Abstract
Nestin is an intermediate filament most related to neurofilaments and expressed predominantly in the developing nervous system and muscles. In the present study we examined the in vivo distribution of nestin in human teeth during embryonic development and in permanent teeth under normal and pathological conditions. The results show that nestin is first expressed at the bell stage and that its distribution is restricted in pulpal cells located at the cusp area of the fetal teeth. In young permanent teeth, nestin is found only in functional odontoblasts, which produce the hard tissue matrix of dentin. Expression is progressively down-regulated and nestin is absent from older permanent teeth. In carious and injured teeth, nestin expression is up-regulated in a selective manner in odontoblasts surrounding the injury site, showing a link between tissue repair competence and nestin up-regulation under pathological conditions. In an in vitro assay system of human dental pulp explants, nestin is up-regulated after local application of bone morphogenic protein-4. A similar effect is seen in cultures of primary pulp cells during their differentiation into odontoblasts. Taken together, these results suggest that nestin plays a potential role in odontoblast differentiation during normal and pathological conditions and that bone morphogenic protein-4 is involved in nestin up-regulation.
The cytoskeleton is formed by three types of filamentous structures: microtubuli, microfilaments, and intermediate filaments (IFs). The IF family consists of more than 50 distinct proteins capable of forming morphologically similar filaments in different cell types. 1,2 The IFs can be divided into six types, based on sequence homology and exon/intron organization in the gene. Keratins belong to the type I and II IFs, expressed mainly in epithelia. Desmin, vimentin, glial fibrillary acidic protein (GFAP), and peripherin form the type III IFs. Type IV comprises the neurofilaments and α-internexin, which are expressed in neuronal tissues. Nuclear lamins belong to the type V IFs, whereas type VI is represented by nestin. However, nestin can be also included in type IV, based on similarities in exon/intron structure.
Little is yet known about the specific functions of individual intermediate filament genes, but there is an emerging picture that IFs are important for the organization and function of cells and tissues. For example, keratin gene mutations are the cause of genetic skin diseases such as epidermolysis bullosa simplex and epidermolytic hyperkeratosis. 3 Similarly, cardiac, skeletal, and smooth muscle phenotypes have been demonstrated in mice with targeted deletion of the desmin gene. 4,5 Furthermore, mutations in the human desmin gene can cause myopathy. 6,7 Functional inactivation of vimentin in mice results in dilated blood vessels, whereas in double-knockout mice for vimentin and GFAP, scar formation after central nervous system injury is compromised. 8
Nestin is mainly expressed at early stages of central nervous system and muscle development and is replaced by neurofilaments and GFAP in nervous tissue 9 and by desmin in muscle 10,11 at more advanced developmental stages. Nestin is also expressed in other tissues, such as in heart, 12 neural crest, 9 and testis. 13 In most of these tissues, nestin is generally expressed early during development and is down-regulated in mature tissues. 9,10 Nestin can, however, become re-expressed in pathological conditions, for example as a result of injury, trauma, or tumors. Nestin is thus up-regulated in reactive astrocytes after brain injury 14-16 and in tumors of the central and peripheral nervous systems. 17-19
Our previous work revealed a strict temporospatial pattern of nestin expression during rodent tooth development. 20 The developing tooth is an excellent model for studying the phenomena of spatial organization, symmetry, morphogenesis, and organ-specific cytodifferentiation. Teeth develop as a result of reciprocal inductive interactions between the oral ectoderm and cranial neural crest-derived mesenchymal cells. During advanced stages of tooth development, some of the ectomesenchymal cells differentiate into odontoblasts, synthesizing the dentin matrix. Odontoblasts form a layer with an epithelial appearance that serves as a protective barrier for the tooth pulp. Nestin is expressed in both epithelial and mesenchymal components of the developing rodent teeth and its expression progressively becomes restricted to differentiating odontoblasts. 20 By contrast to other organs, nestin is not down-regulated in mature functional odontoblasts. These data suggest that nestin may be involved in both odontoblast differentiation and function. It remains uncertain, however, whether nestin is important for tooth homeostasis and regeneration. Here, extending our previous studies to human teeth, we examined the pattern of nestin expression in embryonic and permanent intact teeth, as well as in injured and carious teeth. Furthermore, we studied the regulation of nestin expression in a well-established culture system of human pulp cells in vitro. 21 Our study shows that the distribution of nestin is dependent on the differentiation and functional status of odontoblasts and that its expression is regulated by bone morphogenic protein-4 (BMP4).
Materials and Methods
Materials
Preparation and characterization of the rabbit anti-nestin antiserum, which is raised against the c-terminal region of rat nestin, has been already described. 22 This antiserum specifically identifies nestin in immunohistochemistry and in Western blots 10,17 and does not cross-react with other known IFs. 22,23 Furthermore, this antiserum generates the same pattern in immunohistochemistry and Western blots as the monoclonal antibody against human nestin (no. 4350; Lendahl U, unpublished observations). Vector Vectastain ABC kit was purchased from Biosys (Compiègne, France). For the preparation of the culture media, all materials were purchased from Gibco BRL (Life Technologies Inc., Grand Island, NY). Other chemicals were obtained from Sigma (St. Louis, MO).
For cultures, minimum essential medium (MEM) was supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, 100 UI/ml penicillin, 100 μg/ml streptomycin (Biowhittaker, Gagny, France), and 0.25 μg/ml amphotericin B (Fungizone, Biowhittaker).
Embryonic Tissues
Human fetal tissues were obtained from legal abortions. The material comprised teeth from 5 fetuses aged 6–18 gestational weeks (g.w.). The gestation age was estimated from the fetal foot length and from the date of last menstruation of the mother. Embryos were noninfected, and all tissues were both macroscopically and microscopically normal. The fetuses were fixed immediately by the obstetrician in 10% buffered formalin for 48 hours to 5 days according to the fetus size. Maxillary and mandibular jaws from 7- to 15-week-old embryos and fetuses were embedded in Paraplast at 56°C; the samples from fetuses aged 16 to 18 g.w. were decalcified for 3 weeks in formic acid/10% formalin before embedding in Paraplast. Sections 4 to 6 μm thick were used for immunohistochemistry.
