Abstract
We have generated transgenic mice expressing green fluorescent protein (GFP) driven by 2.453-kb (−2,362 to +91) of the 5′-upstream region of the human vascular endothelial growth factor (VEGF) promoter to monitor changes of VEGF gene transcription in situ. Neonatal transgenic mice exhibited GFP-derived fluorescence in tissues that have been previously reported to express VEGF mRNA expression, including lung, cartilage, and brain. In normal skin during postnatal development, moderate fluorescence was observed in the upper epidermis and, more prominently, in the outer root sheath keratinocytes of hair follicles. Strong up-regulation of GFP fluorescence was observed in the hyperplastic epidermis of the wound edge at 48 hours after wounding, whereas little GFP fluorescence was detected in the dermis. In situ hybridization confirmed an identical expression pattern of VEGF mRNA in these wounds. Topical application of 12-O-tetradecanoylphorbol-13-acetate (TPA) induced strong VEGF-GFP expression in suprabasal epidermis. Little or no fibroblast-derived fluorescence was seen both in the wound model and after TPA application. By confocal laser microscopy, increased GFP fluorescence was detectable in the epidermis of intact mouse ear skin as early as 6 hours after topical TPA treatment. Importantly, GFP fluorescence was also measurable in the skin of living transgenic mice. These results resolve the present controversy regarding the ability of VEGF-GFP transgenic mouse models to correctly reflect established patterns of VEGF expression, and show the model to be a powerful tool for the in vivo monitoring of VEGF gene expression.
Vascular endothelial growth factor (VEGF), also known as vascular permeability factor, plays an important role in the angiogenesis occurring under both physiological and pathological conditions. In particular, up-regulation of VEGF has been observed in the majority of human cancers, as well as in several inflammatory diseases and in ischemia-associated pathologies. 1-6 In the skin, up-regulation of VEGF has been demonstrated in the hyperplastic epidermis of healing wounds, 7,8 psoriatic lesions, 9 viral warts 10 and papillomas, 11 in several blistering diseases, 6 and in squamous cell carcinomas. 10,12 In contrast, adult skin expresses little VEGF under normal conditions. As a general finding, pathological expression of VEGF mRNA has been predominantly detected in the epidermal (epithelial) skin compartment, and little or no VEGF mRNA expression has been found in dermal cells such as fibroblasts or vascular endothelial cells. A number of recent in vitro studies have demonstrated that VEGF expression in epidermal keratinocytes is potently up-regulated by growth factors that also induce epidermal hyperplasia, including transforming growth factor-α, 9,13 epidermal growth factor, 14 keratinocyte growth factor, 14 and the tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA). 15 Based on these findings, it has been suggested that the avascular epidermis satisfies its increased nutritional needs during hyperplasia via a paracrine mechanism that involves secretion of VEGF by keratinocytes and binding of VEGF to its receptors on dermal microvessels, leading to increased skin vascularity. 16 Indeed, selective overexpression of VEGF in the epidermis of transgenic mice resulted in increased numbers of tortuous and hyperpermeable cutaneous microvessels. 17
VEGF is a homodimeric, heparin-binding glycoprotein occurring in at least four isoforms of 121, 165, 189, and 201 amino acids, because of alternative splicing of a single gene. 18,19 VEGF binds to two type III tyrosine kinase receptors on vascular endothelial cells, Flt-1 and KDR/Flk-1. 20,21 Although tissue-specific differences of the relative expression levels of these isoforms have been reported, with predominant expression of VEGF121 and VEGF165 in the skin, the different VEGF isoforms seem to respond equally well to up-regulation by growth factors, TPA, or hypoxia. 15 Increased stability of VEGF mRNA contributes to the enhanced VEGF mRNA expression observed under hypoxic conditions. 5,22 However, the transcriptional regulation of the VEGF gene seems to play the pre-eminent role in the control of VEGF expression, and several response elements within the VEGF promoter region have been characterized in vitro. 13,23-25
Localization of the site of origin of VEGF transcription to distinct cell populations in situ, together with a quantitative temporal analysis of VEGF gene expression, would enable a much more thorough analysis of the role of VEGF during the angiogenic process. However, monitoring of VEGF transcription in vivo has been complicated by the inability to distinguish between the relative contribution of modified transcription or mRNA stability, using standard techniques such as in situ hybridization. Moreover, immunohistochemical studies frequently detect VEGF immunoreactivity at sites distant from VEGF mRNA expression, because of secretion and binding of VEGF protein to its receptors on endothelial cells. To circumvent this problem, transgenic mice expressing green fluorescent protein (GFP), driven by a portion of the VEGF promoter have been generated. 26 Surprisingly, the expression observed deviated significantly from the predicted pattern.
We have also generated a transgenic mouse model in which the gene expression of GFP is controlled by the VEGF promoter (VEGF/GFP mice). Here, we demonstrate a distinct expression pattern of GFP fluorescence in different tissues of these mice, which are in accordance with previously reported patterns of VEGF mRNA expression. Up-regulation of VEGF gene transcription was easily detectable in epidermal keratinocytes after wounding of the skin; these cells also strongly expressed VEGF mRNA as assessed by in situ hybridization. GFP fluorescence was also detected in outer root sheath keratinocytes of hair follicles, but little or no fluorescence was detected in dermal cells. Importantly, TPA-induced up-regulation of VEGF/GFP fluorescence could be detected by confocal laser microscopy in living transgenic mice, thereby allowing real-time monitoring of VEGF gene expression in vivo.
