomas and adenocarcinomas in relation to the nuclear β-catenin expression and in comparison with the embryonic gastrulation in the sea urchin.
Materials and Methods
Materials
The study was comprised of 88 colon adenomas and 45 colon adenocarcinomas. Of five adenocarcinomas, lymph node metastases were also evaluated. Only tubular and tubulovillous adenomas were studied. They were included irrespective of an immunohistochemically detectable nuclear β-catenin expression. Adenocarcinomas had to exhibit clear tubular structures and an immunohistochemically detectable nuclear β-catenin expression for the inclusion into the study. Both findings were present in the majority of colonic adenocarcinomas from the files of our institute.
Immunohistochemistry
Expression of β-catenin was investigated by a mouse anti-β-catenin monoclonal antibody (1:100, clone 14; Transduction Labs., Lexington, KY) and a rabbit anti-β-catenin antiserum (1:750; Sigma, Deisenhofen, Germany). In addition, all tumors were immunostained by mouse anti-cytokeratin 18 (1:200; DAKO, Hamburg, Germany) and mouse anti-E-cadherin monoclonal antibodies (1:100, clone 36; Transduction Labs.). All carcinomas were stained with a rabbit anti-fibronectin antiserum (1:500; DAKO). Single staining on paraffin sections was performed as previously described. 6 For double-immunofluorescence both first antibodies (mouse anti-E-cadherin and rabbit anti-fibronectin antiserum) were incubated together overnight at room temperature. After washing, the slides were incubated with the corresponding secondary antibodies labeled with the fluorescence dyes anti-mouse Alexa 488 (1:500, green) and anti-rabbit Alexa 546 (1:500, red; Molecular Probes, Leiden, Netherlands).
Scoring of Immunostaining and Branching
Normal colonic epithelia adjacent to the adenomas were used as internal control of staining efficiency. The number of neoplastic cells with nuclear expression of β-catenin detected with the monoclonal antibody was never homogeneous, but varied in the adenomas. The following scoring system was used to evaluate the percentage of cells with nuclear staining: no staining, score 0; low-grade staining, score 1 (<20%); medium-grade staining, score 2 (20 to 60%); and high-grade staining, score 3 (>60%). In addition, the branching of tubules was evaluated by a scoring system in the adenomas. Branching tubules were defined as tubules with an obvious tubular ramification or an intensely folded outline because of multiple branches in an immunostaining for cytokeratin 18. The percentage of branching tubules was scored, as follows: no branching, score 0; low-grade branching, score 1 (<20%); medium-grade branching, score 2 (20 to 60%); high-grade branching, score 3 (>60%). For the scoring of nuclear β-catenin immunostaining the adenomas were screened by ×200 and ×400 magnification (ocular, ×10; objective, ×20 and ×40). For the scoring of branching a ×100 and ×200 magnification (ocular, ×10; objective, ×10 and ×20) were used. The areas of highest branching intensity were taken for the score of branching and nuclear β-catenin immunostaining in adenomas with heterogeneous patterns.
Statistics
Statistical analysis was performed using the SPSS software (SPSS Standard version 9.0.0; SPSS Inc., Chicago, IL). The correlation between absolute size (in mm) and branching was evaluated by the Mann-Whitney U-Test. Correlation between branching activity and nuclear β-catenin staining was evaluated by χ-square test (linear-by-linear association).
Comparative Analysis of Patterning
Patterning was analyzed by a comparative analysis of colonic adenomas and adenocarcinomas with the gastrulation of the sea urchin, which exhibits the simplest and best documented patterning of gastrulation. The following steps can be distinguished (see Figure 7 ▶ ): 23 1) epithelio-mesenchymal transition and ingression of the primary mesenchyme: at the center of the vegetal plate of the blastula, some epithelial cells undergo a transition to mesenchymal cells that dissociate from the epithelial layer and migrate as primary mesenchyme into the blastocoel. Together with the secondary mesenchyme, which dissociates during the extension of the primitive gut (archenteron), these cells develop the mesoderm. 2) Endodermal invagination and formation of the primitive gut (archenteron): the epithelial cells of the vegetal plate become the endoderm. They rearrange after the ingression of the mesenchyme and push into the blastocoel as a coherent epithelial layer. After the initial budding of the endodermal cells a primitive tubule (archenteron) is formed by convergent extension. Epithelio-mesenchymal transition during gastrulation is characterized by a loss of membranous E-cadherin and an expression of fibronectin. The cells undergoing this transition (micromeres) exhibit a stronger nuclear β-catenin expression than the cells undergoing endodermal differentiation and invagination (macromeres). 16,17,22
Figure 7.