This study was carried out in compliance with the French legislation, after approval of the Regional Ethics Committee of Development and Reproduction of the Unité de Formation et de Recherche of Medicine of Rheims-France (Department of Developmental Biology, INSERM 314).
Permanent Teeth
The teeth used in this study were of three types: unerupted third molars extracted during normal treatment of 17-year-old patients, premolars of 11- to 15-year-old adolescents, and mature intact and carious teeth of 40-year-old patients. The teeth were freshly extracted and used in this study with the patient’s informed consent. The extracted teeth were fixed in 10% neutral buffered formalin for 24 hours, demineralized in sodium formiate for 21 days, and then embedded in paraffin wax. They were serially sectioned (6-μm-thick sections) and then processed for immunohistochemistry.
Cavities Preparation and Tooth Processing
Cavities were prepared in intact first premolars scheduled for extraction at the Dental Care Center of Marseille. Cavities were prepared first by means of an intermittent application of an Airotor with water coolant to remove the enamel. Cavities 2 to 3 mm wide and 1 to 1.3 mm deep were then cut into the tooth dentin with a bur using the least possible pressure. The pulp chambers were not exposed during the preparation of the cavities. The walls of the cavities were immediately conditioned with a 3% hydrogen peroxide solution and dried with an extremely light stream. The cavities were then restored with the calcium hydroxide product Dycal (Dentsply, York, PA) covered by IRM (De Trey Dentsply, Zurich, Switzerland), a temporary filling material.
After a postoperative interval of 9 weeks, the teeth were extracted using a local anesthetic with the patient’s informed consent.
Teeth with cavities or carious lesions were fixed in 10% neutral buffered formalin for 24 hours, demineralized in sodium formiate for 21 days, and then routinely processed and embedded in paraffin wax.
Explant Culture of Human Pulp
Immediately after extraction of the third molars of the 17-year-old patients, the teeth were swabbed with 70% (v/v) alcohol and the soft tissue was removed with curettes. The teeth were then washed with sterile phosphate-buffered saline (PBS) and transferred into a laminar flow tissue culture hood to perform the rest of the procedures under sterile conditions. The apical part of the teeth was removed with scalpels, and the dental pulps were gently removed with forceps. Each dental pulp was minced separately with scalpels and then rinsed with PBS. After mincing, each tooth pulp explant was cultured in 100-mm diameter culture dishes (Becton Dickinson Labware, Lincoln Park, NJ) in MEM supplemented with 2 mmol/L β-glycerophosphate (Sigma Chemical Co.). The explants were cultured at 37°C in a humidified atmosphere of 5% CO2, 95% air and the culture medium was changed every other day. Confluent cultures were collected by trypsinization (0.2% trypsin and 0.02% EDTA). The cells were plated at 3 × 103/cm 2 on tissue culture-treated 8-chambered glass slides (Becton Dickinson Labware). After 3 weeks of culture, the cells were fixed with 70% ethanol for 1 hour at 4°C and processed for immunohistochemistry.
Recombinant Protein and Treatment of the Beads
BMP4 (a gift of the Genetics Institute, Cambridge, MA) was used to preload agarose beads (1 μl of a solution of 100–250 μg/ml per 10 beads). As a control, we used beads preloaded with 0.2% bovine serum albumin (BSA) in PBS. Beads were transferred on top of human dental pulp explants, and after 24 hours of culture in absence of β-glycerophosphate the explants were fixed in 4% paraformaldehyde and processed for whole mount immunohistochemistry as described previously. 24,25
Immunohistochemistry on Sections and on Cell Cultures
Immunoperoxidase staining on sections was done as previously described. 24,26 Briefly, the sections were deparaffinized, exposed to a 0.3% solution of hydrogen peroxide in methanol, and then incubated overnight at 4°C with the anti-nestin antibody diluted 1:1500 in PBS containing 0.2% BSA. Peroxidase was revealed by incubation with 3,3-diaminobenzidine tetrahydrochloride (DAB) reaction solution. After staining the sections were mounted with Eukitt (Labonord, Villeneuve d’Ascq, France). In control sections the primary antibodies were omitted.
On cell cultures, the cells were permeabilized for 15 minutes with 0.5% Triton X-100 in PBS before immunohistochemistry. Peroxidase was revealed by incubation with 3-amino-9-ethylcarbazole (AEC) reaction solution and then the slides were mounted with Aquamount (BDH Laboratory Supplies, Gurr, UK).
Results
Nestin Expression in the Developing Deciduous Human Tooth Germs
In 5-week-old human embryos, the oral epithelium proliferates into the subjacent mesenchyme and forms a series of epithelial ingrowths into the neural crest-derived mesenchyme at sites corresponding to the position of the future deciduous teeth. From this point tooth development proceeds in three descriptive stages: the bud, cap, and bell stage. At 6 g.w. (Figure 1A) ▶ , the epithelial ingrowth gives rise to the epithelial dental bud. At this stage, immunoreactivity for nestin was not observed in either the epithelial or mesenchyme components (Figure 1D) ▶ . During the cap stage of development (8–15 g.w.), the dental epithelium forms the enamel organ and the mesenchyme condenses to form the dental papilla (Figure 1B) ▶ . At this stage, nestin was absent from cells of the enamel epithelium and the dental papilla (Figure 2E) ▶ .
Figure 1.