Materials and Methods
Transgene Construct
Because polymerase chain reaction (PCR)-based amplification of the entire VEGF promoter was difficult due to the existence of the GC-rich region near the transcriptional starting site, initially, a 1.6-kbp fragment of VEGF genomic DNA (−2362 to −783; GenBank accession #M63971, nucleotide position is numbered from the transcriptional start site; 18 nt 1042 in ref. 42 ) was obtained by genomic PCR amplification. Using this fragment as a probe, a clone containing a 5.0-kb fragment of the VEGF sequence, including the 5′-flanking DNA of human VEGF, was isolated from a human placenta genomic library (Clontech, San Francisco, CA). The sequence was confirmed by multiple restriction enzyme analyses and by direct sequencing. A 2,453-bp EcoRI–AgeI fragment (−2362 to +91) was excised from the agarose gel. This promoter fragment was inserted into a GFP vector (pEGFP-1, Clontech; digested with EcoRI and XbaI) and was designated as pVEGF-GFP. The transgene cassette used for the VEGF-GFP transgene was described previously. 27
In Vitro Transfection Experiments
Primary murine keratinocyte cultures and dermal fibroblast cultures were prepared as previously described. 28,29 Cells were grown to semiconfluence and 2 μg per 60-mm dish of VEGF-GFP vector or control pEGFP-N1 (GFP under cytomegalovirus promoter control) vector plasmid DNA were introduced using the Fugene 6 transfection reagent (Boehringer, Mannheim, Germany). After 48 hours, epifluorescent and phase contrast pictures were taken, using a Nikon microphot-FXA microscope.
Generation of Transgenic Mice
The fragment for pronuclear injection was excised with EcoRI and AflII from the pVEGF-GFP plasmid vector (Figure 1) ▶ . This transgene fragment was injected into fertilized oocytes of DBA2×C57BL/6 (DBF1) mice (Charles River, Wilmington, MA) and the eggs were implanted into pseudopregnant foster mothers. The offspring (F0) were tested for chromosomal integration of the transgene by genomic PCR and Southern blot analysis using a HincII VEGF fragment as a probe following a protocol previously established. 30 All experiments were done with F1 to F3 offspring mice.
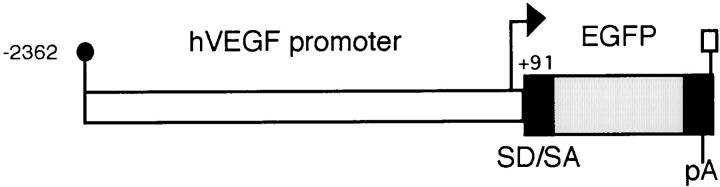
Figure 1.
Construction of the VEGF transgene. VEGF-GFP transgene contains 2,453 bp of human VEGF promoter (open box) linked to the red-shifted variant green fluorescent protein (EGFP) gene (stippled box). SV40 sequences (black boxes) include synthetic splice donor and acceptor sites (SD/SA) and a polyadenylation signal sequence (pA). The transgene was obtained by EcoRI (small black circle) and AflII (small rectangle box) digestion. The number indicates the nucleotide position from transcriptional start site as +1. The arrow indicates the transcriptional start site.
For confocal microscopic observation of intact skin, VEGF-GFP transgenic mice were generated in the hairless genetic background by breeding transgenic founders with SKH-1 hairless mice (Charles River). Male hemizygous transgenic mice (v/-HH) were mated with female hairless mice (−/−hh). Transgene-positive pups (v/-Hh) were selected by genomic PCR and mated back to hairless mice (−/−hh) to obtain the F2 generation of VEGF-GFP hairless mice (v/-hh), which could be easily selected by PCR detection and their hairless phenotype.
Tissue Preparation for GFP Detection and Epifluorescence Microscopic Observation
Freshly dissected, unfixed tissue was snap-frozen and immediately cut into 12-μm thick frozen sections using a Reichert (Leica, Deerfield, IL) cryostat. Epifluorescent microscopic observation was performed immediately after sectioning, using a fluorescein isothiocyanate/tetramethylrhodamine B isothiocyanate double excitation and emission filter (Chroma, Brattleboro, VT). This double-bandpass filter enabled us to distinguish GFP-derived fluorescence from tissue autofluorescence because the GFP signal remained green whereas autofluorescent structures such as hair shafts exhibited orange color under double fluorescence.
Wounding and TPA Treatment
Full-thickness skin wounds were produced with a 4-mm biopsy punch on the shaved back skin of 4- to 6-week-old transgenic mice (n = 5). After 48 hours, normal and wounded tissues were collected and 10-μm cryostat sections were prepared and analyzed as described above.
For induction of VEGF gene expression by TPA, 5 μg of TPA or acetone alone were applied to the dorsal side of transgenic mouse ears (n = 6, mouse age ranged from 6 weeks to 3 months) and skin biopsy samples were collected at 4.5 hours, 6 hours, 12 hours, and 24 hours after treatment. For additional noninvasive in vivo experiments, a single dose of 5 μg of TPA in 50 μl of acetone or acetone alone were topically applied to the back skin of transgenic mice and mice were subjected to confocal microscopic observation after 12 hours. All animal procedures were approved by the Massachusetts General Hospital Committee on Research Animal Care.
