Abstract
Postherpetic neuralgia (PHN) is a debilitating chronic pain condition, yet there is a lack of knowledge regarding underlying brain activity. Here we identify brain regions involved in spontaneous pain of PHN (n = 11) and determine its modulation with Lidoderm therapy (patches of 5% lidocaine applied to the PHN affected body part). Continuous ratings of fluctuations of spontaneous pain during fMRI were contrasted to ratings of fluctuations of a bar observed during scanning, at three sessions: 1) pre-treatment baseline, 2) after 6-hours of Lidoderm treatment, and 3) after 2-weeks of Lidoderm use. Overall brain activity for spontaneous pain of PHN involved affective and sensory-discriminative areas: thalamus, primary and secondary somatosensory, insula and anterior cingulate cortices, as well as areas involved in emotion, hedonics, reward, and punishment: ventral striatum, amygdala, orbital frontal cortex, and ventral tegmental area. Generally, these activations decreased at session 2 and session 3, except right anterior insular activity which increased with treatment. The sensory and affective activations only responded to the short-term treatment (6-hours of Lidoderm); while the ventral striatum and amygdala (reward-related regions) decreased mainly with longer-term treatment (2 weeks of Lidoderm). Pain properties: average magnitude of spontaneous pain, and responses on Neuropathic Pain Scale (NPS), decreased with treatment. The ventral striatal and amygdala activity best reflected changes in NPS, which was modulated only with longer-term treatment. The results show a specific brain activity pattern for PHN spontaneous pain, and implicate areas involved in emotions and reward as best reflecting changes in pain with treatment.
Keywords: Chronic pain, fMRI, ventral striatum, thalamus, anterior cingulate, insula
Introduction
Postherpetic neuralgia (PHN) is characterized with spontaneous pain of various qualities like sharp, stabbing and burning, and commonly accompanied with increased tactile sensitivity (allodynia) (Dworkin and Portenoy 1996;Rowbotham and Fields 1996;Fields et al. 1998;Dworkin 2002;Galer et al. 2002). The condition is considered the prototypical human chronic neuropathic condition, since such patients exhibit multiple peripheral and central signs of neuropathy: decreased innervation density of the epidermis by nociceptive afferents (Oaklander 2001); involvement of large somatic afferents in pain (Nurmikko et al. 1991); loss of cells in the involved dorsal root ganglion and of myelin and axons in the dorsal horn (Watson et al. 1988), as well as spinal cord and brainstem lesions on MRI (Haanpaa et al. 1998). Centrally, there is no clear information regarding brain elements involved in PHN pain. Evidence from animal models for neuropathic pain show peripheral and spinal cord re-organization see reviews (Woolf and Salter 2000;Hunt and Mantyh 2001;Inoue et al. 2004;Devor 2006). Similarly to PHN patients, they exhibit behavioral signs of spontaneous pain, which can be related to increased spontaneous activity of peripheral A- and C-nociceptors (Tal et al. 1999;Wu et al. 2001) and to abnormalities in the expression and trafficking of Na+ channels (Devor 2006).
Chronic pain and PHN in particular that can last from a few months to lifetime greatly diminishes the quality of life, and increases anxiety and depression (Dworkin 2002;Sah et al. 2003). Moreover, chronic back pain is now associated with specific cognitive (Apkarian et al. 2004a), brain chemical (Grachev et al. 2000) and morphologic abnormalities (Apkarian et al. 2004b), all of which are consistent with cortical changes reflecting maladaptive reorganization that may be due to living with chronic pain.
We recently showed that intensity of chronic pain fluctuates spontaneously: PHN patients instructed to indicate their pain intensity on a continuous scale comply readily, and exhibit fluctuations of spontaneous pain with unique properties (Foss et al. 2006). Here we utilize such ratings of fluctuations of spontaneous pain of PHN, performed during fMRI scans, to identify associated brain activity. Moreover, since lidocaine patch (Lidoderm) has been shown to be effective in decreasing PHN pain (Rowbotham et al. 1996;Galer et al. 1999;Galer et al. 2002;Gammaitoni et al. 2003) through the blockade of local Na-channels on peripheral afferents (Persaud and Strichartz 2002;Chevrier et al. 2004), we use the latter as an additional parameter with which we can define brain regions involved in PHN pain by demonstrating the central effects of this therapy. Given the minimal knowledge regarding brain circuitry underlying PHN pain and for spontaneous pain of chronic pain, our starting hypothesis was that it should be distinct from that of acute pain and more similar to other chronic pain conditions, like chronic back pain.
Methods
Patients and Screening Procedures
This study was approved by the Northwestern University Institutional Review Board, and written informed consent was obtained from all participants. Participants were recruited through local media announcements and from local pain clinics. Patients were included if they fulfilled the International Association for the Study of Pain (IASP) criteria for Post Herpetic Neuralgia (PHN) (Merskey and Bogduk 1994). All participants reported a history of shingles, including a sudden episode of rash accompanied with severe pain and sensitivity to touch in the area affected, with at least one visit to a physician where diagnosis of acute infection with varicella-zoster virus was made. All participants reported also a history of persistent pain for at least 3 months after the resolution of the acute shingles episode, with a pain intensity of at least 3 over 10 on a Visual Analog Scale (VAS) (Jung et al. 2004). Patients were tested for the presence of allodynia to brush during the interview, by gently passing a piece of foam over the painful area. The presence of allodynia was asserted if the patient reported an increase of the pain by at least 1 unit above the baseline on VAS scale. Participants were instructed that they can continue using their medications, but cannot change doses for the duration of the study (2 weeks).
Fourteen PHN patients were entered into the study. Only 11 were analyzed: 10 females and one male, ranging in age from 46 to 85 (mean age 67.8). Of the three that were excluded, one dropped out from the study, one had faulty registration of his functional brain scans, and the third was excluded because of excessive head motion. Demographics and clinical characteristics of pain are summarized for the participants in stable 1. Some of these parameters are derived from short-form of McGill Pain Questionnaire (Melzack 1987). Outcomes on this questionnaire did not correlate with Lidoderm treatment effects; as a result its relationship to brain activity was not studied.
Experimental Design and Pain Rating
Patients were asked to report the fluctuations of their spontaneous pain (in the absence of an external stimulus) using a finger-span logging device, task sp (Apkarian et al. 2001a;Foss et al. 2006). In the pre-scan session, patients learned how to use the device to continuously report changes in their spontaneous PHN pain intensity while observing their rating projected on a computer screen as a moving bar (size of the bar is a scale from 0 to 10; 0 = no pain; 10 = worst imaginable pain), or to perform a visual control task (visual control = v) where they rated the length of a bar moving at a rate approximating the variability of spontaneous PHN pain. Only patients who were able to rate properly the fluctuations of the bar during the visual task (v) with the finger-span device were entered into the study (subjects had to score a correlation r-value > + 0.70 between the input and their rating, in two attempts). During fMRI brain imaging, in separate scans patients rated either their spontaneous pain, sp, or performed the visual control task, v. During brain imaging the finger device was synchronized and time locked with the fMRI image acquisition sequence. An example of pain rating taken from one of the participants is shown in Fig.1A.
Figure 1. Characteristics of PHN pain and modulation by Lidoderm treatment.
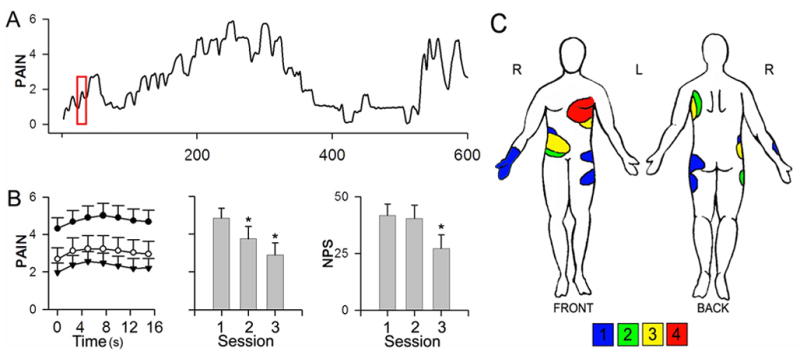
(A) An example rating of fluctuations of spontaneous pain of PHN, collected during a 10-minute fMRI scan (scale is 0 – 10, 0 = no pain, 10 = maximum imaginable pain). (B) Average of spontaneous pain ratings within a 17.5 s time window (red box in A indicates the window size relative to the total rating time) spanning transitions from mean pain to above mean pain ratings , for each session (filled circles = session1 before treatment; empty circles = session 2 after 6 hours of Lidoderm; session 3 = inverted triangles after 2 weeks of Lidoderm use (left), average spontaneous pain at each session (middle), and total score on Neuropathic Pain Scale (NPS) for the seven descriptors showing a treatment effect (right) in all patients (n=11) at each scan session (mean and S.E.M.-s are shown). There was a significant decrease in average fluctuations of spontaneous pain across the three time points (repeated measure ANOVA; F (2,8) = 109, p < 0.01) as well as in the NPS score (repeated measure ANOVA; F(2,20) = 11, p < 0.01). The asterisks indicate a significant decrease from session 1 (paired t-test; p <0.01). (C) Body regions where PHN pain was localized are shown for the 11 participants. Color code is number of patients overlapping with symptoms at indicated body region.