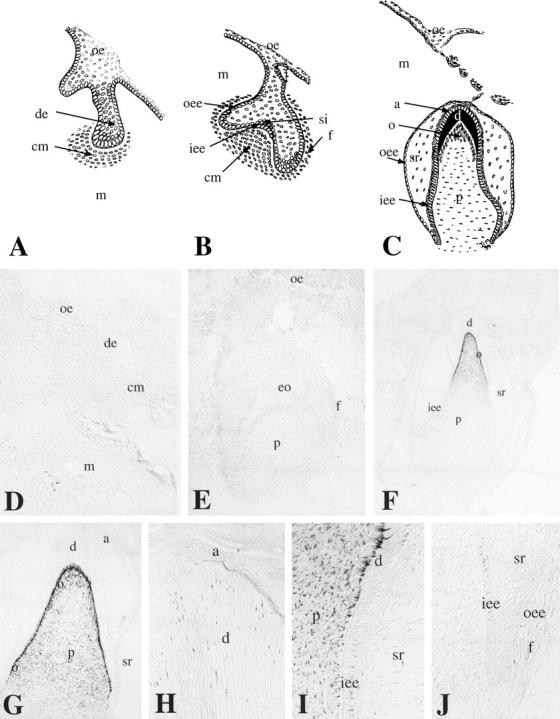
Schematic representation of embryonic human tooth development and immunohistochemical localization of nestin in sections of developing deciduous human teeth. Bud (A), cap (B), and bell (C) stages. D and E: Nestin immunoreactivity is absent from dental tissues at the bud (D) and cap (E) stages. F: At the late bell stage, nestin staining is observed in dental pulp cells of the cusp region and in differentiating odontoblasts. Nestin is absent from dental pulp cells of the cervical region and enamel epithelium. G: Higher magnification of F. Note that gradient of nestin staining in odontoblasts follows their differentiation gradient: the staining is localized at the cusp region. H: Dentin at the cusp area. Note the presence of nestin immunoreactivity in the odontoblast processes. I: Many pulp cells at the cusp region are positive for nestin. Note the gradient of nestin immunoreactivity in cells in close contact with the dentin (differentiating odontoblasts). J: Nestin is absent from the apical region of the tooth. a, ameloblasts; cm, condensed mesenchyme; d, dentin; de, dental epithelium; f, dental follicle; eo, enamel organ; iee, inner enamel epithelium; m, mesenchyme; o, odontoblast; oe, oral epithelium; oee, outer enamel epithelium; p, dental papilla (pulp); si, stratum intermedium; sr, stellate reticulum. Scale bars, 100 μm (D, E, G), 200 μm (F), and 50 μm (H−J).
Figure 2.
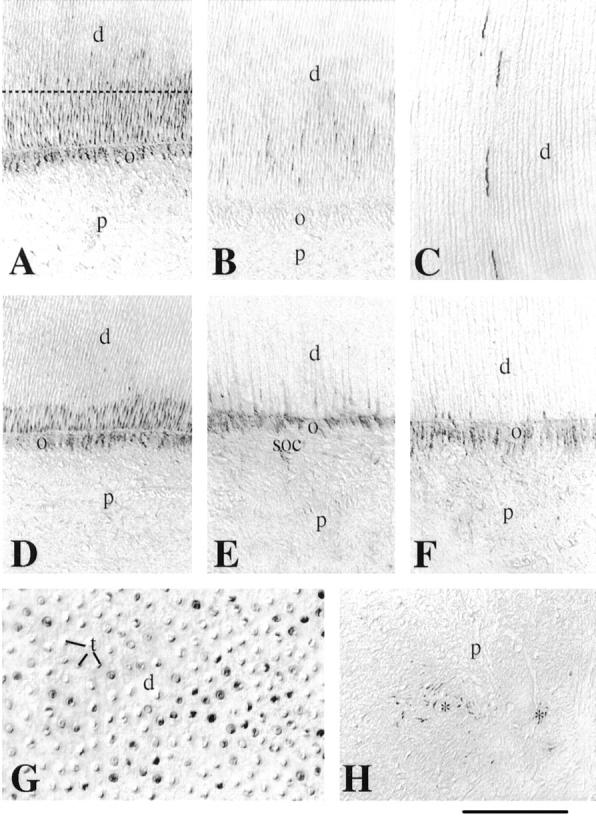
Immunohistochemical localization of nestin in sections of developing permanent human teeth. A: Crown area. Nestin immunoreactivity is observed in the cell bodies and processes of the odontoblasts. Note that the other pulp cells are negative for nestin. B: In the cusp of the crown region nestin staining is seen only in the odontoblastic processes. C: Nestin immunoreactivity is observed in some odontoblast processes near the dentin-enamel junction. D–F: From the middle of the crown (D) until the apical part of the root (F), nestin staining decreases in the odontoblastic processes and is limited in the odontoblastic cell bodies at the apical root area (F). Note the faint immunoreactivity in cells of the subodontoblastic layer at the root area (E). G: Section passing through the dotted line of A. Note the dentinal tubuli and the positive staining representing the odontoblastic processes. H: Immunoreactivity is observed in some pulp cells at the proximity of blood vessels (asterisks). d, dentin; o, odontoblasts; p, pulp; soc, subodontoblastic cells; t, dentinal tubuli. Scale bars, 50 μm (A, B, D–F, H) and 35 μm (C and G).
Continued growth of the tooth germ leads to the bell stage of tooth development (Figure 1C) ▶ . Dentinogenesis is initiated at the tip of the cusp and the tooth shape (crown morphology) is apparent. The pulp cells adjoining the dental epithelium differentiate into odontoblasts, and start to secrete the organic matrix of dentin. After predentin mineralization the inner enamel epithelial cells differentiate into ameloblasts and start to secrete enamel matrix proteins. During the bell stage (18 g.w.), nestin immunoreactivity was observed in the odontoblasts and pulp fibroblasts of the cusp area (Figure 1, F and G) ▶ . Nestin was also expressed in the odontoblastic processes up to the dentin-enamel junction (Figure 1H) ▶ and in pulp cells (Figure 1, G and I) ▶ . A staining gradient was observed in the odontoblasts from the cervical loop to the cusp region: the cervical loop was negative (Figure 1J) ▶ , whereas the immunostaining increased toward the cusp region (Figure 1, F, G, and I) ▶ . Labeling was absent from all dental epithelial cells.
Nestin Expression in Developing and Mature Permanent Teeth
In the 17-year-old developing third molars, nestin expression was restricted to odontoblasts. Distribution was seen in both the odontoblast cell bodies (Figure 2, A and D ▶ –F) and the odontoblast processes (Figure 2, B, C, and G) ▶ . The distribution of nestin in the odontoblasts exhibited a gradient after the maturation state. In more mature odontoblasts, in the cusp region of the crown, nestin immunoreactivity was observed in their processes, but not in their cell bodies (Figure 2B) ▶ . Some of the odontoblast processes reach the dentin-enamel junction and were nestin-positive (Figure 2C) ▶ . In the younger odontoblasts, ie, in the apical root region, nestin immunostaining was restricted to the cell bodies (Figure 2F) ▶ . In odontoblasts situated at the intermediate area, between the cusps and the apical root regions, nestin expression decreased in the odontoblast processes and increased in the cell bodies, following the crown-root direction (Figure 2, A, D, and E) ▶ . Immunoreactivity for nestin was also observed in some pulpal fibroblasts in the proximity of blood vessels (Figure 2H) ▶ .
