Abstract
An imbalance between matrix metalloproteinases (MMPs) and inhibitors of MMPs (TIMPs) may contribute to tissue destruction that is found in various inflammatory disorders. To determine in an in vivo experimental setting whether the inflammatory reaction in the course of lipopolysaccharide (LPS)-induced endotoxemia causes an altered balance in the MMP/TIMP system, we analyzed the expression of a number of MMP and TIMP genes as well as MMP enzymatic activity in the liver, kidney, spleen, and brain at various time points after systemic injection of different doses of LPS in mice. Injection of sublethal doses of LPS led to an organ- and time-specific pattern of up-regulation of several MMP genes and the TIMP-1 gene in the liver, spleen, and kidney, whereas in the brain only TIMP-1 was induced. Injection of a lethal dose of LPS caused similar but more prolonged expression of these MMP genes as well as the induction of additional MMP genes in all organs. In LPS-treated mice in situ hybridization revealed collagenase 3 gene induction in cells resembling macrophages whereas TIMP-1 RNA was detected predominantly in parenchymal cells. Finally, gelatin zymography revealed increased gelatinolytic activity in all organs after LPS treatment. These observations highlight a dramatic shift in favor of increased expression of the MMP genes over the TIMP genes during LPS-induced endotoxemia, and suggest that MMPs may contribute to the development of organ damage in endotoxemia.
Sepsis is a disorder with a high lethality even under appropriate treatment with antibiotics and complete eradication of bacteria. This suggests that ongoing responses of the immune system once started may direct the course of the disease. Lipopolysaccharide (LPS)-induced endotoxemia is a well-established model of an infection with gram-negative bacteria. 1,2 LPS induces symptoms such as fever, hypotension, disseminated intravascular coagulation, and multiple organ system failure and thus mimics sepsis caused by bacteria. 3 LPS binds to several receptors on leukocytes 4,5 resulting in a cascade of events 6 including synthesis and release of cytokines such as tumor necrosis factor-α (TNF-α), interferon-γ, and interleukin-1 (IL-1). 2,7
MMPs are a family of matrix-degrading proteases including collagenases, gelatinase A and B, stromelysins, macrophage metalloelastase, and others. 8-11 A variety of cell types, including leukocytes such as macrophages 12 and lymphocytes, 13 tumor cells, 14 or neural cells 15,16 have been shown to produce MMPs. These proteases are involved in the degradation of otherwise very stable extracellular matrix proteins such as collagens or elastin 13,17,18 as occurs during organogenesis, inflammatory processes, and tumor infiltration. Cytokines, 15,16,19-21 as well as other factors (ie, such as lectins, hormones, viruses, bacteria, and LPS 22-24 ), have been shown to play a crucial role in the regulation of MMP expression in vivo and in vitro. However, cytokine/MMP interactions are bidirectional because further functions of MMPs include the degradation of IL-1β, 25 the cleavage of proTNF-α 26 and proIL-1β 27 to yield their active forms, the shedding of the 80-kd TNF receptor, 28 and the release of cytokines that are bound to the extracellular matrix. 29 Significantly increased MMP activity has been observed in a variety of inflammatory disorders such as rheumatoid arthritis, multiple sclerosis 30 and its experimental counterpart experimental autoimmune encephalomyelitis, 31-33 periodontal disease, 17 and in bacterial sepsis, 24 implicating a role for the MMPs in the tissue injury that accompanies these diseases.
The physiological counterregulators of MMPs are the MMP inhibitors. Besides four specific tissue inhibitors of MMPs (TIMPs) 34 , 35 nonspecific inhibitors such as α2-macroglobulin (α2-M) have been reported. 34 A variety of TIMPs are, albeit at different levels, constitutively expressed in virtually all organs. 36 Similar to the MMP genes, a number of factors are involved in the regulation of TIMP gene expression including cytokines. 37 However, the individual TIMP species are differentially regulated. 38
Ultimately, the biological actions of MMPs are determined by several factors. The first is the level of expression and the type of MMP that is/are expressed. The second is the activation of the inactively secreted zymogens by MT1-MMP, a membrane-bound member of the MMP family, or other proteases. The third is the ratio between MMP protein and inhibiting TIMPs, because this ratio determines the net activity of MMP protein. 39 The fourth is the secretion of MMP-9 from preformed granules of neutrophils under the influence of LPS and chemotactic factors such as IL-8, a mechanism that does not require direct de novo synthesis of MMP-9. 40
In view of their regulation and functions, the MMP/TIMP system is likely to play an important role in LPS-induced endotoxemia. Despite this, most work concerning LPS- or cytokine-induced MMP expression has been done in vitro. 16,41-44 In vitro findings suggest that multiple MMP and TIMP genes could be activated in vivo during endotoxemia. However, to date, this issue has rarely been addressed. 24 In a recent study, Solorzano and coworkers 45 demonstrated an ameliorative effect of the synthetic MMP inhibitor GM-6001 in LPS-induced endotoxemia. The MMP inhibitor increased the survival of LPS-treated mice and inhibited plasma levels of TNF-α. However, the effects of the MMP inhibitor on MMP activity were not studied.