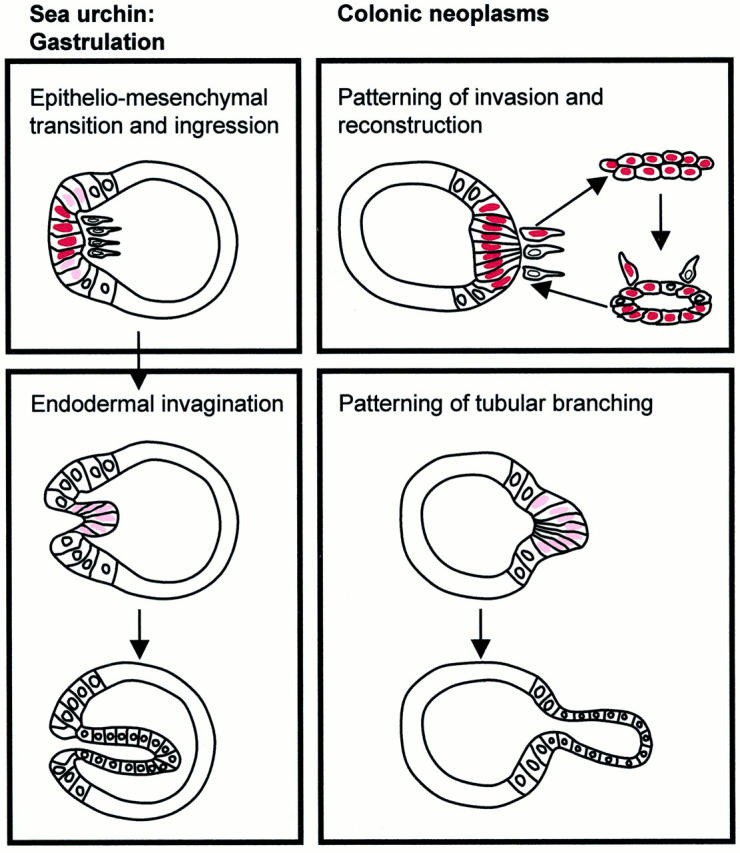
Schematic demonstration of the analogies of patterning and nuclear β-catenin expression in the sea urchin gastrulation and colonic neoplasms. The first phase of gastrulation with epithelio-mesenchymal transition and mesenchymal ingression at the vegetal plate of the blastocyste corresponds with the patterning of invasion with tubular reconstruction in colonic adenocarcinomas. Strong nuclear β-catenin expression (dark red) is found. The second phase of gastrulation with endodermal invagination comprising a budding and tubular extension is similar to the patterning of tubular branching. Here a weaker nuclear β-catenin expression (light red) is seen.
Results
Types of Tumor Patterning
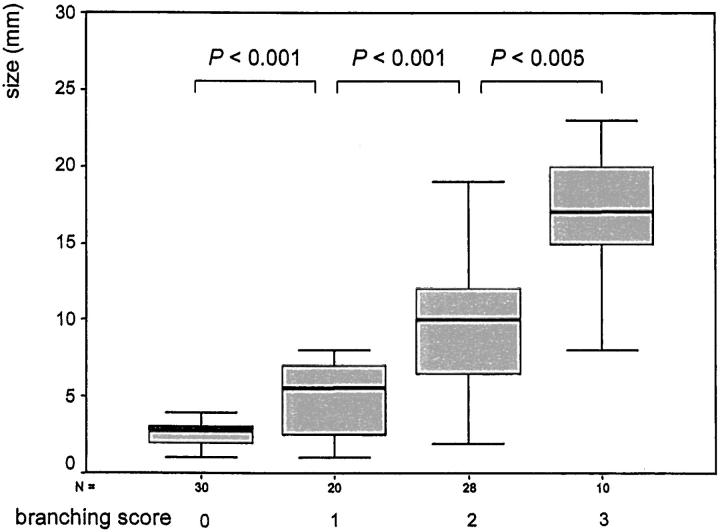
Two types of patterning were distinguished in colonic adenomas and adenocarcinomas: tubular branching and invasion with tubular reconstruction. Tubular branching was characterized by a budding and ramification of epithelial cells from pre-existing tubules (Figures 1 and 3) ▶ ▶ . Tubules with a high degree of branching showed intensely folded outlines (see Figure 3 ▶ ). Branching into the tubules caused cribriform patterns in some tumors. The patterning of tubular branching was found in adenomas and adenocarcinomas. Only 30 very small adenomas (median size, 2.6 mm) exhibited no tubular branching (Figure 2) ▶ . The intensity of branching measured by a score was significantly correlated with the size of adenomas (Figure 2) ▶ .
Figure 1.

The patterning of tubular branching in adenomas is characterized by budding (a) or by ramification and extension (b) of epithelial cells from the tubules in H&E stains. Immunostaining by the monoclonal antibody for β-catenin demonstrates its nuclear expression in most of the budding epithelial cells, whereas nonbudding epithelium in the lower half of the picture contains only few positive cells (c). Original magnifications: ×40 (a and b), ×63 (c).
Figure 3.

