Abstract
Eosinophils are usually associated with parasitic and allergic diseases; however, eosinophilia is also observed in several types of human tumors, including breast carcinomas. In this study we examined several human breast carcinoma cell lines for adhesion molecule expression and the ability to bind and activate eosinophils. MDA-MB-435S and MDA-MB-468 cells constitutively expressed both intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) and this expression was enhanced by treatment with tumor necrosis factor-α (TNF-α). BT-20 and SK-BR-3 cells only expressed ICAM-1 or VCAM-1 after stimulation with TNF-α. Eosinophils constitutively bound to MDA-MB-435S cells, but not to BT-20 cells. Stimulation with TNF-α slightly enhanced eosinophil adhesion to MDA-MB-435S cells and dramatically increased adhesion to BT-20 cells. Greater than 80% of eosinophil adhesion to these cell lines was blocked with an anti-α4-integrin monoclonal antibody. Both MDA-MB-435S and BT-20 cells also released eosinophil activator(s). Supernatants from TNF-α-treated, but not control-treated, cell lines increased eosinophil adhesion to fibronectin and increased eosinophil transmigration across fibronectin-coated transwell plates. Enzyme-linked immunosorbent assays showed that TNF-α-stimulated breast carcinoma cells released the chemokine regulated on activation, T cell expressed and secreted (RANTES). Addition of an anti-RANTES antibody to breast carcinoma cell supernatants partially blocked eosinophil activation suggesting that RANTES in these supernatants was participating in eosinophil activation. These data show that TNF-α-stimulated breast carcinoma cells express mediators that can both bind and activate eosinophils, suggesting a mechanism for eosinophil localization to breast carcinoma sites.
Eosinophils represent a minor fraction of the circulating leukocytes; however, they are key mediators in diseases such as bronchial asthma, allergic dermatitis, and helminthic parasite infections. 1 Eosinophils are also found in many types of human cancers including both hematological cancers such as Hodgkin’s lymphoma 2 as well as solid tumors. 3,4 Deposition of eosinophilic granular proteins has been found in breast, 5 ovarian, and uterine tumors 6 and in some studies the presence of eosinophils is one indicator of increased survival. 7,8 Thus, there is increasing evidence showing that eosinophils infiltrate into tumor sites, suggesting that these cells may play a role in host-tumor interactions. Despite this, little is known about the mechanisms of eosinophil infiltration into tumor sites.
The mechanisms used by eosinophils to adhere to activated endothelium may provide insights into how these cells interact with carcinoma cells. Eosinophils, like other leukocytes, respond to both adhesion and activation signals provided by the endothelium. These signals result in leukocyte adhesion to the vessel wall, activation, and transmigration into the tissue. 9 The first step in this process involves the rapid tethering of eosinophils to P-selectin, 10,11 E-selectin, 10 or vascular cell adhesion molecule-1 (VCAM-1) 11,12 expressed on activated endothelial cells. Once eosinophils have bound to the endothelium, chemoattractants activate eosinophils leading to firm adhesion mediated by α4-integrins binding to VCAM-1 as well as β2-integrins likely interacting with intercellular adhesion molecule-1 (ICAM-1). 12,13 These chemoattractants include chemokines such as eotaxin and regulated on activation, normal T cells expressed and secreted (RANTES) as well as lipid mediators such as platelet-activating factor and leukotrienes. 1 Once bound and activated, eosinophils migrate into inflamed tissue. 1
Many of the adhesion and activation molecules expressed by stimulated endothelium have now been shown to be expressed by tumor cells. P-selectin and E-selectin are associated with many types of tumors; however, expression of the selectins is restricted to the vascular endothelium associated with the tumors. 14,15 Similar observations have been made for VCAM-1, with increased expression found on tumor-associated endothelium. 16,17 Although VCAM-1 is expressed on some mesotheliomas, 18 VCAM-1 expression on tumors of epithelial origin has not been clearly demonstrated. Charpin et al 17 found some focal expression of VCAM-1 on breast carcinoma epithelial cells; yet most of the VCAM-1 expression was found on stromal endothelial cells. In contrast, ICAM-1 is expressed both by tumor endothelium as well as by tumor cells of epithelial origin. 16,19,20 This has been demonstrated using both tissue sections as well as tumor cell lines. Many have speculated that ICAM-1 and VCAM-1 expression may favor adhesive interactions with leukocytes, thus facilitating leukocyte tumor infiltration.
In this study we first examined the synthesis and expression of adhesion proteins by human breast carcinoma cell lines, then determined if expression of these adhesion proteins could be modulated by exposure to tumor necrosis factor-α (TNF-α). Finally we assayed for eosinophil adhesion and activation. We found that human breast carcinoma cells express ICAM-1 and VCAM-1 on their surfaces and bind eosinophils. These cell lines also release eosinophil activators including the CC chemokine RANTES when exposed to TNF-α.
Materials and Methods
Reagents and Antibodies
Human TNF-α and enzyme-linked immunosorbent assay (ELISA) kits for eotaxin, macrophage inflammatory protein-1α (MIP-1α), and RANTES were from R & D Systems, Inc. (Minneapolis, MN). Hanks’ balanced salt solution with Ca2+ and Mg2+ (HBSS), lymphoprep 1077, Dulbecco’s modified Eagle’s medium, Media 199 (M199), fetal bovine serum, Superscript II, and TRIzol reagent were from Life Technologies, Inc. (Grand Island, NY). PCR Master Mix was from Qiagen (Mississagua, Canada). All plasticware was from Becton Dickinson (Franklin Lakes, NJ). Human serum albumin (HSA) was from Immuno US (Rochester, MI). Enhanced chemiluminescence reagents were from Amersham (Buckinghamshire, England). All other chemicals were from BDH, Inc. (Toronto, Canada).
The anti-human P-selectin monoclonal antibodies (mAbs) S12 and G1 (both IgG1-κ) were prepared and characterized as described. 21,22 The blocking anti-human E-selectin mAb ES1 (IgG1-κ) was prepared and characterized as described. 23 Both the P-selectin and E-selectin mAbs were kindly provided by Dr. Rodger McEver (University of Oklahoma, Oklahoma City, OK). Anti-CD16 and anti-CD3 mAbs conjugated to paramagnetic beads were purchased from Miltenyi (Auburn, CA). All other mAbs were purchased from Serotec (Oxford, England). All antibodies were used according to manufacture’s instructions or as described.