In the present study, experiments were performed in LPS-responsive and LPS-resistant mice (C3H/HeJ) 2 to investigate the expression pattern and the regulation of various MMPs and TIMPs in endotoxemia. To elucidate the role of the complex MMP and TIMP network in this model of inflammation, we used RNase protection assays for the simultaneous and semiquantitative determination of MMP and TIMP gene expression. The cellular localization of RNAs was revealed by in situ hybridization. Furthermore, we analyzed the activity of gelatinases in the organs using gelatin polyacrylamide gel electrophoresis (PAGE) zymography and in situ zymography.
Materials and Methods
Mice
C57BL/SJL F1 mice used in this study were maintained under specific pathogen-free conditions in the closed breeding colony of the Scripps Research Institute. Experiments were performed in 8- to 10-week-old mice of both sexes. LPS-resistant C3H/HeJ and LPS-sensitive C3H/FeJ mice as controls were obtained from Jackson Laboratories (Maine, VT) and used at 10 to 14 weeks of age.
Induction of Endotoxemia
Endotoxemia was induced by intraperitoneal injection of different doses of LPS (Escherichia coli 026:B6; Sigma, St. Louis, MO) ranging from 20 μg (sublethal dose) to 1 mg (lethal dose). In an initial experiment, two mice at each time point were injected with 20 μg of LPS and were killed at various times ranging from 15 minutes to 24 hours. In another experiment, a lethal dose of 1 mg of LPS was administered and mice were killed 2, 8, or 16 hours later (three mice at each time point). Experiments with LPS-resistant (C3H/HeJ) mice and the respective LPS-sensitive control (C3H/FeJ) mice were performed with a single injection of 20 μg of LPS and the mice were killed 8 hours later. In all experiments, the brain, kidney, spleen, and liver were removed and immediately snap-frozen in liquid nitrogen and stored at −80°C until RNA isolation or protein extraction. For in situ hybridization, mice were injected with 1 mg of LPS and were killed 8 hours later. Organs were removed, fixed in 4% formaldehyde, and embedded in paraffin. For in situ zymography, mice were injected with 100 μg of LPS and were killed 16 hours later, organs were removed and snap-frozen in liquid nitrogen.
RNase Protection Assay (RPA)
The production of RPA probe sets for the detection of MMP and TIMP gene expression was described previously. 31,46 Briefly, the MMP probe set included probes for stromelysins 1, 2, and 3, matrilysin, metalloelastase, gelatinase A and B, collagenase 3, and membrane type1-MMP (MT1-MMP; MMP probe set). The MMP probe set used for RPAs in the experiment in which 20 μg of LPS was used did not contain the gelatinase A probe. In later experiments, the gelatinase A probe was added to the probe set. These experiments showed that there was no gelatinase A gene expression detectable at the LPS doses tested (20 μg to 1 mg). The probe set for the inhibitors of MMPs (TIMP probe set) included probes for TIMPs 1, 2, and 3, and α2-M. A fragment of the RPL32–4A gene 47 served as an internal loading control. Poly(A)+ RNA was prepared as described. 48 RNase protection assays were performed as described previously. 49
In Situ Hybridization
In situ hybridization was performed as described by Simmons et al 50 with modifications. Briefly, paraffin sections were deparaffinized and rehydrated in graded alcohols. After postfixation in 4% formaldehyde, proteinase K treatment (2.4 mg/100 ml 5× Tris-EDTA buffer at 37°C for 15 minutes), and acetylation in 250 μl acetic anhydride in 100 ml phosphate-buffered saline (PBS) for 10 minutes, the slides were dehydrated in graded alcohol and dried. Templates for the probes for collagenase 3, gelatinase B, stromelysin 1, and TIMP-1 were generated by reverse transcriptase-polymerase chain reaction and cloned into the pGEM-4 plasmid (Promega, Madison, WI). Cloned products were sequenced to confirm their identity with the published sequences. [33P]-labeled sense or antisense probes were hybridized to the tissue overnight at 56 to 59°C. After digestion with RNase A (Promega), slides were washed in decreasing concentrations of standard saline citrate. After dehydration in graded alcohol, slides were air dried and exposed for 5 days to Ultra Vision G film (Sterling, Newark, DE). Then, slides were dipped in Kodak NTB-2 emulsion (Eastman Kodak Co., Rochester, NY), dried, and stored in the dark for 4 weeks. Subsequently, slides were developed, counterstained with Mayer’s hematoxylin, and examined by dark- and bright-field microscopy.
PAGE Zymography
Protease activity was determined as described previously. 31 Briefly, frozen organs were homogenized (1/10, w/v) on ice in Tris buffer (pH 7.6) containing the serine and cysteine protease inhibitors aprotinin, leupeptin, leustatin, and phenylmethyl sulfonyl fluoride. Lysates were centrifuged and supernatants used for zymography. Samples were electrophoresed on 10% polyacrylamide gels containing 0.1% gelatin (Sigma). After electrophoresis, sodium dodecyl sulfate was removed by soaking the gels for 20 minutes each in buffers containing 50 mmol/L Tris (pH 7.6), 10 mmol/L CaCl, and 2.5% Triton X-100 followed by the same buffer with 1% Triton. Protease activity was restored in the same buffer without Triton. Digestion was performed at 37°C for 36 hours. Gels were stained with Coomassie brilliant blue and destained until clear bands became evident.
