Abstract
Elastodysplasia and elastodystrophy are two known manifestations in the conjunctival, ie, pinguecular, part of pterygia. But the mechanisms are still not understood. The purpose of this study is to investigate the mechanism of enhanced elastin gene expression in fibroblasts from the pinguecular part of pterygia, which is related to abnormal elastic fiber expression in the pinguecular part of pterygia. Elastin in surgical specimens of normal conjunctiva and the pinguecular part of pterygia from age-matched patients was detected by immunohistochemical staining. Northern hybridization and quantification of radiolabeled tropoelastin were performed in conjunctival fibroblasts cultured under different doses of ultraviolet (UV) B irradiation, and in cultured pinguecular fibroblasts from pterygia. In vitro translation was also performed to analyze the tropoelastin production in rabbit reticulocyte lysate. The level of tropoelastin in reticulolysates from UV-treated conjunctival and pinguecular fibroblasts of pterygia was higher than in normal conjunctival fibroblasts. The coding sequence and 3′- untranslated region of tropoelastin mRNAs were amplified by reverse transcription-polymerase chain reaction, and mutations were checked by DNA sequencing. Immunohistochemical staining revealed elastin in pinguecular subepithelial connective tissues of pterygia, but not in normal conjunctiva. Tropoelastin mRNA levels were not elevated in cultured pinguecular or conjunctival fibroblasts with or without ultraviolet B irradiation. However, tropoelastin synthesis was enhanced in culture medium of pinguecular and UV-irradiated conjunctival fibroblasts, but not in normal conjunctival fibroblasts. Direct DNA sequencing revealed mutations in the 3′-untranslated region but not in the coding sequence of tropoelastin mRNA, in both pinguecular and UV-irradiated conjunctival fibroblasts. The increased expression of tropoelastin in pinguecular and UV-irradiated fibroblasts is not a result of increased levels of steady-state mRNA, but is a result of posttranscriptional modification of tropoelastin.
The limbus is a transitional zone between the cornea and conjunctiva where corneal epithelial stem cells are located. 1,2 It serves as a barrier that prevents overgrowth of the conjunctiva onto the corneal surface. A pterygium is a triangular growth of fibrovascular tissue onto the cornea and consists of a corneal and a conjunctival part. Like a pinguecula, with which it is associated and from which it may originate, a pterygium is characterized by elastoid degeneration in the subepithelial connective tissue of the conjunctival part. 3 Thus, the conjunctival part is always regarded as the pinguecular part of a pterygium. The corneal part is located on the corneolimbal surface and contains fibroblasts penetrating into the basement membrane of the corneal epithelium, which is located between the fragmented Bowman’s membrane. 4,5 However, the formation of elastoid degeneration in the pinguecular, ie, conjunctival part of a pterygium by fibroblasts is still unclear. Mathematical and epidemiological studies have revealed that UV irradiation is a major environmental predisposing factor for pterygium formation. 6-8 Therefore, it is reasonable to associate the formation of elastoid degeneration with UV irradiation.
Two recent studies showed that transformed limbal epithelium tissue expressing vimentin and fibroblasts with transformed cell characteristics are responsible for the formation of the corneal part in a pterygium. 9,10 But these two studies emphasized the importance of the corneal part in the formation of a pterygium and provided little information on the pinguecular part. Accumulation of elastin in solar elastosis in photodamaged skin has been demonstrated in transgenic mice, by both immunohistochemistry and molecular biology techniques. 11-13 It is likely that the pathological changes in conjunctiva in response to chronic UV irradiation are similar to those in chronically sun-damaged skin. For example, elastodysplasia and elastodystrophy are known to be present in the subepithelial connective tissue of the pinguecular part in a pterygium, and a similar pathological elastin accumulation is found in chronically sun-damaged skin. 14 In the present study, we compared the expression pattern of elastin in the pinguecular part of a pterygium and in UV-damaged conjunctival tissue to determine whether ultraviolet B (UVB) irradiation is associated with the accumulation of elastin in the pinguecular part. Our results indicated that a UV-induced pterygium is characterized by modification of elastin gene expression at the posttranscriptional level.
Materials and Methods
Immunohistochemistry
Surgical specimens of pterygia and normal conjunctival specimens from age-matched cataract patients were obtained from the Department of Ophthalmology, National Taiwan University Hospital, Taipei, Taiwan, according to the tenets of the Declaration of Helsinki. Specimens from the patients, which included seven women and 10 men treated for pterygia and two women and three men treated for cataracts, were used for immunohistochemical stain. Written informed consent was obtained from all patients.
The pinguecular parts of pterygial specimens were aligned parallel to the axis of pterygial movement and the sections were taken perpendicular to this axis, fixed in 3.7% formaldehyde in 0.1 mol/L of phosphate-buffered saline (pH 7.4), and embedded in paraffin after graded dehydration. After deparaffinization and rehydration, serial tissue sections (4-μm thick) were used for immunohistochemical stains and incubated overnight with anti-elastin antibody (1:4,000 dilution; Elastin Products, Ovensville, MO) at 4°C as previously described. 15 The immunoreaction was detected with avidin-biotin-horseradish peroxidase complex (Vector Laboratories, Burlingame, CA), using 4-diamethylaminoazobenzene as a chromogen. Nonimmune mouse IgG was used as the negative control. Routine hematoxylin and eosin (H&E), Masson’s trichrome, and Verhoeff’s elastic stains were also performed for studies of subepithelial connective tissue.