Comparison of the immunostaining for cytokeratin 18 showing different grades (scores) of tubular branching (a –c) with the immunostaining for β-catenin (monoclonal antibody) exhibiting different grades (scores) of positive nuclei (d–f) in three adenomas. The first adenoma contains very few branching tubules (score 1) (arrow) and few nuclei with β-catenin (score 1) (a and d). The second adenoma contains some branching tubules (score 2) and some β-catenin (score 2) expressing nuclei (b and e). The third adenoma contains many and repeatedly branching tubules (score 3) and most of the nuclei are β-catenin-positive (score 3) (c and f). Original magnifications: ×20 (a–c), ×40 (d–f).
Figure 2.
Correlation between adenoma size and tubular branching. Box-plot charts (50% of values within the box; horizontal bar, median; vertical bar, range of values). Included are the P values of the correlations between the indicated branching scores. A P value <0.05 was considered significant.
The patterning of invasion consisted of a dissociation of single neoplastic cells from the tumor mass, an ingression of these cells into the stroma and their rearrangement as epithelial plates leading to a reconstruction of tubules (see Figures 4 and 5 ▶ ▶ ). The tubular reconstruction was accompanied by central apoptoses and outward extensions of epithelial cells (not shown). The patternings of tubular branching and of invasion were spatially organized in the adenocarcinomas. Invasion was usually restricted to the outer front and branching predominated in the central parts of the tumor mass (Figure 4) ▶ .
Figure 4.

Spatial distribution and intensity of β-catenin expression, shown by immunostaining with the polyclonal antibody, correlate with the zonal organization and patterning in colonic adenocarcinoma. Zone of invasion with tubular reconstruction (a, short arrow, and higher magnification in c) with strongest nuclear β-catenin expression contains dissociated single tumor cells (c, small arrows), tumor cell plates and reconstructed tumor tubules. Zone of budding and tubular branching (a, long arrow, and higher magnification in b) exhibits many nuclei with weaker β-catenin expression at the budding sites. In continuity with branching tubules neoplastic epithelial cells with β-catenin at the adherens junctions and without nuclear expression occur (a, arrowhead, and higher magnification in b). Original magnifications: ×10 (a), ×20 (b and c).
Figure 5.
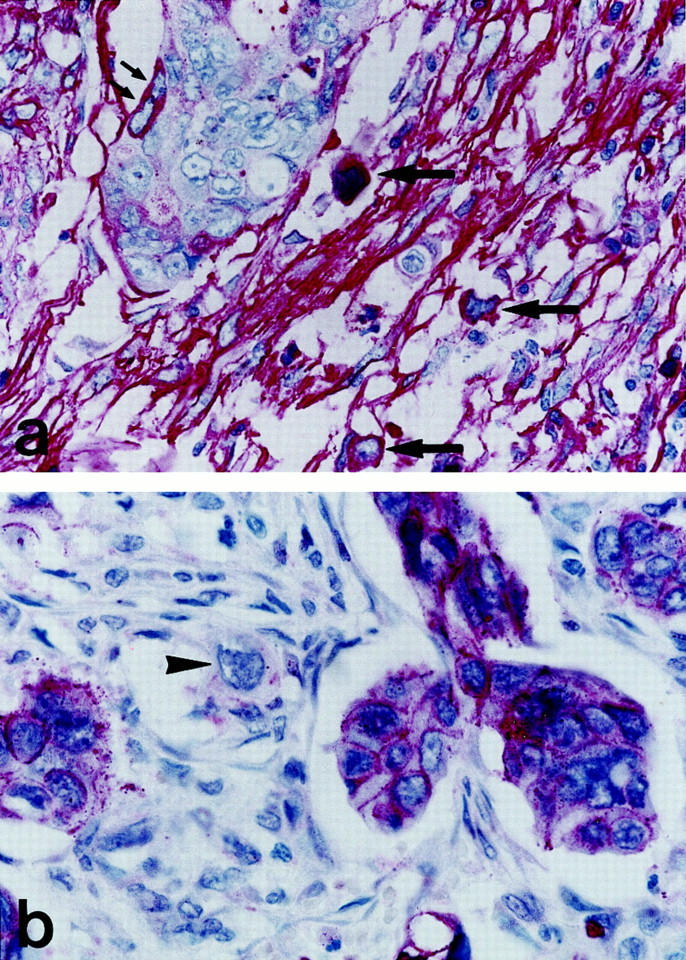
Epithelial-mesenchymal transition at the invasion front of a colonic adenocarcinoma. Immunostaining for fibronectin demonstrates neoplastic cells with cytoplasmatic fibronectin at the outer rim of a tumor cell complex (small arrows) and in dissociated, ingressing single tumor cells (arrows) within a strongly positive stroma (a). Immunostaining for E-cadherin detects strong membranous expression in tumor cell complexes, but loss of membranous positivity and weak cytoplasmic expression of a dissociated single cell in the stroma (arrowhead) (b). Original magnification, ×100.