In Situ Zymography
In situ zymography was performed as described by Oh and co-workers. 51 Briefly, cryostat sections (10 μm) of brain, spleen, liver, and kidney of control mice and of endotoxemic mice were overlaid with assay solution (50 mmol/L Tris-HCl, 5 mmol/L CaCl, 0.2 mmol/L NaN3, pH 7.5) containing 40 μg/ml fluorescein isothiocyanate-labeled DQ gelatin (Molecular Probes, Eugene, OR). Sections were incubated at 37°C for 18 hours and examined by fluorescence microscopy. Gelatinolytic proteases cleave quenched DQ gelatin resulting in fluorescent breakdown products that thereby allow for the localization of net gelatinolytic activity. Addition of 10 mmol/L ethylenediaminetetraacetic acid (EDTA) (a chelating agent that inhibits metal ion dependent proteases such as MMPs) to the assay solution or omission of DQ gelatin served as negative control and abolished the fluorescence.
Results
MMP and TIMP mRNA Expression in LPS-Sensitive Mice
The expression of transcripts for the MMPs: collagenase 3, gelatinase A and B, stromelysins 1, 2, and 3, metalloelastase, matrilysin, and MT1-MMP; and the inhibitors: TIMP-1, 2, and 3 and α2-M was determined after injection of different doses of LPS. After injection of 1 mg of LPS, three of six mice died between 15 and 18 hours. Charts showing the quantitative analysis of MMP and TIMP mRNA expression are given for RNAs that were either induced or were more than twofold up-regulated after challenge with 20 μg or 1 mg of LPS (Figures 1 and 2) ▶ ▶ .
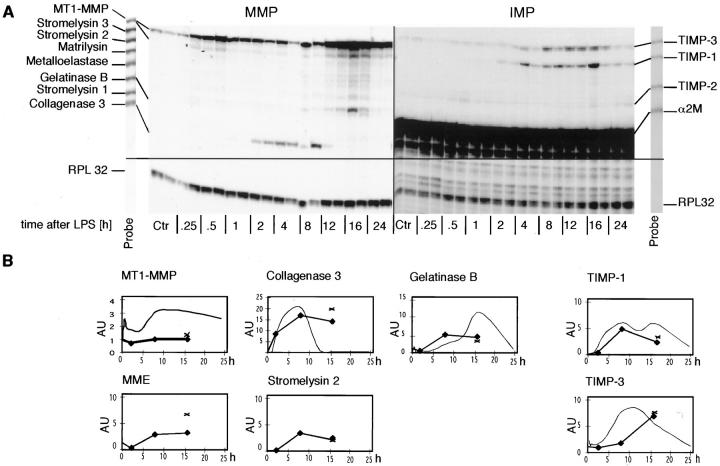
Figure 1.
Time course of MMP and TIMP gene expression in the liver after LPS injection. Mice were given a single i.p. injection of LPS (20 μg or 1 mg in 100 μl of PBS). Poly(A)+ RNA was isolated from the liver at the times shown and 1 μg used to determine MMP or TIMP mRNA levels by RPA as described in Materials and Methods. A: Time course of gene expression after injection of 20 μg of LPS. Autoradiograms showing the RPA-labeled probe sets used in this analysis are shown at the sides. The RPL 32 probe does not appear in the part of the RPA shown here. Note that the hybridized liver target mRNAs that lack the cloning site of the probe migrate at a lower molecular size. Two animals were used for each time point. The autoradiographic exposure times were 16 hours (MMP) and 6 hours (TIMP). B: Quantitative analysis of MMP and TIMP gene expression shown in A (20 μg LPS, fine curve) and after 1 mg of LPS (bold line, three mice were used at each time point, X MMP and TIMP gene expression in three mice that died at 15 to 18 hours after LPS). Charts are given for RNAs that are either induced or up-regulated more than twofold. For constitutively expressed RNAs that are up-regulated after LPS injection, here MT1-MMP and TIMP-3, the y axis label shows the fold up-regulation. For induced genes, the y axis label indicates arbitrary units.
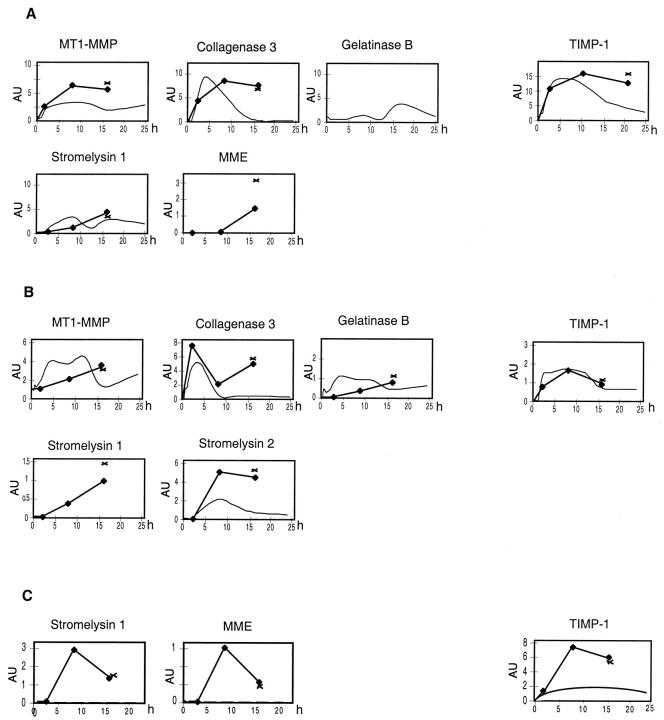
Figure 2.