Anti-Keratin 5 Immunostaining
Freshly cut frozen sections were incubated with a keratin 5-specific polyclonal antibody (1:1000 dilution, kindly provided by Dr. P. Dotto, Harvard Medical School, Boston, MA) for 4 hours, washed briefly with phosphate-buffered saline (PBS), and were then incubated with 1:100 diluted lissamine rhodamine-conjugated secondary antibodies to distinguish the resulting fluorescence signal from the GFP fluorescence signal. After three washes with PBS, slides were coverslipped and were immediately observed under the microscope.
In Situ Hybridization
In situ hybridization was performed on 5-μm thick sections of paraffin-embedded tissue as described. 17 Briefly, slides were processed through xylene to remove paraffin, then passed sequentially through graded alcohols; 0.2 mol/L HCl; Tris/ethylenediaminetetraacetic acid (EDTA) with 3 mg/ml proteinase K; 0.2% glycine; 4% paraformaldehyde in phosphate-buffered saline, pH 7.4; 0.1 mol/L triethanolamine containing 1/200 (v/v) acetic anhydride; and 2× standard saline citrate (SSC). Slides were hybridized overnight at 50°C with 35S-labeled riboprobes in the following mixture: 0.3 mol/L NaCl, 0.01 mol/L Tris, pH 7.6, 5 mmol/L EDTA, 50% formamide, 10% dextran sulfate, 0.1 mg/ml yeast tRNA, and 0.01 mol/L dithiothreitol. Posthybridization washes included 2× SSC/50% formamide/10 mmol/L dithiothreitol at 50°C; 4× SSC/10 mmol/L Tris/1 mmol/L EDTA with 20 μg/ml ribonuclease at 37°C; and 2× SSC/50% formamide/10 mmol/L dithiothreitol at 65°C, and 2× SSC. Slides were then dehydrated through graded alcohols containing 0.3 mol/L ammonium acetate, dried, coated with Kodak NTB 2 emulsion (Eastman Kodak, Rochester, NY) and stored in the dark at 4°C for 2 weeks. The emulsion was developed with Kodak D19 developer and the slides were counterstained with hematoxylin. Antisense and sense single-stranded 35S-labeled RNA probes for VEGF were prepared from a 393-bp rat VEGF cDNA fragment, cloned into pGEM-3Z (Promega, Madison, WI).
Confocal Microscopy In Situ and In Vivo
For confocal microscopy, sections were fixed with 4% paraformaldehyde for 5 minutes. To visualize cell nuclei, sections were counterstained with 7-aminoactinomycin D (7-AAD) (5 μg/ml; Sigma, St. Louis, MO) for 20 minutes at ambient temperature, washed with PBS, and mounted with fluoromount-G (Southern Biotechnology, Birmingham, AL). Five-mm biopsies of ear skin of TPA- or acetone-treated hairless transgenic mice were dissected, placed on glass slides, and were coverslipped for inverted positioning on the confocal microscope plate.
To examine intact skin in living transgenic mice by confocal microscopy, hairless transgenic mice were anesthetized with avertin by intraperitoneal injection and were placed directly on a Petri dish in a dorsal position, and directly examined by confocal microscopy, using a Leica DM IRBE inverted microscope and a Leica TCS NT4D confocal microscopic system (Leica, Heidelberg, Germany) with a 530/30 nm band-pass filter, detecting emission at wavelengths between 515 to 545 nm.
Quantification of Fluorescence Intensity
The images obtained with the confocal laser microscope were digitized and stored as 768 × 512 pixel files. Leica Confocal Microscope System TCS/NT (Version 1.6.568) software program was used to quantify the intensity of GFP fluorescence in each of the files, and the average of pixels with fluorescence intensity was measured. To avoid the signal being saturated because of the bright fluorescence in the TPA-induced ear skin tissue, the same threshold value was chosen for each file and for deducted signal levels.
Results
In Vitro Expression of Transgenic GFP in Epidermal Keratinocytes, but not in Dermal Fibroblasts
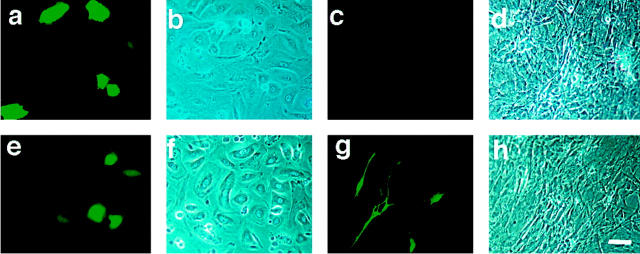
The function of the pVEGF-GFP transgene construct was confirmed by transfection of the pVEGF-GFP plasmid into primary murine keratinocytes, demonstrating strong cell-based fluorescence in keratinocytes (Figure 2, a and b) ▶ . The promoter activity of the VEGF vector was as strong as the activity of a cytomegalovirus promoter vector that was used as a control (Figure 2, e and f) ▶ . In contrast, virtually no signal was observed when the VEGF promoter vector was transfected into dermal fibroblasts (Figure 2, c and d) ▶ , although control transfection with a CMV promoter construct exhibited strong GFP fluorescence in fibroblast cultures (Figure 2, g and h) ▶ . Although the transfection efficiency seemed to be low (∼3 to 5%), flow cytometric analysis also showed that the VEGF promoter gave higher GFP fluorescence signals when transfected into keratinocytes than into fibroblasts (data not shown). These findings suggested that the VEGF promoter activity was stronger in keratinocytes than in fibroblasts in vitro.