Patterning of Tubular Branching and Nuclear β-Catenin Expression
The patterning of tubular branching was correlated with nuclear β-catenin expressions in epithelial tumor cells in the adenomas (Figure 3) ▶ . Most adenomas showed spatial expressions of nuclear β-catenin. Only 10 of 88 adenomas had strong expression of nuclear β-catenin in >60% of the neoplastic cells (Table 1) ▶ . Spatial expressions predominated in tubules with epithelial budding and branching (Figures 1 and 3) ▶ ▶ . Adenomas without tubular branches were negative for nuclear β-catenin. Morphometric analyses demonstrated a significant correlation between the branching score and the score for nuclear β-catenin expression in the adenomas (Table 1) ▶ . The adenocarcinomas usually showed intense tubular branching in the inner zone that was correlated with nuclear β-catenin expression in neoplastic cells (Figure 4, a and b) ▶ . The nuclear staining for β-catenin in branching tubules of adenomas and adenocarcinomas was weaker by the polyclonal than by the monoclonal antibody used in this study.
Table 1.
Correlation between Branching and Nuclear Expression of β-Catenin
| Branching score | β-catenin expression score | Total | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| 0 | 30 (100.0) | 0 | 0 | 0 | 30 |
| 1 | 0 | 15 (75.0) | 5 (25.0) | 0 | 20 |
| 2 | 0 | 3 (10.7) | 22 (71.6) | 3 (10.7) | 28 |
| 3 | 0 | 0 | 5 (50.0) | 5 (50.0) | 10 |
| P < 0.001 | |||||
Table shows absolute numbers and (percentage).
Patterning of Invasion with Reconstruction and Nuclear β-Catenin Expression
The patterning of invasion with reconstruction was associated with nuclear β-catenin expressions. The invasion front showed the strongest spatial nuclear β-catenin immunostaining in most adenocarcinomas (Figure 4, a and c) ▶ . The stronger nuclear β-catenin staining in areas of invasion than in areas of branching was better distinguishable by the polyclonal than by the monoclonal antibody. The strong nuclear β-catenin expressions were found in all patterning steps of invasion, dissociation, and reconstruction of tubules (Figure 4, a and c) ▶ . The neoplastic patterning in lymph node metastases resembled the patterning of invasion with reconstruction and was associated with strong nuclear β-catenin expression, too (not shown).
Patterning of Invasion with Reconstruction and Epithelio-Mesenchymal Transitions
A membranous E-cadherin expression characterizes a phenotype of coherent stationary epithelial cells. Fibronectin production is typical for dissociated migrating cells of mesenchymal phenotype. 8 E-cadherin was detected in the normal cryptal and the coherent neoplastic epithelial cells. Fibronectin was found in the extracellular matrix of the normal colonic mucosa and in the stroma of colonic tumors by immunohistochemistry (Figures 5a and 6) ▶ ▶ .
Figure 6.

Double-immunoflourescence for E-cadherin (green) and fibronectin (red) in normal colonic mucosa (a) and at the invasion front of colonic adenocarcinoma (b). Yellow staining indicates a co-localization of membranous E-cadherin and membrane-bound fibronectin. Lack of cytoplasmic expression of fibronectin with clear separation of yellow-green membranous E-cadherin expressing cryptal epithelial cells and red fibronectin-positive lamina propria mucosae in normal mucosa (a). Green neoplastic cells with membranous and cytoplasmic E-cadherin expression (short arrow), red neoplastic cells with cytoplasmic fibronectin expression (long arrow) and yellow-orange cells with mixed fibronectin and E-cadherin expression (arrowhead) at the invasion front. Original magnification, ×40.
Fibronectin is usually secreted and thus mostly undetectable in cells. Cytoplasmic retention because of disturbed secretion can enable its cellular immunohistochemical detection as a marker of a mesenchymal phenotype. In eight adenocarcinomas of the series, tumor cells with cytoplasmic fibronectin were found at the invasion front. Dissociated single tumor cells with strong cytoplasmic fibronectin expression occurred in the tumor stroma (Figure 5a) ▶ . In addition, dissociated tumor cells had weak and only cytoplasmic expressions of E-cad- herin (Figure 5b) ▶ . This was in contrast to the coherent epithelial aggregates with strong membranous E-cadherin expressions (Figure 5b) ▶ . Double-labeling demonstrated neoplastic cells with cytoplasmic co-expressions of E-cadherin and fibronectin at the invasion front (Figure 6b) ▶ . Thus, coherent tumor cells with strong membranous E-cadherin expression and lack of cytoplasmic fibronectin defining a stationary epithelial phenotype and dissociated single tumor cells with cytoplasmic E-cadherin and/or fibronectin expression defining a transitional mesenchymal phenotype were identified at the invasion front of colonic adenocarcinomas.