Cell Culture and Isolation
MDA-MB-435S, BT-20, SK-BR-3, and MDA-MB-468 were kindly provided by Drs. Patrick Lee and Karl Riabowol (University of Calgary, Calgary, Canada) and were maintained in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum and antibiotics. Granulocytes were isolated from normal human donors by dextran sedimentation, hypotonic lysis, and density centrifugation on lymphoprep 1077 as described. 24 Eosinophils were isolated from granulocytes by negative selection with anti-CD16 and anti-CD3 microbeads using magnetic cell separation as described. 10 Eosinophils isolated in this way maintain an unactivated phenotype 10,12 and were >95% pure as assessed by Kimura staining.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Stimulated cells were washed once with M199 and immediately lysed in TRIzol reagent (Life Technologies, Inc.) and total RNA was extracted according to the manufacturer’s instructions. RNA concentrations were determined using a GeneQuant spectrophotometer (Pharmacia, Pascataway, NJ). Reverse transcription using Superscript II (Life Technologies, Inc.) was performed according to manufacturer’s instructions. PCR was performed with Qiagen Master Mix using 2 μl of the RT reaction as template cDNA and the appropriate primer pairs. The primers were as follows: β-actin forward 5′-CATGGATGATGATATCGCCG-3′ and reverse 5′-ACAG-CCTGGATAGCAACGTA-3′ (417 bp); ICAM-1 forward 5′-GGCAAGAACCTTACCCTACG-3′ and reverse 5′-GAGA-CCTCTGGCTTCGTCAG-3′ (586 bp); and VCAM-1 forward 5′-AGGGGACCACATCTACGCT-3′ and reverse 5′-ACAGAGCTCCCATTCACGA-3′ (1043 bp). Thirty-five PCR cycles with 1 minute at 94°C, 1 minute at 55°C, and 1 minute at 72°C were used for amplification. PCR products were electrophoresed through 2% agarose gels containing 0.5 μg/ml ethidium bromide and were visualized using UV light. β-Actin was used as positive control for mRNA amplification. Control and TNF-α-stimulated endothelial cells were used as positive and negative controls for adhesion molecule mRNA expression.
Determination of Adhesion Protein Expression
Confluent monolayers of breast carcinoma cell lines were washed and stimulated for 4 or 24 hours with either M199 with 0.5% HSA (M199/A) alone or M199/A containing 20 ng/ml TNF-α. Total protein expression in control and TNF-α-stimulated cell lines was determined by Western blotting as described. 25 Surface expression of adhesion proteins on control and TNF-α-stimulated cell lines was determined using a modified ELISA as described. 26 Briefly, stimulated cells were washed once with ice cold HBSS, fixed for 30 minutes at 4°C with 1% paraformaldehyde in HBSS, and then blocked with HBSS/1%HSA for at least 30 minutes at 37°C. Surface expression of adhesion proteins was then determined by adding saturating amounts of mAb in HBSS/1%HSA and incubating for 30 minutes at 37°C. Cells were washed three times with HBSS and then incubated with a 1/1000 dilution of a peroxidase-conjugated goat anti-mouse IgG secondary antibody (Amersham, Buckinghamshire, England) for 30 minutes at 37°C. mAb binding was determined using a trimethyl-benzadine one-step substrate (DAKO, Carpinteria, CA) and quantified by reading absorbance at 450 nm. Background expression was determined by using an isotype-matched nonimmune IgG. Control and TNF-α-stimulated endothelial cells were used as positive and negative controls for adhesion molecule expression.
Adhesion Assay
Cell lines grown in 24-well plates were washed and stimulated for 24 hours with either M199/A alone or M199/A containing 20 ng/ml TNF-α. The supernatant was removed and used in eosinophil activation assays. The cells were washed once with HBSS and 250 μl eosinophils (2 × 106/ml) were added to each well. After 15 minutes at 37°C, the nonadherent eosinophils were removed and the cells were washed twice with HBSS to remove loosely adherent eosinophils. Adherent eosinophils were quantified by counting three random fields using a 20× objective and NIH Image similar to previously described. 12 In eosinophil activation experiments, 24- or 48-well plates were coated with either 0.2% gelatin or 25 μg/ml fibronectin. Two hundred twenty-five microliters of eosinophils (1–2 × 106/ml) were placed in each well and then 25 μl of supernatant from control or TNF-α-stimulated cell lines was added. After 10 minutes at 37°C, the nonadherent eosinophils were removed and the plates were washed twice with HBSS to remove loosely adherent eosinophils. Adherent eosinophils were quantified by assaying for eosinophil peroxidase activity. HBSS/A alone was used as a negative control and HBSS/A containing 10−7 mol/L formyl-Met-Leu-Phe (fMLP) was used as a positive control. In some experiments either cell lines, eosinophils, or both were pretreated for 10 minutes with mAbs. mAbs were used at concentrations previously shown to be optimal. 11,12
Transmigration Assay
Eosinophil transmigration was determined using 3-μm pore transwell plates (Corning Costar Corporation, Cambridge, MA). Briefly, both the membranes and the bottom of the plates were coated with 25 μg/ml fibronectin. Supernatant was diluted 1:10 in HBSS/A and added to the lower chamber. Eosinophils (4 × 106/ml) were added to the upper chamber. After 90 minutes at 37°C, eosinophils that had migrated to the lower chamber were determined by counting three low-powered fields as described above. HBSS/A alone was used a negative control and HBSS/A containing 10−7 mol/L fMLP was used as a positive control.
ELISA for Eotaxin, MIP-1α, and RANTES
Supernatants from control or TNF-α-stimulated cell lines were collected and chemokine levels were determined by ELISA according to manufacturer’s instructions.
Statistics
All experiments were performed at least three times and the data are presented as mean and SEM of those replicates. Statistical differences between experimental groups were evaluated using either paired or unpaired Student’s t-test. P values < 0.05 were considered significant.
Results
Expression of ICAM-1 and VCAM-1 mRNA by Breast Carcinoma Cell Lines
In this study, four human breast carcinoma cell lines were examined under baseline conditions or after stimulation with TNF-α, since this cytokine is a key regulator of adhesion molecule expression in both endothelial cells and epithelial cell lines. 27-30 We first used RT-PCR to determine if these cells synthesized mRNA for various adhesion molecules. MDA-MB-435S and MDA-MB-468 cells both constitutively expressed high levels of mRNA for ICAM-1 (Figure 1A) ▶ . Stimulation with TNF-α did not increase this expression (Figure 1A) ▶ . In contrast, BT-20 and SKBR-3 cells expressed only nominal levels of ICAM-1 under baseline conditions, but stimulation with TNF-α for 24 hours dramatically increased mRNA expression (Figure 1A) ▶ . MDA-MB-468 cells also expressed high constitutive levels of mRNA for VCAM-1; whereas, all of the other cell lines had only low levels of VCAM-1 message with TNF-α stimulation dramatically increasing expression (Figure 1A) ▶ . None of the cell lines examined showed clear mRNA expression for either P-selectin or E-selectin (data not shown).
Figure 1.
Adhesion molecule expression by breast carcinoma cells. Cell lines were treated with M199/A alone (time, 0) or M199/A containing 20 ng/ml TNF-α for 4 or 24 hours (time, 4, 24). A: After the incubation, cells were lysed in TRIzol and total RNA was isolated. RT-PCR was performed as described in Methods. B: Alternatively, cells were lysed in Triton X-100 containing buffer and the soluble proteins were separated on SDS-PAGE. Western blotting was performed using 2 μg/ml of the specified mAbs directed against P-selectin (G1), E-selectin (ES-1), ICAM-1 (BBA 4), or VCAM-1 (1.G11B1). Experiments were performed at least three times with equivalent data. Representative data are shown.