Nestin Expression in Carious Human Teeth
Nestin immunoreactivity was completely absent in intact teeth from a 40-year-old patient (Figure 3A) ▶ , but nestin staining is observed in mature carious teeth. Expression was seen in cells surrounding the carious lesion (Figure 3B) ▶ . Nestin is distributed in the processes of mature odontoblasts situated in the proximity of the carious front (Figure 3, C and D) ▶ , but nestin immunoreactivity was absent in bacteria located at the carious front level (Figure 3D) ▶ . In advanced carious lesion, an inflammation can be observed in the pulp of the teeth. Disintegrated odontoblastic cell bodies and pulp fibroblasts facing the lesion were negative for nestin in such conditions (Figure 3E) ▶ . In some cases, the carious lesions are responsible for the hyperemia of the pulp. During hyperemic conditions, nestin staining is observed in the cell bodies of the odontoblasts facing the lesion and, in pulp fibroblasts, near the dilated blood vessels (Figure 3F) ▶ .
Figure 3.
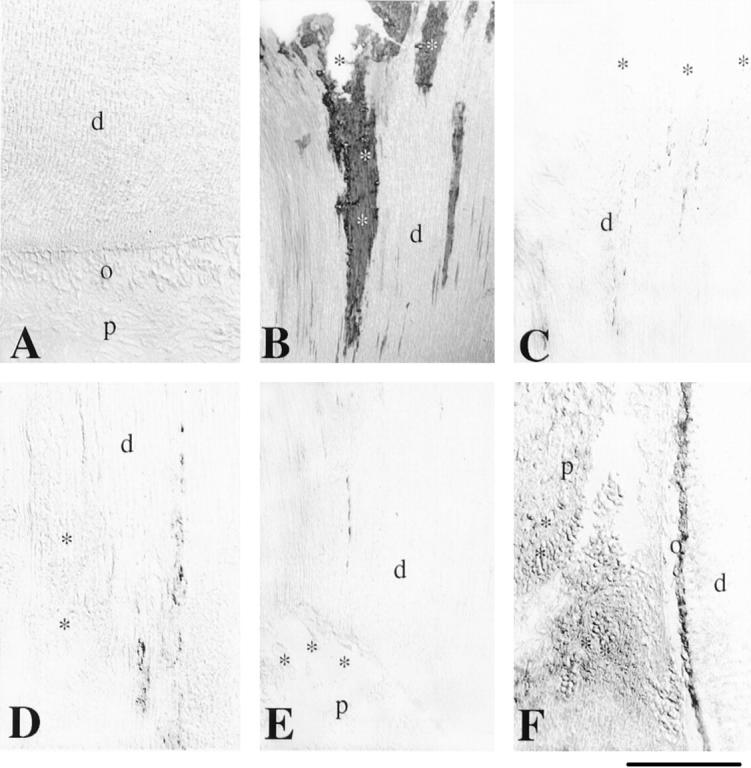
Immunohistochemical localization of nestin in sections of normal and carious mature permanent human teeth. A: Nestin staining is absent from the normal mature teeth. B: In carious teeth, bacterial infiltration of dentin is shown (asterisks) after a gram staining for tissues. C: In carious teeth, nestin reactivity is found in some odontoblastic processes approaching the dentin-enamel junction and near of the carious front (asterisks). D: In the carious front (asterisks), nestin immunostaining is absent, while the staining is strong in the odontoblastic processes near to the carious front. E: Nestin immunoreactivity is absent in the odontoblast bodies (asterisks) of the inflamed pulp. F: Nestin is observed in odontoblast bodies facing the carious irritation and cells in the proximity of the dilated blood vessels (asterisks). d, dentin; o, odontoblasts; p, pulp. Scale bar, 50 μm.
Nestin Expression in the Mature Permanent Human Teeth after Injury
Nine weeks after the cavity preparation, reactionary dentin deposition was seen near to the injury site (Figure 4A) ▶ . This reactionary dentin matrix is synthesized by newly formed odontoblasts (odontoblast-like cells) replacing dying odontoblasts after the injury. Nestin immunoreactivity was not detected at the site of the reactionary dentin production 9 weeks after the lesion (Figure 4B) ▶ , but was evident at a distance from the cavity preparation. Nestin was distributed mainly in the odontoblast processes (Figure 4, C and D) ▶ , whereas a weak staining was found in the odontoblast cell bodies (Figure 4C) ▶ .
Figure 4.
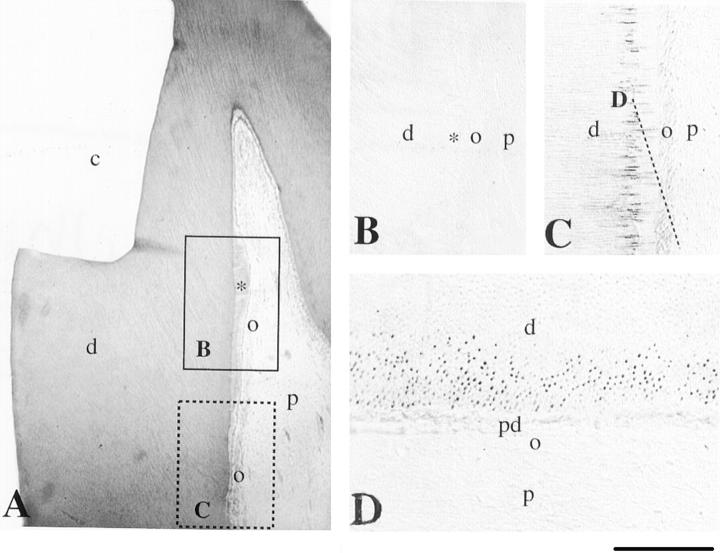
Immunohistochemical localization of nestin in sections of mature permanent human teeth after cavity preparation. A: Hematoxylin-eosin staining. Nine weeks after the cavity preparation, reactionary dentin (asterisk) is seen near to the site of the injury. Frames represent the sites shown in B and C. B: Nestin is absent from in the newly formed odontoblasts. C and D: Nestin reactivity is observed in the odontoblast processes at a site distal of the injury site. Note the weak staining in the odontoblastic bodies. D: Section passing through the dotted line of C. c, cavity; d, dentin; o, odontoblasts; p, pulp; pd, predentin. Scale bars, 80 μm (A) and 50 μm (B--D).