Figure 2.
VEGF-GFP expression in cultured keratinocytes. This figure shows representative pictures of cells transfected with the VEGF-GFP construct. a and b: Primary murine keratinocytes transfected with the pVEGF-GFP vector (a–d) showed bright fluorescence, whereas murine dermal fibroblasts transfected with the same vector showed no expression (c and d). Keratinocytes (e and f) or fibroblasts (g and h), transfected with a control CMV promoter-GFP vector both showed bright fluorescence, indicating that both cell types could be transfected under the conditions used, but only the keratinocytes activated the VEGF promotor. a, c, e, and g: Fluorescence microscopy; b, d, f, and h: phase contrast microscope. Scale bar, 50 μm.
Expression of GFP in Transgenic Mice
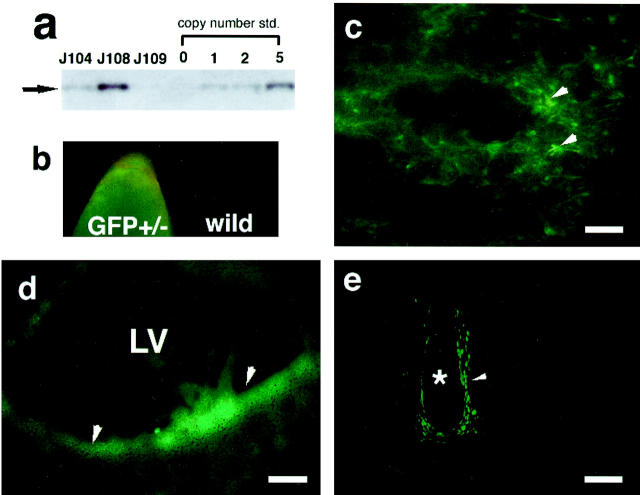
Out of 30 F0 mice, three founders positive for chromosomal transgene insertion were detected by PCR (data not shown) and Southern analysis (Figure 3a) ▶ . The number of integrated transgene copies varied from a single insertion to >10 copies as measured by comparison with a copy number standard (Figure 3a) ▶ . These lines showed GFP-derived fluorescence in the epidermis as assessed by epifluorescence microscopy of intact tail tissue. In contrast, no fluorescence was detected in wild-type littermates (Figure 3b) ▶ . The expression pattern of VEGF promoter activity was evaluated in neonatal F1 mice of these three lines. The lung, kidney, and brain, previously shown to express VEGF by in situ hybridization studies 31,32 were chosen for initial examination of GFP fluorescence. Fluorescence was observed in lung alveoli (Figure 3c) ▶ and in the lateral ventricle wall in the brain (Figure 3d) ▶ . Detection of fluorescence in the glomeruli of the kidney was complicated by strong autofluorescence (data not shown). Strong GFP fluorescence was also detectable in chondrocytes of developing cartilage tissue (Figure 3e) ▶ . All three transgenic lines showed similar expression patterns, but the intensities of the fluorescent signals differed (data not shown). Further analyses were performed in line J108, which gave the most intense GFP signal and had multiple transgene integrations (Figure 3a) ▶ .
Figure 3.
VEGF-GFP expression in newborn transgenic mice. a: Southern blotting analysis of founder transgenic line. The copy number standards from the same transgene plasmid DNA were loaded on the same gel with transgenic mouse genomic DNA. b: Fluorescence micrograph of intact tail tissue of a newborn VEGF-GFP transgenic mouse (left) and of a wild-type litter mate (right). The orange color in wild-type indicates background autofluorescence. c–e: Fluorescence micrographs of tissue sections taken from neonatal VEGF-GFP transgenic mice: lung (c; arrowhead indicates fluorescence in alveoli; note autofluorescence can be distinguished by its orange color); lateral ventricle (LV) of the brain (d; arrowhead indicates fluorescence in lateral epithelium); vertebral cartilage (e; note that the chondrocytes in mineralizing regions were GFP-positive, indicated by arrowhead). *, intervertebral disk. Scale bar, 100 μm.
Expression of GFP in the Skin
VEGF-GFP expression was further evaluated in the skin of young and adult transgenic mice. Unfixed frozen sections of skin from 7-day-old transgenic mice revealed strong GFP fluorescence in the outer root sheath of growing hair follicles, mostly at the lower part (Figure 4A) ▶ . The dermal papilla, derived from condensed mesenchyme in the embryonic hair germ, was GFP-negative (Figure 4A) ▶ . Also, weak GFP signals were occasionally observed in the epidermis (Figure 4B) ▶ . The hair follicle-specific fluorescence was also observed in 4-week-old transgenic mice (Figure 4B) ▶ . However, hair follicle fluorescence was not seen in the skin of 7-week-old animals (data not shown). Higher magnification of confocal microscopic images revealed that the follicular fluorescence was likely confined to the nucleus, as assessed by double labeling with 7-AAD that stains nuclei (Figure 4C) ▶ . Double labeling with keratin 5-specific antibodies showed that hair follicle-derived GFP signals partially overlapped with K5 expression. GFP fluorescence was localized within the K5 signal (Figure 4D) ▶ .
