Abstract
Benign prostatic hyperplasia (BPH) is an extremely common disease of older men in which there is benign overgrowth of the prostatic transition zone, leading to obstruction of urine outflow. FGF7, a potent growth factor for prostatic epithelial cells, is increased by threefold in BPH and is correlated with increased epithelial proliferation in this condition. Immunohistochemistry of normal and hyperplastic prostate revealed that FGF7-expressing fibroblastic cells were present in higher numbers near the epithelial acini, implying that epithelial cells may express a factor that induces FGF7 expression by stromal cells. Conditioned medium (CM) from primary cultures of prostatic epithelial cells was capable of inducing a two- to sixfold increase in the expression of FGF7 by primary stromal cultures. Blocking experiments with neutralizing anti-interleukin-1α (Il-1α) antibodies and IL-1Ra, an Il-1α receptor antagonist, show that this inducing activity was due to the presence of Il-1α in the epithelial CM. Analysis of normal prostatic peripheral zone and BPH tissue by enzyme-linked immunoabsorption assay reveal that Il-1α is present at increased levels in hyperplastic prostate and that levels of Il-1α correlate strongly with tissue FGF7 concentration in BPH. Therefore Il-1α is produced by prostatic epithelial cells and can induce FGF7, a potent epithelial growth factor, which can in turn lead to further epithelial growth and increased Il-1α secretion, thus establishing a double paracrine loop that is functionally equivalent to an autocrine growth loop. This double paracrine loop may play a key role in the abnormal proliferation of the transition zone, which is critical to the pathogenesis of BPH.
Benign prostatic hyperplasia (BPH) is an extremely common condition of older men. 1 This benign growth of the prostatic transition zone leads to obstruction of urine outflow and in this manner causes considerable morbidity. The annual cost of treating BPH is more than $3 billion, and the importance of this problem will only increase as the number of elderly men continues to grow. 2 Thus BPH is of considerable medical importance, and yet its pathogenesis is still obscure.
Prostate growth is controlled by a variety of polypeptide growth factors, including members of the fibroblast growth factor (FGF) gene family. 3,4 FGF2, FGF7, and FGF9 are all present in high concentrations in normal human prostate. 5-7 All of these growth factors can stimulate proliferation of primary prostatic epithelial cells in culture. These FGFs are produced by prostatic stromal cells, and appropriate receptors are present on prostatic epithelial cells, so that these FGFs can act as paracrine growth factors for prostatic epithelial cells in vivo.
The concentrations of both FGF7 and FGF2 are significantly increased in hyperplastic prostate in comparison to normal peripheral and transition zone tissue, and this overexpression is not due to alterations in the percentage of stroma in the tissue. 5 Interestingly, the normal transition zone tissues had higher levels of both of these FGFs than the normal peripheral zone, indicating a fundamental difference in the biology of the two prostatic zones, and is consistent with the idea that increased expression of FGF2 and FGF7 in the transition zone may be important in the eventual development of BPH. FGF7 is a potent mitogen for prostatic epithelial cells, and quantitative analysis of cellular proliferation by Ki67 immunohistochemistry of frozen sections of the tissue taken before protein extraction revealed a strong correlation of epithelial proliferation with FGF7 content in BPH tissue, consistent with a key role for this growth factor in driving the abnormal epithelial proliferation in BPH. This correlation of the FGF7 content with epithelial proliferation is the first demonstration that alterations of growth factor concentration in BPH in vivo can be linked directly to increased proliferation.
Given that FGF7 protein is increased in BPH and that this overexpression has functional consequences for the proliferation of prostatic epithelial cells, we sought to determine what factors control FGF7 expression in the human prostate. We report here that interleukin-1α (IL-1α) is produced by prostatic epithelial cells and can act as a paracrine inducer of FGF7 production by prostatic stromal cells in vitro. In addition, we have found that IL-1α is present in substantial quantities in human prostate in vivo and that IL-1α concentration correlates with FGF7 concentration in BPH tissue. Thus epithelial proliferation in BPH is controlled, at least in part, by a double paracrine loop in which epithelial cells produce IL-1α, which stimulates FGF7 production by stromal cells, which in turn induces epithelial proliferation and further production of IL-1α. This double paracrine loop ultimately leads to increased tissue mass in the prostatic transition zone, which is critical in the pathogenesis of BPH.
Materials and Methods
Tissue Acquisition and Analysis
Samples of the benign peripheral zone of the prostate or hyperplastic transition zone were taken from radical prostatectomies. Tissues were received fresh, and portions were snap-frozen in liquid nitrogen or used to establish primary cell cultures (see below). The frozen tissues were then analyzed by frozen section to confirm the absence of carcinoma or high-grade prostatic intraepithelial neoplasia. Samples of hyperplastic transition zone were also harvested from suprapubic prostatectomies performed for the treatment of severe BPH.