Quantitative analysis of MMP and TIMP gene expression the organs of mice after injection of different doses of LPS (20 μg LPS, fine curve) and after 1 mg of LPS (bold line, three mice were used at each time point, X MMP and TIMP gene expression in three mice that died at 15 to 18 hours after LPS). A: MMP and TIMP gene expression in the spleen after different doses of LPS. B: Quantitative analysis of MMP and TIMP gene expression in the kidney after different doses of LPS. C: Quantitative analysis of MMP and TIMP gene expression in the brain after different doses of LPS. The RPAs and quantitative analysis was performed as described in Materials and Methods and the legend to Figure 1 ▶ .
In control liver, expression of MT1-MMP and low levels of gelatinase B was observed (Figure 1) ▶ . After injection of 20 μg of LPS, changes in MMP gene expression first became evident by 1 hour. At 2 hours there was induction of collagenase 3 mRNA that peaked at 8 hours and then declined rapidly during the next 8 hours back to below detectable levels. In contrast, expression of gelatinase B mRNA showed a more delayed response that peaked by 16 hours. MT1-MMP gene expression showed an up-regulation that peaked by 12 hours. A different time course of MMP gene expression was observed after injection of 1 mg of LPS (Figure 1) ▶ . Whereas the levels of collagenase 3 and gelatinase B gene expression during the first 8 hours was similar to that seen after injection of 20 μg LPS, the expression of these genes then remained elevated until the mice died. Moreover, injection of 1 mg LPS induced the expression of additional MMP genes including macrophage metalloelastase and stromelysin 2 that peaked by 8 hours and also remained elevated until the mice died. There was no significant change in MT1-MMP gene expression after 1 mg of LPS.
Analysis of the TIMP gene expression, revealed constitutive expression at very high levels of α2-M with no detectable change after LPS challenge (Figure 1) ▶ . In contrast, TIMP-1 mRNA, which was not detectable in controls, was induced 2 hours after LPS injection and increased further to maximum levels between 8 and 16 hours before declining by 24 hours. TIMP-3 mRNA, which was expressed constitutively, increased by 4 hours and peaked by 12 hours before declining to baseline levels by 24 hours. Similar changes of TIMP-1 and -3 mRNA were observed after 1 mg of LPS (Figure 1) ▶ . At 8 hours there were no differences observed in the TIMP mRNA induction between the different LPS doses.
Constitutive expression of MT1-MMP and gelatinase B mRNAs and to a lesser extent, metalloelastase mRNA was observed in control spleen. After LPS injection, MT1-MMP mRNA showed a prolonged increase (Figure 2) ▶ . In addition, a rapid induction of collagenase 3 mRNA expression was observed which peaked by 4 hours and declined slowly to baseline levels by 16 hours (20 μg), whereas after 1 mg LPS, these mRNA levels remained high until the mice died. After 20 μg LPS, gelatinase B mRNA expression showed a modest up-regulation between 4 hours to 12 hours, followed by a stronger up-regulation that peaked by 16 hours. After 1 mg of LPS there was a similar modest up-regulation as seen after 20 μg of LPS, however there was no further increase of gelatinase B expression after 12 hours. In contrast to the liver, in the spleen stromelysin 1 mRNA levels were significantly elevated after LPS treatment. Stromelysin 1 mRNA, which was induced 1 hour after 20 μg LPS increased slowly to peak by 8 hours and then declined slowly by 24 hours (Figure 2) ▶ . After treatment with 1 mg of LPS, stromelysin 1 mRNA expression reached similar levels as after 20 μg of LPS, however, expression remained at elevated levels until the animals died. Additionally, injection of 1 mg LPS caused an induction of metalloelastase mRNA by 8 hours that increased until the animals died.
TIMP-2 and TIMP-3 mRNAs were constitutively expressed in the spleen. After LPS injection only minor changes were observed with less than twofold increases of TIMP-2 and 3 mRNAs (Figure 2) ▶ . However, after LPS injection, expression of TIMP-1 mRNA was induced in this organ with peak expression by 4 hours which then decreased slowly (20 μg) or remained elevated until the mice died (1 mg). Peak expression of TIMP-1 mRNA was similar after treatment with 20 μg and 1 mg LPS.
Constitutive expression of low levels of MT1-MMP mRNA was observed in the kidney. After LPS injection the earliest changes in MMP expression were observed in the kidney (Figure 2) ▶ . Expression of MT1-MMP mRNA was up-regulated during the entire experimental period. Induction of collagenase 3 mRNA was detectable 15 minutes after LPS challenge, then increased rapidly to peak by 4 hours, followed by a rapid decrease to low levels. After injection of 1 mg of LPS there was a second peak of collagenase 3 mRNA expression at 16 hours at which time some of the mice died. Induction of gelatinase B mRNA was observed after 15 minutes; however, in contrast to collagenase 3 expression, the expression of gelatinase B was lower and more prolonged (Figure 2) ▶ . Stromelysin 2 mRNA was also induced with detectable mRNA levels 2 hours after LPS-injection which peaked by 8 hours before declining slowly to 24 hours (20 μg), while remaining at high levels after 1 mg LPS (Figure 2) ▶ . Low levels of metalloelastase mRNA were also induced between 4 and 8 hours after LPS injection. Only after the injection of 1 mg of LPS was an induction of stromelysin 1 mRNA observed.