Figure 4.
GFP fluorescence in transgenic mouse skin. Fluorescence photomicrographs of VEGF-GFP transgenic mouse skin. A: Seven-day-old skin. Strong fluorescence is observed in the outer root sheath of the hair follicles as shown by the arrowhead). The orange color in the hair shaft is because of autofluorescence which is easily distinguishable from GFP-derived fluorescence with double band-pass filter. B: Four-week-old skin. Hair follicles (arrowheads) are fluorescence-positive This fluorescence is considerably weaker in 6-week-old mice. *, Indicates occasional GFP signals in the epidermis. C: Confocal image of the outer root sheath of the hair follicle with nuclear counter staining. Note the yellow signal indicating that the GFP fluorescent signal co-localized with the nuclear staining (arrowheads). D: Confocal image of the hair follicle double-labeled with K5 immunostaining with rhodamine. E, epidermis; D, dermis. Scale bars: 100 μm (A, B) and 25 μm (C, D).
GFP Is Expressed in Epidermal Keratinocytes at the Edge of Healing Wounds
Because normal epidermal keratinocytes showed only weak GFP fluorescence, we tested the inducibility of VEGF-driven GFP during wound healing. Forty-eight hours after wounding, the new epidermis beneath the scab showed a very strong and distinct fluorescent signal as compared with the adjacent unwounded epidermis (Figure 5a) ▶ . For nuclear counterstaining, fresh cryostat sections were briefly fixed and incubated with 7-AAD and were examined by laser confocal microscopy (Figure 5b) ▶ . Only little up-regulation of VEGF-specific fluorescence was observed in the granulation tissue of full-thickness wounds, mainly localized to single cells. A similar VEGF mRNA expression pattern was found in the epidermis by in situ hybridization (Figure 5, c and d) ▶ .
Figure 5.
GFP expression induced by skin wound healing. Four- to six-week-old VEGF-GFP transgenic mice were wounded by 4-mm punch biopsy. a: Healing wound edge 48 hours after biopsy. Strong fluorescence is observed in the neoepithelium. A dotted line indicates unwounded epidermis. E, epidermis; D, dermis. Nonspecific autofluorescence in the scabs (*) was distinguishable from the GFP signal. b: Confocal imaging with nuclear counterstaining clearly showed GFP induction in the neoepithelium in wound site and in hair follicles. In the wound, fluorescence is strongly evident in the neoepithelium (arrowhead) and in the hair follicles (arrows). Red indicates 7-AAD nuclear staining. E, epidermis; D, dermis. c and d: In situ hybridization for VEGF mRNA. The strongest signals overlie the epithelial cells. Bright-field (c) and dark-field (d) photograph. *, Indicates scab; arrows, hair follicles; large arrowhead, wound edge; small arrowheads, neoepithelium; E, epidermis; D, dermis. Scale bars: 50 μm (a); 100 μm (b–d).
Topical TPA Application Enhances Keratinocyte GFP Expression
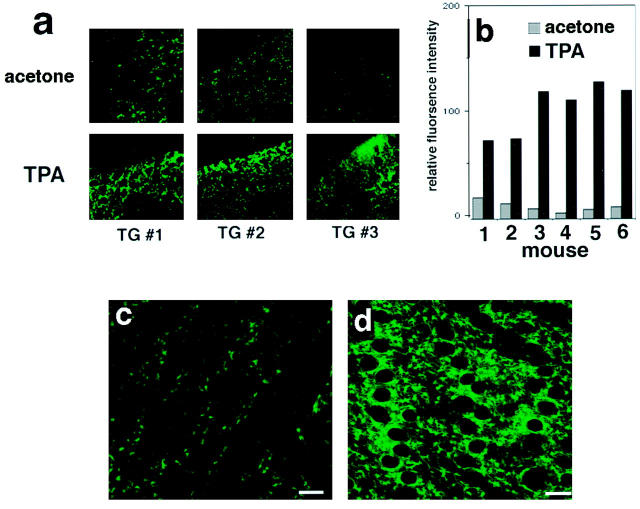
To confirm that TPA can induce GFP expression in the epidermis via the VEGF promoter used, and to establish a noninvasive monitoring technique for changes of VEGF gene expression, we studied the effect of topical application of TPA on the skin. Acetone or TPA were applied to the dorsal ear skin of transgenic hairless mice, and ear skin samples were examined by confocal imaging at 4.5 hours, 6 hours, 12 hours, and 24 hours after treatment. The first and highest induction of GFP expression was observed at 6 hours. At 6 hours, the induction of VEGF was significantly higher compared with the acetone-treated control side of the same mouse (Figure 6a) ▶ and this induction could be quantified by image analysis (Figure 6b) ▶ . Background levels of GFP fluorescence differed between individual mice.
Figure 6.