Nestin Expression in Human Tooth Pulp Cells in Vitro
After 3 weeks of culture of human pulp explants, fiber-like structures started to appear from the explant border and extended toward the peripheral parts. This was followed by the deposition of mineral crystals along and within the fibrous structures in pulp explants cultured in presence of β-glycerophosphate. This mineralization front continued to expand during the 8-week culture procedure; furthermore, new nodules were formed during this culture period. The cells in direct contact with the nodules exhibited a polarized morphology similar to that observed in vivo (Figure 5A) ▶ . Mineral nodules were not observed in pulp explants cultured in absence of β-glycerophosphate (Figure 5F) ▶ . Nestin immunoreactivity was observed in pulp cells treated with β-glycerophosphate (Figure 5, B ▶ -E). The staining was very strong in polarized cells contacting the mineralization nodules (Figure 5, C and D) ▶ , whereas a faint staining was observed in cells at a distance from the nodules. Pulp cells cultured in absence of β-glycerophosphate were nestin-negative (Figure 5G) ▶ .
Figure 5.
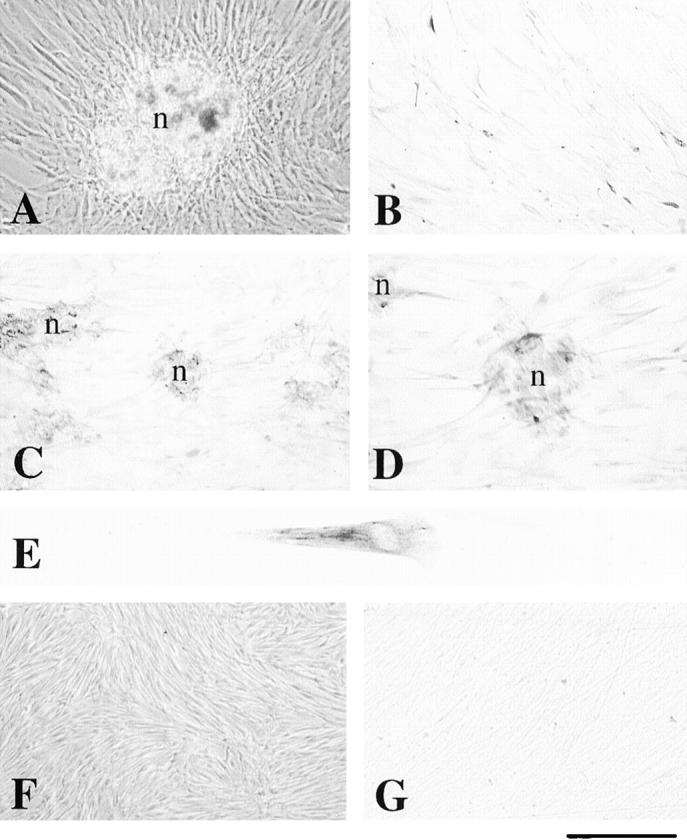
Nestin immunoreactivity in human dental pulp cells in vitro after β-glycerophosphate treatment. A: Phase contrast microscopy showing multilayered cells and the mineralization nodule formation after three weeks of culture in presence of β-glycerophosphate. B: Confluent pulp cells treated with β-glycerophosphate demonstrate weak nestin immunoreactivity. C and D: A strong staining for nestin is observed in polarized cells implicated in the formation of the nodules. E: High magnification of a polarized dental pulp cell showing nestin staining in its cytoskeleton. F: Phase contrast microscopy showing confluent pulp cells after three weeks of culture in absence of β-glycerophosphate. Note the absence of mineralized nodules. G: Nestin immunoreactivity is absent in pulp cells cultured without β-glycerophosphate. n, nodule. Scale bars, 100 μm (A and F); 50 μm (B, C, and G), 35 μm (D), and 10 μm (E).
BMP4 Up-Regulates Nestin Expression in Human Dental Pulp Cells
It has been shown that BMPs are involved in odontoblast differentiation, 27 suggesting that these signaling molecules may play a role in the regulation of nestin expression in the dental pulp mesenchyme. To test this, we placed beads releasing BMP4 on top of dental pulp explants from a 17-year-old patient and followed the expression of nestin by whole mount immunohistochemistry. Analysis of the explants showed nestin immunoreactivity in pulp cells surrounding beads containing BMP4 (Figure 6, A ▶ -C). BMP4 up-regulated nestin expression in a wide area of cells surrounding the bead. As a control, we used beads soaked in BSA, and in no case was nestin expression induced (Figure 6, D and E) ▶ .
Figure 6.