Patients were scanned at three different sessions to investigate the effect of treatment of the PHN condition with Lidoderm patches (topical 5% lidocaine) after acute and long-term use: brain scan session 1 was done at baseline before start of treatment; session 2 after 6 hours of use of the Lidoderm patch, and session 3 after 2 weeks of continuous use of the Lidoderm patch. The procedure consisted of asking the patients to wear the patches for 6 hours after finishing session 1 scans, and then to remove the patches and perform the second scanning session. Then the participant was instructed to wear the patches continuously during the day (12 hours) for two weeks and return and undergo the last, session 3, brain scan. A registered nurse instructed each patient on how to use the patch. The patients kept on using their entire physician prescribed medications, including those for PHN, with no change during the course of the study. At each session, Washington Neuropathic Pain Scale (NPS) (Galer and Jensen 1997), and short form of McGill Pain Questionnaire (MPQ) were used to determine sensory and negative-affective dimensions of PHN pain.
Functional Magnetic Resonance Data Acquisition
Functional MRI data was acquired with a 3T Siemens Trio whole-body scanner with echo-planar imaging (EPI) capability using the standard radio-frequency head coil. Multi-slice T2*-weighted echo-planar images were obtained with the following parameters: repetition time TR = 2.5 s, echo time TE = 30 ms, flip angle = 90°, slice thickness = 3 mm, in-plane resolution = 3.475 × 3.475 mm2. The 36 slices covered the whole brain, from cerebellum to the vertex. Two hundred and forty volumes were acquired per scan. At each scan session, a given patient performed first a maximum of 3 repetitions of the sp task, with one patient having one sp task scan only at session 2, and another two having no sp task scans at session 3, leaving us with a total of 47 sp task scans. After the sp task scans, each patient performed only one v task scan. We could not however obtain a v task scan at each session for all patients. This was due either to time limitation, to head motion during the scan, or to bad performance on the control task (r < 0.40, p < 0.0001). Thus, each participant had at least one such scan across the three sessions, with a total of 24 task v scans. A T1-weighted anatomical MRI image was also acquired for each subject using the following parameters: TR = 2.1 s, TE = 4.38 ms, flip angle = 8°, FOV = 220 mm, slice thickness = 1 mm, in-plane resolution = 0.86 × 0.86 mm2 and number of sagittal slices = 160.
fMRI data Analysis
Patients with high levels of pain invariably move during fMRI scans, and such motion degrades brain activity. To compensate for these artifacts we performed head motion correction in two steps. The first step consisted in standard correction for head motion using MCFLIRT (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl, (Jenkinson and Smith 2001;Jenkinson et al. 2002)). In the second step, the motion corrected data was again analyzed for head motion artifacts running Independent Component Analysis (see Supplementary Methods).
The following pre-processing was applied to the motion corrected data: slice-timing correction using Fourier-space time-series phase-shifting; non-brain removal using BET (Smith 2002); spatial smoothing using a Gaussian kernel of FWHM 5 mm; global (volumetric) multiplicative mean intensity renormalization; and registration to standard space using each subject’s high-resolution anatomic images as an intermediate step, using FLIRT (Jenkinson and Smith 2001;Jenkinson et al. 2002).
Five of our subjects had their lesion on the right side of their body. In order to investigate whether the side of the disease affected the group brain activity, the orientation of the raw data was flipped for those five subjects in a separate analysis, so that their activity came to be as if they had the lesion on the left side. The orientation of the data, which is radiological by default, was changed using a subroutine in FSL software (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). The rest of the analysis was performed as with the non-flipped data.
Spontaneous pain (sp) and visual (v) ratings were convolved with a canonical hemodynamic response function and used in a general linear model as the primary vectors of interest (FEAT Version 5.4). Two of our patients (patient 7 and 14 in STable 1) had minimal or no pain at session 3 and therefore, they did not report any pain fluctuations, making impossible the identification of BOLD events with their pain rating time series. Therefore, those scans were dropped from session 3. Given the fact that sp time series spanned a wide spectrum of frequency ranges (Foss et al. 2006), different high-pass temporal filters were used for each scan, so as to eliminate 15% of the low frequency range of the power spectrum. Hence, our high pass temporal filters ranged from 50s to 300s (see Supplementary Methods).
fMRI statistical Analysis
BOLD time series statistical analysis was carried out using FILM with local autocorrelation correction (Woolrich et al. 2004). Z-statistic images were thresholded at z > 2.3 and a (repeated-measures corrected) cluster significance threshold of P = 0.01 (Worsley et al. 1992). We also tested a higher threshold cutoff (cluster threshold P = 0.005) but this did not reveal any important changes in activity patterns. The following second level contrasts were computed sp1, sp2, sp3 and spall (average activity for sp for each session and across all treatment sessions) and v1, v2, v3 and vall (average activity for v for each session and across all treatment sessions), and were run with age and pain duration as covariates of no interest, thus removing the contribution of these parameters, which in fact did reduce variability of activations. We will report the effects of these parameters in a separate communication. At this analysis level, both increased as well as decreased activations were determined in separate contrasts. To specifically isolate brain activity for pain, third-level contrasts were performed, for each session and across all scans: (sp – v)1, (sp – v)2, (sp– v)3, and (sp –v)all. Only positive activity was determined for these contrasts, after masking each with the map generated for spall at a threshold of z > 0. This mask avoids false positive activations due to decreased activity in v, by limiting the outputs to only brain regions where spontaneous pain resulted in positive activity. Treatment effect was studied by a one-way ANOVA (using the model: 1, 0, −1, for session 1, 2 and 3 scans respectively) to determine brain regions with decreased activity with treatment. This test was performed on the higher-level contrasts, (sp – v), across treatment sessions masked with (sp-v)all at a threshold of z > 0. All higher-level contrasts are random effects analyses.
Multiple covariate analyses were performed. At second level analysis, spall and vall were covaried with mean pain intensity, calculated from the mean rating of pain in corresponding sp scans (together with age and pain duration as covariates of no interest). At third -level contrasts (sp-v)all, covariates with pain intensity, or with descriptors from NPS did not yield significant clusters.
We examined relationships between pain parameters and brain activity for specific regions of interest using linear correlation, for which parameter estimates (P.E., betas from general linear model) were extracted for a 1 cc (3x3x3 voxels) volume at specific coordinates of interest. Brain activity change across sessions was correlated to pain parameter changes with sessions.
Temporal BOLD signal Plots
The BOLD signal was extracted, using our own Matlab script, based on the pain rating sp time series. Epochs where pain was higher than the average of a given time series were identified using patients own pain ratings. At the transition from mean to higher pain ratings a fixed-size window, 17.5 s wide and spanning the upstroke (two TRs backwards and 5 TRs forwards; TR =2.5s; 17.5 s in total), was used to average across all such epochs. The width of this window was determined by the observation that the mode of the amount of time when the PHN pain is higher than average is one TR across all the sp runs, and by the need to show the slow return to baseline. The corresponding brain activity BOLD signal was averaged within each session for each subject, and averaged across subjects. Resultant BOLD signal is in arbitrary MR units. The same approach was also used on the pain ratings to delineate the time profile of the ratings in the same window as for determining BOLD responses.
Results
Influence of lidocaine treatment on pain
Treatment with Lidoderm patch led to a significant decrease in pain intensity (mean rating of fluctuations of spontaneous pain), and in the NPS score in 9 out of 11 patients, both after 6 hours (session 2) and after 2-weeks of treatment (session 3), a response rate similar to published clinical trials (Gammaitoni et al. 2003). Figure 1B (left panel) shows the group-average time course of pain-rating signal in absolute scale, mean pain ratings (mid panel) and NPS score (right panel) are also shown as a function of session. Average pain intensity for all patients at session 1 was 4.9 ± 0.5 (mean ± S.E.M.), 3.7 ± 0.7 at session 2, and 2.9 ± 0.6 at session 3 (F(2,8) = 109;P < 0.01; session 2 and session 3 mean pain is significantly less than in session 1, P < 0.01 for both; on a 0–10 scale). Moreover, 7 out of 10 pain descriptors in NPS (Galer and Jensen 1997) showed a significant improvement with treatment, except for dull, cold, and deep (STable 2). Total NPS score, summed over descriptors that showed treatment effect, across all patients decreased primarily with 2weeks of treatment: 41.7 ± 5.1 at session 1; 40.5 ± 5.7 at session 2; and 27.1± 6.2 at session 3 (F(2, 20) = 11 p < 0.01). The majority of our patients had their lesion on the trunk. The distribution of the affected areas is shown in Figure 1C.