Detection of GFP expression in intact skin of living hairless transgenic mice by confocal microscopy. The epidermis of intact ear skin from a hairless transgenic mouse (11 weeks old) was treated with TPA or acetone and scanned using laser confocal microscopy. Horizontal optical sections of the epidermis of the acetone treated control skin (a; top) show basal levels of fluorescence. The epidermis of the same mouse treated with TPA (a; bottom) showed significantly increased fluorescence after 6 hours. Large holes are utricles, which are characteristic premature follicle structures of the hairless mouse. b: Quantification of fluorescence intensity. The epidermis of a living hairless transgenic mouse was scanned using laser confocal microscopy. Horizontal optical sections of the epidermis of acetone treated control skin (c) show detectable basal levels of fluorescence, whereas the epidermis of the same mouse (d) showed significantly increased fluorescence at 12 hours after TPA treatment. Scale bar, 25 μm (c, d).
We next examined whether the detection of GFP signal could be directly detected in the skin of living mice. A horizontal optical view through the living epidermis treated with acetone (Figure 6c) ▶ or TPA (Figure 6d) ▶ revealed a similar induction as that seen in ear skin sections. In contrast, wild-type littermates showed no detectable specific fluorescence (data not shown).
Discussion
In this study, we show that the GFP fluorescent signal resulting from VEGF promoter activity was readily detectable in live cultured keratinocytes, in sectioned transgenic mouse skin, and in the epidermis of living mice. Furthermore, up-regulation of VEGF gene expression either induced by wound healing or by TPA treatment was readily detected. Summarized findings for VEGF mRNA induction in skin tissue in vivo using this transgenic model are as follows: 1) in normal skin, VEGF/GFP fluorescence message was observed in the follicular epithelium up to ∼6 weeks of age; 2) VEGF induction by TPA took place in the suprabasal epidermis and was detectable as early as 6 hours after treatment; and 3) induction of VEGF/GFP fluorescence mainly occurred in epidermal keratinocytes during wound healing, whereas no significant increase was detected in fibroblasts and stromal cells.
Previously, prominent VEGF mRNA expression has been reported in lung alveoli 31 and in the lateral ventricle of the brain 32 by in situ hybridization. Evaluation of neonatal VEGF-GFP transgenic mice confirmed these findings. Recently, a similar tissue distribution pattern of β-galactosidase activity was reported in a VEGF-LacZ “knock-in” mouse model. 33 The detection of VEGF promoter activity in developing cartilage is also consistent with the recent findings of VEGF immunoreactivity in human neonatal cartilage. 34 The failure to detect fluorescence in the kidney glomeruli was most likely because of the strong autofluorescence of this tissue, although we cannot exclude the lack of a tissue-specific response element in the 2.3 kbp of 5′-flanking region of VEGF DNA used for injection. However, this region includes the TGF-α responsive region (−88 to −65), 13 the presumable hypoxia-inducible responsive element (−2013 to −2006), 23 the platelet-derived growth factor-responsive element (−85 to −50), 35 and the von Hippel-Lindau responsive element (−194 to −50). 25 Nucleotide positions are numbered from the transcriptional start site.
In skin from 1- or 4-week-old transgenic mice, normal epidermal keratinocytes showed basal levels of GFP expression, whereas bright fluorescence was observed in the outer root sheath of hair follicles. This GFP signal overlapped with keratin 5 expression which is a characteristic marker for keratinocytes, 36 confirming that the source of GFP signal in hair follicles did not originate from the surrounding dermal fibroblast sheath. The skin of older mice (7 weeks old) did not show equivalent GFP expression in the hair follicles, suggesting that VEGF expression in the follicles may be correlated with the hair cycle. Because of the stability of GFP protein (∼2 days half-life), 26 determining the precise timing of the turn-off of VEGF gene expression during the hair cycle may be difficult. Although VEGF expression in hair dermal papilla cells (specialized mesenchymal cells in the hair follicle) has been previously reported by immunohistochemistry and by PCR-based detection methods, 37-39 we could not detect a significant VEGF signal assessed by GFP fluorescence in this transgenic model.
We have shown the acute inducibility of GFP during wound healing (within 48 hours) and after TPA treatment (within 6 hours), confirming that the GFP expression pattern is highly VEGF promoter-dependent. 8,11 These data exclude a possible delay of fluorescent signal production because of slow chromophore formation as reported in Drosophila embryos. 40 In anticipation of this difficulty, we had selected the red-shifted variant of GFP for these studies, and the correct folding of the chromophore appears to occur in skin as well as in internal tissues.
In both the wound model and after TPA treatment, our transgenic model showed little or no fibroblast-derived VEGF expression. These findings differ from the previously reported fibroblast-derived GFP fluorescence in VEGF promoter-driven GFP transgenic mice, 26 but are in accordance with previous studies showing primarily epithelial expression of VEGF. 6,8-10,14,17,31 This discrepancy may be because of the different promoter regions used to generate the transgenic mice. The promoter we used for the studies described above includes an additional 500-bp sequence of the 5′-upstream region of the VEGF promoter which was absent in the previously reported transgenic model. This region may contain cell type-specific suppressor elements.