Cell Culture, Production of Epithelial Conditioned Medium, and Assay for FGF7 Induction
Primary epithelial and stromal cell cultures were established from prostatic tissue samples from the peripheral zone as described previously. 8 To prepare conditioned medium (CM), primary epithelial cells were plated in 10-cm tissue culture dishes. When the cells were subconfluent, epithelial growth medium was replaced with 8 ml of MCDB 153 medium containing insulin, transferrin, selenium, bovine serum albumin (BSA), and oleic acid (1% ITS+2; Sigma Chemical, St. Louis, MO). Conditioned medium (CM) was collected after 72 hours. The epithelial cells tolerated this treatment well and appeared to be healthy after this period. To assay for FGF7 induction, 1 × 10 6 primary stromal cells were plated in 10-cm tissue culture dishes. The following day cells were placed in 7.2 ml RPMI 1640 with 1% ITS+2. Cells were then treated with either 800 μl of epithelial conditioned medium or MCDB153 with 1% ITS+2 as a control. At 24-hour intervals aliquots were removed for the analysis of FGF7 content by enzyme-linked immunoabsorption assay (ELISA) (see below).
To determine whether IL-1α could induce FGF7 release, stromal cultures were plated as above and treated with either 2.5 or 6 pg/ml of recombinant IL-1α (R&D Systems, Minneapolis, MN) in RPMI 1640 with 1% ITS+2, and the FGF7 content was determined by ELISA on aliquots removed at 24-hour intervals. To block IL-1α activity, 800 μl of CM was preincubated with 5 μg of neutralizing anti-IL-1α antibody (MAB200; R&D Systems) for 2 hours at 37°C. The negative control antibody was 5 μg of anti-VEGF antibody (AF-293-NA; R&D Systems). A second approach to blocking IL-1α activity was to use recombinant IL-1Ra, a naturally occurring antagonist of IL-1 activity. Before the addition of CM, stromal cultures were pretreated with 400 ng of recombinant human IL-1Ra (R&D Systems) and incubated for 2 hours. For both blocking experiments FGF7 concentration was determined by ELISA of stromal medium removed after 48 hours of treatment.
Immunohistochemistry
Frozen tissue sections were fixed in acetone for 10 minutes and stored at −80°C. All sections were treated with Autoblocker (R&D Systems) to inhibit endogenous peroxidase and avidin/biotin (Vector Laboratories, Burlingame, CA) to block endogenous biotin. The sections were incubated with 2.5 ng/ml of anti-FGF7 mouse monoclonal antibody (R&D Systems) at 4°C for 12 hours. After liberal washing with phosphate-buffered saline (pH 7.4) (PBS), sections were then incubated with biotinylated horse anti-mouse antibody at 7.5 μg/ml for 45 minutes at room temperature (Vector Laboratories). Sections were then washed with PBS containing 0.1% Tween 20 and incubated with avidin-biotin complex (Vectastain Elite, Vector Laboratories) for 15 minutes. The antigen-antibody reaction was demonstrated, using diaminobenzidine as the substrate, and the sections then counterstained with hematoxylin.
Enumeration of FGF7-Expressing Cells
To determine the number of FGF7 cells that were located near epithelial acini or in stromal areas without adjacent epithelium, a point-counting protocol was used. After immunohistochemistry with anti-FGF7 antibody, random fields in each slide were examined at 200×, using a Nikon Labphot-2 microscope with an ocular micrometer. If epithelium was present in the field, then the center of the ocular micrometer was placed approximately 50 μm from the nearest epithelial unit and aligned parallel to its base. Any positively staining cell that intersected the ocular micrometer was counted. A total of 10 such periacinar fields were counted, and the result was summed. Fields without epithelium were counted directly in a manner similar to that of periacinar fields, and a total of 10 stromal fields were counted and summed. The ocular micrometer corresponded to a distance of approximately 400 μm at 200×. A total of six slides from the normal peripheral zone and 11 from hyperplastic transition zone were examined.
Enzyme-Linked Immunoabsorption Assay
Cell extracts were prepared from snap-frozen tissues as described previously. 7 Determination of the FGF7 and IL-1α concentrations in the media and cell extracts was carried out by ELISA, based on a quantitative sandwich immunoassay technique. Typically, 96-well enzyme immunoassay plates (Nunc, Rochester, NY) were coated with 1 μg/ml of capture antibody (MAB 251 for FGF7 and MAB 200 for IL-1α; R&D Systems) for either 12 hours (FGF7) or 24 hours (IL-1α) at room temperature. The plates were washed with PBS with 0.05% Tween 20 (wash buffer) to remove unbound antibodies. Plates were then blocked with PBS containing 1% BSA, 5% sucrose, and 0.05% sodium azide for 1 hour at room temperature. This was followed by the addition of 100 μl of culture medium or 25 μl of tissue extract (prepared as described previously) 7 made to 100 μl with diluent (0.1% BSA, 0.05% Tween 20, 10 mM Tris, and 150 mmol/L sodium chloride, pH 7.3) per well and incubation at room temperature for 2 hours. Recombinant FGF7 or IL-1α (R&D Systems) was used as the standard. After multiple washes with wash buffer, the plates were incubated with either biotinylated anti-human FGF7 (BAF251, R&D Systems) at 100 ng/ml or biotinylated anti-human IL-1α (BAF200, R&D Systems) at 50 ng/ml for 2 hours at room temperature. Plates were washed liberally with wash buffer, and 100 μl of diluted peroxidase conjugated streptavidin (1:4000; Zymed Laboratories, San Francisco, CA) was aliquoted per well and incubated for 30 minutes at room temperature. The plates were then washed with wash buffer and incubated with 100 μl per well of substrate consisting of a 1:1 mixture of peroxidase solution and tetramethyl benzidine substrate (KPL, Gaithersburg, MD) for 20 minutes at room temperature. The enzymatic activity was stopped with 5 N sulfuric acid, and the optical density was determined at 450 nm in a microplate reader. The FGF7 ELISA was linear between 60 and 2000 pg/ml, whereas the IL-1α ELISA was linear between 2 and 200 pg/ml.