Cell Cultures
Because of the limited amount of tissue available for further studies, we used explant cultures, established from normal conjunctival and pterygial tissue samples obtained from surgical specimens from age-matched patients for the following experiments. The subepithelial connective tissue in the pinguecular part of the pterygium was dissected out and used for the following studies. Twenty specimens used for explant culture were different from those used for immunohistochemistry. Five strains of conjunctival fibroblasts, five strains of normal conjunctival fibroblasts with 3-mJ/cm 2 UV irradiation, five strains with 6-mJ/cm 2 UV irradiation, and five strains of pterygial fibroblasts were used for protein analysis. Three strains from each group were also used for in vitro translation. From these strains, we chose randomly three of five normal conjunctival fibroblasts, two of five pterygial fibroblasts, and another two strains of normal conjunctival fibroblasts with UV irradiation for the Northern hybridization. Furthermore, four strains of control fibroblasts, four strains with 3 mJ/cm2, four strains with 6 mJ/cm2, and five strains of pterygial fibroblasts were used for reverse transcriptase-polymerase chain reaction (RT-PCR) and DNA sequencing. The epithelial layer and subepithelial connective tissue layer of pinguecular tissue from pterygia and normal conjunctiva were dissected under a surgical microscope. Subepithelial connective tissues of the pinguecular part of pterygia and subconjunctival connective tissue of normal conjunctiva, were cut into 0.5 × 0.5-mm fragments, and then cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and 2 mmol/L l-glutamine (all from Life Technologies, Inc., Grand Island, NY) at 37°C for 1 week. Fibroblasts that migrated outside the tissue fragments were subcultured in the same Dulbecco’s modified Eagle’s medium culture medium at 37°C. The early passages (passages 1 to 4) of pinguecular and conjunctival fibroblasts were used for the subsequent experiments. Contamination of epithelial cells was prevented by meticulous surgical excision and almost disappeared after the first passage because of their limited replicative capacity and culture medium used.
UV Source
UVB (312 nm) light was supplied by a Westinghouse Fs-40 sunlamp (Westinghouse Electric Corp., Pittsburgh, PA), which delivers uniform irradiation at a distance of 38 cm. The output of the Fs-40 sunlamp is 23.4 units UVB light/hour UVB at a distance of 38 cm, where each unit is equivalent to 21 mJ/cm 2 of erythema-effective energy. For the following experiments, the total energy used ranged from 0 mJ/cm 2 to 6 mJ/cm2. Cells were irradiated after immediately reaching confluence and the culture medium was replaced with phosphate-buffered saline (PBS). After irradiation, phosphate buffered saline was substituted with fresh culture medium.
RNA Extraction, Preparation of Elastin cDNA, and Northern Hybridization
Total RNA was isolated from cell cultures with an Ultraspec RNA isolation kit according to the manufacturer’s instructions (Biotecx Laboratories, Houston, TX).
The RT-PCR was performed according to a published method. 16 A pair of 23-nucleotide residue primers (upstream primer, 5′-TTTCTCCCCGAGATGGCGGGTCT-3′; downstream primer, 5′-TCTCTTCCGGCCACAAGCTTTCC-3′) were designed for RT-PCR amplification of the full-length coding sequence of elastin mRNA. 13 After initial denaturation for 6 minutes at 94°C, 34 amplification cycles were performed as follows: first cycle at 92°C for 2 minutes, annealing at 60°C for 2 minutes, and extension at 72°C for 2 minutes. The PCR products were cloned into a TA-cloning vector (pGEM4Z-T, Promega, Madison, MI). The sequence of the cDNA insert was confirmed with an ABI PRISMTM dRhodamine Terminator Cycle Sequencing Ready Reaction kit and an ABI Prism 310 Genetic Analyzer (Perkin-Elmer-Cetus, Emeryville, CA) after purification of the insert through a spin column (Qiagen Inc., Valencia, CA).
32P-labeled probes were made by means of a random-priming labeling method (Boehringer Mannheim, Indianapolis, IN). Twenty micrograms of RNA sample was mixed with loading buffer and heated at 65°C for 10 minutes. The mixture was then chilled in an ice bath and electrophoresed though 1% agarose/formaldehyde (Amresco, Solon, OH) at 100 V. After electrophoresis, the gel was equilibrated twice in 20× standard saline citrate for 15 minutes. Capillary transfer of the RNA onto a nylon membrane (Amersham, Arlington Heights, IL) that had been prepared by washing in methanol and distilled water was performed at 4°C overnight or 4 hours at room temperature. The transferred RNA was cross-linked to the membrane in a UV cross-linking box. Glyceraldehyde-3-phosphate dehydrogenase cDNA contained in a plasmid (pHcGAP; American Type Culture Collection, Rockville, MD) served as an internal control.
Prehybridization was performed in prehybridization buffer (5× Denhardt’s solution, 5× sodium chloride/sodium phosphate/ethylenediaminetetraacetic acid, 50% formamide, 1% sodium dodecyl sulfate, 100 μg/ml tRNA) at 42°C for 90 minutes. Hybridization was performed at 42°C overnight. The membrane was washed twice with washing buffer 1 (2× standard saline citrate and 0.2% sodium dodecyl sulfate) at room temperature for 15 minutes and twice in washing buffer 2 (0.2× standard saline citrate and 0.5% sodium dodecyl sulfate) at 75°C for 30 minutes, and then blotted onto 3 mol/L paper. X-ray film (Amersham) was exposed to the membrane in a dark cassette at −70°C for 2.5 days. The quantities of RNA were determined with a FUJIX PhosphoImager scanner (FUJI Inc., Tokyo, Japan).
Quantification of Radiolabeled Tropoelastin
Radiolabeled tropoelastin was quantified according to the methods described by Schwartz et al. 17 Four days after treatment with UV irradiation, the cell cultures were incubated with fresh medium containing 5 μCi [14C]-proline (Amersham) per 75-cm 2 dish. After 24 hours, protease inhibitors (phenylmethylsulfonyl fluoride, 2 mmol/L; N-ethyl maleimide, 1 mmol/l; aprotinin, 1 μg/ml; pepstatin, 1 μg/ml; and leupeptin, 1 μg/ml, final concentration; all from Sigma Chemical Co., St. Louis, MO) were added to the medium. The medium was decanted and stored at −20°C until use. The cell layers were then rinsed with PBS containing proteolytic inhibitors and extracted at 4°C overnight with PBS containing 0.05% Tween-20 and protease inhibitors. The cell lysate was centrifuged at 12,000 × g for 5 minutes and the residue was extracted overnight with 1% sodium dodecyl sulfate, 0.05 mol/l Tris-HCl (pH7.4), 0.33 mol/L mercaptoethanol, and protease inhibitors. An aliquot was taken for the determination of proteins by the method of Bradford. 18 The accumulation of radiolabeled tropoelastin was determined by the amount bound to an affinity column (Aminolink; Pierce Chemical Co., Rockford, IL) prepared with IgG directed against human α elastin (Elastin Products).