Analogies of the Tumor Patternings with Embryonic Gastrulation
The patterning of invasion with reconstruction showed analogies with the epithelio-mesenchymal transition, the ingression of the primary mesenchyme and the rearrangement of endodermal cells in the first phase of gastrulation in the sea urchin (Figure 7) ▶ . The analogies were: 1) the transition of epithelial tumor cells to a mesenchymal phenotype; 2) the dissociation and ingression of single tumor cells with the transient mesenchymal phenotype into the stroma; and 3) the rearrangement of tumor cells as new tubules at the invasion front (Figures 4 and 7) ▶ ▶ . Similar to the zone of epithelio-mesenchymal transition and mesenchymal ingression at the vegetal plate of the sea urchin, the strongest nuclear β-expressions were associated with the patternings of invasion with reconstruction in the adenocarcinomas (Figure 4) ▶ . The patterning of tubular branching exhibited analogies with the endodermal invagination during the second phase of the gastrulation in the sea urchin (Figure 7) ▶ . The budding of epithelial cells from neoplastic tubules resembled the initial invagination of endodermal cells in gastrulation. The extension of tubular branches corresponded to the convergent extension of the primitive gut (Figure 1, a–c) ▶ . The observation of a weaker nuclear β-catenin expression at branching than at invasion (Figure 4) ▶ is equivalent to a weaker nuclear β-catenin expression in the zone of invaginating endodermal cells than the zone of epithelio-mesenchymal transition and ingression at the vegetal plate of the sea urchin. 22
Discussion
The study demonstrates analogies of the patterning in colonic adenomas and adenocarcinomas with the embryonic gastrulation in the sea urchin. Firstly, the patterning of invasion with reconstruction in adenocarcinomas resembles the epithelio-mesenchymal transition, ingression of the mesenchymal cells, and the rearrangement of endodermal cells at the vegetal plate during the first phase of gastrulation. Secondly, the patterning of tubular branching in adenomas and adenocarcinomas is similar to the endodermal invagination and extension during the second phase of gastrulation. Thus, two main patterning processes that determine the growth and progression of colonic neoplasms, apparently repeat a basic morphogenetic program of embryogenesis. This program involves the WNT pathway with nuclear β-catenin expression in the gastrulation as well as the neoplasms. Here the spatial distribution of nuclear β-catenin expression is significantly related with the presence and activity of tubular branching and with the patterns of invasion. Even the heterogeneous intensity of nuclear β-catenin expression resembles that of gastrulation. The strongest nuclear β-catenin expression in gastrulation is found in the micromeres undergoing the epithelio-mesenchymal transition and mesenchymal ingression, whereas weaker nuclear β-catenin expression is seen in the macromeres leading to the endoderm formation and the invagination of the primitive gut. 22 Similarly invasion is associated with the strongest and tubular branching is related with a weaker staining. Therefore the analogies with gastrulation elucidate the complexity of the self-organizing context in colonic adenocarcinomas and suggest two zones of organization: an outer zone of invasion with reconstruction reflecting the epithelio-mesenchymal transition, ingression of mesenchymal cells, and rearrangement of endodermal cells during the first phase of gastrulation, and an inner zone of tubular branching corresponding to the endodermal invagination during the second phase.
In contrast, the adenomas grow by tubular branching without invasion and repeat only the second phase without the first phase of gastrulation. Nuclear β-catenin expression and tubular branching were absent in very small adenomas. This lack of nuclear β-catenin goes along with an absence of tubular branching. Wasan et al 24 previously described that microadenomas in human familial adenomatosis polyposis and in the multiple intestinal neoplasia mouse enlarge by elevated rates of crypt fission, which is the process for new crypt formation during the extension of the colon in development. In larger adenomas they described a high percentage of abnormal and heterogeneous crypt fissions, which morphologically corresponds to the tubular branching described here. Studies in both epithelial cell culture 25 and intestinal epithelial cells in mice 26 demonstrated that forced overexpression of β-catenin leads to polyp formation by increased abnormal branching. These findings fit well to our observations, that the presence and increasing number of cells with nuclear β-catenin are significantly related to the presence and increasing rate of tubular branching in the adenomas. The opposite direction of tubular invagination in adenomas and in gastrulation (see Figure 7 ▶ ) might be because of the localization of the basal membrane, which is outside in neoplastic tubules, but inside in the blastocoel.