Statistical Analysis
Data were analyzed by paired or unpaired t-tests for comparison of two groups as appropriate. ANOVA was used for the comparison of multiple groups. Calculations were performed using SigmaPlot and SigmaStat programs.
Results
Increased Numbers of FGF7-Expressing Stromal Cells Near Epithelial Acini
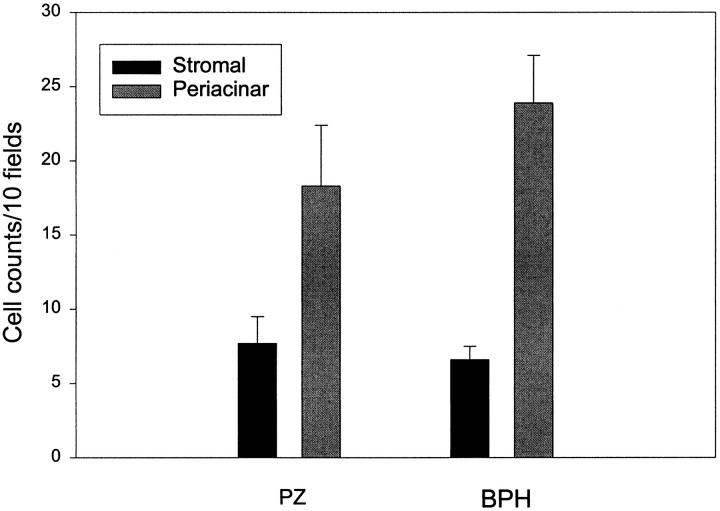
To determine the localization of the increased FGF7 in BPH we examined tissue sections from normal peripheral zone and BPH tissues after immunohistochemistry with anti-FGF7 monoclonal antibodies. FGF7 was expressed in stromal cells with fibroblastic morphology. In these sections we noted a definite tendency for the FGF7-positive cells to be located near the epithelial acini. Examples of such concentrations of FGF7-positive cells near epithelial acini are shown in Figure 1 ▶ , as is a control photomicrograph of an adjacent stromal area. It should be noted that this distribution was not uniform, in that some epithelial acini had large numbers of adjacent positive cells, whereas others had relatively few. To try and quantitate this phenomenon, a simple point-counting protocol was used to enumerate the number of cells staining positively on FGF7 IHC in periacinar and stromal areas. At ×200 magnification, an ocular micrometer was aligned parallel to the base epithelial acini chosen at random, and all positively staining cells crossing the ocular micrometer were counted for a total of 10 measurements per slide. The same measurement was then performed with the ocular micrometer placed in a stromal area without adjacent epithelium. Six slides from normal peripheral zone and 11 BPH tissues were evaluated in this manner without knowledge of their origin. The results are shown in Figure 2 ▶ . For each type of tissue the number of FGF7-positive cells was significantly higher in the periacinar area (paired t-test, P < 0.03). In addition, higher cell counts were present in the periacinar areas of the BPH tissues compared with the peripheral zone tissue, although this difference is not statistically significant (P = 0.31, t-test). The increase in cell number in the periacinar tissue of BPH (one- to threefold) is not proportional to the increase in FGF7 protein content in BPH (threefold). The most likely explanation for this discrepancy is that in BPH there are both increased numbers of FGF7-expressing stromal cells and increased expression of FGF7 per cell in BPH. We could not identify any obvious association of these areas of increased FGF7-positive cells with atrophy, metaplasia, or inflammatory infiltrate
Figure 1.
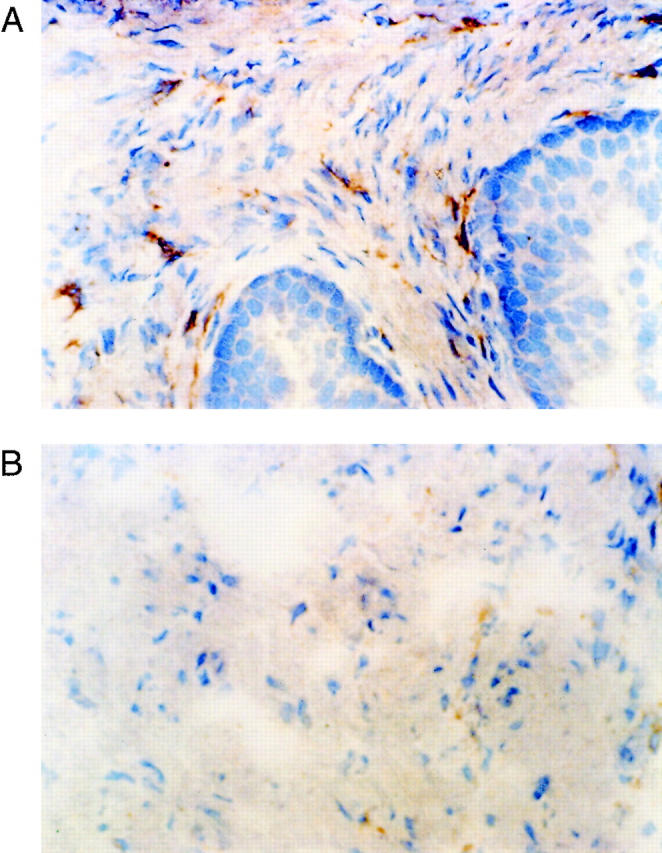
Localization of FGF7 expression in prostatic tissue by immunohistochemistry. Frozen sections of hyperplastic prostatic tissue were analyzed by immunohistochemistry, using anti-FGF7 mouse monoclonal antibodies. A: High-power view of periacinar area. Original magnification, ×400. B: High-power view of adjacent stromal area. Original magnification, ×400. FGF7-expressing cells are present, but the number of such cells is less than in the periacinar area.