Laser confocal microscopic evaluation of living transgenic mouse skin indicates that GFP is readily detectable within the epidermis. These results suggest the potential use of GFP transgenic mice for noninvasive monitoring of long-term gene expression in vivo. When the transgenic GFP reporter gene is combined with confocal microscopy, the system has a great advantage over conventional transgenic reporter gene methods in animals because it reduces the number of animals used, utilizes a simple monitoring system, and allows long-term monitoring of changes in gene expression in the same individual. However, several limitations of this model should be mentioned. 1) Posttranscriptional control mechanisms of VEGF expression act by stabilizing VEGF mRNA, 22,41,42 a process that is not detected in our transgenic system. 2) Reporter molecules might not completely represent endogenous gene expression correctly in all tissues and cell types, because regulatory elements for transcriptional control might exist far from the promoter region, and such factors might be present within intronic regions, making it nearly impossible to insert the complete transcriptional control unit into the transgene cassette. 3) This type of transgenic model does not allow protein localization of the target gene product in tissue.
In conclusion, the detection of GFP in transgenic mouse skin will facilitate the examination of the regulation of VEGF gene expression in inflammatory and neoplastic skin diseases, providing a powerful in vivo monitoring and screening tool.
Acknowledgments
We thank Nanyan Jiang and Dr. Lin Wu for pronuclear injections to generate transgenic mice.
Footnotes
Address reprint requests to Jiro Kishimoto, Ph.D., Cutaneous Biology Research Center, Massachusetts General Hospital–East, Building 149, 13th Street, Charlestown, MA 02129. E-mail: jiro.kishimoto@cbrc2. mgh.harvard.edu.
Supported by the Cutaneous Biology Research Center, Massachusetts General Hospital; by Shiseido Co., Ltd., Tokyo, Japan; and by National Institutes of Health/National Cancer Institute grant CA69184.
References
- 1.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z: Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999, 13:9-22 [PubMed] [Google Scholar]
- 2.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF: Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219:983-985 [DOI] [PubMed] [Google Scholar]
- 3.Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, Dvorak HF: Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev 1993, 12:303-324 [DOI] [PubMed] [Google Scholar]
- 4.Potgens AJ, Lubsen NH, van Altena MC, Schoenmakers JG, Ruiter DJ, de Waal RM: Vascular permeability factor expression influences tumor angiogenesis in human melanoma lines xenografted to nude mice. Am J Pathol 1995, 146:197-209 [PMC free article] [PubMed] [Google Scholar]
- 5.Claffey KP, Brown LF, del Aguila LF, Tognazzi K, Yeo KT, Manseau EJ, Dvorak HF: Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res 1996, 56:172-181 [PubMed] [Google Scholar]
- 6.Brown LF, Harrist TJ, Yeo KT, Stahle-Backdahl M, Jackman RW, Berse B, Tognazzi K, Dvorak HF, Detmar M: Increased expression of vascular permeability factor (vascular endothelial growth factor) in bullous pemphigoid, dermatitis herpetiformis, and erythema multiforme. J Invest Dermatol 1995, 104:744-749 [DOI] [PubMed] [Google Scholar]
- 7.Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA: Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol 1998, 152:1445-1452 [PMC free article] [PubMed] [Google Scholar]
- 8.Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L: Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 1992, 176:1375-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, Berse B, Dvorak HF: Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med 1994, 180:1141-1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weninger W, Uthman A, Pammer J, Pichler A, Ballaun C, Lang IM, Plettenberg A, Bankl HC, Sturzl M, Tschachler E: Vascular endothelial growth factor production in normal epidermis and in benign and malignant epithelial skin tumors. Lab Invest 1996, 75:647-657 [PubMed] [Google Scholar]
- 11.Larcher F, Robles AI, Duran H, Murillas R, Quintanilla M, Cano A, Conti CJ, Jorcano JL: Up-regulation of vascular endothelial growth factor/vascular permeability factor in mouse skin carcinogenesis correlates with malignant progression state and activated H-ras expression levels. Cancer Res 1996, 56:5391-5396 [PubMed] [Google Scholar]
- 12.Raleigh JA, Calkins-Adams DP, Rinker LH, Ballenger CA, Weissler MC, Fowler WC, Jr, Novotny DB, Varia MA: Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res 1998, 58:3765-3768 [PubMed] [Google Scholar]
- 13.Gille J, Swerlick RA, Caughman SW: Transforming growth factor-alpha-induced transcriptional activation of the vascular permeability factor (VPF/VEGF) gene requires AP-2-dependent DNA binding and transactivation. EMBO J 1997, 16:750-759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S: Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem 1995, 270:12607-12613 [DOI] [PubMed] [Google Scholar]
- 15.Tober KL, Cannon RE, Spalding JW, Oberyszyn TM, Parrett ML, Rackoff AI, Oberyszyn AS, Tennant RW, Robertson FM: Comparative expression of novel vascular endothelial growth factor/vascular permeability factor transcripts in skin, papillomas, and carcinomas of v-Ha-ras Tg.AC transgenic mice and FVB/N mice. Biochem Biophys Res Commun 1998, 247:644-653 [DOI] [PubMed] [Google Scholar]