Brain activity for sp and v tasks
Spontaneous PHN pain shows characteristic fluctuations over the 10-minutes span of the scanning run (Foss et al. 2006). One such example of rating taken from one patient is shown in Fig.1A. The brain activity identified for such ratings constitutes the main outcome for spontaneous PHN pain, sp; similarly rating the size of the bar presented visually, v, is the motor/cognitive/visual control for sp.
We show the activation pattern for both the rating of the spontaneous PHN pain (spall) (Figure 2A) and the visual rating (vall) (Figure 2B), after averaging across all sessions. Brain regions activated in the spall (see STable 3) can be grouped in three main clusters, and are reminiscent of the acute pain map in normal subjects (Apkarian et al. 2005), mainly, left thalamus, bilateral striatum, left secondary somatosensory cortex (SII), bilateral insula, left anterior cingulate cortex (ACC), right orbitofrontal cortex (OFC) (BA11), and cerebellum.
Figure 2. Brain activity maps for rating spontaneous pain of PHN, and the control visual task.
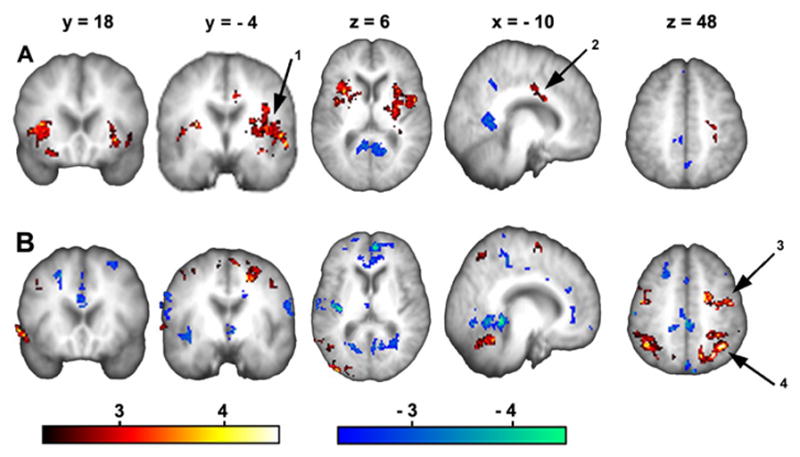
(A) Average brain activity across the three sessions (spall) shows significant increased brain activity in multiple brain areas. Arrow 1 indicates L SII/insula, arrow 2 points to ACC activity. (B) Average brain activity across all three sessions for the visual control task (vall). Arrow 3 indicates L PPC activity, and arrow 4 indicates activity in L SI/MI. Stables 3 and 4 provide the complete list of regions activated in A and B respectively. (For abbreviations see table 1)
In the visual control task, v, the patients attempt to mimic their own pain rating, with the difference that in v they estimate the magnitude for a visual stimulus instead of their own pain. Hence, the areas activated are mainly visuospatial attention areas such as the right visual cortex, bilateral posterior superior parietal cortices, right primary motor area (MI) and left primary motor (MI) and somatosensory area (SI), in addition to the cerebellum, the right mid frontal gyrus (BA9/46), and the right inferior temporal gyrus (TG) (See STable 4).
Average brain activity for the visual control task at each session increased from v1 to v2 to v3 (see Sfigure 1). A similar but smaller increase was also seen in the pain-rating task (Sfigure 2). For this reason we performed a correlation analysis for both tasks with mean spontaneous pain. We determined the modulation of brain activity for sp and v tasks with pain intensity by using mean pain intensity as a covariate. Figure 3 shows the influence of pain intensity on across-sessions averaged brain activity for tasks, spall and vall. The resultant map is generally similar for both tasks: activity in medial and lateral prefrontal regions was positively correlated, while posterior parietal attentional areas were negatively correlated with mean pain intensity. This result shows that brain activity for both tasks is influenced by the level of spontaneous pain, implying that the level of pain influences task performance in general. This idea is further corroborated by the observation that in the visual task there is a trend in improvement in rating ability with sessions (mean correlation between the visual input and the actual rating shows a borderline session dependence; mean r-value = 0.66, SD = 0.19; 1-way ANOVA comparing r-values across the three sessions, F2,22= 3.3, p < 0.06). This in turn reinforces the need for correcting brain activity by a control condition performed at the same pain level, that is the necessity of subtracting v from sp at each treatment session. Our finding indicates that the intensity of spontaneous pain impacts brain activity for any task that the subject attempts to perform. Therefore, the decreased brain activity reported for pain tasks in many clinical pain conditions (Derbyshire 1999;Peyron et al. 2000;Apkarian et al. 2005) is most likely a reflection of the presence of the spontaneous pain, and is not specific to the task being investigated.
Figure 3. Pain intensity has a large effect on brain activity for rating spontaneous pain of PHN, and for rating the visual control task.
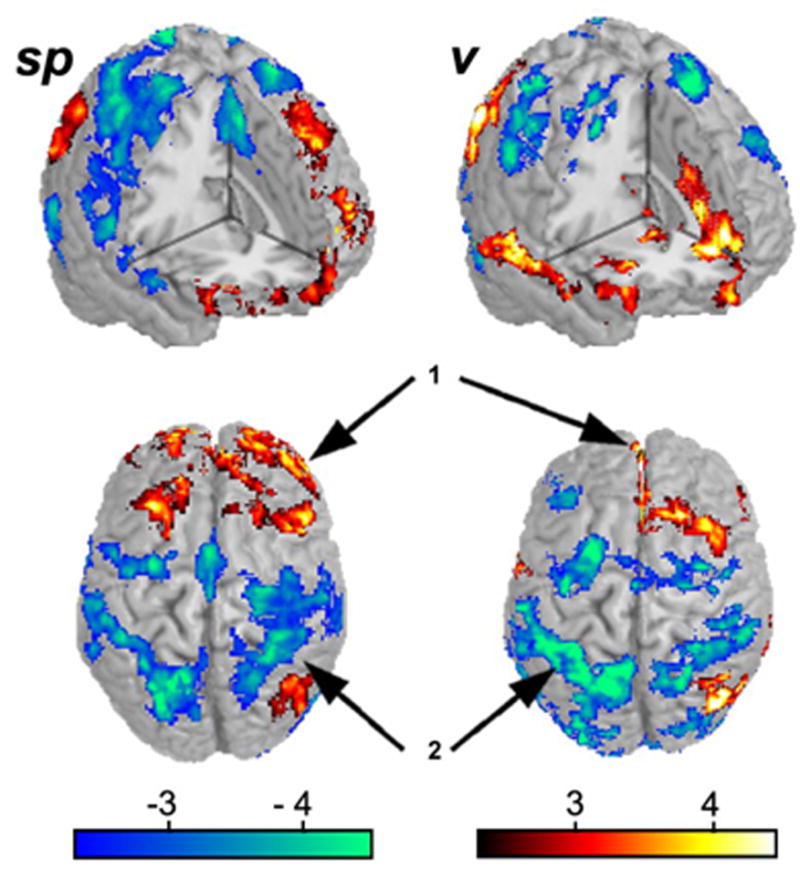
Brain activity for rating spontaneous pain (sp) or rating visual control (v) task were correlated with the average pain rating at the time of the scan. Generally, in both tasks similar regions were modulated with average spontaneous PHN pain: medial and lateral prefrontal areas were positively modulated (arrow 1), while posterior parietal attentional areas (arrow 2) were negatively modulated..
Brain activity for spontaneous pain of PHN
To isolate brain activity specifically for spontaneous pain of PHN we examine the contrast between sp and v (sp-v), for each session and for the average across all sessions. Resultant activations are shown in figure 4, and corresponding coordinates are in table 1. Overall, spontaneous PHN pain maps to areas involved in sensory-discriminative and, affective processing as well as hedonic experience, while treatment decreases this activity. In session 1, (sp-v)1 (Figure 4A), activity is seen in left thalamus and bilateral SII/insula (sensory areas); ACC (BA 24/32) (affective); and bilateral ventral striatum, amygdala, and OFC (BA 11) (hedonic); as well as in hypothalamus, left inferior temporal gyrus (BA 22), and left visual /precuneus cortices (BA17/7) within the occipitoparietal sulcus. Brain activity decreases markedly in session 2, (sp-v)2 (Figure 4B), where bilateral insula, right SII and right amygdala are still activated. In session3, (sp-v)3 (Figure 4C), only left SII, right amygdala and the cerebellum are active after 2 weeks of Lidoderm use. Across-sessions average brain activity for the spontaneous PHN pain, (sp-v)all (SFigure 3A), resulted in the same significantly active areas seen in session 1, except for the thalamus which was not activated for across-sessions average. Flipping the brain orientation in the 5 patients who had a right sided lesion does not change the map for the (sp-v)all , and still do not show activity in the thalamus (SFigure 3B). Statistical subtraction of the flipped average from the non-flipped one (map in sfigure 3B – map in sfigure 3A) does not yield any significant clusters.