The kidney showed high constitutive expression of TIMP-3 mRNA and low levels of TIMP-2 and α2-M mRNAs which remained unaltered after LPS (Figure 2) ▶ . After LPS injection, rapid induction of TIMP-1 mRNA was observed within an hour reaching maximum levels by 12 hours, before slowly declining to a third of these levels at 24 hours. Similar changes were seen with both doses of LPS tested.
In the brain, constitutive expression of MT1-MMP and lower levels of gelatinase B mRNAs were observed (Figure 2) ▶ . Although injection of 20 μg of LPS caused no significant alterations in the expression of MMP mRNAs, injection of 1 mg LPS resulted in an induction of stromelysin 1 and metalloelastase mRNAs.
In control brain, constitutive expression of high levels of TIMP-2 and of TIMP-3 mRNAs was found. In contrast to the MMP mRNAs, injection of 20 μg LPS induced TIMP-1 mRNA, which became detectable after 4 hours, peaked at 8 hours, with a slow decline thereafter. A similar change in TIMP-1 mRNA expression was seen after 1 mg LPS.
MMP and TIMP Expression in LPS-Resistant Mice
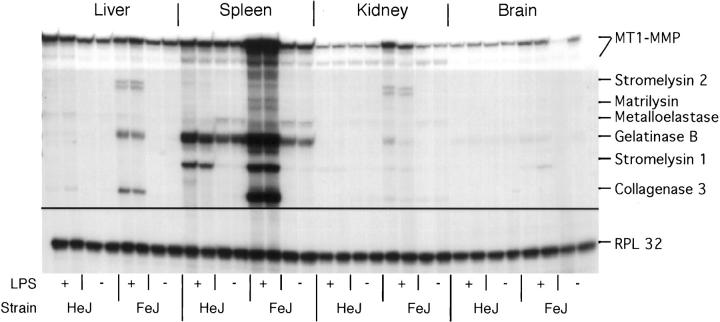
In the basal state, expression patterns of the MMP and TIMP genes in LPS-sensitive C3H/FeJ and LPS-resistant C3H/HeJ mice were similar to C57BL/SJL mice (Figure 3) ▶ . After LPS injection in LPS-responsive mice, with some exceptions, similar changes in MMP and TIMP gene expression were observed to those seen in C57BL/SJL mice. Stromelysin 2 mRNA was induced in the liver, a change that was not observed in the C57BL/SJL mice. In contrast with LPS-sensitive mice, and with the exception of stromelysin 1 mRNA induction and slight up-regulation of gelatinase B in the spleen, LPS-resistant C3H/HeJ mice showed no significant alterations in MMP gene expression after LPS administration (Figure 3) ▶ .
Figure 3.
Comparison of MMP gene expression in LPS-resistant C3H/HeJ and LPS-sensitive C3H/FeJ mice. Mice were given an i.p. injection of LPS (20 μg) and were killed 8 hours later. RPA was performed using 1 μg poly(A)+ RNA from each organ as described in Materials and Methods.
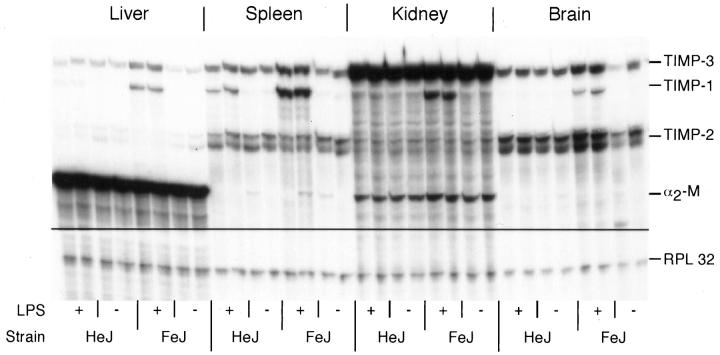
The expression of the TIMP mRNAs in both strains of C3H mice showed similar patterns to those found in C57B/SJL mice (Figure 4) ▶ . After LPS injection, in LPS-sensitive mice, TIMP-1 mRNA was up-regulated in a similar fashion to C57B/SJL mice, whereas in LPS-resistant mice, similar to the MMP gene expression, the only change in TIMP mRNA expression was found in the spleen. Here an induction of TIMP-1 mRNA was observed, although this was not as pronounced as in the LPS-sensitive control animals.
Figure 4.
Comparison of TIMP gene expression in LPS-resistant C3H/HeJ and LPS-sensitive C3H/FeJ mice. Mice were given an i.p. injection of LPS (20 μg) and were killed 8 hours later. RPA was performed using 1 μg poly(A)+ RNA from each organ as described in Materials and Methods.
Cellular Localization of MMP and TIMP RNAs in LPS-Sensitive Mice
The cellular source of MMP and TIMP gene expression during LPS-induced endotoxemia is unknown. Therefore, here we performed in situ hybridization experiments to determine the cell types that express these genes.