Translation in Vitro
In vitro translation was performed by using a rabbit reticulocyte lysate (FlexiLysate, Promega) for 60 minutes at 30°C. The translation reaction (50 μl) contained 35 μl of reticulocyte lysate, 1 μl of 0.5 mmol/L amino acids (lacking methionine), 1 μl of 16.5 mmol/L of MgCl2, 2 μl of 1 mol/L KCl, 0.25 μl of ascorbic acid (5 mg/ml), 5 μCi of [14C]-proline (Amersham), 2 μl of translation cocktails, and 2 μg of transcribed RNA. After 60 minutes of translation, cycloheximide was added to 5 mmol/L to stop the reaction. The translated products were measure as above.
Detection of Mutations in the 3′-Untranslated Region (UTR) of Tropoelastin mRNA
Total RNA was extracted as described above from normal conjunctival fibroblasts, UV-irradiated conjunctival fibroblasts, and pinguecular fibroblasts from pterygial tissue. Twenty microliters of RT mixture containing 400 ng of total RNA was used for RT-PCR. The PCR reaction was performed with SuperTaq DNA polymerase as described above. Amplification of a 660-bp portion of the 3′-UTR of human tropoelastin mRNA was done using a pair of primers: upstream primer, 5′-CCCTGACTCACGACTCATCAACG-3′; downstream primer, 5′-AACTGGGACATGGATGGACGGACC-3′. PCR was performed in a Perkin-Elmer PCR thermocycler for 40 cycles at the following conditions: denaturation for 1 minute at 94°C, annealing at 63°C for 1 minute, and primer extension at 72°C for 2 minutes. The products were separated on 2% agarose gels and visualized by ethidium bromide staining. The PCR products were purified with a PCR purification kit (Qiagen), and the sequence of the 660-bp 3′-UTR was determined with an ABI Prism dRhodamine Terminator Cycle Sequencing Ready Reaction kit and ABI Prism 310 Genetic Analyzer (Perkin-Elmer-Cetus).
Statistical Analysis
Data from quantification of radiolabeled tropoelastin and Northern hybridization were analyzed by using analysis of variance and Student’s t-test. Means were considered to be significantly different from controls when P < 0.05.
Results
Immunohistochemistry
On H&E staining, the subepithelial connective tissues and vessels of the pinguecular part in pterygia were more prominent than those of normal conjunctiva tissue (Figure 1, A and B) ▶ . An amorphous subepithelial superficial hyalinized zone and coarse eosinophilic granular materials were observed in the pinguecular parts of pterygia, but not in normal conjunctiva specimens (Figure 1, A and B) ▶ . Coarse fibers were visible only in the deeper subepithelial connective tissues of pterygial samples (Figure 1, A and B) ▶ . On Masson’s trichrome staining, the dense staining of collagen fibers was also more prominent in the pinguecular part of pterygia than in the subepithelial connective tissues of conjunctiva (Figure 1, C and D) ▶ . On Verhoeff’s elastic staining, the dark coarse elastic fibers were found only in the subepithelial deep connective tissues of pinguecula in the pterygia but not in the conjunctiva (Figure 1, E and F) ▶ .
Figure 1.
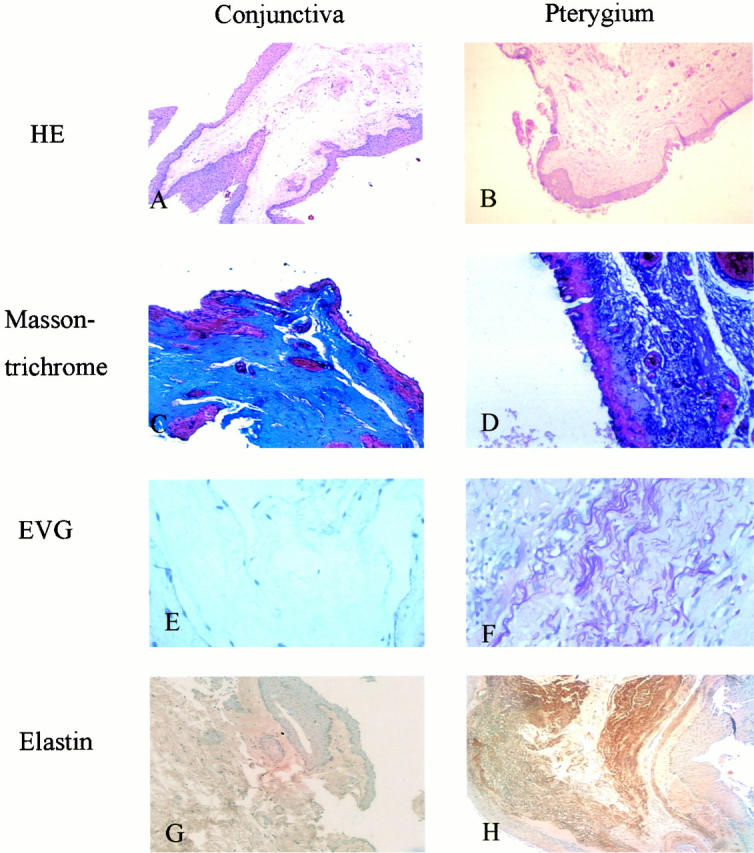
Histological studies of pterygia. A: H&E stain of pterygia showing prominent subepithelial connective tissue in the pinguecular part of pterygia (original magnification, ×100). B: H&E stain of normal conjunctiva (original magnification, ×100). C: Masson’s trichrome stain of the pinguecular part in pterygia shows strong staining of collagen in the subepithelial connective tissue (original magnification, ×400). D: Masson’s trichrome stain of normal conjunctiva reveals weak staining of collagen in the subepithelial connective tissue (original magnification, ×400). E: Verhoeff’s elastic stain of the pinguecular part in pterygia reveals prominent coarse elastic fibers in the subepithelial layer (original magnification, ×400). F: Verhoeff’s elastic stain of normal conjunctiva shows no elastic fibers (original magnification, ×200). G: Immunohistochemical staining of elastin in the pinguecular part of pterygium is positive in deeper subepithelial connective tissue (original magnification, ×100). H: Immunohistochemical staining of elastin is negative in normal conjunctiva (original magnification, ×100).