Figure 4. Brain activity maps for rating spontaneous pain of PHN, after subtracting the control visual task.
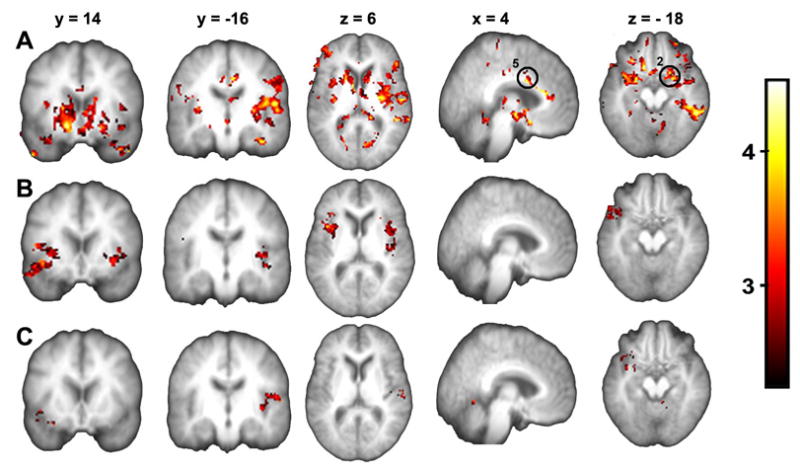
(A – C) Brain activity for spontaneous PHN pain vs. the visual control task (sp-v) for group averages for sessions 1–3, respectively. Note brain activations (only increases were determined) generally decrease from A to C. . Coordinates and activity probabilities are in Table 1. (Circled regions refer to Figure 6; 2 = L Amygdala ; 5 = ACC)
Table 1.
Brain regions activated for spontaneous pain of PHN, sp - v contrast, within and across sessions
| Region | Coordinates† | Z-value | Region | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (Sp-v)all | X | Y | Z | (Sp-v)1 | (Sp-v)2 | (Sp-v)3 | (Sp-v)all | (Sp-v)all flipped | Index ‡ | |
| L OFC (11) | −24 | 50 | −12 | 3.13 | NS | NS | 3.28 | 3.51 | + Mid FG(10) | 1 |
| L Vis/Precun (17/7) | −16 | −72 | 28 | 3.57 | NS | NS | 3.21 | NS | 2 | |
| R Mid Frontal G (10) | 44 | 48 | 8 | 3.37 | NS | NS | 3.81 | 3.65 | L Mid FG (10) | 3 |
| Mid ACC (32) | −2 | 8 | 40 | 2.66 | NS | NS | 3.59 | 3.95 | same | 4 |
| Rostral ACC (9/32) | −4 | 24 | 20 | 3.90 | NS | NS | 3.84 | 2.95 | same | 4 |
| L Inf Temporal G (37) | −54 | −44 | −18 | 4.25 | NS | NS | 3.84 | 3.6 | same | 5 |
| L Cerebellum | −20 | −56 | −26 | 2.77 | NS | 3.76 | 4.00 | 3.5 | same | 6 |
| L Insula | −42 | 4 | 0 | 2.90 | 2.80 | NS | 2.80 | 4.08 | same | 7 |
| R Insula | 38 | 2 | 6 | 2.93 | 2.47 | NS | 3.61 | 3.03 | same | 7 |
| L Amygdala | −24 | 2 | −18 | 3.97 | NS | NS | 3.71 | 2.47 | same | 7 |
| R Amygdala | 22 | 2 | −18 | 3.95 | 2.70 | 3.11 | 2.78 | 2.55 | same | 7 |
| Hypothalamus | 2 | −8 | −8 | 3.83 | NS | NS | 2.97 | NS | 7 | |
| L V Striatum | −12 | 20 | 6 | 2.72 | NS | NS | 3.10 | 2.81 | same | 7 |
| R V Striatum | 16 | 14 | −12 | 4.13 | 2.44 | NS | 4.16 | 3.08 | same | 7 |
| L SII | −42 | −20 | 16 | 3.95 | NS | 2.82 | 3.99 | 3.10 | same | 7 |
| R SII | 52 | −18 | 20 | 2.41 | 2.85 | NS | 2.86 | 2.76 | same | 7 |
| R OFC (11) | 28 | 48 | −12 | 3.15 | NS | NS | 3.27 | NS | 7 | |
Activated region names and coordinates are shown for (sp – v)all. Only peak z-values in each brain region are shown for each treatment session. For the flipped data, (Sp-v)all flipped, z-values and brain regions are shown; regions with no activity are left blank. The (Sp-v)all contrast contained 13 clusters: cluster 1, 102 voxels, p < 0.01; cluster 2, 110 voxels, p < 0.01; cluster 3, 151 voxels, p <10−3; cluster 4, 281 voxels, p < 10−6; cluster 5, 282 voxels, p < 10−6; cluster 6, 673 voxels, p < 10−12; cluster 7, 5588 voxels, p < 10−12.
Abbreviations: L = left; R = right; Inf = inferior; G = gyrus; V =ventral; Mid = middle; m = medial; ACC = anterior cingulated cortex; BS = brainstem; Cun = cuneus; DLPFC = dorsolateral prefrontal cortex; MI = primary motor cortex; OFC = orbitofrontal cortex; PFC = prefrontal cortex; PPC = posterior parietal cortex; Precun = precuneus; SI = primary somatosensory cortex; SII = secondary somatosensory cortex; SMA = supplementary motor cortex. Numbers in parenthesis = Brodmann areas.
= Coordinates taken from peak voxels of (Sp-v)all contrast.
= Cluster index for (Sp-v)all
Effect of Lidoderm treatment on brain activity
The above results demonstrate that brain activity for spontaneous PHN pain is decreasing with treatment. However, the analysis does not indicate which areas are statistically significantly modulated with treatment. We tested the latter by modeling treatment effect as a monotonic decrease with sessions. The hypothesis is based on the decrease in intensity of spontaneous pain in sessions 2 and 3, the decrease in NPS in session 3, and on the assumption that continued Lidoderm use will result in further decrease in brain activity in regions involved in pain. This model was tested only for brain regions identified to be positively active for spontaneous PHN pain across sessions (that is areas where (sp - v)all z-values > 0), using a one-way ANOVA statistical analysis. Figure 5 and table 2 summarize this result. Regions decreasing in activity with treatment included: left thalamus, hypothalamus, left SI/MI (BA3/4), left SII/insula, bilateral ventral striatum, and precuneus (BA7), and right OFC (BA11). The right inferior frontal gyrus/anterior insula (BA 47) was the only region showing increased activity with treatment.
Figure 5. Treatment effect on brain activity for spontaneous pain of PHN.
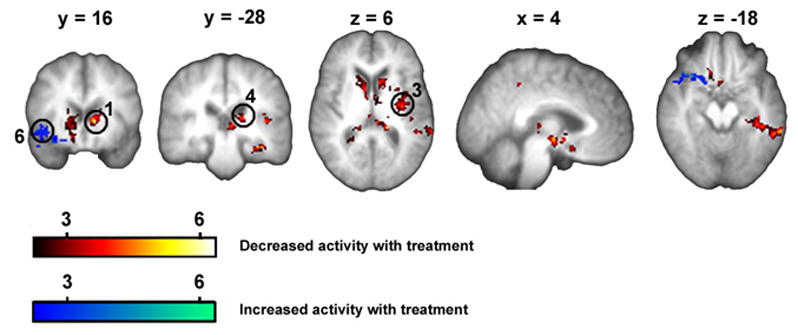
Lidoderm effect on brain activity was modeled as a monotonic decrease with treatment sessions. This model was tested for brain regions with positive activity in the contrast of spontaneous pain ratings vs. visual ratings, over all three sessions (one-way ANOVA for treatment model masked with (sp - v)all > 0; n = 11 patients). Coordinates and activity probabilities are in table 2. (Circled regions refer to Figure 6; 1 = L ventral striatum; 3 = L mid insula; 4 = L posterior thalamus 6 = R anterior insula)
Table 2.