- 16.Detmar M: Molecular regulation of angiogenesis in the skin. J Invest Dermatol 1996, 106:207-208 [DOI] [PubMed] [Google Scholar]
- 17.Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK: Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol 1998, 111:1-6 [DOI] [PubMed] [Google Scholar]
- 18.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA: The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem 1991, 266:11947-11954 [PubMed] [Google Scholar]
- 19.Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW: The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol 1991, 5:1806-1814 [DOI] [PubMed] [Google Scholar]
- 20.Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Bohlen P: Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun 1992, 187:1579-1586 [DOI] [PubMed] [Google Scholar]
- 21.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT: The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992, 255:989-991 [DOI] [PubMed] [Google Scholar]
- 22.Stein I, Neeman M, Shweiki D, Itin A, Keshet E: Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genes. Mol Cell Biol 1995, 15:5363-5368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy AP, Levy NS, Wegner S, Goldberg MA: Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem 1995, 270:13333-13340 [DOI] [PubMed] [Google Scholar]
- 24.Finkenzeller G, Technau A, Marme D: Hypoxia-induced transcription of the vascular endothelial growth factor gene is independent of functional AP-1 transcription factor. Biochem Biophys Res Commun 1995, 208:432-439 [DOI] [PubMed] [Google Scholar]
- 25.Mukhopadhyay D, Knebelmann B, Cohen HT, Ananth S, Sukhatme VP: The von Hippel-Lindau tumor suppressor gene product interacts with Sp1 to repress vascular endothelial growth factor promoter activity. Mol Cell Biol 1997, 17:5629-5639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B: Tumor induction of VEGF promoter activity in stromal cells. Cell 1998, 94:715-725 [DOI] [PubMed] [Google Scholar]
- 27.Kishimoto J, Ehama R, Wu L, Jiang S, Jiang N, Burgeson RE: Selective activation of the versican promoter by epithelial-mesenchymal interactions during hair follicle development. Proc Natl Acad Sci USA 1999, 96:7336-7341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamimura J, Lee D, Baden HP, Brissette J, Dotto GP: Primary mouse keratinocyte cultures contain hair follicle progenitor cells with multiple differentiation potential. J Invest Dermatol 1997, 109:534-540 [DOI] [PubMed] [Google Scholar]
- 29.Weinberg WC, Goodman LV, George C, Morgan DL, Ledbetter S, Yuspa SH, Lichti U: Reconstitution of hair follicle development in vivo: determination of follicle formation, hair growth, and hair quality by dermal cells. J Invest Dermatol 1993, 100:229-236 [DOI] [PubMed] [Google Scholar]
- 30.Katsuki M, Yokoyama M, Nozawa S, Kobayashi K, Kimura M, Sato M: Experimental Manual for Developmental Engineering: How to Generate a Transgenic Mouse. Katsuki M eds. (In Japanese). 1987, :pp 128-167 Kodansha, Ltd., Tokyo [Google Scholar]
- 31.Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR: Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell 1992, 3:211-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breier G, Albrecht U, Sterrer S, Risau W: Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 1992, 114:521-532 [DOI] [PubMed] [Google Scholar]
- 33.Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A: Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol 1999, 212:307-322 [DOI] [PubMed] [Google Scholar]
- 34.Horner A, Bishop NJ, Bord S, Beeton C, Kelsall AW, Coleman N, Compston JE: Immunolocalisation of vascular endothelial growth factor (VEGF) in human neonatal growth plate cartilage. J Anat 1999, 194:519-524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finkenzeller G, Sparacio A, Technau A, Marme D, Siemeister G: Sp1 recognition sites in the proximal promoter of the human vascular endothelial growth factor gene are essential for platelet-derived growth factor-induced gene expression. Oncogene 1997, 15:669-676 [DOI] [PubMed] [Google Scholar]
- 36.Foitzik K, Paus R, Doetschman T, Dotto GP: The TGF-beta2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev Biol 1999, 212:278-289 [DOI] [PubMed] [Google Scholar]
- 37.Lachgar S, Moukadiri H, Jonca F, Charveron M, Bouhaddioui N, Gall Y, Bonafe JL, Plouet J: Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J Invest Dermatol 1996, 106:17-23 [DOI] [PubMed] [Google Scholar]
- 38.Kozlowska U, Blume-Peytavi U, Kodelja V, Sommer C, Goerdt S, Majewski S, Jablonska S, Orfanos CE: Expression of vascular endothelial growth factor (VEGF) in various compartments of the human hair follicle. Arch Dermatol Res 1998, 290:661-668 [DOI] [PubMed] [Google Scholar]
- 39.Lachgar S, Charveron M, Gall Y, Bonafe JL: Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br J Dermatol 1998, 138:407-411 [DOI] [PubMed] [Google Scholar]
- 40.Davis I, Girdham CH, O’Farrell PH: A nuclear GFP that marks nuclei in living Drosophila embryos: maternal supply overcomes a delay in the appearance of zygotic fluorescence. Dev Biol 1995, 170:726-729 [DOI] [PubMed] [Google Scholar]
- 41.Claffey KP, Shih SC, Mullen A, Dziennis S, Cusick JL, Abrams KR, Lee SW, Detmar M: Identification of a human VPF/VEGF 3′ untranslated region mediating hypoxia-induced mRNA stability. Mol Biol Cell 1998, 9:469-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy AP, Levy NS, Goldberg MA: Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem 1996, 271:2746-2753 [DOI] [PubMed] [Google Scholar]