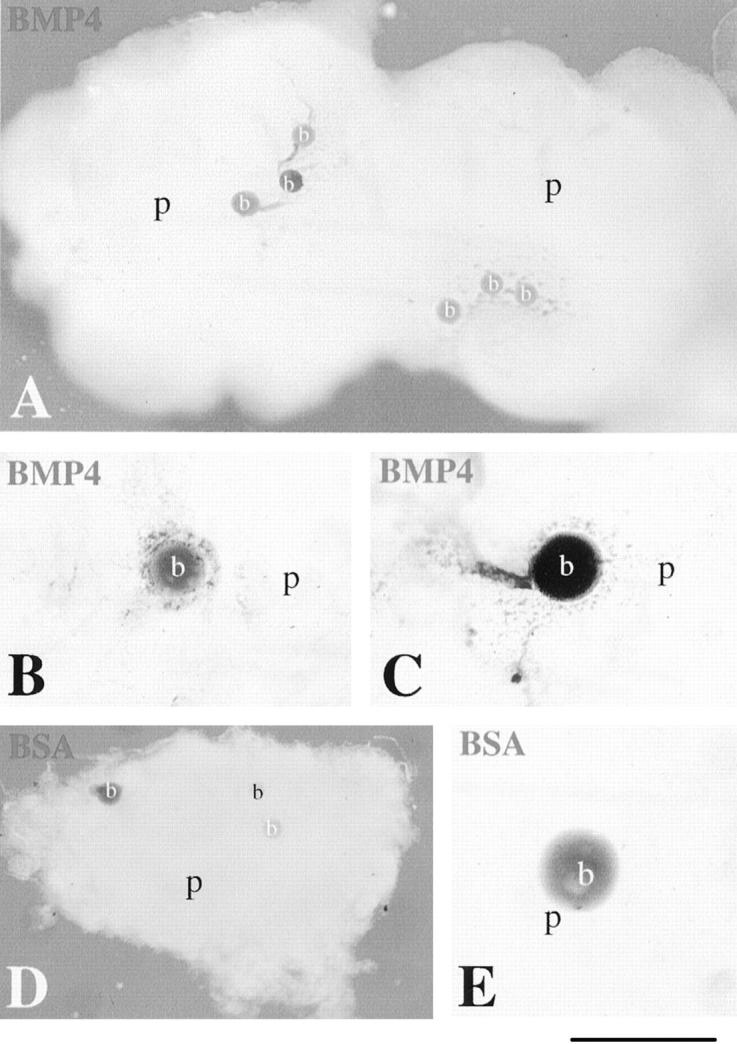
Effects of BMP4 on nestin up-regulation in human dental pulp explants. The explants were cultured for 24 hours. Whole mount immunostaining using the anti-nestin antiserum is shown. A–C: Beads soaked in 100 μg/ml of BMP4 were placed on top of 17-year-old dental pulp explants and then were cultured together. Nestin immunoreactivity is found in the pulpal cells surrounding the BMP4 beads. D and E: The BSA bead does not induce nestin expression in pulp cells. b, bead; p, pulp. Scale bars, 500 μm (A), 200 μm (B, C, and E), and 600 μm (D).
Discussion
Nestin in Developing and Intact Adult Human Teeth
In this report, we show that the IF protein nestin is expressed during human tooth development, is down-regulated in adult teeth, and finally is up-regulated under pathological conditions such as carious lesions and dental wound healing. The nestin expression pattern in human teeth differs from that previously reported in the teeth of rodents. 20 Nestin is first expressed at the bell stage of the developing human teeth, whereas in the rat molars nestin was first detected at the bud stage. Nestin expression is down-regulated in odontoblasts of mature permanent human teeth, whereas nestin expression is maintained in odontoblasts of adult rats. Finally, nestin expression in the human teeth is restricted to the odontoblasts and pulp fibroblasts, whereas in the rat molars nestin is also expressed in cells of the dental epithelium. The reason for this species difference in expression is not known, but may be the difference in life span between rodents and human, the single continuously erupting dentition of rodents, or the different use of teeth. The expression pattern of nestin in embryonic human teeth, ie, a transient expression during development of the organ, is quite similar to nestin expression seen in other tissues, eg, the nervous system and muscle. 9,10
In the developing deciduous tooth germs, nestin expression in the odontoblasts increases from the apical to the cusp area following a cytodifferentiation gradient. In the developing permanent teeth, the inverse gradient is observed: nestin expression is maintained only in the processes, but not in the cell bodies, of the odontoblasts of the cusp area, where they have completed their maturation. Nestin distribution decreases in the odontoblast processes and increases in the cell bodies toward the apical area. The exact function of nestin has not been established, but its expression in the cell bodies suggests a role in the dentin matrix synthesis, while its presence in the odontoblast processes is not clear. Odontoblast processes are accompanied by small nerve fibers in the dentinal tubuli, but direct contacts between these two structures have not been reported. It has been shown recently that the distribution and expression of nestin in myofibers are regulated by innervation, 28 suggesting a similar effect in odontoblasts.
Although nestin can form homodimers, 29 it requires the presence of other IFs for proper filament formation. 2,29,30 Nestin transfected into IF-negative cells fails to generate an IF network, 30 and, in mice targeted for both vimentin and GFAP, the nestin protein is diffusely spread in reactive astrocytes. 8 Nestin is not the only IF protein expressed in odontoblasts and pulp cells of the teeth. Desmin 31 and vimentin 32 are expressed in dental pulp cells, but only vimentin is detected in odontoblasts. 31-33 Taken together, these data suggest that nestin may copolymerize with vimentin to form IFs in the cell body and process of the odontoblastic cell.
Nestin in Irritated Adult Human Teeth
Microorganisms are involved in both decalcification and proteolysis of the dentin during the process of dental caries. The pulp defends itself against the lesion of caries; in response to the oncoming process of decay, the tubuli of the dentin gradually become calcified, provided that the odontoblasts remain vital. In the event that the irritation increases, the odontoblasts degenerate and form dead tracts. In response to further irritation, the secretory activity of the remaining vital odontoblasts is stimulated to elaborate reactionary dentin. In effect, the pulp volume is reduced with the elaboration of reactionary dentin, and the aging process of the pulp is accelerated. Nestin is re-expressed in the processes of odontoblasts surrounding the carious lesion, suggesting a role for nestin in the elaboration of the reactionary dentin (hypercalcification). The pulp does not become inflamed until wide areas of dentinal tubuli are decalcified. When the caries progress more rapidly than the elaboration of reactionary dentin, the blood vessels of the pulp dilate, and scattered inflammatory cells become evident in the pulp. Nestin is expressed in dying odontoblasts facing the irritation front and forming the dead tracts, as well as in inflammatory cells close to the dilated blood vessels. This indicates a correlation between nestin up-regulation and inflammatory events.