Brain regions modulated with treatment
| Region | X | Y | Z | Z-value | Index |
|---|---|---|---|---|---|
| Contrast 1. Regions showing decreased activity | |||||
| R Parahipocampal G (30/35) | 24 | − 40 | 0 | 5.56 | 1 |
| L Fusiform G (20) | − 34 | − 28 | − 20 | 5.06 | 2 |
| L V Striatum | −12 | 16 | 6 | 3.97 | 3 |
| L Thalamus | −16 | −28 | 12 | 4.1 | 3 |
| V Tegmentum | 0 | −10 | − 10 | 3.65 | 4 |
| R V Striatum | 20 | 22 | 0 | 4.8 | 4 |
| Hypothalamus | 4 | − 8 | − 6 | 4.77 | 4 |
| L Sup Temporal G (22) | − 52 | − 42 | 16 | 5.61 | 5 |
| L MI/SI (3/4) | −62 | − 12 | 40 | 5.13 | 5 |
| L SII | −44 | − 20 | 20 | 4.17 | 5 |
| L Insula | − 40 | − 8 | 8 | 4.6 | 5 |
| Contrast 2. Region showing increased activity | |||||
| R Inf frontal G/Insula (47) | 38 | 20 | −18 | 4.13 | 1 |
Index is cluster index. Contrast 1 contained 5 clusters: cluster 1, 243 voxels; p < 10 3, cluster 2, 404 voxels; p < 10−4; cluster 3, 572 voxels, p < 10−6; cluster 4, 652 voxels, p < 10−6; cluster 5, 1167 voxels, p < 10−11; Contrast 2 contained one cluster with 464 voxels, 10−5. For abbreviations see table 4.
We investigated the treatment effect further by examining activity patterns for specific brain regions of interest derived from across-session average activity map (Figure 4A) and from the treatment map (Figure 5). Extracted activities were related to treatment sessions, and to pain intensity and NPS descriptors (Figure 6, Table 3). Table 3 lists correlations between regional brain activities on the one hand, and mean ratings of spontaneous PHN pain (pain intensity) or the 7 NPS descriptors modulated with treatment on the other hand. The correlations were calculated for across session changes in these parameters. All regions investigated are listed. All three anterior cingulate regions examined show a negative correlation; insular regions show both positive and negative correlations; while left amygdala, and especially left and right ventral striatum are positively correlated with change in pain parameters (Table 3).
Figure 6. Characteristics of brain regional activity in relation to treatment response.
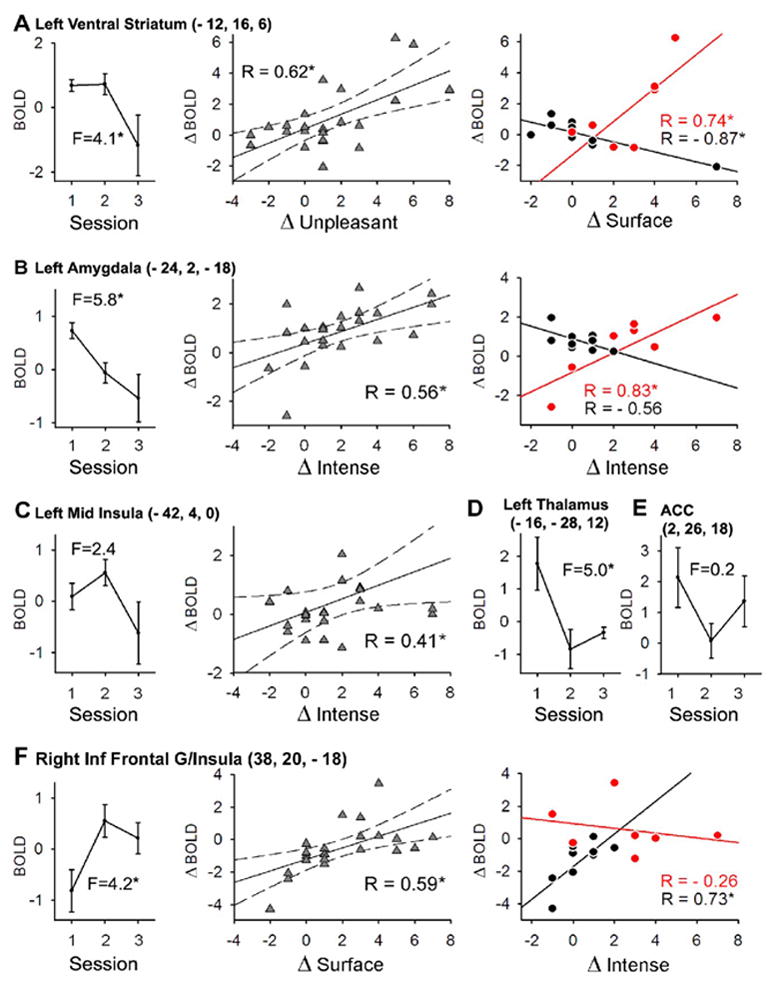
Six brain regions of interest extracted from either the treatment analysis (Figure 5) or from mean activity for spontaneous pain (Figure 4A) are shown in relation to treatment (one-way ANOVA across the three treatment sessions; n = 9 patients; all F-values have 2 and 22 d.f.; * = p < 0.05) and to various descriptors from the Neuropathic Pain Scale (see table 3) (A) Activity in striatum (circled as 1 in figure 5; LVS in Table 3) is significantly modulated by treatment. The change in activity across sessions (ΔBOLD) is significantly positively correlated with change in unpleasantness (ΔUnpleasant) between sessions (second panel). Last panel shows the correlation between ΔBOLD and ΔSurface (another NPS descriptor), between sessions 1 and 2 (acute treatment, black), and between sessions 2 and 3 (long-term treatment, red). The change in correlation coefficient is significant. (B) The same analysis as in A for amygdala (circled as 2 in figure 4A; L Amyg in Table 3). Note that the cluster did not reach significance in whole brain statistical analysis (Figure 5), but there is a significant effect of treatment. The ΔBOLD is significantly positively correlated with ΔIntense (second panel). This correlation is not significant after acute treatment (black; last panel), but changes significantly at session 3 after 2-weeks of treatment (red; last panel). (C) Left mid insula (circled as 3 in Figure 5; L Ins3 in Table 3) shows no overall treatment effect, but activity decreases most at session 3. The ΔBOLD is significantly correlated with ΔIntense, across sessions. (D) Treatment effect was also observed in left thalamus (circled 4 in Figure 5; L Tha in Table 3); there was no significant correlation however between across session activity change and NPS descriptors. (E) No overall treatment effect was observed in ACC (BA 24) (circled as 5 in Figure 4A; ACC3 in Table 3). However, both left thalamus and ACC do show a significant decrease in activity with acute treatment. (F) Activity in right anterior insula (indicated by number 6 on Fig.5; R insula/Inf Frontal G in Table 3) increases significantly with treatment, and ΔBOLD is significantly correlated to Δsurface. The correlation of ΔBOLD to ΔIntense shows an opposite pattern than that in ventral striatum and amygdala (last panels in A and B) after acute vs. long-term treatment (positive correlation for acute treatment, black, and negative correlation with long-term treatment, red). Correlations with significant r = *, p < 0.05.
Table 3.
Correlation matrix for change in regional BOLD and change in pain intensity and 7 NPS descriptors across sessions
| ACC1 | ACC2 | ACC3 | L Ins1 | L Ins2 | L Ins3 | R Ins | R OFC | L VS | R VS | L Amyg | R Amyg | L Tha | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain Intensity | −0.43* | −0.56* | −0.45* | 0.52* | 0.03 | 0.39 | −0.06 | 0.07 | 0.28 | 0.40* | −0.08 | 0.09 | 0.08 |
| Intense | −0.12 | −0.16 | −0.31 | 0.22 | −0.31 | 0.41* | 0.23 | 0.03 | 0.55* | 0.62* | 0.56* | 0.13 | 0.13 |
| Sharp | −0.13 | −0.29 | −0.32 | 0.07 | −0.31 | 0.28 | 0.12 | −0.01 | 0.48* | 0.53* | 0.41* | −0.01 | 0.16 |
| Hot | 0.14 | −0.10 | −0.21 | −0.10 | −0.47* | 0.20 | 0.18 | −0.02 | 0.15 | 0.46* | 0.54* | −0.28 | −0.07 |
| Sensitive | −0.40* | −0.34 | −0.33 | 0.39 | −0.03 | 0.32 | 0.27 | 0.03 | 0.41* | 0.42* | 0.16 | 0.12 | 0.02 |
| Itch | 0.21 | 0.02 | 0.24 | 0.07 | 0.07 | −0.16 | −0.37 | 0.31 | −0.11 | −0.38 | 0.22 | −0.20 | 0.2 |
| Unpleasant | −0.22 | −0.30 | −0.49* | 0.12 | −0.35 | 0.54* | 0.34 | −0.06 | 0.62* | 0.70* | 0.36 | 0.01 | 0.08 |
| Surface | −0.45* | −0.31 | −0.51* | 0.38 | −0.26 | 0.51* | 0.59* | −0.03 | 0.42* | 0.65* | 0.16 | 0.21 | −0.13 |
Correlations are calculated for change across session 1 and 2, session 1 and 3, and session 2 and 3 in BOLD and in each parameter (n = 9). Pain Intensity is mean rating of spontaneous PHN pain. Subscripts for each brain area are the specific regions investigated, with coordinates: ACC1 (− 4, 6, 32); ACC2 (−2, 22, 20); ACC3 (2, 26, 18); L Ins1 (− 38, − 2, 0); L Ins2 (− 46, − 16, 12); L Ins3 (− 42, 4, 0); R Ins = R insula/inferior Frontal gyrus (38, 20, − 18), R OFC (14, 22, − 18); L VS (− 12, 16, 6); R VS (20,10, 6); L Amyg (− 24, 2, − 18); R Amyg (22, 2, −18); L Tha (− 16; − 28; 12); Amyg = amygdala; ACC = anterior cingulated cortex; Ins = insula; OFC = orbitofrontal cortex; Significant correlations = *, p <0.05 uncorrected. Bonferroni correction for repeated measures renders only r-values > 0.6 significant, observed only in VS. Repeat-measure correction is not necessary since most regions examined already show whole-brain repeat-measure corrected treatment effects.