Figure 2.
Quantitation of the localization of FGF7-expressing cells in prostate tissue. Frozen sections of six normal peripheral zone and 11 BPH tissues were analyzed by immunohistochemistry with anti-FGF7 monoclonal antibody. The number of cells located adjacent to epithelial acini (periacinar) was compared to the number of cells in stromal areas, using a point counting protocol. The mean counts in the 10 fields (± SEM) in each location for the normal peripheral zone (PZ) and hyperplastic transition zone (BPH) is shown. For both types of tissue the difference between the periacinar and stromal tissues is statistically significant (P < 0.03, paired t-test).
Prostatic Epithelial Cells Secrete a Paracrine Factor Which Stimulates FGF7 Secretion by Stromal Cells
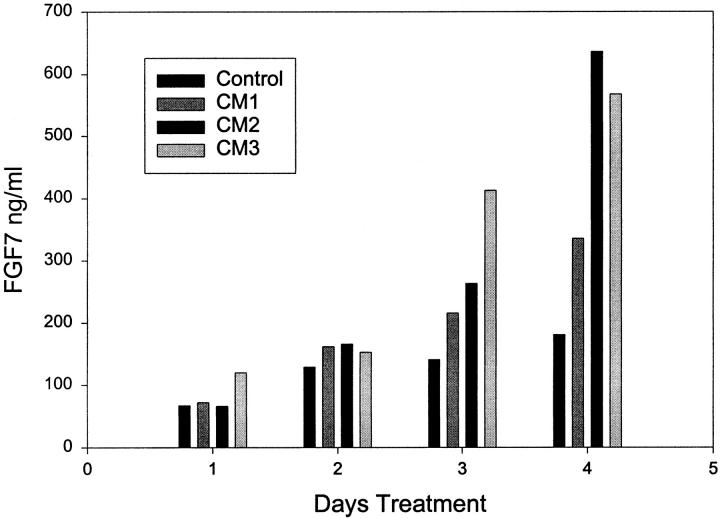
Given that the FGF7-positive cells are more common closer to the epithelial acini, we wished to determine whether there was a paracrine factor or factors secreted by epithelial cells that induced FGF7 production by prostatic stromal cells. Such paracrine factors could play a role in normal growth if they are secreted into the stroma or might be released into the surrounding tissue if the epithelium is disrupted during inflammation. To determine whether such a factor(s) is secreted in vitro we collected conditioned medium (CM) from primary cultures of prostatic epithelial cells after 72 hours of incubation. The collection medium contained insulin as the only added growth factor, to avoid the effect of EGF and/or FGFs, which are normally present in the epithelial media, on the stromal cells. The CM was then added at a 1:10 dilution to primary cultures of prostatic stromal cells in RPMI 1640 containing insulin, and aliquots were removed at 24-hour intervals over 96 hours. The aliquots were then assayed by ELISA for FGF7 content. Results of several such experiments are shown in Figure 3 ▶ . FGF7 accumulates in the medium of the untreated stromal cells over the four day incubation, and the diluted CM induces a two- to fourfold increase in the FGF7 content relative to untreated controls after 4 days. We have repeated this experiment with multiple batches of conditioned medium and have found a consistent, two- to sixfold stimulatory effect of the conditioned medium on FGF7 production. To determine whether the increased concentration of FGF7 in the conditioned medium was due to an increase in cell number or increased production of FGF7 per cell, the number of prostatic stromal cells was determined after 5 days of incubation with or without treatment with diluted conditioned medium. There was no significant difference in cell number between the treated and untreated stromal cells (data not shown). We have repeated these experiments with both a low-passage and high-passage number of stromal cells and have found a similar response, although the low-passage cells consistently have a higher basal (unstimulated) FGF7 expression, so that the fold increase in FGF7 production for any given CM was altered by the passage number of the stromal cells used for testing (data not shown). It should be noted that the induction seen (two- to sixfold) is on the same order of magnitude as the increase in FGF7 in BPH (relative to normal PZ) that was correlated with increased epithelial proliferation.
Figure 3.
Stimulation of FGF7 secretion by epithelial conditioned medium. Primary cultures of prostatic stromal cells were plated in RPMI 1640 with insulin, transferrin, and selenium and supplemented with a 1:10 dilution of conditioned medium (CM) prepared from primary epithelial cultures or were not supplemented. At 24-hour interval aliquots of stromal media were removed and assayed in duplicate for FGF7 content by ELISA. Results with three different epithelial conditioned media are shown.