ICAM-1 and VCAM-1 Proteins Are Present and Expressed on the Surface of Breast Carcinoma Cell Lines
mRNA expression of these adhesion molecules led us to examine protein expression. In these experiments, cell lines were treated with buffer alone or with buffer containing TNF-α for either 4 or 24 hours. The cells were then scraped and the Triton X-100 soluble proteins were separated by SDS-PAGE and Western blotted using the appropriate mAbs. We found robust, constitutive expression of both ICAM-1 and VCAM-1 in MDA-MB-435S and MDA-MB-468 cells but not on the other cell lines examined (Figure 1B) ▶ . Treatment of MDA-MB-435S and MDA-MB-468 cells with TNF-α led to a slight increase in the expression of both ICAM-1 and VCAM-1 (Figure 1B) ▶ . Although BT-20 and SK-BR-3 cells did show low level constitutive expression of mRNA for ICAM-1 and VCAM-1, neither protein was expressed in these cell lines under control conditions (Figure 1B) ▶ . Stimulation of these cells with TNF-α for 24 hours dramatically induced the expression of both ICAM-1 and VCAM-1 by BT-20 and SKBR-3 cells (Figure 1B) ▶ . These data represent the first description of VCAM-1 expression by tumor cell lines of epithelial origin.
Figure 1 ▶ shows that breast carcinoma cells can express constitutive or induced protein for ICAM-1 and VCAM-1; however, Western blots cannot assess whether these proteins are present on the cell surface. We addressed this question by using a modified ELISA technique to evaluate adhesion molecule surface expression. Similar to the Western blotting, both ICAM-1 and VCAM-1 were constitutively expressed on the surface of MDA-MB-435S and MDA-MB-468 cells (Figure 2A ▶ and data not shown). Although Western blotting showed an increase in ICAM-1 and VCAM-1 expression with TNF-α treatment, no significant change in the surface expression of these proteins was observed after TNF-α stimulation (Figure 2A) ▶ . Neither ICAM-1 or VCAM-1 were constitutively expressed on the surface of BT-20 (Figure 2B) ▶ or SK-BR-3 cells (data not shown). Instead there was a dramatic increase in the surface expression of both of these proteins when cells were stimulated with TNF-α (Figure 2B ▶ and data not shown). These data were consistent with the Western blotting results.
Figure 2.
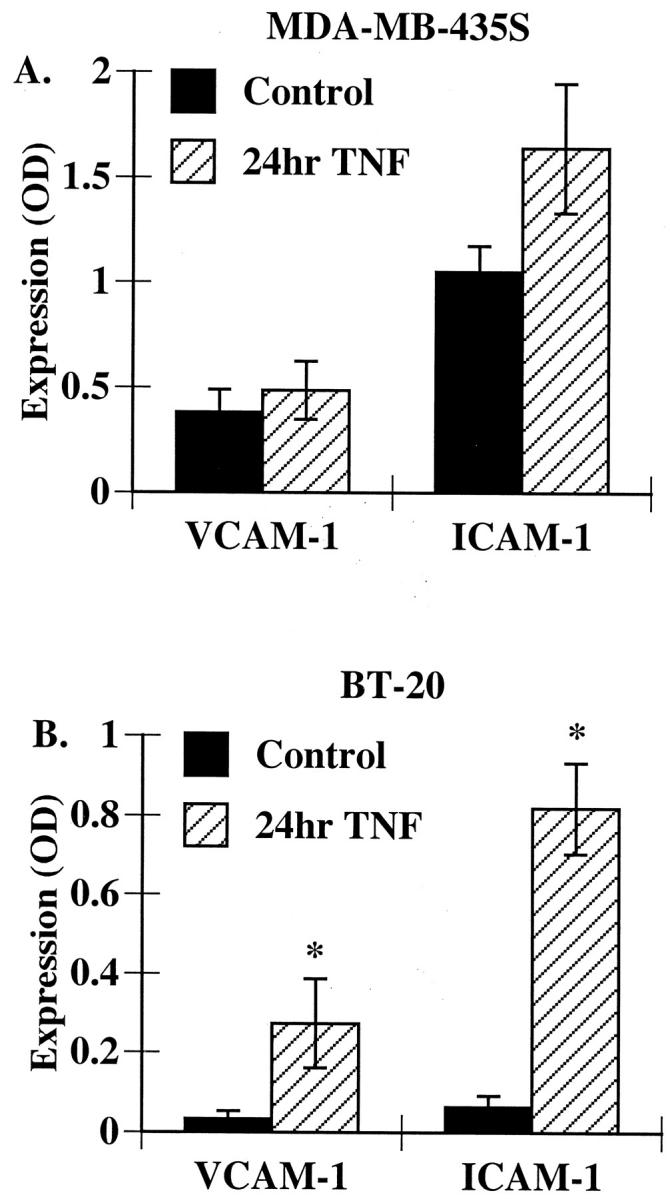
VCAM-1 and ICAM-1 are surface expressed on breast carcinoma cells. MDA-MB-435S (A) or BT-20 (B) cells were treated with TNF-α as described in Figure 1 ▶ . Cell surface expression of VCAM-1 or ICAM-1 was determined using a modified ELISA as described in Methods. Data represent the mean ± SEM of at least three experiments. * P < 0.05 as compared to control.
Many investigators have shown increased levels of soluble adhesion proteins present in the serum of patients with certain types of cancer as well as during other inflammatory diseases. 31,32 This suggests that adhesion proteins can be released or shed, as opposed to exclusively being expressed on the cell surface after synthesis. We assayed the supernatant from these cells using either ELISA techniques or Western blotting. There was no evidence that either VCAM-1 or ICAM-1 was being released/shed from these cells (data not shown).