Regional activity in left ventral striatum, left amygdala, and the left thalamus again show a significant decrease with treatment; where the striatal response occurs only during long-term treatment, thalamic response only during short-term treatment, while amygdala response during both short and long-term treatments (Figure 6A and B first panels, and Figure 6D). No treatment effect is seen in the left mid insula (Figure 6C, first panel), and a significant decrease is only seen for acute treatment in the ACC (Figure 6E). Similarly to ACC and left thalamus, left SI and left SII regional activity decreased only with acute treatment (data not shown). On the other hand, the right inferior frontal gyrus/anterior insula shows a significant increase in activity with treatment, but only with acute treatment (Figure 6F, first panel). The change in regional activity in relation to the change in NPS descriptors as a function of treatment sessions is also shown in Figure 6. The correlation coefficients change significantly between acute and long-term treatment for left ventral striatum (for surface descriptor), left amygdala (for intense descriptor), and right inferior frontal gyrus/anterior insula (for intense descriptor) (Figures 6A, B, and F, last panels). There is an important difference in these changes between the first two brain regions in comparison to right inferior frontal gyrus/anterior insula. In striatum and amygdala regions, pain descriptor to activity relationship switches from a negative correlation to a positive one, while in the right inferior frontal gyrus/anterior insula the opposite pattern is seen, with 6-hour in contrast to 2-week Lidocaine treatment, indicating that these areas are differentially modulated by acute and long-term treatment. Left thalamus, SI, SII, OFC, and ACC regions examined did not correlate or only negatively correlated with pain parameters.
We did examine the temporal properties of BOLD in a number of regions. Only the temporal pattern of left ventral striatum BOLD decreased with treatment (Figure 7, right). In comparison, the magnitude of change in spontaneous pain fluctuations (Figure 7, left) shows a systematic, but non-significant, decrease with treatment. Therefore, the BOLD signal decrease is more likely a reflection of the change in absolute value magnitude in spontaneous pain rather than in the temporal pattern of the change in spontaneous pain.
Figure 7. Temporal profile for pain ratings and for BOLD signal in ventral striatum.
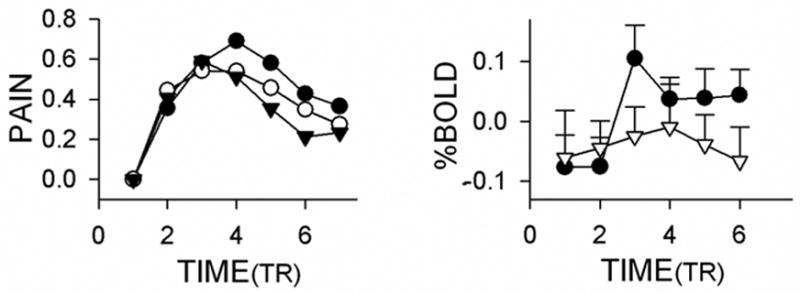
Left panel shows the average change in pain ratings from baseline (same data as in Figure 1B, left panel, after removing baselines, expressed in TR timescale rather than in seconds), across patients and in session 1 (filled circles), session 2 (empty circles) and session 3 (inverted triangles). Overall, the pain ratings show a small, statistically non-significant, decrease in magnitude and duration. Right panel shows the percentage change in BOLD signal in the left ventral striatum (from Figure 6A), extracted for the same time windows as in left panel. This is the only brain region where we can identify a decrease in BOLD temporal pattern with treatment; before treatment (filled circles) to after treatment (inverted triangles, sessions 2 and 3, bars are S.E.M.). Time is in TR = 2.5s.
Discussion
The results show that by relating fluctuations of spontaneous pain to fMRI, and using a proper control task, we can identify brain regions involved in spontaneous PHN pain, which maps to sensory (thalamus, SI, SII, insula), affective (anterior cingulate, insula), as well as hedonic regions (ventral striatum, amygdala, ventral tegmentum, and orbital frontal cortex). We use responses to Lidoderm therapy and correlations with changes in neuropathic pain scale to functionally subdivide the identified brain areas. It should be noted that the lack of a placebo group obviates making specific statements as to the mechanisms by which Lidoderm is modulating pain perception and related brain activity. Only a subset of the areas involved in PHN pain respond to Lidoderm therapy, which in turn can be subdivided to regions that respond to short term therapy, and others that respond to longer-term therapy. The acute responses to therapy are observed mainly in sensory and affective regions, while longer-term responses are observed in brain areas implicated more in hedonic and emotional processing. Bilateral ventral striatum seems the region that most tightly reflects the spontaneous pain of PHN, since it is highly activated in the baseline session, decreases in activity with treatment, and the change in activity across sessions is correlated to almost all NPS descriptors that decreased with treatment.
This is the first study documenting brain activity for spontaneous pain in PHN patients. There are very few studies regarding brain activity for human neuropathic pain conditions, the majority of these examine responses to external stimuli (Hsieh et al. 1995;Hsieh et al. 1999;Apkarian et al. 2001b;Gracely et al. 2002;Giesecke et al. 2004;Peyron et al. 2004;Maihofner et al. 2006), and in many of these cases perception or stimulus conditions are not matched between contrasts, which complicates interpretation of obtained results. Therefore, our study is comparable only to few others (Hsieh et al. 1995;Hsieh et al. 1999) where brain activity for ongoing pain was contrasted before and after a therapeutic manipulation. However, the lack of an internal control in these studies confounds the results, the necessity of which is illustrated in figure 3.
The methodology used here involved online ratings of perception. A similar approach has been used to identify brain activity abnormalities in bowel disorder patients with chronic pain (Kwan et al. 2005) . We have used this approach for assessing stimulus-evoked pain and spontaneous pain in various chronic pain groups and in healthy subjects (Baliki et al. 2004;Baliki et al. 2005). In healthy subjects and using thermal painful stimuli (Baliki et al. 2004), we observe that ratings of perceived pain activate brain regions commonly seen during acute pain (Apkarian et al. 2005). Therefore, we can state that the activations observed in the current study for PHN spontaneous pain are not a reflection of the method.
Many of the brain regions observed involved in spontaneous pain of PHN are areas commonly seen for acute pain in normal subjects, especially thalamus, SI, SII, parts of insula, and anterior cingulate regions (Apkarian et al. 2005). Thalamus, SI, SII, and parts of insula, code sensory-discriminative components of acute pain both in intensity and in somatotopy (for example see, (Derbyshire et al. 1997;Davis et al. 1998;Coghill et al. 1999;Casey et al. 2001;Buchel et al. 2002), and (Bingel et al. 2003;Bingel et al. 2004;Brooks et al. 2005;Henderson et al. 2006)). Of these areas, left thalamus, SI and SII decreased in activity with 6-hour Lidoderm treatment but not with longer-term treatment, and were not related to NPS pain descriptors. The lack of relationship between NPS descriptors and these activations is consistent with the observation that NPS measures were not affected with short-term therapy. The anterior cingulate activity had a similar response pattern. Mid-anterior cingulate activity, at a site closely approximating the brain region specifically shown to be modulated by pain unpleasantness rather than pain intensity for acute pain (Rainville et al. 1997;Hofbauer et al. 2001), was in fact negatively correlated with changes in mean spontaneous PHN pain intensity, and was not correlated with changes in NPS descriptors as a result of treatment. Thus, although these regions are active during spontaneous PHN pain and decrease with short-term treatment, given the lack of a positive relationship with reported pain, we cannot assert that they are directly involved in the reported PHN pain. In contrast, some insular regions are positively correlated with mean pain, while other areas are positively correlated to various NPS descriptors and also decrease in activity during long-term treatment. Thus, these insular regions satisfy all the conditions to be involved in spontaneous PHN pain. Generally the lack of involvement of thalamus, SI, and SII in the long-term treatment is consistent with earlier observations of decreased cortical activity in clinical pain conditions in contrast to acute pain, as has been pointed in systematic reviews of the literature (Derbyshire 1999;Peyron et al. 2000;Apkarian et al. 2005).