IL-1α Is the Paracrine Factor Secreted by Prostatic Epithelial Cells That Stimulates FGF7 Secretion
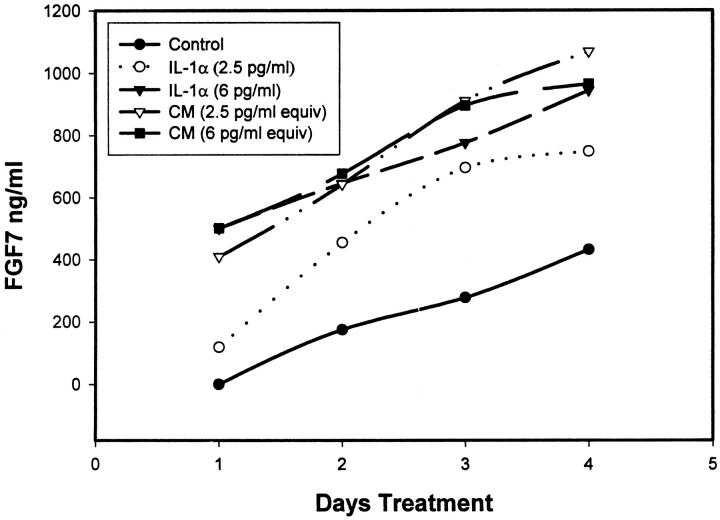
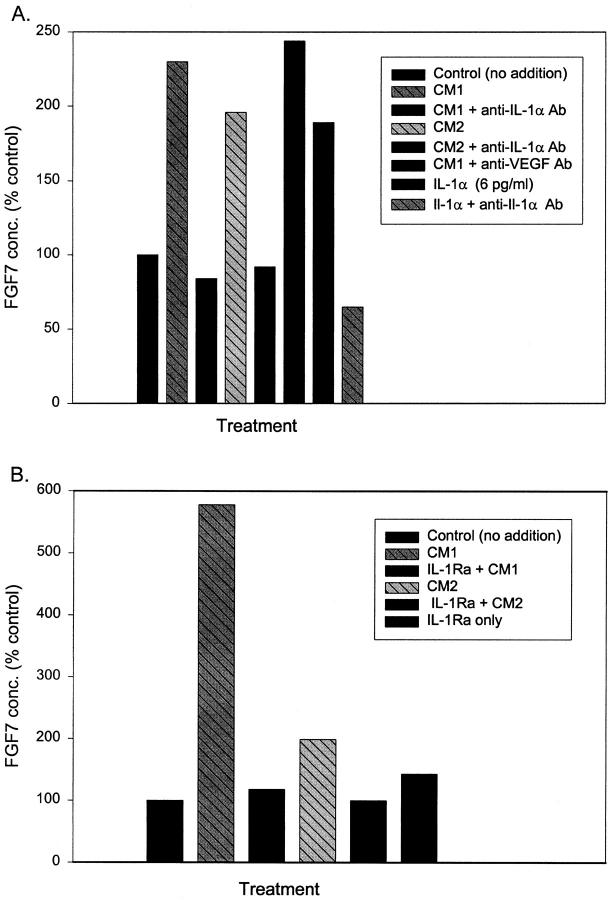
To determine the identity of the factor responsible for the FGF7 accumulation, we assayed the epithelial CM for Il-1α, because it is known to induce FGF7 transcription. 9-12 Il-1α was present at an average concentration of 33.9 ± 6.9 pg/ml (SEM, n = 12) in the CMs from the prostatic epithelial cells. Using recombinant Il-1α at 2.5 or 6 pg/ml, roughly equivalent to the concentration after a 1:10 dilution of the CM, we found a two- to threefold increase in accumulation of FGF7 production by prostatic stromal cells (Figure 4) ▶ , similar to the level of induction by the CM. When compared to CM containing an equal amount of Il-1α, the pure Il-1α induced 60–98% as much FGF7, indicating that the majority of FGF7-inducing activity in the CM can be attributed to Il-1α. To verify that the FGF7-inducing activity of the conditioned medium was due to Il-1α, we pretreated the CM with anti-Il-1α-neutralizing antibody. As can be seen in Figure 5A ▶ , this pretreatment essentially eliminated the response of the stromal cells to the CM. As expected, similar treatment also completely blocks the response to added recombinant Il-1α. Treatment with control anti-VEGF antibody had no effect. Il-1Ra is a secreted Il-1 antagonist that blocks Il-1α and Il-1β activity. 13 When cells were pretreated with recombinant Il-1Ra before the addition of the CM, the FGF7-inducing activity was again completely abrogated (Figure 5B) ▶ . IL-1β, which is also blocked by IL-1Ra, was almost undetectable in the epithelial CM by ELISA (less than 1 pg/ml; data not shown). It should also be noted that no Il-1α was detected in medium collected from stromal cells, indicating that little or no Il-1α is produced by these cells. Thus Il-1α is the major paracrine inducer of FGF7 production present in epithelial CM.
Figure 4.
Stimulation of FGF7 secretion by IL-1α. Primary cultures of prostatic stromal cells were plated in RPMI 1640 with insulin, transferrin, and selenium and supplemented with a dilution of conditioned medium (CM) prepared from primary epithelial cultures calculated to contain 2.5 or 6 pg/ml of Il-1α (based on the concentration of Il-1α determined by ELISA) or 2.5 or 6 pg/ml of pure recombinant Il-1α, or they were not supplemented. The concentration of FGF7 in the stromal media was then determined by ELISA of aliquots removed at 24-hour intervals. •, control (not supplemented), Il-1α; ○, 2.5 pg/ml, Il-1α; ▾, 6 pg/ml; ▿, CM equivalent to 2.5 pg/ml Il-1α; ▪, CM equivalent to 6 pg/ml Il-1α.
