Abstract
Several studies have emphasized the significance of neoangiogenesis for tumor growth and progression, but few have focused on malignant hematological disorders. We studied vascular density and architecture in bone marrow samples of patients with chronic myeloproliferative disease (MPD). Vascular structures were immunostained (for von Willebrand factor/FVIII-RAG, CD 31/PECAM or Ulex europeus I for vessels and for vascular endothelial growth factor, VEGF) in samples from patients with polycythemia vera (PV) (n = 7), chronic myelocytic leukemia (CML) (n = 9), and myelofibrosis (MF) (n = 6) when diagnosed and were compared with normal bone marrow specimens (n = 9). We observed that the mean (± SD) vessel count per high-power microscopy field (HPF) was 5.3 (± 2.1) in normal bone marrow, 5.9 (± 2.1) in PV, 10.8 (± 3.2) in CML, and 14.4 (± 5.5) in MF (P < 0.001 for CMP and MF versus controls). Confocal microscopy, including three-dimensional reconstructions of the blood vessel architecture, confirmed this increased vessel density and revealed tortuous vessel architecture and increased branching in the MPD, particularly in CML and MF. Furthermore, the number of VEGF-positive bone marrow cells was increased in CML and, particularly, in MF. Numbers of VEGF-positive cells and vessels per HPF correlated significantly (r = 0.41; P = 0.037). Thus the myeloproliferative diseases PV, CML, and MF exhibit neoangiogenesis that is related to diagnosis.
Several studies of solid tumors, such as breast cancer and malignant melanoma, 1 have shown that a tumor, to grow beyond the size of a few millimeters and to metastasize, needs a rich vascular network. The formation of this network of blood vessels, called neoangiogenesis, is induced by the production and release of angiogenetic factors, which is triggered by tumor cells. 2 These factors, of which the best described mitogens for endothelial cells (ECs) are basic fibroblast growth factor (bFGF) and vascular endothelial cell growth factor (VEGF), stimulate bystanding ECs to migrate, proliferate, and form a vascular network in the tumor. It has also been shown that the degree of vascular activity in a tumor correlates well with its growth and its capacity to metastasize. 3 The vascular density of a given tumor also may predict the risk of recurrent disease after treatment. 4
Malignant hematological diseases, such as leukemias, have traditionally been regarded as “liquid tumors” because of the terminology and reflection of the disease process in the peripheral blood. There is now some evidence 5,6 for the concept that malignant hematological diseases may be considered to be dependent on vascular support in the bone marrow like solid tumors in other tissues. We therefore studied angiogenesis in the myeloproliferative diseases (MPDs) polycythemia vera (PV), chronic myelocytic leukemia (CML), and myelofibrosis (MF).
Materials and Methods
Patients
Selection criteria for this study were as follows. Patients were selected based on the availability of well-preserved bone marrow biopsy specimens, suitable for staining. Among the patients diagnosed and treated for PV, CML, and MF at Stockholm Söder Hospital, Stockholm, over the last 25 years, we identified seven patients with PV (mean age 63 years, with a male/female ratio of 1:6), nine with CML (mean age 59, male/female ratio 5:4), and six with MF (mean age 66, three males and three females). One patient with CML progressing to blastic phase/acute myelocytic leukemia was also found. The diagnosis of PV was made according to the criteria by the PV Study Group. 7 The diagnosis of CML was supported by chromosome or cytogenetic analyses; however, because this study includes patients diagnosed up to 25 years ago, detailed information is not always available. All patients with myelofibrosis had previously been diagnosed with PV and had been followed at the Hematology Section, Department of Medicine, Stockholm Söder Hospital.
A control group (n = 9, mean age 78 years, five males and four females) consisted of patients who had been investigated because of anemia or leukocytosis, and who had normal bone marrow according to routine examination by a pathologist and no clinical evidence of a malignant condition. The control group was also selected to try to match the study group by age and sex but was slightly older than the patients; because we studied cell-dense hot spots of the bone marrow, possible age-related differences in bone marrow cellularity were not believed to play a role in the assessment of microvessel density.
The study was performed according to the guidelines of the local Board of Ethics. After identification, bone marrow samples from patients and controls were retrieved; they were a mix of aspiration and true-cut biopsies, which all had been fixed in formaldehyde and embedded in paraffin. We analyzed the sample on which the diagnosis was made. The patients with myelofibrosis and acute myelocytic leukemia had previously been treated with a variety of drugs (eg, busulphan, hydroxyurea, 32P).