Odontoblasts are directly damaged by cavity preparations involving the dentin. The degenerated odontoblasts may affect the neighboring odontoblasts, which, in turn, die. Disturbance of the odontoblastic layer affects the elaboration of dentin: the quantity and the quality of the deposited dentin matrix change. In this case, newly formed odontoblast-like cells, originating from dental pulp cells, 34,35 replace the injured odontoblasts and elaborate the reparative dentin. 36-38 Despite the absence of nestin at the site of the injury, which may be due to the delay between cavity preparation and tooth extraction, nestin is localized to the processes of odontoblasts located at a distance from the injury site. These results suggest that nestin may be involved in reparative processes, most notably in the secretory activity of the odontoblastic cell after dental injury. It is worth noting that nestin is often re-expressed under pathological conditions. Nestin is, for example, up-regulated in reactive astrocytes after central nervous system injury 14,15 and in different types of tumors in the nervous system. 17-19 Similarly, nestin is up-regulated in pathological conditions such as Duchenne’s and Becker’s muscular dystrophy 22 and in tumors of muscle origin. 39
Regulation of Nestin Expression in Dental Pulp
BMPs are good candidates for involvement in signaling within the dental mesenchyme. During the late bell stage of mouse tooth development, BMP2 and BMP4 are expressed in differentiating odontoblasts. 40,41 Furthermore, BMPs have been shown to induce odontoblast differentiation in vitro. 27 Here, we show that BMP4 up-regulates nestin expression in the mesenchyme when applied to dental pulp explants. This is the first example of a signaling molecule directly influencing nestin expression, and may explain some aspects of nestin expression during embryonic development, for example, in migrating neural crest cells. 42,43
Nestin is also up-regulated in dental pulp cells which have differentiated to odontoblast-like cells and secreted the matrix of the mineralization nodules in an in vitro assay system. 21 This confirms the re-expression of nestin in the secretory odontoblast-like cells and the potential of pulpal cells to differentiate into dentin secretory cells. Dentin matrix can be seen as a reservoir for signaling molecules such as BMPs and fibroblast growth factors. During dentin decalcification, after a carious irritation, these signaling molecules are released from the matrix and can diffuse to reach adjecent cells. Dental pulp cells in the vicinity of the lesion, under the influence of the diffused BMP4, could then differentiate into odontoblast-like cells and start the secretion and deposition of the reparative dentin matrix.
In conclusion, the present results demonstrate that nestin is expressed not only during tooth development but also under pathological conditions such as carious and operative lesions of the tooth. The limited nestin distribution remaining in differentiated odontoblasts may be associated with some IF functions during dentin matrix deposition. Finally, nestin expression in dental pulp cells and odontoblasts is up-regulated by BMP4.
Acknowledgments
We thank Ms. Mireille Remusat for skillful technical assistance.
Footnotes
Address reprint requests to Tim Mitsiadis, Laboratoire IMEB, Faculté d’Odontologie, 27 Boulevard Jean Moulin, 13385 Marseille, France. E-mail: mitsiadis.e@odontologie.univ-mrs.fr.
Supported by institutional grants from the Université de la Méditerranée and by specific grants of the Ligue Nationale Contre le Cancer and the Association pour la Recherche sur le Cancer (ARC).
References
- 1.Fuchs E, Weber K: Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem 1994, 63:345-382 [DOI] [PubMed] [Google Scholar]
- 2.Parry DAD, Steinert PM: Intermediate filament structure. 1995. R. G. Landes and Co., Austin TX
- 3.Fuchs E, Coulombe PA: Of mice and men: genetic skin diseases of keratin. Cell 1992, 69:899-902 [DOI] [PubMed] [Google Scholar]
- 4.Capetanaki Y, Milner DJ, Weitzer G: Desmin in muscle formation and maintenance: knockouts and consequences. Cell Struct Funct 1997, 22:103-116 [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Colucci-Guyon E, Pincon-Raymond M, Mericskay M, Pournin S., Paulin D, Babinet C: Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev Biol 1996, 175:362–366 [DOI] [PubMed]
- 6.Munoz-Marmol AM, Strasser G, Isamat M, Coulombe PA, Yang Y, Roca X, Vela E, Mate JL, Coll J, Fernandez-Figueras MT, Navas-Palacios JJ, Ariza A, Fuchs E: A dysfunctional desmin mutation in a patient with severe generalized myopathy. Proc Nat Acad Sci USA 1998, 95:11312-11317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldfarb LG, Park KY, Cervenakova L, Gorokhova S, Lee HS, Vasconcelos O, Nagle JW, Semino-Mora C, Sivakumar K, Dalakas MC: Missense mutations in desmin associated with familial cardiac and skeletal myopathy. Nat Genet 1998, 19:402-403 [DOI] [PubMed] [Google Scholar]
- 8.Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallen A, Perlmann T., Lendahl U, Betsholtz C, Berthold CH, Frisen J: Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol 1999, 145:503–514 [DOI] [PMC free article] [PubMed]
- 9.Lendahl U, Zimmerman LB, McKay RDG: CNS stem cells express a new class of intermediate filament protein. Cell 1990, 60:585-595 [DOI] [PubMed] [Google Scholar]
- 10.Sejersen T, Lendahl U: Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci 1993, 106:1291-1300 [DOI] [PubMed] [Google Scholar]
- 11.Kachinsky AM, Dominov JA, Miller JB: Myogenesis and the intermediate filament protein, nestin. Dev Biol 1994, 165:216-228 [DOI] [PubMed] [Google Scholar]
- 12.Kachinsky AM, Dominov JA, Miller JB: Intermediate filaments in cardiac myogenesis: nestin in the developing mouse heart. J Histochem Cytochem 1995, 43:843-847 [DOI] [PubMed] [Google Scholar]
- 13.Frojdman K, Pelliniemi LJ, Lendahl U, Virtanen I, Eriksson JE: The intermediate filament protein nestin occurs transiently in differentiating testis of rat and mouse. Differentiation 1997, 61:243-249 [DOI] [PubMed] [Google Scholar]