Multiple insular areas are activated for acute pain in contrast to non-painful touch (Price 2000;Apkarian et al. 2005), in anticipation of pain (Ploghaus et al. 1999), pain empathy (Singer et al. 2004;Saarela et al. 2006), unpleasantness of pain (Schreckenberger et al. 2005), insular lesions can lead to pain neglect-like behavior (Berthier et al. 1988), and stimulation within insula evokes painful experiences (Ostrowsky et al. 2002). Thus, insula is regarded as critically involved in encoding sensory as well as affective components of acute pain. The role of the insula in spontaneous PHN pain seems complex and heterogeneous with distinct portions showing opposite responses to treatment. This is not peculiar to chronic pain, since a similar observation has been made in acute pain in normal subjects as the pain intensity was hypnotically manipulated (Hofbauer et al. 2001). The increased activity in right anterior insula suggests that gustatory processing in PHN patients should be changing with Lidoderm treatment, paralleling enhanced gustatory processing observed in chronic back pain (Small and Apkarian 2006). It has been suggested that insula responds to ‘interoceptive’ states (Craig 2002;Critchley et al. 2004). However, other recent studies suggest that anterior insula may be involved in more general cognitive processing (Kong et al. 2005;Dosenbach et al. 2006). Similarly to the insula, mid and rostral anterior cingulate has also been implicated in other function besides pain: activated for motivational drive, reward, gain or loss, conflict-monitoring or error prediction, and attentional changes or anticipation (Barch et al. 2001;Ridderinkhof et al. 2004), as well as in pain empathy (Singer et al. 2004;Saarela et al. 2006). Thus, relative changes along these dimensions between short and long-term therapy may commonly explain the distinct responses of both anterior cingulate and parts of insula..
Orbital frontal cortex, amygdala, ventral tegmentum, and ventral striatum were active during baseline spontaneous pain of PHN, and decreased with treatment; most importantly, bilateral ventral striatum reflected changes in almost all NPS descriptors that decreased with treatment. These regions together are part of the reward, addiction, hedonic and emotional circuitry of the brain (Schultz et al. 2000;Volkow and Fowler 2000;Kringelbach 2005). They are only rarely observed activated in acute pain and only in the early phase for painful thermal stimuli in normal subjects (Becerra et al. 2001). The role of this circuitry in pleasure and rewarding conditions has been extensively documented, yet little is known regarding its role in aversive conditions (Jensen et al. 2003;Seymour et al. 2005), and the present study and our study in chronic back pain (Baliki et al. 2004) are the first to directly implicate this ‘reward’ circuitry in chronic clinical pain states. Sustained high pain for spontaneous chronic back pain involves a unique activation of this reward circuitry, with the exception that prefrontal activity seems more prominent and localized more superiorly within the medial prefrontal cortex (Baliki et al. 2004). The similarity of involvement of reward circuitry between the two chronic pain conditions is striking, while the differences need further studies. The ventral striatum has access to representations of reward and thereby processes information regarding motivational control of goal-directed behavior (Schultz et al. 2000), and in a conditioned electrical shock paradigm it exhibits an anticipatory response to the aversive stimulus (Jensen et al. 2003). More relevant to the present study are observations of the responses of components of reward circuitry in a Pavlovian learned pain relief from a tonic painful condition, where various components exhibit either reward or aversion prediction activity, and both signals appear co-expressed in ventral striatum (Seymour et al. 2005). In spontaneous pain of PHN, we presume that the involvement of reward circuitry implies that the condition is more emotional than acute pain and induces decreased motivated behavior, as commonly observed in chronic pain patients.
A recent anatomical study shows that the ventral striatum and amygdala receive direct nociceptive projections from non-peptidergic IB4 neurons terminating in spinal cord lamina II (Braz et al. 2005). In contrast, the spinothalamic pathways, composed mainly of SP/CGRP peptidergic afferents, is the pathway thought to be mediating nociceptive inputs to lateral thalamus, SI, SII, and parts of insula. The results of the present study suggest that Lidoderm therapy affects the latter pathway with short-term treatment and the IB4-pathway with longer-term treatment, and only by affecting the IB4-pathway related responses does it give rise to perceptually detectable decrease in spontaneous PHN pain. Therefore, at least for spontaneous pain and in PHN and chronic back pain there seems to be a shift in the brain pain-related circuitry, away from sensory-representational cortical areas, favoring instead hedonic/reward sub-cortical areas, making the condition perhaps more sub-conscious and impacting on motivational drives.
Supplementary Material
Acknowledgments
The study was funded by Endo Pharmaceuticals and by NIH NINDS 35115. We thank Marivi Centeno and Rami Jabakhanji for technical assistance.
Footnotes
Note Supplementary Material is provided.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Krauss BR, Fredrickson BE, Szeverenyi NM. Imaging the pain of low back pain: functional magnetic resonance imaging in combination with monitoring subjective pain perception allows the study of clinical pain states. Neurosci Lett. 2001a;299:57–60. doi: 10.1016/s0304-3940(01)01504-x. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden R, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004a;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RE, Harden R, Parrish T, Gitelman D. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004b;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Thomas PS, Krauss BR, Szeverenyi NM. Prefrontal cortical hyperactivity in patients with sympathetically mediated chronic pain. Neurosci Lett. 2001b;311:193–197. doi: 10.1016/s0304-3940(01)02122-x. [DOI] [PubMed] [Google Scholar]
- Baliki M, Chialvo DR, Levy RE, Harden R, Parrish T, Apkarian AV. Chronic pain captures the emotional mind: Unique spatiotemporal brain activity pattern associated with spontaneous fluctuations of chronic back pain. Neuron. 2006 doi: 10.1523/JNEUROSCI.3576-06.2006. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M, Katz J, Chialvo DR, Apkarian AV. Single subject pharmacological-MRI (phMRI) study: Modulation of brain activity of psoriatic arthritis pain by cyclooxygenase-2 inhibitor. Mol Pain. 2005 doi: 10.1186/1744-8069-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 2001;11:837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Tensorial extensions of independent component analysis for multisubject FMRI analysis. Neuroimage. 2005;25:294–311. doi: 10.1016/j.neuroimage.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Berthier M, Starkstein S, Leiguarda R. Asymbolia for pain: a sensory-limbic disconnection syndrome. Ann Neurol. 1988;24:41–49. doi: 10.1002/ana.410240109. [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Glauche V, Knab R, Glascher J, Weiller C, Buchel C. Somatotopic organization of human somatosensory cortices for pain: a single trial fMRI study. Neuroimage. 2004;23:224–232. doi: 10.1016/j.neuroimage.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Single trial fMRI reveals significant contralateral bias in responses to laser pain within thalamus and somatosensory cortices. Neuroimage. 2003;18:740–748. doi: 10.1016/s1053-8119(02)00033-2. [DOI] [PubMed] [Google Scholar]
- Bowsher D. The lifetime occurrence of Herpes zoster and prevalence of post-herpetic neuralgia: A retrospective survey in an elderly population. Eur J Pain. 1999;3:335–342. doi: 10.1053/eujp.1999.0139. [DOI] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201–209. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Buchel C, Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: a parametric single-trial laser functional magnetic resonance imaging study. J Neurosci. 2002;22:970–976. doi: 10.1523/JNEUROSCI.22-03-00970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KL, Morrow TJ, Lorenz J, Minoshima S. Temporal and spatial dynamics of human forebrain activity during heat pain: analysis by positron emission tomography. J Neurophysiol. 2001;85:951–959. doi: 10.1152/jn.2001.85.2.951. [DOI] [PubMed] [Google Scholar]