Regular Light Microscopy
From the bone marrow samples of each patient and control we made several 5-μm-thick sections. Some sections from each patient and control were stained for von Willebrand/factor VIII-related-antigen (Dako A/S, Glostrup, Denmark) to highlight vascular structures, using standard immunohistochemical techniques. Counterstaining was made with hematoxylin. Other sections from each group of diseases and controls were stained for CD31/PECAM (Dako A/S), Ulex europeus I (Dako A/S), or VEGF (R&D Systems, Abingdon, UK). For negative controls we used the same staining procedure, omitting the primary antibody.
Using light microscopy, we counted the number of vessels per 200× high-power field (HPF) in the area of most dense vascularization (hot spot), taking the average of four randomly chosen areas. During the study we observed that three independent reviewers, blinded to the diagnoses and other clinical issues, identified the same number of vessels, irrespective of the staining method or of the biopsy technique; the separate evaluations did not differ from each other by more than one or two vessels per HPF. Similar conclusions were made in another study. 6 The mean results of these independent evaluations were subsequently used for calculations. In VEGF-stained specimens the number of stained cells in 400× HPF was counted in the most intensely stained area; counting was made by two independent reviewers blinded to the diagnosis.
Because an earlier study 6 suggested that the expanded vasculature in leukemic bone marrow has a pathological appearance, with more tortuous and branched vascular architecture than in normal marrow, we wanted see whether this was also true for PV, MF, and CML. To this end we used a confocal microscopic technique.
Confocal Microscopy
A Leica TCSNT confocal laser scanning microscope (Leica, Exton, PA) fitted with air-cooled argon and krypton lasers recorded confocal images from specimens, using a 40× objective. Microvessel number and branch points were recorded from fields of view that contained the highest density of microvessels in each biopsy. Serial optical sections were recorded at 1-μm or 3-μm intervals, beginning at the top surface of the prepared specimen. Stacks of serial sections as well as three-dimensional reconstructions were then rendered in three dimensions with ImageSpace (Molecular Dynamics, Sunnyvale, CA) or VoxelView 2.5.1 (Vital Images, Fairfield, IA) to gain insight into microvessel architecture. In some cases blood vessels were extracted from serial section stacks by manual segmentation. A red intensity-mapped lookup table was then applied to give the structures of interest a red appearance. Confocal micrographs are presented without filtering as black and white.
Statistical Analyses
Statistical analyses were performed with Student’s t-test and linear regression analysis.
Results
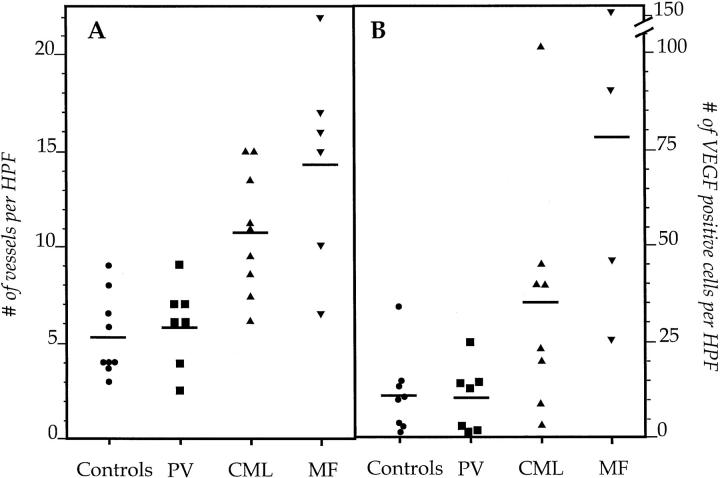
Specimens (5-μm-thick sections) from patients with a normal bone marrow biopsy showed, in areas with hematopoietic marrow, an average (± SD) of 5.3 vessels (± 2.1). Patients with PV had a mean of 5.9 vessels (± 2.1), which was not significantly more than the number for the controls. In contrast, patients with CML displayed significantly more vessels than controls (10.8 ± 3.2; P = 0.0006). Even more vessels were observed in the specimens from the MF patients (14.4 ± 5.5; P = 0.0005 when compared to controls) (Figure 1A) ▶ . The patient with a blastic crisis, transforming from chronic to acute myelocytic leukemia, exhibited 40 vessels per HPF. There was no difference in vessel counts for specimens stained for von Willebrand factor/factor VIII-related antigen, CD34/PECAM, or Ulex europeus, but the best technical results were obtained with staining for von Willebrand factor; thus the figures given above stem from those specimens. The mean values (± SD) for numbers of cells staining positively for VEGF/400× HPF were 12 ± 10 for controls, 11 ± 8 for PV, 36 ± 30 for CML (P = 0.053 when compared to controls) and 78.3 ± 55 for MF (P = 0.006 for the difference from controls) (Figure 1B) ▶ . Moreover, numbers of VEGF-positive cells and vessels per HPF correlated significantly (r = 0.41; P = 0.037).