The subepithelial connective tissues of pinguecular parts of pterygia stained strongly for elastin in the surgical specimens from all 17 patients (Figure 1G) ▶ , whereas the subepithelial connective tissues of the normal conjunctival specimens from the five cataract patients did not stain positively for elastin (Figure 1H) ▶ . The staining of elastin by anti-elastin monoclonal antibodies was localized in massive quantities of thickened, tangled, coarse and tortuous fibers, which is characteristic of elastic fibers.
Northern Hybridization
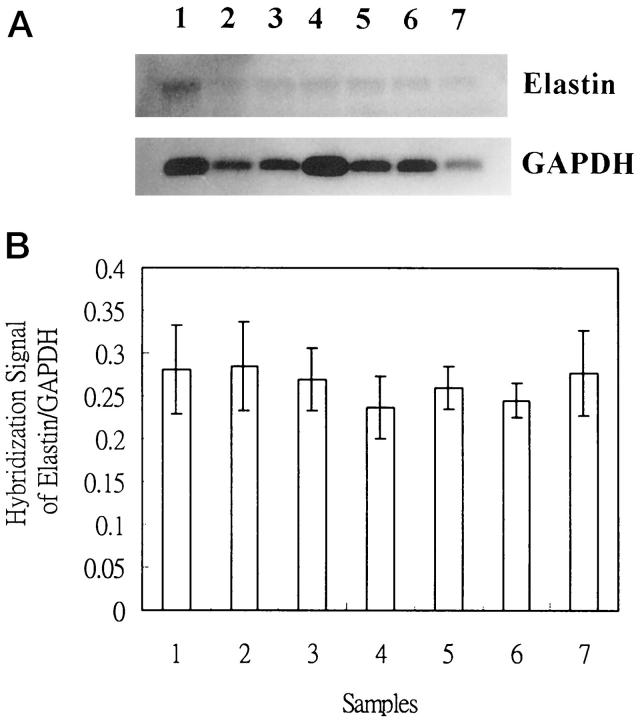
The quantities of tropoelastin mRNA in cultured cells were determined by Northern hybridization. Tropoelastin mRNA levels did not increase in pinguecular or UV-treated conjunctival fibroblasts, as compared with glyceraldehyde-3-phosphate dehydrogenase mRNA levels (one-way analysis of variance, P > 0.05) (Figure 2, A and B) ▶ . The amounts of mRNA in steady-state and UV-treated conjunctival fibroblasts were also similar.
Figure 2.
Tropoelastin and glyceraldehyde-3-phosphate dehydrogenase mRNA levels at steady-state and after UV irradiation in conjunctival fibroblasts and pinguecular fibroblasts of pterygia. A: Samples 1, 2, and 3 represent normal conjunctival fibroblasts; samples 4 and 5 represent conjunctival fibroblasts which received 3 and 6 mJ/cm 2 of UVB irradiation, respectively; samples 6 and 7 represent pinguecular fibroblasts. B: Quantitative study with the PhosphoImager scanner reveals no differences in tropoelastin mRNA expression between these groups (five repeated measurements in each mRNA, one-way analysis of variance, P > 0.05).
Increased Accumulation of Tropoelastin in Culture Media of Pterygial and UV-Treated Conjunctival Fibroblasts
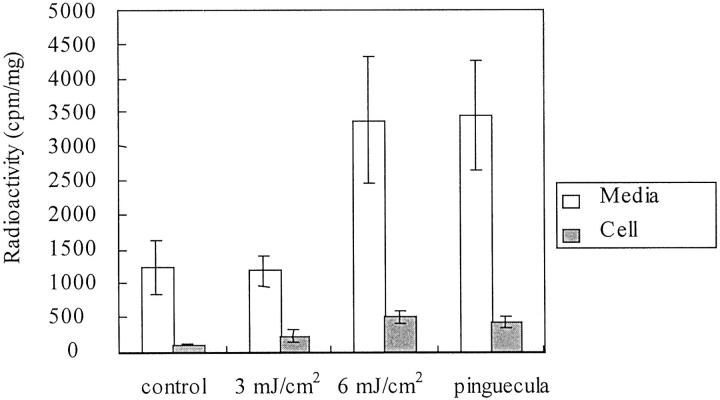
When the UV irradiation dose increased to 6 mJ/cm2, a large quantity of radiolabeled tropoelastin in the culture media of UV-treated conjunctival fibroblasts was found to be gradually increased, as determined by the use of affinity chromatography (n = 5; Student’s t-test; P < 0.05) (Figure 3) ▶ . The quantity did not increase at 3 mJ/cm 2 of UVB irradiation (n = 5; Student’s t-test; P > 0.05). The quantity of tropoelastin in culture medium of pinguecular fibroblasts was increased and had a quantity similar to that of conjunctival fibroblasts treated with 6.0 mJ/cm 2 UV irradiation (n = 5; Student’s t-test; P > 0.05). The levels of tropoelastin in cellular extracts did not increase in any group (n = 5; one-way analysis of variance; P > 0.05).
Figure 3.