Eosinophils Bind to Breast Carcinoma Cell Lines via α4- and β2-Integrins
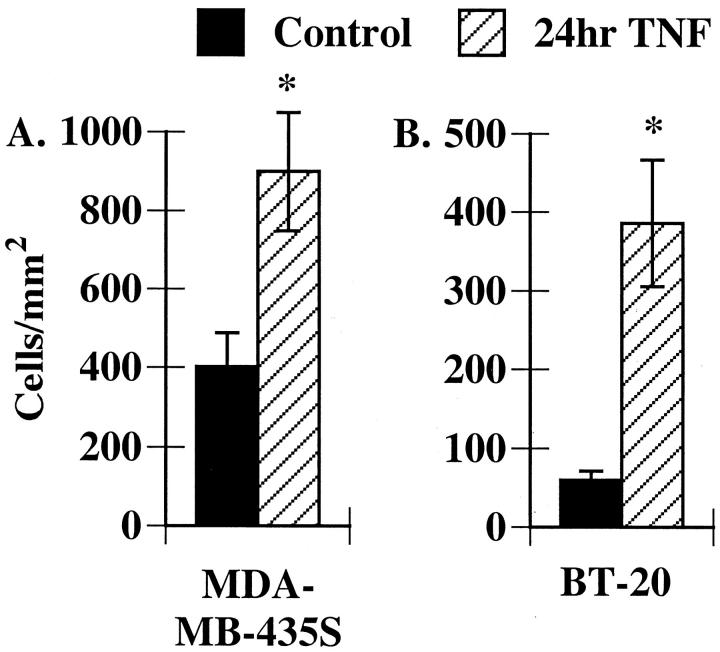
The remaining experiments in this study focus on the MDA-MB-435S cells and the BT-20 cells, since they represented the two phenotypes observed. We next determined if these breast carcinoma cell lines were able to support eosinophil adhesion. Freshly isolated eosinophils were added to either control or TNF-α-treated carcinoma cells. After 15 minutes the nonadherent and loosely adherent eosinophils were removed and the adherent eosinophils were counted using microscopy. We found that eosinophils constitutively bound both to MDA-MB-435S cells and to the surrounding extracellular matrix. Stimulation with TNF-α resulted in a modest, but significant increase in eosinophil adhesion (Figure 3) ▶ . In contrast, few eosinophils bound to unstimulated BT-20 cells (Figure 3) ▶ ; however, there was a significant increase in eosinophil adhesion to BT-20 cells stimulated with TNF-α, with virtually all adhesion occurring directly on the cells (Figure 3) ▶ . Thus eosinophils can bind directly to breast carcinoma cells.
Figure 3.
Eosinophils bind to breast carcinoma cells. Freshly isolated eosinophils (2 × 106/ml) were added to MDA-MB-435S (A) or BT-20 (B) cells treated with buffer alone or TNF-α as described in Figure 1 ▶ . After 15 minutes, nonadherent and loosely adherent cells were removed and eosinophil adhesion was determined as described in Methods. Data represent the mean ± SEM of at least three experiments. * P < 0.05 as compared to control.
We next used a series of blocking mAbs to identify the molecules involved in eosinophil adhesion to these cell lines, focusing first on the MDA-MB-435S cells. The anti-α4-integrin mAb was the only antibody that alone reduced constitutive eosinophil adhesion to MDA-MB-435S cells (Figure 4A) ▶ . Furthermore, an anti-α4-integrin mAb also blocked most eosinophil adhesion to TNF-α-stimulated MDA-MB-435S cells (Figure 4B) ▶ . A β2-integrin mAb attenuated adhesion to TNF-α-stimulated cells; however, neither ICAM-1 nor VCAM-1 mAbs alone had any effect on eosinophil adhesion (Figure 4B) ▶ . The lack of an effect of the anti-ICAM-1 and anti-VCAM-1 mAbs may reflect the observation that eosinophil adhesion was occurring both to the breast carcinoma cells as well as to the exposed matrix. Alternatively, both molecules may be required for optimal adhesion and blocking either one alone would have no effect on adhesion.
Figure 4.
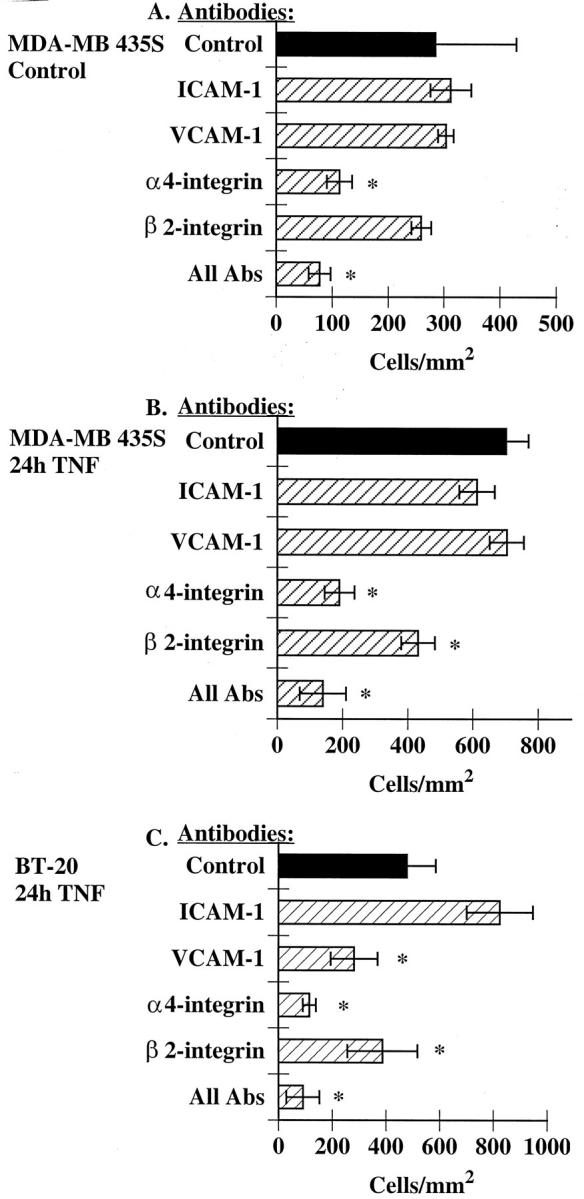
Eosinophil adhesion to MDA-MB-435S and BT-20 cells requires α4-integrins. MDA-MB-435S cells were treated with buffer alone (A) or TNF-α (B) for 24 hours as described in Figure 1 ▶ . Alternatively, BT-20 cells were treated TNF-α for 24 hours as described in Figure 1 ▶ (C). MDA-MB-435S (A and B) or BT-20 cells (C) were pretreated for 15 minutes at 37°C with 5 μg/ml of the specified mAbs directed against ICAM-1 (BBA4) or VCAM-1 (1.G11B1). Alternatively, eosinophils were preincubated with α4-integrin mAb (H2/1) or β2-integrin mAb (7E4) for 15 minutes before addition to the cell monolayers. Eosinophil adhesion was determined in the continued presence of the specified mAbs as described in Methods. Data are mean ± SEM of at least three experiments. * P < 0.05 as compared to control.
We next examined the effect of these mAbs on eosinophil adhesion to BT-20 cells. mAbs directed against either VCAM-1 or α4-integrins both attenuated eosinophil adhesion to TNF-α-stimulated BT-20 cells, although inhibition was greater with the anti-α4-integrin mAb (Figure 4C) ▶ . ICAM-1 mAb had no effect on adhesion, despite high expression of ICAM-1 on these cells (Figures 4C and 2B) ▶ ▶ , yet a β2-integrin mAb did attenuate eosinophil adhesion. These data suggest that eosinophils are binding to β2-integrin ligands other than ICAM-1 or that eosinophils may need to be further activated, possibly by a chemokine, to effectively bind to ICAM-1 expressed on the BT-20 cells.