Figure 1.
A: The number of blood vessels/HPF in normal, PV, CML, and MF bone marrows at diagnosis. Five-micrometer-thick bone marrow sections were stained with antibodies against vWF. The horizontal bars denote the mean value. B: The number of VEGF-positive cells/HPF in 5-μm-thick bone marrow specimens in the same patients.
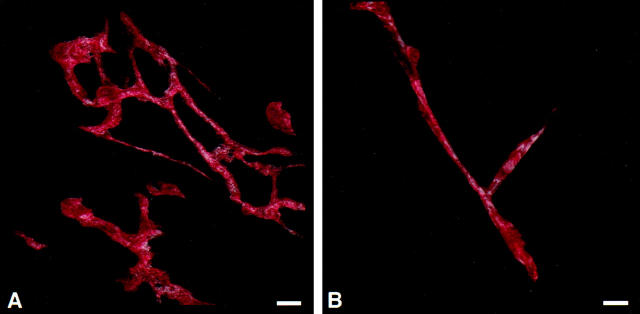
Examination of the bone marrow vessels with serial section confocal microscopy (Figure 2) ▶ revealed that vessels in normal bone marrow were of regular diameter and were not found in nests. Vessels appeared to be straight, occasionally branched, but not tortuous in nature (Figure 3B) ▶ . Vessels in bone marrow from patients with PV appeared to be relatively straight and more highly branched than vessels in bone marrow from normal patients. Bone marrow vessels from patients with CML appeared to be relatively straight with numerous branches. These vessels were similar in appearance to bone marrow vessels from patients with PV. Vessels from patients with MF appeared in extensive localized vascular nests consisting of numerous short vessels that were highly branched and tortuous (Figure 3A) ▶ . In the patient with acute myelocytic leukemia there was a pattern of extensive localized vascular nests.
Figure 2.

Confocal micrographs of bone marrow biopsy specimens from normal bone marrow or from patients with PV, CML, or MF. Specimens were sectioned to thicknesses of 25–200 μm, then immunostained to reveal microvessels. Stacks of serial optical sections were recorded from regions with the highest vessel density. Single optical sections from four representative serial sections are presented. Bar = 20 μm. A: Confocal micrograph (section 6 of 18) of normal bone marrow shows occasional immunostained microvessels (black), generally straight in nature, occasionally branched, and very rarely tortuous in appearance. B: Confocal micrograph (section 4 of 9) of bone marrow from a patient with polycythemia vera (PV) shows relatively straight, branched immunostained microvessels (black). C: Confocal micrograph (section 7 of 15) of bone marrow from a patient with myelofibrosis (MF), showing numerous short microvessels (black) that appear to be highly branched and tortuous. D: Confocal micrograph (section 6 of 14) of bone marrow of from a patient with chronic myelocytic leukemia (CML), showing immunostained microvessels (black) with numerous branches.
Figure 3.
A three-dimensional projection made from segmented confocal image data reveals blood vessel architecture in myelofibrosis marrow (A) and in normal bone marrow (B). The three-dimensional projection was made from 38 or 43 (respectively) serial optical sections recorded at 1-μm intervals. A red hue intensity-mapped look-up table was applied to the blood vessels extracted from the surrounding marrow (not shown).
Discussion
Angiogenesis is a basic physiological phenomenon in many naturally occurring processes such as wound healing and embryogenesis. Its role in pathological conditions such as retinopathy in diabetes and in inflammatory conditions such as the pannus of rheumatoid arthritis has been well documented. 8 The concept of tumor induction of as well as tumor dependence on angiogenesis is widely accepted, confirming its importance in tumor growth and progression to metastatic disease. 9,10 Much of the evidence for tumor dependence on angiogenesis is based on animal studies.
However, hematological malignancies, originating in the bone marrow, have rarely been studied in relation to angiogenesis. This is may be due to the historical concept of leukemia as a “liquid tumor,” because many of the diagnostic and therapeutic approaches rely on results from peripheral blood counts.