Quantification of radiolabeled tropoelastin. The amounts of radiolabeled tropoelastin in fibroblasts of normal conjunctival cells increased after irradiation with UVB at 6 mJ/cm 2 (Student’s t-test; P < 0.05 compared with normal conjunctival fibroblasts). The levels of radiolabeled tropoelastin in fibroblasts of normal conjunctival cells irradiated with UVB at 6 mJ/cm 2 are the same as in pinguecular fibroblasts (n = 5; Student’s t-test; P > 0.05). In the cellular extracts, the amounts of radiolabeled tropoelastin were similar to fibroblasts of normal conjunctiva cells, UVB-irradiated conjunctival fibroblasts, and pinguecular fibroblasts (n = 5; one-way analysis of variance; P > 0.05).
Translation in Vitro
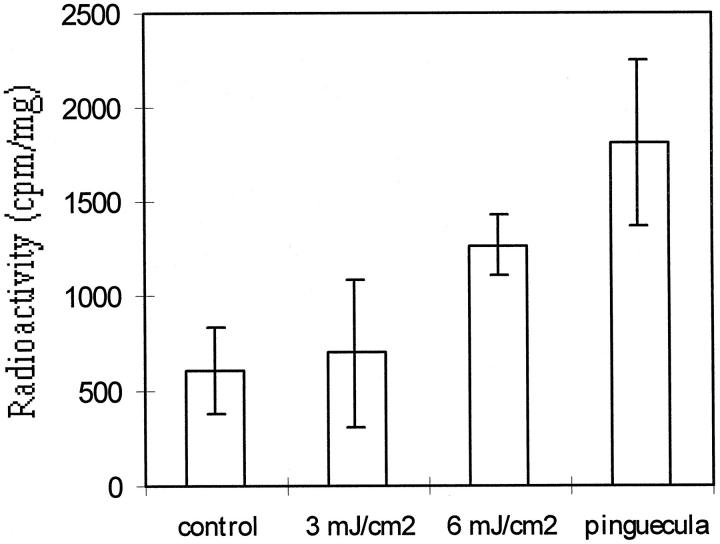
The amount of radiolabeled tropoelastin after in vitro translation was consistent with the results of cellular cultures (Figure 4) ▶ . Levels of radiolabeled tropoelastin in reticulolysates from cytoplasmic mRNA from conjunctival fibroblasts were not different from fibroblasts irradiated with UVB at 3 mJ/cm 2 (n = 3; Student’s t-test; P > 0.05), but increased after irradiation with UVB at 6 mJ/cm 2 (n = 3; Student’s t-test; P < 0.05 compared with normal conjunctival fibroblasts). The levels of radiolabeled tropoelastin in reticulolysates from cytoplasmic mRNA irradiated with UVB at 6 mJ/cm 2 were less than those in pinguecular fibroblasts (n = 3; Student’s t-test; P < 0.05).
Figure 4.
Quantification of radiolabeled tropoelastin after in vitro translation. The amounts of radiolabeled tropoelastin in reticulocyte lysates from cytoplasmic RNA of normal conjunctival fibroblasts increased after irradiation with UVB at 6 mJ/cm 2 (n = 3; Student’s t-test; P < 0.05 compared with normal conjunctival fibroblasts), but are not different from fibroblasts irradiated with UVB at 3 mJ/cm 2 (n = 3; Student’s t-test; P > 0.05). The levels of radiolabeled tropoelastin in reticulolysates from cytoplasmic mRNA irradiated with UVB at 6 mJ/cm 2 are less than in pterygial fibroblasts (n = 3; Student’s t-test; P < 0.05).
Mutations in the 3′-UTR of Tropoelastin mRNA
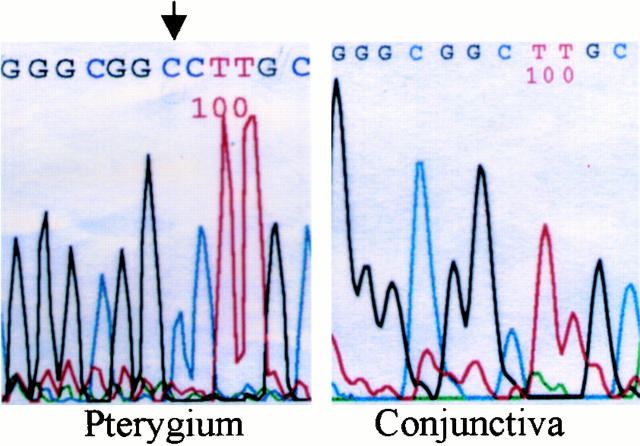
We checked the mutations of 3′-UTR and coding sequences in five strains of pinguecular fibroblasts, four strains of 3-mJ/cm 2 UV-irradiated conjunctival fibroblasts, four strains 6-mJ/cm 2 UV-irradiated conjunctival fibroblasts, and four stains of normal conjunctival fibroblasts (Table 1) ▶ . There was no mutation in the 3′-UTR of normal conjunctival fibroblasts. We found that there were 10 mutations in the 3-mJ/cm 2 group, 20 mutations in the 6-mJ/cm 2 group, and 29 mutations in five strains of pinguecular fibroblasts. There were on average 2.5, 5, and 5.8 mutations in the 3-mJ/cm2, 6-mJ/cm2, and pinguecular groups, respectively. We also found that insertion occurred more frequently in pinguecular fibroblasts. For example, in one strain of pinguecular fibroblasts, there were two nucleotide insertions in the 3′- UTR of tropoelastin mRNA: a C insertion after nucleotide 2409 and an A insertion after nucleotide 2832. In another strain, we found the same C insertion after nucleotide 2409 (Figure 5) ▶ .
Table 1.