Figure 5.
Inhibition of stimulation of FGF7 secretion by CM with inhibitors of IL-1 α action. A: Primary cultures of prostatic stromal cells were plated in RPMI 1640 with insulin, transferrin, and selenium and supplemented with a 1:10 dilution of conditioned medium (CM) prepared from two separate primary epithelial cultures (CM1 and CM2); diluted CMs preincubated with excess anti-Il-1α neutralizing antibody; diluted CM1 preincubated with anti-VEGF (vascular endothelial cell growth factor) antibody; Il-1α at 6 pg/ml; or Il-1α at 6 pg/ml preincubated with anti-Il-1α neutralizing antibody or were not supplemented (control). FGF7 concentration was determined by ELISA after 48 hours of treatment. B: Primary cultures of prostatic stromal cells were plated in RPMI 1640 with insulin, transferrin, and selenium and supplemented with a 1:10 dilution of conditioned medium (CM) prepared from two separate primary epithelial cultures (CM1 and CM2); diluted CMs after cells were pretreated with recombinant IL-1Ra or Il-1Ra only, or were not supplemented (control). FGF7 concentration was determined by ELISA after 48 hours of treatment.
Expression of IL-1α in Human Prostate in Vivo
Having established that IL-1α can act as an epithelial-derived paracrine inducer of FGF7 production by stromal cells in vitro, we sought evidence that this paracrine stimulation occurs in vivo. We therefore assayed tissue extracts from normal peripheral zone and BPH tissue for IL-1α content by ELISA. The tissue content of IL-1α was quite variable, ranging from undetectable (<5 pg/g wet weight) to 954 pg/g. The mean IL-1α content of the peripheral zone was 200 ± 108 pg/g wet weight (SEM, n = 9), whereas BPH tissue contained 451 ± 99 pg/g (SEM, n = 26). Although this difference is not statistically significant (P = 0.12, t-test), the two- to fivefold higher Il-1α concentration in BPH tissue is similar to the threefold higher level of FGF7 in BPH. When the IL-1α and FGF7 contents were determined in the same BPH tissue extract, we found a strong correlation between IL-1α and FGF7 content, as shown in Figure 6A ▶ . The correlation coefficient by linear regression is 0.73, which is a very good correlation, particularly when dealing with human tissue samples from a histologically heterogeneous tissue. When the tissue samples are divided into four groups based on their IL-1α content (<200, 200–400, 400–600, and >600 pg/g), there is a clear correlation of increased IL-1α and FGF7 content (Figure 6B) ▶ . The difference between these four groups is highly significant statistically (P = 0.001; ANOVA). In summary, IL-1α is expressed at relatively high levels in prostate tissue, and the tissue content of IL-1α is strongly correlated with FGF7 content in BPH.
Figure 6.
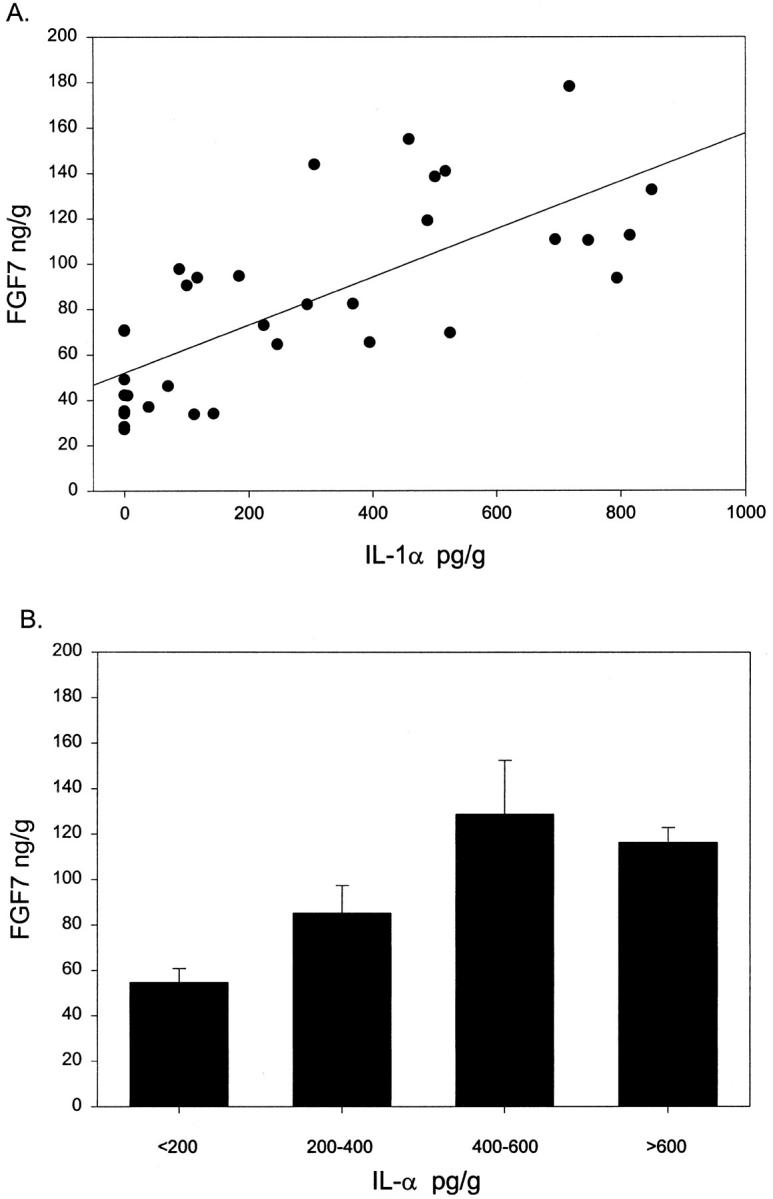
Correlation of tissue concentration of IL-1α and FGF7 in hyperplastic prostate. Proteins were extracted from snap-frozen BPH tissue samples, and the concentration of Il-1α and FGF7 was determined by ELISA in each extract. A: Scatter diagram with best fit linear regression shown (r = 0.73). B: Data are expressed as the mean FGF7 concentration (± SEM) for BPH tissue in four groups: <200 pg/ml (n = 17); 200–400 pg/ml (n = 6); 400–600 pg/ml (n = 5); and >600 pg/ml (n = 6).