There are now a couple of studies, however, one on children with acute lymphocytic leukemia and some on adults with multiple myeloma, showing an increased vascular density in the bone marrow and elevated levels of angiogenetic peptides in serum or urine. 5,6 Another study demonstrated the increased presence of VEGF and its receptors flt-1 and KDR in the bone marrow of other hematopoietic malignancies such as myeloma. 13
We demonstrate here that the chronic myeloproliferative diseases chronic myelocytic leukemia and myelofibrosis are similarly associated with an increased vascular density in the bone marrow compared to the bone marrow of healthy subjects. We also observed a distinct increase in the number of cells with positive staining for VEGF in CML and a significant increase in MF versus controls, suggesting that VEGF might be an important signaling molecule for angiogenesis in these conditions. Moreover, the architecture of the vasculature clearly differs from the normal architecture in that the vessels of the three myeloproliferative disorders are more tortuous and branched. Thus, although PV did not exhibit higher microvessel density, the architecture was abnormal. This type of architecture is similar to what has been reported for various solid malignant tumors. Finally, the number of vessels and branches shows increases parallel to the general prognosis for each diagnostic entity. PV, having in general the most favorable prognosis, did not differ much from the normal marrows with respect to vessel counts and number of branches. In contrast, myelofibrosis and the case with acute myelocytic leukemia, with the shortest general life expectancy, had a significantly higher number of vessels and branches. These results can be compared to findings of increased blood flow in the bone marrow of patients with PV 11 and certain abnormalities of blood vessels in relation to megakaryocytes in MF. 12
We therefore suggest that these myeloproliferative diseases, like solid tumors, may be dependent on angiogenesis to expand. The exact pathophysiological mechanisms by which this process works, as well as the diagnostic and prognostic place of this method in clinical medicine, warrant further study.
Acknowledgments
We thank Jenny Roberts and Bi Hui Zhong for assistance in specimen staining, Mike Roberts for confocal microscopy, and Jean Lai for expert three-dimensional rendering of confocal image data.
Footnotes
Address reprint requests to Dr. Jan Palmblad, Department of Medicine M54, Huddinge University Hospital, S-141 86 Huddinge, Sweden. E-mail: jan.palmblad@hematol.hs.sll.se.
Supported by grants from the Swedish Medical Research Council (19X-05991, 71XS-13135), the Stockholm Cancer Society, the Swedish Association against Rheumatism, Karolinska Institutet, Huddinge University Hospital, and Stockholm Söder Hospital.
References
- 1.Weidner N, Folkman J: Tumor vascularity as a prognostic factor in cancer. deVita VT Hellman S Rosenberg SA eds. Important Advances in Oncology. 1997, :pp 167-190 Lippincott-Raven, Philadelphia [PubMed] [Google Scholar]
- 2.Folkman J: Tumor angiogenesis. Mendelsohn J Howley MJ Israel MA Liotta LA eds. The Molecular Basis of Cancer. 1995, :pp 206-232 Saunders, Philadelphia [Google Scholar]
- 3.Folkman J, Shing Y: Angiogenesis. J Biol Chem 1992, 267:10931-10934 [PubMed] [Google Scholar]
- 4.Weidner N: Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol 1995, 147:9-19 [PMC free article] [PubMed] [Google Scholar]
- 5.Vacca A, Ribatti D, Presta M, Minischetti M, Iurlaro M, Ria R, Albini A, Bussolino F, Dammacco F: Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood 1999, 93:3064-3073 [PubMed] [Google Scholar]
- 6.Perez-Atayde AR, Sallan SR, Tedrow U, Connors S, Allred E, Folkman J: Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol 1997, 3:815-821 [PMC free article] [PubMed] [Google Scholar]
- 7.Berlin NI: Diagnosis and classification of the polycythemias. Semin Hematol 1975, 12:339-351 [PubMed] [Google Scholar]
- 8.Firestein GS: Starving the synovium: angiogenesis and inflammation in rheumatoid arthritis. J Clin Invest 1999, 103:3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheresh DA: Death to a blood vessel, death to a tumor. Nat Med 1998, 4:395-396 [DOI] [PubMed] [Google Scholar]
- 10.Boehm T, Folkman J, Browder T, O’Reilly MS: Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 1997, 390:404-407 [DOI] [PubMed] [Google Scholar]
- 11.Lahtinen R, Lahtinen T, Hyodynmaa S: Increased bone marrow blood flow in polycythemia vera. Eur J Nucl Med 1983, 8:19-22 [DOI] [PubMed] [Google Scholar]
- 12.Kvasnicka HM, Thiele J, Amend T, Fischer R: Three-dimensional reconstructions of histologic structures in human bone marrow from serial sections of trephine biopsies. Anal Quant Cytol Histol 1994, 16:159-166 [PubMed] [Google Scholar]
- 13.Bellamy WT, Richter L, Frutiger Y, Grogan TM: Expression of vascular endothelial growth factor and its receptors in hematopoietic malignancies. Cancer Res: 1999, 59:728–733 [PubMed]