Nucleotides Changes in the 3′-UTR Domain of UV-Treated Conjunctival Fibroblasts and Pinguecular Fibroblasts of Pterygia
| Base Change | Number of Mutations | |||
|---|---|---|---|---|
| UV (0 mJ/cm2) (n = 4) | UV (3 mJ/cm2) (n = 4) | UV (6 mJ/cm2) (n = 4) | Pterygia (n = 5) | |
| G-C:A-T | 0 | 1 | 4 | 5 |
| G-C:T-A | 0 | 0 | 3 | 1 |
| G-C:C-G | 0 | 1 | 0 | 2 |
| A-T:G-C | 0 | 0 | 1 | 4 |
| A-T:C-G | 0 | 0 | 3 | 3 |
| A-T:T-A | 0 | 1 | 3 | 1 |
| C insertion | 0 | 0 | 0 | 2 |
| A insertion | 0 | 0 | 0 | 3 |
| T insertion | 0 | 2 | 1 | 1 |
| G insertion | 0 | 1 | 0 | 0 |
| C deletion | 0 | 1 | 1 | 2 |
| A deletion | 0 | 1 | 3 | 4 |
| T deletion | 0 | 2 | 0 | 1 |
| G deletion | 0 | 0 | 2 | 2 |
| Total | 0 | 10 | 20 | 29 |
Figure 5.
DNA sequence of 3′-UTR of one strain of pinguecular fibroblasts of pterygia and one strain of conjunctival fibroblasts. The C insertion after nucleotide 2409 is shown (arrow).
Discussion
Elastin is a major component of connective tissue in large arteries, lung, skin, and ligmenetum, and provides elasticity in these tissues. 19,20 In normal skin, elastic fibers are found in the dermal connective tissue in only small amounts. 13 In photodamaged skin, on the other hand, elastic fibers are the most prominent component of the extracellular matrix and are located in the superficial to mid-dermis as an amorphous blue staining area on routine H&E staining. 21 They also stain intensely with elastic tissue stains such as the Verhoeff-van Gieson stain. 22,23 The main component of the amorphous materials in the dermis of photodamaged skin was confirmed by immunohistochemistry as elastin. 23,24 Furthermore, there is a fourfold increase of desmosine content, a component of elastin, in sun-exposed skin compared with nonexposed skin in the same individual. 25 This evidence suggests that elastin is a major component of the extracellular matrix in sun-exposed dermis.
The mechanisms of the development of conjunctival lesions and their growth onto the cornea are unknown, but the relationship between chronic sun exposure and the formation of pterygia is well established. 8,26,27 Chronic exposure to sunlight, especially UV irradiation, is considered to be the main cause of pterygia as well as solar elastosis. In the present study we used a model of solar elastosis to investigate the mechanism of abnormal elastin expression or elastoid degeneration in pterygia.
Electron microscopy studies have revealed elastogenesis in the substantia propria as well as in deeper episcleral and Tenon’s-level connective tissues in normal conjunctiva. 14 Ultrastructural examination also disclosed evidence of elastogenesis in the pinguecular part of pterygia, but the morphogenetic sequence of fiber formation was distorted. 14 Therefore, elastodysplasia (immature formation of elastic fibers) and elastodystrophy (degenerative changes of elastic fibers and formation of electron-dense inclusions) were found in the substantia propria. 14 The amorphous materials in the pinguecular parts of pterygia are composed of excessive numbers of hollow-centered microfibrils, precursors of elastic fibers that tend to clump centrally in large aggregated sheets and to acquire electron-dense inclusions. 14 In previous studies, these changes were considered to reflect an increased accumulation of elastic fibers in normal bulbar conjunctiva and were thought to result from degeneration of collagen fibers in response to sun exposure and subsequent insensitivity to elastase digestion. 28-30 However, Austin et al 14 reported that these abnormal elastic fibers exist in the substantia propria of the pinguecular parts in pterygia, and that pinguecular fibroblasts in pterygia might play a role in their formation. In the present study, we used immunohistochemical staining to confirm that these abnormal tortuous fibers are composed of elastin; these fibers are elastic and do not result from elastotic degeneration of collagen fibers. In contrast, there was no expression of elastin in the substantia propria of normal conjunctiva. The accumulation of abnormal elastic fibers was the same as that reported in solar elastosis of photodamaged skin. 12,13 These findings confirm the appropriateness of our model of photodamaged or photoaged skin for studies of the pathological changes in the pinguecular part of a pterygium. An increase in elastin mRNA levels has been demonstrated in fibroblasts of photoaged skin, compared with those from healthy skin. 31,32 This increased level of elastin mRNA in sun-damaged skin results from enhanced elastin promoter activity, as shown by transient transfections of fibroblasts with a DNA construct composed of the human elastin promoter linked to the chloramphenicol acetyl transferase reporter gene. 31 This finding was further confirmed in transgenic mice carrying a human elastin promoter exposed to UV radiation in vivo and in vitro. 11 However, the study of Schwartz et al 17 indicated that the increased tropoelastin accumulation that results from UV radiation is because of a posttranscriptional mechanism rather than increased synthesis of mRNA. There are multiple base substitutions in the noncoding domain of tropoelastin mRNA that may be responsible for the posttranscriptional increase in tropoelastin accumulation after UV irradiation. In addition, other reports describing the posttranscriptional regulation of tropoelastin mRNA, as in cultured bovine chondrocytes treated with phorbol ester or vitamin D3, provide strong evidence to support posttranscriptional regulation of tropoelastin mRNA under specific conditions. 33,34 In the present study the levels of elastin mRNA were similar in normal conjunctival fibroblasts, UV-irradiated conjunctival fibroblasts, and pinguecular fibroblasts of pterygia, whereas tropoelastin gene expression was enhanced in pinguecular and UV-irradiated conjunctival fibroblasts. These observations suggest that the increased elastin accumulation in pinguecular or UV-damaged conjunctival fibroblasts is not because of elevated steady-state levels of mRNA.