Taken together the colonic adenoma-carcinoma sequence seems to reflect a step-wise return to the patternings of the second and first phase of the gastrulation, which goes along with an increasing extension and intensity of nuclear β-catenin expression in the neoplastic epithelial cells. The distribution and amount of nuclear β-catenin expression in colonic neoplasms has consequently to be regulated and this regulation or misregulation could be decisive for the neoplastic progression. The question therefore is, what regulates and forces the nuclear translocation of β-catenin? The APC gene mutation is probably the gatekeeper, but itself not a sufficient cause for the accumulation of nuclear β-catenin. This is supported by observations that small adenomas lack detectable nuclear β-catenin, despite APC gene mutations. 10 Additional dominant mutations in signal pathways, eg, in the ras or src oncogene, that might influence the β-catenin function, would cause genetically fixed nuclear expressions, but cannot explain the regulation and spatial nuclear translocation in carcinomas. We speculate that signals from the tumor microenvironment, eg, by soluble factors or extracellular matrix molecules, are important regulators. For instance stimulation by epidermal growth factor 27,28 or trefoil factors 29 was shown do release E-cadherin-bound β-catenin by a tyrosine phosphorylation. Also an activation of integrins by extracellular matrix molecules can influence intracellular β-catenin distribution, as it was demonstrated for the integrin β1 and the associated integrin-linked kinase. 30,31
The increase of nuclear β-catenin in neoplastic cells could finally reach a critical level that is necessary for epithelio-mesenchymal transitions and ingressions of cells with mesenchymal phenotype at the invasion front. The critical level is probably powerfully contraregulated, because it initiates a decisive alteration and new rearrangement of the cellular context. However, when that level is crossed the patterning of invasion could start and perpetuate itself by ongoing epithelio-mesenchymal and mesenchymo-epithelial transitions in the primary tumors and in metastases. Particularly the second retransition step, enabling a tubular reconstruction, can explain the metastatic growth with an identical differentiated morphology as the primary tumor after the dissemination of isolated tumor cells. Based on the present observations we suggest that nuclear β-catenin could be a prerequisite for the transition processes to drive tumor cells toward either a stationary epithelial or a migratory mesenchymal phenotype. Tumors could thus be considered as dynamic epithelial-mesenchymal equilibriums that are balanced by contrarotating signal gradients, as in developmental biology (Figure 8) ▶ .
Figure 8.

A hypothetical model for the morphogenesis of colonic adenocarcinoma derived from the analogy of the patterning with the gastrulation in developmental biology. Nuclear β-catenin might initiate a signal and transcriptional susceptibility of tumor cells for differentiation programs either toward an epithelial phenotype for position formation and tubular patterning or toward a mesenchymal phenotype with the properties for invasion with migration and metastasis. The direction of transcriptional activity and differentiation could be determined by contrarotating signaling gradients that could regulate the equilibrium of epithelio-mesenchymal transitions and thus the tumor behavior.
In this context the molecular role of β-catenin can be explained by its recently identified features: nuclear β-catenin together with members of the TCF-family, is not a typical transcriptional activator, but a regulator that derepresses its target genes. Thereby it competes with repressors like groucho for binding to TCF factors, 32,33 and a set of additional specific transcription factors is necessary to activate the characteristic target genes. For instance target gene promoters, like matrix metalloproteinase-7 6,7 and fibronectin, 8 possess binding sites for ets factors and the AP-1 transcription factor that are activated by additional signals. However, the E-cadherin gene, which also can be regulated by β-catenin/TCF, 34 is activated through different additional promoter elements by the transcription factors AP-2, c-myc, 35 and CREB. 36 This could explain why both the promoters of E-cadherin and fibronectin can have β-catenin/TCF binding sites, but are nevertheless differentially expressed. The β-catenin/TCF binding sites could even function in the promoter context either as a repressive or a derepressive element depending on the signaling by the microenvironment and the subsequent transcription factor activation. Alternatively, concentration-dependent opposite effects of nuclear β-catenin on different targets are discussed in endoderm formation, 20 which could be relevant during colorectal carcinogenesis, too.
How can the additional signaling from the microenvironment be defined? During gastrulation the transforming growth factor-β-like molecule BMP4 counteracts the functions of nuclear β-catenin in the endo-mesoderm formation. 21 Interestingly mutations inactivating the transforming growth factor-β pathway, eg, in the DPC4 gene 37 or transforming growth factor-β type II receptor, 38 are found in late dysplastic adenomas, where a strong increase in nuclear β-catenin is observed. 10 Moreover, some factors are known that can drive epithelio-mesenchymal transitions. For instance the hepatocyte growth factor can initiate a mesenchymal transition of epithelial cells by activating the Ets-1 transcription factor. 39 In contrast the leukemia inhibitory factor was shown to initiate a mesenchymo-epithelial conversion. 40 Because extracellular matrix proteins can also have different effects via integrin binding on tumor cell phenotypes, 41 the spatial heterogeneities of the extracellular matrix composition in tumors and metastases could have important impacts. For example experimental mouse mammary tumors demonstrate, that metalloproteases produced by tumor cells can induce epithelio-mesenchymal transitions by a remodeling of the extracellular matrix. 42,43 Intratumorous signaling gradients could consequently be built up by combinations of soluble morphogens and modified extracellular matrix components. In conclusion the present study provides some intriguing implications for colonic carcinogenesis: 1) the adenoma-carcinoma sequence can be considered as a sequential return of neoplastic cells to a transcriptional susceptibility of embryonic gastrulation. 2) The return could be because of a step-wise deregulation of the β-catenin turnover and translocation to the nucleus that primarily starts by a decrease of β-catenin degradation because of APC loss of function mutations. 3) The intratumorous nuclear β-catenin expression might enable a step-wise transcriptional activation and initiation of patternings repeating basic morphogenic processes of embryonic gastrulation. 4) The microenvironmental regulation of such processes could be the driving force of tumor growth and progression.