In the liver from control mice, there was no detectable expression in any cells of the collagenase 3, gelatinase B, or TIMP-1 RNAs. However, after LPS injection strong expression of collagenase 3 RNA was detected in parenchymal cells with smaller nuclei than hepatocytes that resembled liver macrophages (von Kupffer cells) (Figure 5) ▶ . Some of the cells hybridizing with the collagenase 3 probe were also found around small vessels. These cells also resembled cells of the macrophage phagocyte system. In contrast to RPA analysis that showed induction of gelatinase B in endotoxemia, we were not able to detect signals above background for gelatinase B RNA by in situ hybridization. After LPS treatment, hybridization with a probe for TIMP-1 revealed a diffuse signal in a majority of liver cells including hepatocytes (not shown).
Figure 5.
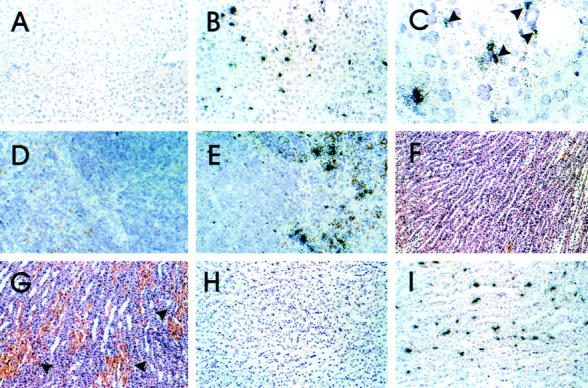
Cellular localization of collagenase 3 RNA in various organs of control and LPS-treated mice. In situ hybridization was performed as described in Materials and Methods. Liver: control (A), LPS-injected (B and C). The probe hybridizes to cells resembling von Kupffer-cells (arrowheads). Original magnification: ×20 (A and B), ×60 (C). Spleen: control (D); LPS-injected (E). Original magnification ×20. Kidney: control (F); hemorrhage after LPS injection (G; arrowheads). H&E; original magnification, ×10. Control (H); LPS-injected (I). Original magnification, ×10.
In spleens from control mice, constitutive expression of gelatinase B and stromelysin 1 RNA was detected in the red pulp in cells resembling macrophages (not shown). Although there were many cells positive for gelatinase B only a few cells expressed stromelysin 1 RNA. After LPS injection there was severe hyperemia in the red pulp. Furthermore, the number of cells expressing gelatinase B and stromelysin 1 RNAs was increased. In addition, expression of the collagenase 3 (Figure 5) ▶ and TIMP-1 (Figure 6) ▶ RNAs was detectable in cells in the vicinity of the sinus endothelia that resembled phagocytic cells.
Figure 6.
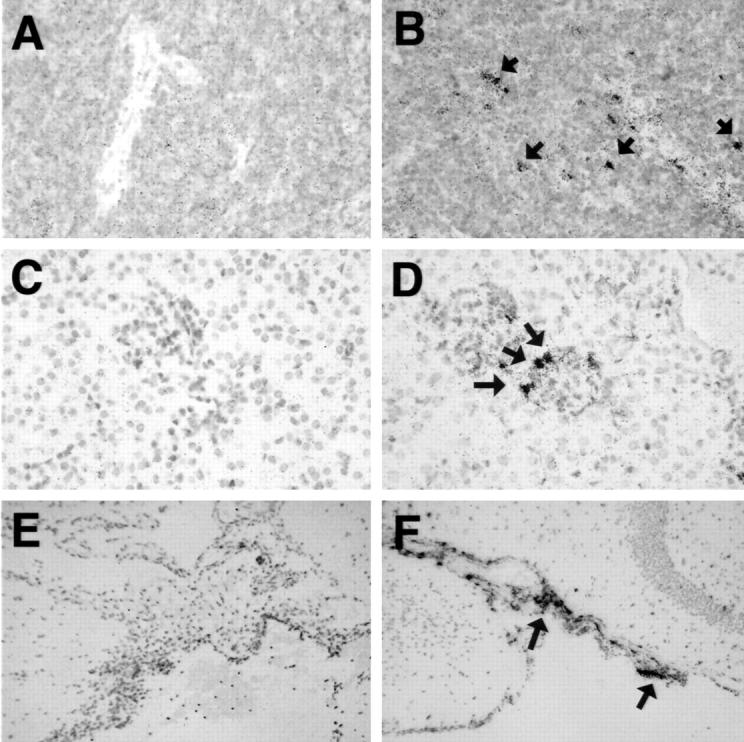
Cellular localization of TIMP-2 RNA in various organs of control and LPS-treated mice. In situ hybridization was performed as described in Materials and Methods. Spleen: control (A); LPS-injected (B). Original magnification, ×40. Kidney: control (C); glomerulum, LPS-injected, note the labeled cells around a glomerulum (D, arrows). Original magnification, ×40. Brain: control (E); expression of TIMP-1 RNA in the meninges after LPS-injection (F; arrows). Original magnification, ×10.