Breast Carcinoma Cell Lines Stimulated with TNF-α Release Eosinophil Activators
Deposition of eosinophil granule proteins at tumor sites suggests that mechanisms exist not only for eosinophil adhesion, but also for their activation. 5,33 Furthermore, the observation that eosinophils were adhering both to MDA-MB-435S cells as well as to the exposed matrix suggested a role for eosinophil activators. This issue was addressed by harvesting the supernatants from either MDA-MB-435S or BT-20 cells treated with buffer alone or treated with TNF-α, then determining if there was a mediator in these supernatants that could increase eosinophil adhesion to either gelatin- or fibronectin-coated surfaces. These surfaces were used to assess increased adhesivness of the β2-integrins or the α4-integrins, respectively. Freshly isolated eosinophils were added to plates coated with gelatin or fibronectin. Eosinophils were then stimulated with a 1:10 dilution of supernatants from control or TNF-α-treated breast carcinoma cell lines.
Supernatants from 24-hour TNF-α-treated, but not control-treated, MDA-MB-435S cells increased eosinophil adhesion to fibronectin with little increase in binding to gelatin (Figure 5A) ▶ . This was not because of the presence of TNF-α alone as neither TNF-α alone (data not shown) nor the 4-hour TNF-α supernatant increased eosinophil adhesion in this assay (Figure 5A) ▶ . As expected, an anti-α4-integrin mAb blocked eosinophil adhesion to fibronectin (88.2 ± 3.3% inhibition; n = 3). Thus, MDA-MB-435S cells constitutively express adhesion proteins on their surface and can bind eosinophils, but only after stimulation with TNF-α for 24 hours do these cells release mediator(s) that can activate eosinophils.
Figure 5.
Both MDA-MB-435S and BT-20 cells release eosinophil activator(s). Supernatant from either MDA-MB-435S (A) or BT-20 (B) cells treated with buffer alone treated with TNF-α for 4 or 24 hours were collected. Forty-eight-well plates were coated with either 0.2% gelatin or 25 μg/ml fibronectin (Fn) for 2 hours at 37°C. Plates were washed once with HBSS and then freshly isolated eosinophils (1–2 × 106/ml) were added. Eosinophils were stimulated with a 1:10 dilution of the specified supernatants. After 10 minutes, the nonadherent and loosely adherent eosinophils were removed and adhesion was quantified using eosinophil peroxidase activity as described in Methods. Data are mean ± SEM of at least three experiments. * P < 0.05 as compared to control.
We extended these experiments to examine the supernatant from the BT-20 cells both before and after TNF-α stimulation. Unlike the MDA-MB-435S cells, the supernatants from BT-20 cells treated with TNF-α increased eosinophil adhesion to both gelatin and fibronectin (Figure 5B) ▶ . Interestingly, supernatant from both the 4-hour and the 24-hour TNF-α supernatants were able to increase eosinophil adhesion on fibronectin; whereas only the 24-hour supernatant had pro-adhesive effects on gelatin (Figure 5B) ▶ . As with the supernatants from MDA-MB-435S, an anti-α4-integrin blocked adhesion to fibronectin (91.6 ± 4.2% inhibition; n = 3). In addition, an anti-β2-integrin blocked most adhesion to gelatin (79.4 ± 1.9% inhibition; n = 3). These data suggest that mediators released from BT-20 cells activate both the β2- and α4-integrins on eosinophils promoting adhesion to both gelatin and fibronectin, whereas the mediators released from MDA-MB-435S cells only activate the α4-integrins promoting adhesion only to fibronectin.
We used an eosinophil transmigration assay to determine if the mediators present in these supernatants were also chemotactic for eosinophils. Eosinophils were placed in the upper chamber of a transwell plate and supernatant from control or TNF-α-treated cells was diluted 1:10 and placed in the lower chamber. Supernatants from buffer-treated cells had no effect on eosinophil transmigration (Figure 6) ▶ . In contrast, supernatants from both MDA-MB-435S and BT-20 cells treated with TNF-α elicited eosinophil transmigration (Figure 6) ▶ . TNF-α alone at the same concentration present in the diluted supernatant (2 ng/ml) induced only minimal transmigration (Figure 6) ▶ . Thus supernatants from TNF-α-stimulated breast carcinoma cells contain eosinophil chemotactic factors.
Figure 6.
Breast carcinoma cells release eosinophil chemoattractant(s). Supernatants from MDA-MB-435S or BT-20 cells were collected as described in Figure 5 ▶ . Freshly isolated eosinophils (4 × 106/ml) were added to the upper chamber of a 3-μm pore transwell dish coated with 25 μg/ml of fibronectin. Supernatants were placed in the lower chamber at a 1:10 dilution. M199/A or M199/A with an equivalent amount of TNF-α was used as controls (control). Eosinophils that had migrated into the lower chamber after 90 minutes were counted as described in Methods. Experiments were performed three times with equivalent data. Data represent the mean ± range of a single experiment.
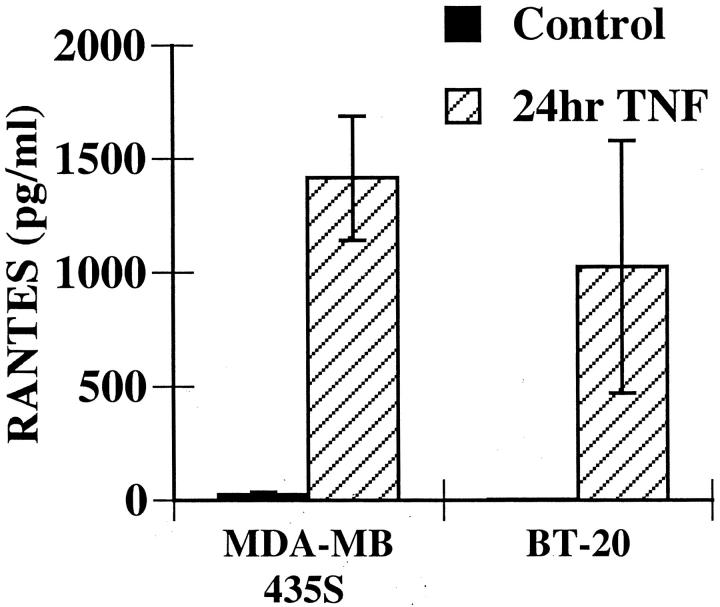
ELISAs for several chemokines were performed to identify the eosinophil activators released by breast carcinoma cells. We found that supernatants from TNF-α-stimulated MDA-MB-435S and BT-20 cells contained the CC chemokine RANTES (Figure 7) ▶ . In contrast neither eotaxin nor MIP-1α was released by these breast carcinoma cell lines (data not shown). To determine whether the RANTES present in the supernatant was participating in eosinophil activation, we examined the ability of an anti-RANTES antibody to block eosinophil adhesion to fibronectin. An anti-RANTES antibody was able to attenuate eosinophil adhesion in response to both MDA-MB-435S supernatants (30.0 ± 3.3% inhibition, n = 3) and to BT-20 supernatants (40.6 ± 14.4% inhibition, n = 3). Experiments using 10-fold excess antibody resulted in similar inhibition, suggesting that this antibody was used at saturating concentrations. These data show that RANTES participates in eosinophil activation in response to breast carcinoma supernatants; however, the incomplete inhibition with this antibody suggests that additional eosinophil activators are likely present. Studies are ongoing to identify these additional mediators.