Discussion
BPH is due to the slow but progressive growth of the prostatic transition zone tissue over many years. Quantitative immunohistochemical studies have shown that both increased cellular proliferation and decreased apoptosis contribute to this excess growth. 14 FGF7, a strong mitogen for prostatic epithelial cells, is increased by threefold in BPH, and epithelial proliferation is correlated with FGF7 concentration in BPH tissue. 5 FGF2 is elevated by approximately twofold in BPH tissue, 5,15 and FGF2 concentrations are correlated with stromal proliferation. 5 These alterations in FGF levels are independent of alterations in the epithelial-to-stromal ratio. 5 It should be noted that although earlier work indicated that stroma may be disproportionately increased in BPH, more recent work by Shapiro et al 16 indicates that the epithelial-to-stromal ratio in BPH is actually the same as in the normal prostate. Thus any changes in FGF expression are unlikely to be due to changes in the absolute stromal content of BPH tissues. In addition, these findings indicate that both epithelial proliferation and stromal proliferation are important in the pathogenesis of BPH, and benign prostatic hyperplasia is not due exclusively to increased stromal content.
We have found that FGF7-producing stromal cells tend to be located near epithelial acini in the prostate and that epithelial cells release IL-1α, which can induce FGF7 secretion in vitro. At the present time we do not know the mechanism by which IL-1α increases FGF7 secretion, but it is likely that it reflects, at least in part, increased FGF7 transcription, because IL-1α and other cytokines have been shown to increase FGF7 transcription in other systems. 9-12 IL-1α lacks a signal peptide but is secreted by a defined mechanism, 17 so that it can act as a paracrine factor in vivo. The strong correlation between IL-1α and FGF7 levels in vivo argue that this is the case. This in vivo correlation, in conjunction with our morphological observations and in vitro data, is consistent with the idea that IL-1α plays an important role in controlling FGF7 production in BPH. In turn, FGF7 appears to be a major factor in the increased epithelial proliferation in BPH.
Given the highly variable levels of IL-1α in vivo, it is likely that control of IL-1α expression in the prostate is complex. We have shown that IL-1α is produced and secreted by prostatic epithelial cells. We do not know the extent of variability of IL-1α expression by epithelial cells in vivo. It is highly likely that inflammatory cells, such as macrophages and T lymphocytes, also contribute to IL-1α levels in vivo, because IL-1α is produced by inflammatory cells, 13 and it is well known that normal prostate and BPH tissue are often subject to chronic inflammation, which can be extensive. 18,19 However, we did not observe any obvious correlation between FGF7-expressing cells detected by immunohistochemistry and infiltrates of chronic inflammatory cells, so the extent to which inflammation contributes to IL-1α and FGF7 expression in vivo is unclear. In addition, we do not know the extent to which the IL-1α produced by the epithelial cells is normally released to the stroma and whether inflammation and atrophy may increase such release. Thus, whereas the strong correlation of IL-1α and FGF7 levels argues that IL-1α plays an important role in driving FGF7 production in BPH, the control of IL-1α expression and the manner in which it comes to interact with prostatic stromal cells need further investigation.
Although our data indicate that FGF7 secretion is induced by IL-1α, there are almost certainly other factors that also control FGF7 expression. Some of our tissue samples from normal and BPH tissue did not contain detectable IL-1α and yet had significant, although lower, FGF7 expression. One factor that may be important is androgens, which have been shown to induce FGF7 expression by prostatic stromal cells in vitro. 20 More recent observations in castrated rats indicate that FGF7 expression is not decreased in such animals. 21 Thus the role of androgens in the control of FGF7 expression in the prostate is controversial. Further investigations are needed to determine the role of androgens and other factors in the control of prostatic FGF7 expression and the interactions of these factors with IL-1α.
In this report we have demonstrated that FGF7-expressing stromal cells tend to be located near epithelial acini and that epithelial cells can release IL-1α, which can induce FGF7 expression. Thus it is possible that a double paracrine loop can be established in which IL-1α is secreted by epithelial cells, which then induces FGF7 expression by stromal cells, which can then induce epithelial proliferation and further increases in Il-1α expression, which in turn lead to increased FGF7 expression, and so forth. Our in vivo observations showing a correlation between IL-1α and FGF7 levels support the existence of such a double paracrine loop in vivo. This double paracrine loop is functionally equivalent to an autocrine loop and can result in increased and poorly controlled proliferation, which can explain, in part, the increased tissue mass of the prostatic transition zone that is characteristic of BPH. Interruption of this double paracrine loop is an attractive target for therapeutic interventions aimed at preventing BPH and its associated morbidity in older men.