The present data for these conclusions are observational and their limitations have to be overcome by additional molecular and functional studies. Moreover, the study is representative just for the common type of colon carcinoma, exhibiting clear tubular structures as well as nuclear β-catenin expression. However, the novel approach to link tumor patternings with developmental biology already offers remarkable new insights into the complexity of colon cancer. It might be fruitful for other cancers, too.
Acknowledgments
We thank Drs. A. Jung, T. Papadopoulos, and K. Plate for helpful discussions and C. Egerer-Sieber, G. Herbig, C. Knoll, U. Suchy, and C. Winkelmann for expert technical assistance.
Footnotes
Address reprint requests to Dr. Thomas Kirchner, Department of Pathology, University of Erlangen-Nuremberg, Krankenhausstr. 8-10, D-91054 Erlangen, Germany. E-mail: sekretariat@patho.imed.uni-erlangen.de.
References
- 1.Fearon ER, Vogelstein B: A genetic model for colorectal tumorigenesis. Cell 1990, 61:759-767 [DOI] [PubMed] [Google Scholar]
- 2.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H: Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997, 275:1784-1787 [DOI] [PubMed] [Google Scholar]
- 3.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW: Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997, 275:1787-1790 [DOI] [PubMed] [Google Scholar]
- 4.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW: Identification of c-MYC as a target of the APC pathway. Science 1998, 281:1509-1512 [DOI] [PubMed] [Google Scholar]
- 5.Tetsu O, McCormick F: Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999, 398:422-426 [DOI] [PubMed] [Google Scholar]
- 6.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T: β-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 1999, 155:1033-1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford HC, Fingleton BM, Rudolph-Owen LA, Heppner-Goss KJ, Rubinfeld B, Polakis P, Matrisian LM: The metalloproteinase Matrilysin is a target of β-catenin transactivation in intestinal tumors. Oncogene 1999, 18:2883-2891 [DOI] [PubMed] [Google Scholar]
- 8.Gradl D, Kuhl M, Wedlich D: The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol 1999, 19:5576-5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brabletz T, Jung A, Hermann K, Gunther K, Hohenberger W, Kirchner T: Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol Res Pract 1998, 194:701-704 [DOI] [PubMed] [Google Scholar]
- 10.Brabletz T, Herrmann K, Jung A, Faller G, Kirchner T: Expression of nuclear beta-catenin and c-myc is correlated with tumor size but not with proliferative activity of colorectal adenomas. Am J Pathol 2000, 156:865-870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao X, Tomlinson I, Ilyas M, Palazzo JP, Talbot IC: Reciprocity between membranous and nuclear expression of beta-catenin in colorectal tumours. Virchows Arch 1997, 431:167-172 [DOI] [PubMed] [Google Scholar]
- 12.Potten CS, Kellett M, Rew DA, Roberts SA: Proliferation in human gastrointestinal epithelium using bromodeoxyuridine in vivo: data for different sites, proximity to a tumour, and polyposis coli. Gut 1992, 33:524-529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura S, Kino I, Baba S: Nuclear DNA content of isolated crypts of background colonic mucosa from patients with familial adenomatous polyposis and sporadic colorectal cancer. Gut 1993, 34:1240-1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura S, Kino I: Morphogenesis of minute adenomas in familial polyposis coli. J Natl Cancer Inst 1984, 73:41-49 [PubMed] [Google Scholar]
- 15.Park HS, Goodlad RA, Wright NA: Crypt fission in the small intestine and colon. A mechanism for the emergence of G6PD locus-mutated crypts after treatment with mutagens. Am J Pathol 1995, 147:1416-1427 [PMC free article] [PubMed] [Google Scholar]
- 16.Miller JR, McClay DR: Changes in the pattern of adherens junction-associated beta-catenin accompany morphogenesis in the sea urchin embryo. Dev Biol 1997, 192:310-322 [DOI] [PubMed] [Google Scholar]
- 17.Miller JR, McClay DR: Characterization of the role of cadherin in regulating cell adhesion during sea urchin development. Dev Biol 1997, 192:323-339 [DOI] [PubMed] [Google Scholar]
- 18.Schneider S, Steinbeisser H, Warga RM, Hausen P: Beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev 1996, 57:191-198 [DOI] [PubMed] [Google Scholar]
- 19.Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, Kimelman D, Moon RT: Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J Cell Biol 1997, 136:1123-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wikramanayake AH, Huang L, Klein WH: Beta-catenin is essential for patterning the maternally specified animal-vegetal axis in the sea urchin embryo. Proc Natl Acad Sci USA 1998, 95:9343-9348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angerer L, Angerer R: Regulative development of the sea urchin embryo: signalling cascades and morphogen gradients. Semin Cell Dev Biol 1999, 10:327-334 [DOI] [PubMed] [Google Scholar]