Figure 7.
RANTES is released by TNF-α-stimulated breast carcinoma cells. Supernatants from MDA-MB-435S or BT-20 cells were collected as described in Figure 5 ▶ . RANTES present in the supernatants was assayed by ELISA according to the manufacturer’s instructions. Data represent the mean ± SEM of at least three experiments.
Discussion
Eosinophils represent only a few percent of the peripheral blood leukocytes; however they are the predominant cell type found at sites of parasitic and allergic inflammation. 1 It is now clear that eosinophils are also found in and around many types of tumors including breast, colon, gastric small cell, and cervical carcinomas. 4 In vitro, eosinophils can enhance the tumoricidal activity of macrophages 34 and in vivo, blood eosinophilia or eosinophil infiltration into some tumors is associated with improved prognosis. 3,7,8,35 Despite the overwhelming data showing that eosinophils are associated with numerous types of cancer, little is known about the molecular mechanisms required for localization of eosinophils to these sites.
Several families of adhesion molecules are involved in the localization of eosinophils into sites of inflammation. Endothelial cells stimulated with either TNF-α 11 or interleukin-4 12 have increased expression of both selectins and Ig superfamily proteins that act to tether and firmly bind eosinophils to these surfaces. Once eosinophils are bound to the endothelium, directed localization of eosinophils into tumor sites likely involves the combined actions of chemoattractants and adhesion molecules present on tumor cells themselves. Increased expression of selectins has been observed at tumor sites as compared to normal tissue samples; however, this expression is associated with the tumor endothelium. 14,15 In contrast, ICAM-1 is expressed both by the tumor endothelium as well as by tumor cells themselves. 16,19,20 Increased expression of ICAM-1 has been seen in tissue samples of breast carcinomas and is associated with an improved prognosis. 36 The mechanisms by which ICAM-1 expression leads to decreased tumor size are unknown, but may involve localization of leukocytes to the tumor.
In this study we used several human breast carcinoma cell lines including MDA-MB-435S, BT-20, SK-BR-3, and MDA-MB-468 to determine whether the expression of known adhesion molecules may result in eosinophil adhesion. All of the cell lines used in this study are adenocarcinomas of the breast, meaning that the tumors arose from glandular epithelial cells. The major difference between the four cell lines lies in their tumorigenicity. With MDA-MB-435S as the exception, all of the cell lines used are capable of forming tumors in nude mice. Morphologically, MDA-MB-435S also differ by growing in spindle shape, whereas the other cell lines form uniform monolayers. All of these cell lines are extensively used in breast cancer research and concentrating on the MDA-MB-435S and BT-20 cell lines allowed us to identify the differences in adhesion molecule expression, cell activation, and chemokine expression between these two characteristically different cell lines.
We examined adhesion molecule expression before and after stimulation with TNF-α. TNF-α is a multifunctional cytokine that has been shown to be produced by tumor-associated macrophages. 37,38 TNF-α has many effects on tumors cells in vitro including the induction of either necrotic or apoptotic cell death in some types of tumors. TNF-α, along with interleukin-1 and interleukin-6, has also been demonstrated to induce ICAM-1 expression on several types of epithelial tumors including breast carcinomas. 29,30,39 In contrast, VCAM-1 has not been examined with respect to induction with TNF-α; however, in other studies VCAM-1 has not been shown to be expressed by breast carcinoma cells. 14
RT-PCR, Western blotting, and cell surface ELISAs were used to examine adhesion molecule expression on several breast carcinoma cell lines. Two of the cell lines examined, MDA-MB-435S and MDA-MB-468, constitutively expressed ICAM-1 and VCAM-1 (Figure 1) ▶ . This expression was enhanced after exposure to TNF-α. The other cell lines examined only expressed these adhesion molecules after stimulation with TNF-α. Although ICAM-1 expression was expected based on previous studies, the expression of VCAM-1 was not expected. Using RT-PCR we amplified a band for VCAM-1 at the expected size (1043 bp). Since mRNA levels do not always correlate with protein expression, we also performed Western blots and detected a protein with a molecular weight of ≈110 kd. One explanation for the differences between our data (this study) and others who have examined VCAM-1 protein expression 14,17 may lie in the immunogenicity of the VCAM-1 expressed by breast carcinoma cells. This may be attributable to either differential splicing or posttranslational modifications of VCAM-1. VCAM-1 can be alternatively spliced resulting in either a 6 domain or 7 domain form of the molecule in endothelial cells. 40 We found evidence for this type of splicing with VCAM-1 expressed by MDA-MB-435S cells by Western blotting where a doublet was clearly observed (Figure 1B) ▶ . Furthermore, Abe et al 41 have demonstrated that endothelial cells treated with a combination of interleukin-4 and TNF-α express VCAM-1 based on Western blotting and ELISAs, but this VCAM-1 does not recognize its ligand on circulating T cells. To further support this hypothesis, we found that only one of three commercially available mAbs to VCAM-1 recognized the molecule expressed on breast carcinoma cells, whereas, all three recognized VCAM-1 present on TNF-α-stimulated endothelial cells (data not shown).
Breast carcinoma cells bind eosinophils through interactions with α4-integrins. Although α4-integrins bind to BT-20 cells via VCAM-1 this may not be the case with MDA-MB-435S cells, because our VCAM-1 mAb has no effect on eosinophil adhesion. Other ligands for the α4-integrins include alternate epitopes on VCAM-1 not recognized by our mAb as well as extracellular matrix components such as fibronectin. Interestingly, we found that eosinophils did bind to the exposed matrix of MDA-MB-435S cells. This adhesion was partially dependent on β2-integrins, suggesting that the cell line was releasing an eosinophil activator. By testing the ability of breast carcinoma cell supernatants for the ability to stimulate eosinophil adhesion to extracellular matrix proteins, we showed that these cell lines release potent eosinophil activators including the CC chemokine RANTES. Although RANTES is a potent eosinophil chemoattractant, it also exerts its effects on T cells and monocytes. Thus these breast carcinoma cells release mediators that are potentially able to bring several cell types into the tissue. An anti-RANTES antibody only blocked 30 to 40% of eosinophil adhesion suggesting that other mediators are released by these breast carcinoma cell lines. Studies are currently underway to identify and characterize these additional mediators.