Acknowledgments
We gratefully acknowledge Dr. Douglas Mann for the use of his microplate reader.
Footnotes
Address reprint requests to Dr. Michael M. Ittmann, Research Service (151), Houston Department of Veterans Affairs Medical Center, 2002 Holcombe Blvd., Houston, TX 77030. E-mail: mittmann@bcm.tmc.edu.
Supported by National Institutes of Health grant R01 DK54170.
References
- 1.Glenn R, Campaign E, Bouchard G, Silbert J: The development of benign prostatic hyperplasia among volunteers in the Normative Aging Study. Am J Epidemiol 1985, 121:78-83 [PubMed] [Google Scholar]
- 2.Cotton P: Case for prostate therapy wanes despite more treatment options. JAMA 1991, 266:459-460 [DOI] [PubMed] [Google Scholar]
- 3.Culig Z, Hobisch A, Cronauer M, Radmayr C, Hittmair A, Zhang J, Thurnher M, Bartsch G, Klocker H: Regulation of prostatic growth and function by peptide growth factors. Prostate 1996, 28:392-405 [DOI] [PubMed] [Google Scholar]
- 4.Davies P, Eaton C: Regulation of prostate growth. J Endocrinol 1991, 131:5-17 [DOI] [PubMed] [Google Scholar]
- 5.Ropiquet F, Giri D, Lamb D, Ittmann M: FGF7 and FGF2 are increased in benign prostatic hyperplasia and are associated with increased proliferation. J Urol 1999, 162:595-599 [PubMed] [Google Scholar]
- 6.Giri D, Ropiquet F, Ittmann M: Alterations in expression of FGF2 and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res 1999, 5:1063-1071 [PubMed] [Google Scholar]
- 7.Giri D, Ropiquet F, Ittmann M: FGF9 is an autocrine and paracrine prostatic growth factor expressed by prostatic stromal cells. J Cell Physiol 1999, 180:53-60 [DOI] [PubMed] [Google Scholar]
- 8.Ittmann M, Mansukhani A: Expression of fibroblast growth factors (FGFs) and FGF receptors in human prostate. J Urol 1997, 157:351-356 [PubMed] [Google Scholar]
- 9.Finch PW, Lengel C, Chedid M: Cloning and characterization of the promoter region of the human keratinocyte growth factor gene. J Biol Chem 1995, 270:11230-11237 [DOI] [PubMed] [Google Scholar]
- 10.Tang A, Gilchrest B: Regulation of keratinocyte growth factor gene expression in human skin fibroblasts. J Dermatol Sci 1996, 11:41-50 [DOI] [PubMed] [Google Scholar]
- 11.Brauchle M, Angermeyer K, Hubner G, Werner S: Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene 1994, 9:3199-3204 [PubMed] [Google Scholar]
- 12.Chedid M., Rubin J, Csaky K, Aaronson S: Regulation of keratinocyte growth factor gene expression by interleukin 1. J Biol Chem 1994, 269:10753–10757 [PubMed]
- 13.Dinarello C: Interleukin-1. The Cytokine Handbook, ed 3. Edited by A Thomson. New York, Academic Press, 1998, pp 35–72.
- 14.Kyprianou N, Tu H, Jacobs S: Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum Pathol 1996, 27:668-675 [DOI] [PubMed] [Google Scholar]
- 15.Begun F, Story M, Hopp K, Shapiro E, Lawson R: Regional concentration of basic fibroblast growth factor in normal and benign hyperplastic prostates. J Urol 1995, 153:839-843 [PubMed] [Google Scholar]
- 16.Shapiro E, Hartanto V, Perlman E, Tang R, Wang B, Lepor H: Morphometric analysis of pediatric and nonhyperplastic glands: evidence that BPH is not a unique stromal process. Prostate 1997, 33:177-182 [DOI] [PubMed] [Google Scholar]
- 17.Watanabe N, Kobayashi Y: Selective release of a processed form of interleukin 1 alpha. Cytokine 1994, 6:597-601 [DOI] [PubMed] [Google Scholar]
- 18.Anim JT, Udo C, John B: Characterization of inflammatory cells in benign prostatic hyperplasia. Acta Histochem 1998, 100:439-449 [DOI] [PubMed] [Google Scholar]
- 19.Nickel JC, Downey J, Young I, Boag S: Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. Br J Urol Int 1999, 84:976-981 [DOI] [PubMed] [Google Scholar]
- 20.Yan G, Fukabori Y, Nikolaropoulos S, Wang F, McKeehan W: Heparin-binding keratinocyte growth factor is a candidate stromal to epithelial cell andromedin. Mol Endocrinol 1992, 6:2123-2128 [DOI] [PubMed] [Google Scholar]
- 21.Nemeth J, Zelner D, Lang S, Lee C: Keratinocyte growth factor in the rat ventral prostate: androgen-independent expression. J Endocrinol 1998, 156:115-125 [DOI] [PubMed] [Google Scholar]