- Chevrier P, Vijayaragavan K, Chahine M. Differential modulation of Nav1.7 and Nav1.8 peripheral nerve sodium channels by the local anesthetic lidocaine. Br J Pharmacol. 2004;142:576–584. doi: 10.1038/sj.bjp.0705796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauw DJ, Williams D, Lauerman W, Dahlman M, Aslami A, Nachemson AL, Kobrine AI, Wiesel SW. Pain sensitivity as a correlate of clinical status in individuals with chronic low back pain. Spine. 1999;24:2035–2041. doi: 10.1097/00007632-199910010-00013. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol. 1998;80:1533–1546. doi: 10.1152/jn.1998.80.3.1533. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW. Meta-Analysis of Thirty-Four Independent Samples Studied Using PET Reveals a Significantly Attenuated Central Response to Noxious Stimulation in Clinical Pain Patients. Curr Rev Pain. 1999;3:265–280. doi: 10.1007/s11916-999-0044-7. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006;7:S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18:343–349. doi: 10.1097/00002508-200211000-00001. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Portenoy RK. Pain and its persistence in herpes zoster. Pain. 1996;67:241–251. doi: 10.1016/0304-3959(96)03122-3. [DOI] [PubMed] [Google Scholar]
- Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis. 1998;5:209–227. doi: 10.1006/nbdi.1998.0204. [DOI] [PubMed] [Google Scholar]
- Foss JM, Apkarian AV, Chialvo DR. Dynamics of pain: fractal dimension of temporal variability of spontaneous pain differentiates between pain States. J Neurophysiol. 2006;95:730–736. doi: 10.1152/jn.00768.2005. [DOI] [PubMed] [Google Scholar]
- Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology. 1997;48:332–338. doi: 10.1212/wnl.48.2.332. [DOI] [PubMed] [Google Scholar]
- Galer BS, Jensen MP, Ma T, Davies PS, Rowbotham MC. The lidocaine patch 5% effectively treats all neuropathic pain qualities: results of a randomized, double-blind, vehicle-controlled, 3-week efficacy study with use of the neuropathic pain scale. Clin J Pain. 2002;18:297–301. doi: 10.1097/00002508-200209000-00004. [DOI] [PubMed] [Google Scholar]
- Galer BS, Rowbotham MC, Perander J, Friedman E. Topical lidocaine patch relieves postherpetic neuralgia more effectively than a vehicle topical patch: results of an enriched enrollment study. Pain. 1999;80:533–538. doi: 10.1016/S0304-3959(98)00244-9. [DOI] [PubMed] [Google Scholar]
- Gammaitoni AR, Alvarez NA, Galer BS. Safety and tolerability of the lidocaine patch 5%, a targeted peripheral analgesic: a review of the literature. J Clin Pharmacol. 2003;43:111–117. doi: 10.1177/0091270002239817. [DOI] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, Clauw DJ. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- Grachev ID, Fredrickson BE, Apkarian AV. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain. 2000;89:7–18. doi: 10.1016/S0304-3959(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Haanpaa M, Dastidar P, Weinberg A, Levin M, Miettinen A, Lapinlampi A, Laippala P, Nurmikko T. CSF and MRI findings in patients with acute herpes zoster. Neurology. 1998;51:1405–1411. doi: 10.1212/wnl.51.5.1405. [DOI] [PubMed] [Google Scholar]
- Helgason S, Petursson G, Gudmundsson S, Sigurdsson JA. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective study with long term follow up. BMJ. 2000;321:794–796. doi: 10.1136/bmj.321.7264.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LA, Bandler R, Gandevia SC, Macefield VG. Distinct forebrain activity patterns during deep versus superficial pain. Pain. 2006;120:286–296. doi: 10.1016/j.pain.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain. J Neurophysiol. 2001;86:402–411. doi: 10.1152/jn.2001.86.1.402. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Belfrage M, Stoneelander S, Hansson P, Ingvar M. Central Representation of Chronic Ongoing Neuropathic Pain Studied Positron Emission Tomography. Pain. 1995;63:225–236. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Meyerson BA, Ingvar M. PET study on central processing of pain in trigeminal neuropathy. Eur J Pain. 1999;3:51–65. doi: 10.1053/eujp.1998.0101. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- Hyvarinen A, Pajunen P. Nonlinear independent component analysis: Existence and uniqueness results. Neural Netw. 1999;12:429–439. doi: 10.1016/s0893-6080(98)00140-3. [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M, Koizumi S. ATP- and adenosine-mediated signaling in the central nervous system: chronic pain and microglia: involvement of the ATP receptor P2X4. J Pharmacol Sci. 2004;94:112–114. doi: 10.1254/jphs.94.112. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Jung BF, Johnson RW, Griffin DR, Dworkin RH. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62:1545–1551. doi: 10.1212/01.wnl.0000123261.00004.29. [DOI] [PubMed] [Google Scholar]
- Katz J, McDermott MP, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Psychosocial risk factors for postherpetic neuralgia: a prospective study of patients with herpes zoster. J Pain. 2005;6:782–790. doi: 10.1016/j.jpain.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2005 doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost RG, Straus SE. Postherpetic neuralgia--pathogenesis, treatment, and prevention. N Engl J Med. 1996;335:32–42. doi: 10.1056/NEJM199607043350107. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kwan CL, Diamant NE, Pope G, Mikula K, Mikulis DJ, Davis KD. Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology. 2005;65:1268–1277. doi: 10.1212/01.wnl.0000180971.95473.cc. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Handwerker HO, Birklein F. Functional imaging of allodynia in complex regional pain syndrome. Neurology. 2006;66:711–717. doi: 10.1212/01.wnl.0000200961.49114.39. [DOI] [PubMed] [Google Scholar]
- Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Merskey H, Bogduk N. Classification of chronic pain. 1994 [Google Scholar]
- Nurmikko T, Wells C, Bowsher D. Pain and allodynia in postherpetic neuralgia: role of somatic and sympathetic nervous systems. Acta Neurol Scand. 1991;84:146–152. doi: 10.1111/j.1600-0404.1991.tb04923.x. [DOI] [PubMed] [Google Scholar]
- Oaklander AL. The density of remaining nerve endings in human skin with and without postherpetic neuralgia after shingles. Pain. 2001;92:139–145. doi: 10.1016/s0304-3959(00)00481-4. [DOI] [PubMed] [Google Scholar]
- Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguiere F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb Cortex. 2002;12:376–385. doi: 10.1093/cercor/12.4.376. [DOI] [PubMed] [Google Scholar]
- Persaud N, Strichartz GR. Micromolar lidocaine selectively blocks propagating ectopic impulses at a distance from their site of origin. Pain. 2002;99:333–340. doi: 10.1016/s0304-3959(02)00163-x. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Peyron R, Schneider F, Faillenot I, Convers P, Barral FG, Garcia-Larrea L, Laurent B. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology. 2004;63:1838–1846. doi: 10.1212/01.wnl.0000144177.61125.85. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC, Davies PS, Verkempinck C, Galer BS. Lidocaine patch: double-blind controlled study of a new treatment method for post-herpetic neuralgia. Pain. 1996;65:39–44. doi: 10.1016/0304-3959(95)00146-8. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC, Fields HL. The relationship of pain, allodynia and thermal sensation in post-herpetic neuralgia. Brain. 1996;119 ( Pt 2):347–354. doi: 10.1093/brain/119.2.347. [DOI] [PubMed] [Google Scholar]
- Saarela MV, Hlushchuk Y, Williams AC, Schurmann M, Kalso E, Hari R. Cereb Cortex. 2006. The Compassionate Brain: Humans Detect Intensity of Pain from Another's Face. [DOI] [PubMed] [Google Scholar]
- Sah DW, Ossipo MH, Porreca F. Neurotrophic factors as novel therapeutics for neuropathic pain. Nat Rev Drug Discov. 2003;2:460–472. doi: 10.1038/nrd1107. [DOI] [PubMed] [Google Scholar]
- Schreckenberger M, Siessmeier T, Viertmann A, Landvogt C, Buchholz HG, Rolke R, Treede RD, Bartenstein P, Birklein F. The unpleasantness of tonic pain is encoded by the insular cortex. Neurology. 2005;64:1175–1183. doi: 10.1212/01.WNL.0000156353.17305.52. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, Dolan R. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat Neurosci. 2005;8:1234–1240. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Small DM, Apkarian AV. Increased taste intensity perception exhibited by patients with chronic back pain. Pain. 2006;120:124–130. doi: 10.1016/j.pain.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M, Wall PD, Devor M. Myelinated afferent fiber types that become spontaneously active and mechanosensitive following nerve transection in the rat. Brain Res. 1999;824:218–223. doi: 10.1016/s0006-8993(99)01190-7. [DOI] [PubMed] [Google Scholar]
- Truini A, Haanpaa M, Zucchi R, Galeotti F, Iannetti GD, Romaniello A, Cruccu G. Laser-evoked potentials in post-herpetic neuralgia. Clin Neurophysiol. 2003;114:702–709. doi: 10.1016/s1388-2457(03)00009-9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Watson CP, Morshead C, Van der KD, Deck J, Evans RJ. Post-herpetic neuralgia: post-mortem analysis of a case. Pain. 1988;34:129–138. doi: 10.1016/0304-3959(88)90158-3. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;21:RC140. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