The expression of adhesion and activation molecules by breast carcinoma cells may provide a mechanism for localizing eosinophils to tumor sites. Once localized at these sites, the role for eosinophils is unclear. There are several studies that suggest that eosinophil tumor infiltration is associated with improved prognosis. 3,7,35 There are an equal number of studies showing either no role for eosinophils or an association with a poor prognosis. 42-44 Recently it has been suggested that eosinophils may not play a direct role in tumor cell clearance, but may instead participate in tissue remodeling. 4,45 Eosinophils can synthesize cytokines, growth factors, and proteases that are associated with both tissue remodeling and angiogenesis. These include transforming growth factors-α and -β1, 46-48 vascular endothelial cell growth factor, 49 and matrix metalloprotease-9. 50,51 Thus, the role of eosinophils in tumor biology may be directed less toward direct tumoricidal activity and more to modulation of the tumor microenvironment.
Acknowledgments
We thank Evelyn Lailey for her excellent technical assistance, Drs. Rodger McEver, Patrick Lee, and Karl Riabowol for their generous gifts of reagents, and Dr. Randy Johnston for critical reading of this manuscript.
Footnotes
Address reprint requests to Dr. Kamala D. Patel, Dept. of Physiology and Biophysics, University of Calgary, 3330 Hospital Dr. NW, Calgary, Alberta, Canada T2N 4N1. E-mail: kpatel@ucalgary.ca.
Supported by grants from the Canadian Breast Cancer Foundation, Alberta Chapter, the Honda Run for the Cure and the Medical Research Council of Canada (MT-14180). Dr. K. D. Patel is supported both by a scholarship from the Medical Research Council of Canada and a scholarship from the Alberta Heritage Foundation for Medical Research.
References
- 1.Wardlaw AJ, Moqbel R, Kay AB: Eosinophils: biology and role in disease. Adv Immunol 1995, 60:151-266 [DOI] [PubMed] [Google Scholar]
- 2.Samoszuk M, Ramzi E: IgE, Reed-Sternberg cells, and eosinophilia in Hodgkin’s disease. Leuk Lymphoma 1993, 9:315-319 [DOI] [PubMed] [Google Scholar]
- 3.Goldsmith MM, Belchis DA, Cresson DH, Merritt WD, Askin FB: The importance of the eosinophil in head and neck cancer. Otolaryngol Head Neck Surg 1992, 106:27-33 [DOI] [PubMed] [Google Scholar]
- 4.Samoszuk M: Eosinophils and human cancer. Histol Histopathol 1997, 12:807-812 [PubMed] [Google Scholar]
- 5.Samoszuk MK, Nguyen V, Gluzman I, Pham JH: Occult deposition of eosinophil peroxidase in a subset of human breast carcinomas. Am J Pathol 1996, 148:701-706 [PMC free article] [PubMed] [Google Scholar]
- 6.Samoszuk M, Nguyen V, Rim P, Strathearn G: A new marker for blood vessels in human ovarian and endometrial cancers. Clin Cancer Res 1996, 2:1867-1871 [PubMed] [Google Scholar]
- 7.Bethwaite PB, Holloway LJ, Yeong ML, Thornton A: Effect of tumour associated tissue eosinophilia on survival of women with stage IB carcinoma of the uterine cervix. J Clin Pathol 1993, 46:1016-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ownby HE, Roi LD, Isenberg RR, Brennan MJ: Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer 1983, 52:126-130 [DOI] [PubMed] [Google Scholar]
- 9.Springer TA: Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 1994, 76:301-314 [DOI] [PubMed] [Google Scholar]
- 10.Patel KD, McEver RP: Comparison of tethering and rolling of eosinophils and neutrophils through selectins and P-selectin glycoprotein ligand-1. J Immunol 1997, 159:4555-4565 [PubMed] [Google Scholar]
- 11.Kitayama J, Fuhlbrigge RC, Puri KD, Springer TA: P-selectin, L-selectin, and alpha 4 integrin have distinct roles in eosinophil tethering and arrest on vascular endothelial cells under physiological flow conditions. J Immunol 1997, 159:3929-3939 [PubMed] [Google Scholar]
- 12.Patel KD: Eosinophil tethering to interleukin-4-activated endothelial cells requires both P-selectin and vascular cell adhesion molecule-1. Blood 1998, 92:3904-3911 [PubMed] [Google Scholar]
- 13.Kitayama J, Mackay CR, Ponath PD, Springer TA: The C-C chemokine receptor CCR3 participates in stimulation of eosinophil arrest on inflammatory endothelium in shear flow. J Clin Invest 1998, 101:2017-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox SB, Turner GD, Gatter KC, Harris AL: The increased expression of adhesion molecules ICAM-3, E- and P-selectins on breast cancer endothelium. J Pathol 1995, 177:369-376 [DOI] [PubMed] [Google Scholar]
- 15.Charpin C, Bergeret D, Garcia S, Andrac L, Martini F, Horschowski N, Choux R, Lavaut M: ELAM selectin expression in breast carcinomas detected by automated and quantitative immunohistochemical assays. Int J Oncol 1998, 12:1041-1048 [DOI] [PubMed] [Google Scholar]
- 16.Regidor PA, Callies R, Regidor M, Schindler AE: Expression of the cell adhesion molecules ICAM-1 and VCAM-1 in the cytosol of breast cancer tissue, benign breast tissue and corresponding sera. Eur J Gynaecol Oncol 1998, 19:377-383 [PubMed] [Google Scholar]
- 17.Charpin C, Garcia S, Andrac L, Horschowski N, Choux R, Lavaut MN: VCAM (IGSF) adhesion molecule expression in breast carcinomas detected by automated and quantitative immunocytochemical assays. Hum Pathol 1998, 29:896-903 [DOI] [PubMed] [Google Scholar]
- 18.Ruco LP, de Laat PA, Matteucci C, Bernasconi S, Sciacca FM, van der Kwast TH, Hoogsteden HC, Uccini S, Mantovani A, Versnel MA: Expression of ICAM-1 and VCAM-1 in human malignant mesothelioma. J Pathol 1996, 179:266-271 [DOI] [PubMed] [Google Scholar]
- 19.Mayer B, Lorenz C, Babic R, Jauch KW, Schildberg FW, Funke I, Johnson JP: Expression of leukocyte cell adhesion molecules on gastric carcinomas: possible involvement of LFA-3 expression in the development of distant metastases. Int J Cancer 1995, 64:415-423 [DOI] [PubMed] [Google Scholar]
- 20.Dippold W, Wittig B, Schwaeble W, Mayet W, Meyer zum Buschenfelde KH: Expression of intercellular adhesion molecule 1 (ICAM-1, CD54) in colonic epithelial cells. Gut 1993, 34:1593–1597 [DOI] [PMC free article] [PubMed]
- 21.McEver RP, Martin MN: A monoclonal antibody to a membrane glycoprotein binds only to activated platelets. J Biol Chem 1984, 259:9799-9804 [PubMed] [Google Scholar]
- 22.Geng JG, Bevilacqua MP, Moore KL, McIntyre TM, Prescott SM, Kim JM, Bliss GA, Zimmerman GA, McEver RP: Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature 1990, 343:757-760 [DOI] [PubMed] [Google Scholar]