- 14.Frisen J, Johansson CB, Torok C, Risling M, Lendahl U: Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol 1995, 131:453-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin RCS, Matesic DF, Marvin M, McKay RD, Brustle O: Re-expression of the intermediate filament nestin in reactive astrocytes. Neurobiol Dis 1995, 2:79-85 [DOI] [PubMed] [Google Scholar]
- 16.Holmin S, Almqvist P, Lendahl U, Mathiesen T: Adult nestin-expressing subependymal cells differentiate to astrocytes in response to brain injury. Eur J Neurosci 1997, 9:65-75 [DOI] [PubMed] [Google Scholar]
- 17.Dahlstrand J, Collins PV, Lendahl U: Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res 1992, 52:5334-5341 [PubMed] [Google Scholar]
- 18.Tohyama T, Lee VMY, Rorke LB, Marvin M, McKay RDG, Trojanowski J: Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest 1992, 66:303-313 [PubMed] [Google Scholar]
- 19.Florenes VA, Holm R, Myklebost O, Lendahl U, Fodstad O: Expression of the neuroectodermal intermediate filament nestin in human melanomas. Cancer Res 1994, 54:354-356 [PubMed] [Google Scholar]
- 20.Terling C, Rass A, Mitsiadis TA, Fried K, Lendahl U, Wroblewski J: Expression of the intermediate filament nestin during rodent tooth development. Int J Dev Biol 1995, 39:947-956 [PubMed] [Google Scholar]
- 21.Hao JJ, Shi JN, Niu ZY, Xun WX, Yue L, Xiao MZ: Mineralized nodule formation by human dental papilla cells in culture. Eur J Oral Sci 1997, 105:318-324 [DOI] [PubMed] [Google Scholar]
- 22.Sjöberg G, Edström L, Lendahl U, Sejersen T: Myofibers from Duchenne/Becker muscular dystrophy and myositis express the intermediate filament nestin. J Neuropathol Exp Neurol 1994, 53:416-423 [DOI] [PubMed] [Google Scholar]
- 23.Sjöberg G, Jiang WO, Ringertz NR, Lendahl U, Sejersen T: Colocalization of nestin and vimentin/desmin in skeletal muscle cells demonstrated by three-dimensional fluorescence digital imaging microscopy. Exp Cell Res 1994, 214:447-458 [DOI] [PubMed] [Google Scholar]
- 24.Mitsiadis TA, Muramatsu T, Muramatsu H, Thesleff I: Midkine (MK), a heparin-binding growth/differentiation factor, is regulated by retinoic acid and epithelial-mesenchymal interactions in the developing mouse tooth, and affects cell proliferation and morphogenesis. J Cell Biol 1995, 129:267-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsiadis TA, Hirsinger E, Lendahl U, Goridis C: Delta-notch signaling in odontogenesis: correlation with cytodifferentiation and evidence for feedback regulation. Dev Biol 1998, 204:420-431 [DOI] [PubMed] [Google Scholar]
- 26.Mitsiadis TA, Dicou E, Joffre A, Magloire H: Immunohistochemical localization of nerve growth factor (NGF) and NGF receptor (NGF-R) in the developing first molar tooth of the rat. Differentiation 1992, 49:47-61 [DOI] [PubMed] [Google Scholar]
- 27.Ruch JV, Lesot H, Bègue-Kirn C: Odontoblast differentiation. Int J Dev Biol 1995, 39:51-68 [PubMed] [Google Scholar]
- 28.Vaittinen S, Lukka R, Sahlgren C, Rantanen J, Hurme T, Lendahl U, Eriksson JE, Kalimo H: Specific and innervation-regulated expression of the intermediate filament protein nestin at neuromuscular and myotendinous junctions in skeletal muscle. Am J Pathol 1999, 154:591-600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinert PM, Chou YH, Prahlad V, Parry DAD, Marekov LN, Wu KC, Jang SI, Goldman RD: A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. J Biol Chem 1999, 274:9881-9890 [DOI] [PubMed] [Google Scholar]
- 30.Marvin MJ, Dahlstrand J, Lendahl U, McKay RD: A rod end deletion in the intermediate filament protein nestin alters its subcellular localization in neuroepithelial cells of transgenic mice. J Cell Sci 1998, 111:1951-1961 [DOI] [PubMed] [Google Scholar]
- 31.Lombardi T, Samson J, Mühlhauser J, Fiore-Donno G, Maggiano N, Castellucci M: Expression of intermediate filaments and actins in human dental pulp and embryonic dental papilla. Anat Rec 1992, 234:587-592 [DOI] [PubMed] [Google Scholar]
- 32.Lesot H, Meyer JM, Ruch JV, Weber K, Osborn M: Immunofluorescent localization of vimentin, prekeratin, and actin during odontoblast and ameloblast differentiation. Differentiation 1982, 21:133-137 [DOI] [PubMed] [Google Scholar]
- 33.Sigal MJ, Aubin JE, TenCate AR: An immunocytochemical study of the human odontoblast process using antibodies against tubulin, actin and vimentin. J Dent Res 1985, 12:1348-1355 [DOI] [PubMed] [Google Scholar]
- 34.Sveen OB, Hawes RR: Differentiation of new odontoblasts and dentine bridge formation in rat molar teeth after tooth grinding. Arch Oral Biol 1968, 13:1399-1412 [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald M, Chiego DJ, Jr, Heys DR: Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol 1990, 35:707-715 [DOI] [PubMed] [Google Scholar]
- 36.Bergenholz G: Inflammatory response of the dental pulp to bacterial irritation. J Endodont 1981, 7:100-104 [DOI] [PubMed] [Google Scholar]
- 37.Trowbridge HO: Pathogenesis of pulpitis resulting from dental caries. J Endodont 1981, 7:52-60 [DOI] [PubMed] [Google Scholar]
- 38.Smith AJ, Cassidy N, Perry H, Begue-Kirn C, Ruch JV, Lesot H: Reactionary dentiogenesis. Int J Dev Biol 1995, 39:273-280 [PubMed] [Google Scholar]
- 39.Kobayashi M, Sjoberg G, Soderhall S, Lendahl U, Sandstedt B, Sejersen T: Pediatric rhabdomyosarcomas express the intermediate filament nestin. Pediatr Res 1998, 43:386-392 [DOI] [PubMed] [Google Scholar]
- 40.Vainio S, Karavanova I, Jowett A, Thesleff I: Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 1993, 75:45-58 [PubMed] [Google Scholar]
- 41.Åberg T, Wozney J, Thesleff I: Expression patterns of bone morphogenetic proteins (Bmps) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev Dyn 1997, 210:383-396 [DOI] [PubMed] [Google Scholar]
- 42.Stemple DL, Anderson DJ: Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell 1992, 71:973-985 [DOI] [PubMed] [Google Scholar]
- 43.Lothian C, Lendahl U: An evolutionarily conserved region in the second intron of the human nestin gene directs gene expression to CNS progenitor cells and to early neural crest cells. Eur J Neurosci 1997, 9:452-462 [DOI] [PubMed] [Google Scholar]