In kidneys from control mice, expression of the collagenase 3, stromelysin 1, gelatinase B, and TIMP-1 RNAs was not detectable by in situ hybridization. In contrast, strong expression of collagenase 3 RNA was observed in stromal cells of the renal medulla of endotoxemic mice (Figure 5) ▶ . Labeled cells had spindle-shaped nuclei indicating that these cells were likely vascular endothelial cells or phagocytic cells. Interestingly, expression of collagenase 3 RNA co-localized with areas of renal hemorrhage (Figure 5) ▶ . TIMP-1 RNA expression was found in the glomeruli, in perivascular macrophages, and overlapped with areas of collagenase 3 RNA expression in the renal medulla (Figure 6) ▶ .
In brain from control mice, there were no cells positive for gelatinase B, collagenase 3, stromelysin 1, and TIMP-1 RNAs. In the brain of endotoxemic mice expression of collagenase 3 RNA was detectable in a few cells in the meninges and in the choroid plexus (Figure 5) ▶ . Expression of the gelatinase B or stromelysin RNAs was not detectable by this method. TIMP-1 RNA-expressing cells were found in the meninges, the choroid plexus, and scattered through the parenchyma (Figure 6) ▶ .
MMP Enzyme Activity in Organs
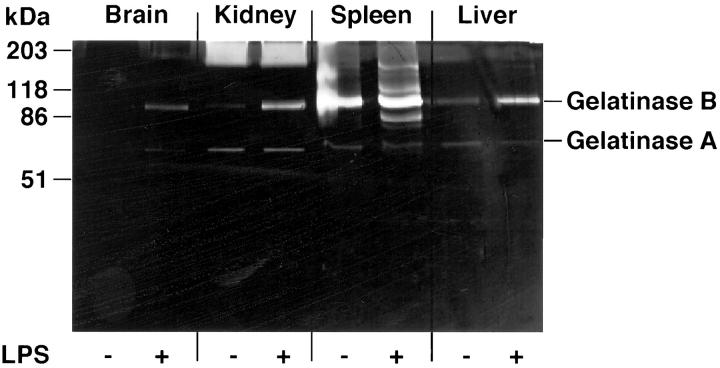
To determine whether the increased expression of MMP mRNAs after LPS injection correlated with MMP enzymatic activity in the respective organs, gelatin PAGE-zymography was performed. Figure 7 ▶ shows the gelatinolytic activity in the different organs of control or LPS-injected animals (note the different protein loading for the different organs). All organs showed low constitutive gelatinolytic activity at ∼60 kd. This activity was not altered by LPS treatment. In contrast to the 60-kd gelatinase, a second activity with a molecular weight of 92 kd corresponding to the inactive form of gelatinase B was increased in all organs from LPS-treated mice. However, major differences between the organs were observed. The brain showed only very low activity whereas spleen and liver had high activity of the 92-kd gelatinase. The 92-kd gelatinase activity in the spleen remained unchanged after LPS treatment, however, several additional activities with molecular weights of approximately 80 kd, 110 kd, and 160 kd were apparent after LPS treatment. All organs had diffuse gelatinolytic activity of different levels at a molecular weight of ∼200 kd.
Figure 7.
MMP activity in different organs of mice after LPS-injection. Mice were killed 12 hours after i.p. LPS injection (20 μg) and organs were frozen in liquid nitrogen. Frozen organs were homogenized in lysis buffer and protein content determined as described in Materials and Methods. Unreduced samples were fractionated on 10% acrylamide gels containing 1 mg/ml gelatin. Subsequently, gelatin zymography was performed as described in Materials and Methods. Protein loading was 50 μg for brain, 20 μg for kidney, 6 μg for spleen, and 7 μg for liver. The constitutive gelatinolytic activity in the brain is not easily visible in this reproduction.
To identify the cellular source of gelatinolytic activity, in situ zymography was performed. In control liver a diffuse gelatinolytic activity was observed that could not be localized to particular cells (Figure 8) ▶ . In good correlation with the findings in PAGE zymography strongest constitutive gelatinolytic activity was observed in the spleen. Gelatinase-producing cells that resembled macrophages were found dispersed in the red pulp. In the kidney, no constitutive gelatinolytic activity was observed. In control brain, gelatinolytic activity was observed in neurons of the hippocampus, the cerebellum, and the frontal cortex. In LPS-treated mice, an increased gelatinolytic activity was observed in spleen, liver, and occasionally in the kidney whereas in the brain no alterations were detected (Figure 8) ▶ . In the spleen, plaques of gelatinolytic activity became apparent in the follicles, whereas the pattern of gelatinolytic activity in the red pulp remained similar to control spleens. In the liver, perivascular cells resembling macrophages showed gelatinolytic activity. In the kidney, blood vessel walls occasionally showed fluorescence, indicative of the presence of gelatinolytic activity.
Figure 8.