- 23.Patel KD, Moore KL, Nollert MU, McEver RP: Neutrophils use both shared and distinct mechanisms to adhere to selectins under static and flow conditions. J Clin Invest 1995, 96:1887-1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmerman GA, McIntyre TM, Prescott SM: Thrombin stimulates the adherence of neutrophils to human endothelial cells in vitro. J Clin Invest 1985, 76:2235-2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel KD, Nollert MU, McEver RP: P-selectin must extend a sufficient length from the plasma membrane to mediate rolling of neutrophils. J Cell Biol 1995, 131:1893-1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinhardt PH, Elliott JF, Kubes P: Neutrophils can adhere via alpha4beta1-integrin under flow conditions. Blood 1997, 89:3837-3846 [PubMed] [Google Scholar]
- 27.Zimmerman GA, Prescott SM, McIntyre TM: Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today 1992, 13:93-100 [DOI] [PubMed] [Google Scholar]
- 28.Springer TA: Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol 1995, 57:827-872 [DOI] [PubMed] [Google Scholar]
- 29.Budinsky AC, Brodowicz T, Wiltschke C, Czerwenka K, Michl I, Krainer M, Zielinski CC: Decreased expression of ICAM-1 and its induction by tumor necrosis factor on breast-cancer cells in vitro. Int J Cancer 1997, 71:1086-1090 [DOI] [PubMed] [Google Scholar]
- 30.Hutchins D, Steel CM: Regulation of ICAM-1 (CD54) expression in human breast cancer cell lines by interleukin 6 and fibroblast-derived factors. Int J Cancer 1994, 58:80-84 [DOI] [PubMed] [Google Scholar]
- 31.Liu CM, Shun CT, Cheng YK: Soluble adhesion molecules and cytokines in perennial allergic rhinitis. Ann Allergy Asthma Immunol 1998, 81:176-180 [DOI] [PubMed] [Google Scholar]
- 32.Velikova G, Banks RE, Gearing A, Hemingway I, Forbes MA, Preston SR, Jones M, Wyatt J, Miller K, Ward U, Al-Maskatti J, Singh SM, Ambrose NS, Primrose JN, Selby PJ: Circulating soluble adhesion molecules E-cadherin, E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in patients with gastric cancer. Br J Cancer 1997, 76:1398-1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samoszuk MK, Nathwani BN, Lukes RJ: Extensive deposition of eosinophil peroxidase in Hodgkin’s and non-Hodgkin’s lymphomas. Am J Pathol 1986, 125:426-429 [PMC free article] [PubMed] [Google Scholar]
- 34.Spessotto P, Dri P, Bulla R, Zabucchi G, Patriarca P: Human eosinophil peroxidase enhances tumor necrosis factor and hydrogen peroxide release by human monocyte-derived macrophages. Eur J Immunol 1995, 25:1366-1373 [DOI] [PubMed] [Google Scholar]
- 35.Iwasaki K, Torisu M, Fujimura T: Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer 1986, 58:1321-1327 [DOI] [PubMed] [Google Scholar]
- 36.Ogawa Y, Hirakawa K, Nakata B, Fujihara T, Sawada T, Kato Y, Yoshikawa K, Sowa M: Expression of intercellular adhesion molecule-1 in invasive breast cancer reflects low growth potential, negative lymph node involvement, and good prognosis. Clin Cancer Res 1998, 4:31-36 [PubMed] [Google Scholar]
- 37.Tracey KJ, Cerami A: Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med 1994, 45:491-503 [DOI] [PubMed] [Google Scholar]
- 38.Beutler B. TNF, immunity and inflammatory disease: lessons of the past decade. J Invest Med 1995, 43:227–235 [PubMed]
- 39.Bajaj P, Lawry J, Shenton G, Rees RC: Interleukin-6 and tumour necrosis factor alpha synergistically block S-phase cell cycle and upregulate intercellular adhesion molecule-1 expression on MCF7 breast carcinoma cells. Cancer Lett 1993, 71:143-149 [DOI] [PubMed] [Google Scholar]
- 40.Osborn L, Vassallo C, Benjamin CD: Activated endothelium binds lymphocytes through a novel binding site in the alternately spliced domain of vascular cell adhesion molecule-1. J Exp Med 1992, 176:99-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abe Y, Ballantyne CM, Smith CW: Functions of domain 1 and 4 of vascular cell adhesion molecule-1 in alpha4 integrin-dependent adhesion under static and flow conditions are differentially regulated. J Immunol 1996, 157:5061-5069 [PubMed] [Google Scholar]
- 42.Leighton SE, Teo JG, Leung SF, Cheung AY, Lee JC, van Hasselt CA: Prevalence and prognostic significance of tumor-associated tissue eosinophilia in nasopharyngeal carcinoma. Cancer 1996, 77:436-440 [DOI] [PubMed] [Google Scholar]
- 43.Fisher ER, Paik SM, Rockette H, Jones J, Caplan R, Fisher B: Prognostic significance of eosinophils and mast cells in rectal cancer: findings from the National Surgical Adjuvant Breast and Bowel Project (protocol R-01). Hum Pathol 1989, 20:159-163 [DOI] [PubMed] [Google Scholar]
- 44.van Driel WJ, Hogendoorn PC, Jansen FW, Zwinderman AH, Trimbos JB, Fleuren GJ: Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum Pathol 1996, 27:904-911 [DOI] [PubMed] [Google Scholar]
- 45.Kadin M, Butmarc J, Elovic A, Wong D: Eosinophils are the major source of transforming growth factor-beta 1 in nodular sclerosing Hodgkin’s disease. Am J Pathol 1993, 142:11-16 [PMC free article] [PubMed] [Google Scholar]
- 46.Elovic AE, Ohyama H, Sauty A, McBride J, Tsuji T, Nagai M, Weller PF, Wong DT: IL-4-dependent regulation of TGF-alpha and TGF-beta1 expression in human eosinophils. J Immunol 1998, 160:6121-6127 [PubMed] [Google Scholar]
- 47.Minshall EM, Leung DY, Martin RJ, Song YL, Cameron L, Ernst P, Hamid Q: Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 1997, 17:326-333 [DOI] [PubMed] [Google Scholar]
- 48.Eisma RJ, Allen JS, Lafreniere D, Leonard G, Kreutzer DL: Eosinophil expression of transforming growth factor-beta and its receptors in nasal polyposis: role of the cytokines in this disease process. Am J Otolaryngol 1997, 18:405-411 [DOI] [PubMed] [Google Scholar]
- 49.Horiuchi T, Weller PF: Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am J Respir Cell Mol Biol 1997, 17:70-77 [DOI] [PubMed] [Google Scholar]
- 50.Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM: Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol 1997, 17:519-528 [DOI] [PubMed] [Google Scholar]
- 51.Ohno I, Ohtani H, Nitta Y, Suzuki J, Hoshi H, Honma M, Isoyama S, Tanno Y, Tamura G, Yamauchi K, Nagura H, Shirato K: Eosinophils as a source of matrix metalloproteinase-9 in asthmatic airway inflammation. Am J Respir Cell Mol Biol 1997, 16:212-219 [DOI] [PubMed] [Google Scholar]