In the present study, UV-irradiation of conjunctival fibroblasts at 3 and 6 mJ/cm 2 resulted in, on average, 2.5 and five mutations, respectively. There were on average 5.8 mutations in pinguecular fibroblasts of pterygia. The mutations increased with UV dosage and in pinguecular fibroblasts. Furthermore, we found that the insertion occurred more frequently either in pinguecular fibroblasts or after UV-irradiation than in control fibroblasts. For example, we found that there was a constant C insertion after nucleotide 2409 in two strains of pinguecular fibroblasts in pterygia and there was another A insertion after nucleotide 2832 in one strain of pinguecular fibroblasts in pterygia. The total base substitution rates in our study were <5%. The regulation of gene expression at the level of translation is important for the control of protein synthesis, and specific sequences in the 5′- and 3′-UTRs are known to control translation. 35-38 Stable secondary structures (stem loops) in the 5′-UTR can interfere with the initiation of translation. 39 The regulation of cytoplasmic polyadenylation in the 3′-UTR is controlled by two unique sequences, AAUAAA and the poly (U) tract. 40 Mutations in these sequences can cause deadenylation and decay of mRNA. In addition, RNA-binding proteins can interact with specific sequences in the 3′-UTR and therefore regulate mRNA stability and translational efficiency. 41 Our findings suggest that mutations in the 3′-UTR can alter the translational efficiency of tropoelastin mRNA in pterygial and UV-treated conjunctival fibroblasts. We also speculate that the number of mutations will affect the translational efficiency of tropoelastin mRNA. When the number of mutations increase, the secondary structure of 3′-UTR will be destabilized more heavily. Therefore, we found that the quantity of tropoelastin did not increase in the 3-mJ/cm 2 group, but did increase in the 6-mJ/cm 2 group and in pinguecular fibroblasts of pterygia.
Although UVB irradiation has been reported to specifically target G-C bp, resulting in G-C to A-T transversion mutations, we found that UVB irradiation resulted in both transition and transversion mutations. 42 Furthermore, insertion of nucleotides was found only in pinguecular fibroblasts of pterygia, although there were no base substitutions in pinguecular fibroblasts of pterygia. Although the patterns of mutations in the 3′-UTR in our study were not completely consistent with those of previous reports of UVB-induced mutations, we could not find any mutation in the conjunctival fibroblasts and these mutations are still significant in the control of tropoelastin mRNA expression. These multiple mutations result from gap filling by an error-prone DNA polymerase activity during repair. 43 One possible explanation for the lack of mutations in the coding region is that the structure of mRNA in the coding region is protected from UV radiation. Alternatively, another repair mechanism responsible for the coding region, such as the transcription-coupled DNA repair system, may be involved. 43,44
Footnotes
Address reprint requests to Fung-Rong Hu, Department of Ophthalmology, National Taiwan University Hospital, 7, Chung-Shan S. Road, Taipei 100, TAIWAN. E-mail: fungrong@ha.mc.ntu.edu.tw.
Supported in part by Grants NSC-86-2314-B-002-076, NSC-87-2314-B-002-071 and NSC-88-2314-B-002-380 from the National Science Council, Taiwan.
References
- 1.Schermer A, Galvin S, Sun TT: Differentiation-related expression of a major 64K keratins in vivo and in cell culture suggests limbal location of cornealepithelial stem cell. J Cell Biol 1986, 103:49-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng SCG, Chen JJY, Huang AJW, Kruse FE, Maskin SL, Tsai RJF: Classification of conjunctival surgeries for corneal diseases based on stem cell concept. Ophthalmol Clin North Am 1990, 3:595-610 [Google Scholar]
- 3.Klintworth GK: Chronic actinic keratopathy: a condition associated with conjunctival elastosis (pingeculae) and typified by characteristic extracellular concretions. Am J Pathol 1972, 67:327-348 [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan MJ, Alvarado J: Pterygium and pigecula: electron microscopic study. Arch Ophthalmol 1967, 78:174-186 [DOI] [PubMed] [Google Scholar]
- 5.Cameron ME: Histology of pterygium: an electron microscopic study. Br J Ophthalmol 1983, 67:604-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maloof JA, Ho A, Coroneo MT: Influence of corneal shape on limbal light focusing. Invest Ophthalmol Vis Sci 1994, 35:2592-2598 [PubMed] [Google Scholar]
- 7.Coroneo MT: Pterygium as an early indicator of ultraviolet insolation: an hypothesis. Br J Ophthalmol 1993, 77:734-739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor HR, West SK, Rosenthal FS, Munoz B, Newland HS, Emmett EA: Corneal changes associated with chronic UV irradiation. Arch Ophthalmol 1989, 107:1481-1484 [DOI] [PubMed] [Google Scholar]
- 9.Chen JK, Tsai RF, Lin SS: Fibroblasts isolated from human pterygia exhibit transformed cell characteristics. In Vitro Cell Dev 1994, 30A:243-248 [DOI] [PubMed] [Google Scholar]
- 10.Dushku N, Reid TW: Immunohistochemical evidence that human pterygia originates from an invasion of vimentin-expression altered limbal epithelial basal cells. Curr Eye Res 1994, 13:472-481 [DOI] [PubMed] [Google Scholar]
- 11.Bernstein EF, Brown DB, Urbach F, Forbes D, Del Monaco M, Wu M, Katchman SD, Uitto J: Ultraviolet radiation activates the human elastin promoter in transgenic mice: a novel in vivo an in vitro model of cutaneous photoaging. J Invest Dermatol 1995, 105:269-273 [DOI] [PubMed] [Google Scholar]
- 12.Montagna W, Kirchner S, Carlisle K: Histology of sun-damaged skin. J Am Acad Dermatol 1989, 21:907-918 [DOI] [PubMed] [Google Scholar]