- 22.Logan CY, Miller JR, Ferkowicz MJ, McClay DR: Nuclear beta-catenin is required to specify vegetal cell fates in the sea urchin embryo. Development 1999, 126:345-357 [DOI] [PubMed] [Google Scholar]
- 23.Gilbert SF: Developmental Biology. 1997. Sinauer Associates, Inc. Publishers, Sunderland
- 24.Wasan HS, Park HS, Liu KC, Mandir NK, Winnett A, Sasieni P, Bodmer WF, Goodlad RA, Wright NA: APC in the regulation of intestinal crypt fission. J Pathol 1998, 185:246-255 [DOI] [PubMed] [Google Scholar]
- 25.Pollack AL, Barth AIM, Altschuler Y, Nelson WJ, Mostov KE: Dynamics of beta-catenin interactions with APC protein regulate epithelial tubulogenesis. J Cell Biol 1997, 137:1651-1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong MH, Rubinfeld B, Gordon JI: Effects of forced expression of an NH2-terminal truncated beta-catenin on mouse intestinal epithelial homeostasis. J Cell Biol 1998, 141:765-777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi K, Suzuki K, Tsukatani Y: Induction of tyrosine phosphorylation and association of beta-catenin with EGF receptor upon tryptic digestion of quiescent cells at confluence. Oncogene 1997, 15:71-78 [DOI] [PubMed] [Google Scholar]
- 28.Kuwada SK, Lund KA, Li XF, Cliften P, Amsler K, Opresko LK, Wiley HS: Differential signaling and regulation of apical vs. basolateral EGFR in polarized epithelial cells. Am J Physiol 1998, 275:C1419-C1428 [DOI] [PubMed] [Google Scholar]
- 29.Liu D, el-Hariry I, Karayiannakis AJ, Wilding J, Chinery R, Kmiot W, McCrea PD, Gullick WJ, Pignatelli M: Phosphorylation of beta-catenin and epidermal growth factor receptor by intestinal trefoil factor. Lab Invest 1997, 77:557-563 [PubMed] [Google Scholar]
- 30.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S: Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA 1998, 95:11211-11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novak A, Hsu SC, Leung-Hagesteijn C, Radeva G, Papkoff J, Montesano R, Roskelley C, Grosschedl R, Dedhar S: Cell adhesion and the integrin-linked kinase regulate the LEF-1 and beta-catenin signaling pathways. Proc Natl Acad Sci USA 1998, 95:4374-4379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A: Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature 1998, 395:604-608 [DOI] [PubMed] [Google Scholar]
- 33.Barker N, Morin PJ, Clevers H: The Yin-Yang of TCF/beta-catenin signaling. Adv Cancer Res 2000, 77:1-24 [DOI] [PubMed] [Google Scholar]
- 34.Huber O, Bierkamp C, Kemler R: Cadherins and catenins in development. Curr Opin Cell Biol 1996, 8:685-691 [DOI] [PubMed] [Google Scholar]
- 35.Batsche E, Muchardt C, Behrens J, Hurst HC, Cremisi C: RB and c-Myc activate expression of the E-cadherin gene in epithelial cells through interaction with transcription factor AP-2. Mol Cell Biol 1998, 18:3647-3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrigo I, Cato AC, Cano A: Regulation of E-cadherin gene expression during tumor progression: the role of a new Ets-binding site and the E-pal element. Exp Cell Res 1999, 248:358-371 [DOI] [PubMed] [Google Scholar]
- 37.Duff E, Clarke A: Smad4 (DPC4)—a potent tumour suppressor? Br J Cancer 1998, 78:1615-1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grady WM, Rajput A, Myeroff L, Liu DF, Kwon K, Willis J, Markowitz S: Mutation of the type II transforming growth factor-beta receptor is coincident with the transformation of human colon adenomas to malignant carcinomas. Cancer Res 1998, 58:3101-3104 [PubMed] [Google Scholar]
- 39.Fafeur V, Tulasne D, Queva C, Vercamer C, Dimster V, Mattot V, Stehelin D, Desbiens X, Vandenbunder B: The ETS1 transcription factor is expressed during epithelial-mesenchymal transitions in the chick embryo and is activated in scatter factor-stimulated MDCK epithelial cells. Cell Growth Differ 1997, 8:655-665 [PubMed] [Google Scholar]
- 40.Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, Tempst P, Parravicini E, Malach S, Aranoff T, Oliver JA: Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell 1999, 99:377-386 [DOI] [PubMed] [Google Scholar]
- 41.Boudreau N, Bissell MJ: Extracellular matrix signaling: integration of form and function in normal and malignant cells. Curr Opin Cell Biol 1998, 10:640-646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lochter A, Sternlicht MD, Werb Z, Bissell MJ: The significance of matrix metalloproteinases during early stages of tumor progression. Ann NY Acad Sci 1998, 857:180-193 [DOI] [PubMed] [Google Scholar]
- 43.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z: The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 1999, 98:137-146 [DOI] [PMC free article] [PubMed] [Google Scholar]