- 13.Uitto J, Christiano AM: Elastic fiber. Fitzpatuck TB Eisen AZ Wolff K Freedberg IM Austen KF eds. Dermatology in General Medicine, 1993, vol. 1.:pp 339-349 McGraw-Hill, New York [Google Scholar]
- 14.Autstin P, Jakobiec FA, Iwamoto T: Elastodysplasia and elastodystrophy as the pathologic bases of ocular pterygia and pingecula. Ophthalmology 1983, 90:96-109 [DOI] [PubMed] [Google Scholar]
- 15.Lin CT, Lin CR, Tan GK, Chen W, Dee AN, Chan WY: The mechanism of Epstein-Barr virus infection in nasopharyngeal carcinoma cells. Am J Pathol 1997, 150:1745-1756 [PMC free article] [PubMed] [Google Scholar]
- 16.Ausubel FM, Brent R, Kingston RE: Current Protocols in Molecular Biology. 1988. Chichester, Brisbane, Toronto, Singapore, Greene Publishing Associates and Wiley-Interscience, New York
- 17.Schwartz E, Feinberg E, Lebwohl M, Mariani TJ, Boyd C: Ultraviolet radiation increases tropoelastin accumulation by a post-transcriptional mechanism in dermal fibroblasts. J Invest Dermatol 1995, 105:65-69 [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72:248-254 [DOI] [PubMed] [Google Scholar]
- 19.Rosenbloom JC, Bashir M, Yeh H, Rosenbloom J, Ornstein-Goldstein N, Fazio M, Kahari TM, Uitto J: Regulation of elastin gene expression. Ann NY Acad Sci 1991, 624:116-136 [DOI] [PubMed] [Google Scholar]
- 20.Rosenbloom J, Abrams WR, Mecham R: Extracellular matrix 4: the elastic fiber. FASEB J 1993, 7:1208-1218 [PubMed] [Google Scholar]
- 21.Kligman AM: Early destructive effects of sunlight of human skin. JAMA 1969, 210:2377-2380 [PubMed] [Google Scholar]
- 22.Mitchell RE: Chronic solar elastosis: a light and electron microscopic study of the dermis. J Invest Dermatol 1967, 43:203-230 [DOI] [PubMed] [Google Scholar]
- 23.Chen VL, Fleischmajer R, Schwartz E, Palia M, Timpl R: Immunochemistry of elastotic material in sun-damaged skin. J Invest Dermatol 1986, 87:334-337 [DOI] [PubMed] [Google Scholar]
- 24.Mera SL, Lovell CR, Jones RR, Davies JD: Elastic fibers in normal and sun-damaged skin: an immunohistochemical study. Br J Dermatol 1987, 117:21-27 [DOI] [PubMed] [Google Scholar]
- 25.Uitto J, Matsuoka LY, Kornberg RL: Elastic fibers in cutaneous elastosis. Rudolph R eds. Problems in Aesthetic Surgery. 1986, :pp 307-338 The CV Mosby Co., St. Louis [Google Scholar]
- 26.Cameron ME: Pterygium Throughout the World. 1965. Charles C. Tomas, Springfield
- 27.Moran DJ, Hollows FC: Pterygium and ultraviolet radiation: a positive correlation. Br J Ophthalmol 1984, 68:343-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugars HS: A surgical treatment of pterygium based on new concepts as to its nature. Am J Ophthalmol 1949, 32:912-916 [DOI] [PubMed] [Google Scholar]
- 29.Ansari MW, Rahi AHS, Shukla BR: Pseudoelastic nature of pterygium. Br J Ophthalmol 1970, 54:473-476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vass P, Tapaszto I: The histochemical examination of the fibers of pterygium by elastase. Acta Ophthalmol 1964, 42:849-854 [DOI] [PubMed] [Google Scholar]
- 31.Bernstein FF, Chen YQ, Tamai K, Shepley KJ, Resnik KS, Zhang H, Tuan R, Mauviel A, Uitto J: Enhanced elastin and fibrillin gene expression in chronically photodamaged skin. J Invest Dermatol 1994, 103:182-186 [DOI] [PubMed] [Google Scholar]
- 32.Bernstein FF, Fisher LW, Li K, LeBaron RG, Tan E, Uitto J: Differential expression of versican and decorin genes in photoaged and sun-protected skin. Lab Invest 1995, 72:662-669 [PubMed] [Google Scholar]
- 33.Parks WC, Kolodziej ME, Pierce RA: Phorbol ester-mediated downregulation of tropoelastin expression is controlled by a posttranscriptional mechanism. Biochemistry 1992, 31:6639-6645 [DOI] [PubMed] [Google Scholar]
- 34.Pierce RA, Kolodziez ME, Parks WC: 1,25-dihydroxyvitamin D3 represses tropoelastin expression by a posttranscriptional mechanism. J Biol Chem 1992, 267:11593-11599 [PubMed] [Google Scholar]
- 35.Hershey JWB: Translational control in mammalian cells. Ann Rev Biochem 1991, 60:717-750 [DOI] [PubMed] [Google Scholar]
- 36.Pesole G, Fiormarino G, Saccone C: Sequence analysis and compositional properties of untranslated regions of human mRNAs. Gene 1994, 140:219-225 [DOI] [PubMed] [Google Scholar]
- 37.Sachs AB: Messenger RNA degradation in eukaryocytes. Cell 1993, 74:413-421 [DOI] [PubMed] [Google Scholar]
- 38.Gallie DR: The cap and poly A tail function synergistically to regulate mRNA translational efficiency. Genes Dev 1991, 5:2108-2116 [DOI] [PubMed] [Google Scholar]
- 39.Manzella JM, Blackshear PJ: Regulation of rat ornithine decarboxylase mRNA translation by its 5′ untranslated region. J Biol Chem 1990, 265:11817-11822 [PubMed] [Google Scholar]
- 40.Wickens M: In the beginning is the end: regulation of poly A addition and removal during early development. Trends Biol Sci 1990, 15:320-324 [DOI] [PubMed] [Google Scholar]
- 41.Bohjanen PR, Petryniak B, June CH, Thompson CB, Linsten T: AU RNA-binding factors differ in their binding specificities and affinities. J Biol Chem 1992, 267:6301-6309 [PubMed] [Google Scholar]
- 42.Keyse SM, Amaudruz F, Tyrrell RM: Determination of the spectrum mutations induced by defined-wavelength solar UVB (313-nm) radiation in mammalian cells by use of a shuttle vector. Mol Cell Biol 1988, 8:5425-5431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seidman MM, Bredberg A, Seetharam S, Kraemer KH: Multiple point mutations in a shuttle vector propagated in human cells: evidence for an error prone DNA polymerase activity. Proc Natl Acad Sci USA 1987, 84:4944-4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanawalt PC: Transcription-coupled repair and human disease. Science 1994, 266:1957-1958 [DOI] [PubMed] [Google Scholar]