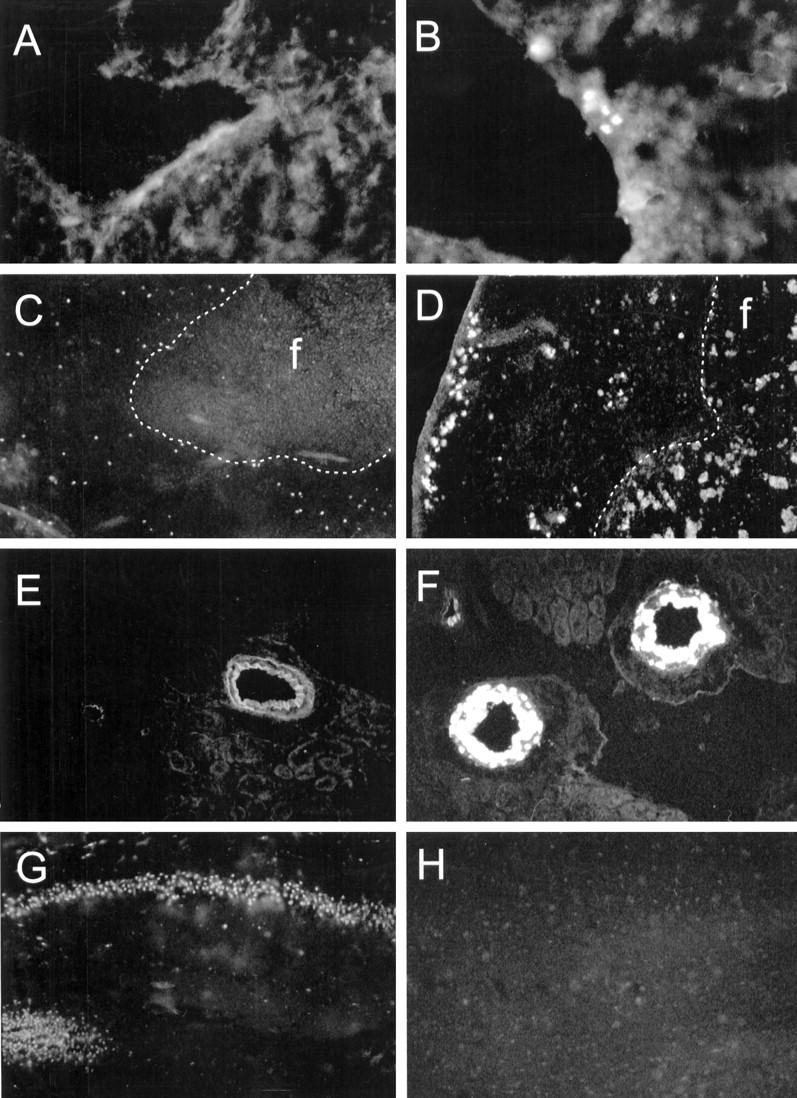
Localization of gelatinolytic activity in different organs of mice after LPS-injection. Mice were killed 16 hours after i.p. LPS-injection (100 μg) and organs were frozen in liquid nitrogen. Cryostat sections were over-laid with a solution containing quenched fluorescent gelatin and sections were examined 16 to 20 hours later. Gelatinolytic activity becomes apparent as fluorescence. Liver: control (A); LPS-injected (B). Original magnification, ×40. Spleen: control (C); follicle (f), the border between the follicle and red pulp is demarcated by the dotted white line; LPS-injected (D). Note the fluorescent plaques in the follicle (f) after LPS injection (arrows). Original magnification, ×40. Kidney: control (E), note the autofluorescence of the vessel walls; LPS-injected (F). Original magnification, ×20. Brain (dentate gyrus): control (G); control, addition of 10 mmol/L EDTA to the assay solution abolished the neuronal fluorescence (H). Original magnification, ×20.
Discussion
A number of reports have demonstrated the effects of LPS on MMP and TIMP expression in different cell types in vitro. 15,16,41,52 However, there have been few, if any reports that have examined the spatiotemporal regulation of MMP and TIMP gene expression in vivo after LPS administration. Here we showed that in mice, after LPS administration, increased expression of various MMP and the TIMP-1 genes was observed in kidney, liver, spleen, and brain. Compared with nonlethal doses, lethal doses of LPS were associated with the induction of a larger repertoire of MMP genes for a more prolonged period of time. Furthermore, we showed that the increased MMP mRNA expression was associated with elevated MMP enzymatic activity in the different organs. Finally, we demonstrated localization of the MMP gene expression to parenchymal cells in many tissues, which in some cases (eg, kidney) co-localized with sites of tissue injury. Together these findings suggest that the MMP/TIMP system may play an important role in the pathogenesis of endotoxemia.
Control animals showed organ-specific patterns of constitutive expression of MMP and TIMP genes. These findings confirm previous reports on the constitutive expression of gelatinase B, MT1-MMP, and TIMP-1, -2, and -3 9,31,36,37,53-55 . MT1-MMP is a membrane-bound MMP that activates gelatinase A and collagenase 3, which, in common with most other MMPs, are secreted as inactive zymogens. 14,56 Furthermore, activated gelatinase A has been shown to activate progelatinase B. 57 Thus, the constitutive expression of MT1-MMP in all organs tested highlights a possible alert state of the MMP system that would permit the immediate activation of newly synthesized MMPs as they are secreted. In liver, kidney, and brain there was a strong constitutive expression of different TIMP and α2-M mRNAs. Similar to the brain, the kidney had a high constitutive expression of TIMP-3. The glomerular basement membrane which is an important structure for the ultrafiltration function of the kidney is composed of matrix proteins that can be degraded by MMPs. 58 Breakdown of the glomerular basement membrane leads to loss of the ultrafiltration function of the kidney resulting in proteinuria. Therefore, the tendency toward low MMP activity observed in both the brain and the kidney would favor protection of important structures against injury, thus maintaining undisturbed function. 59
After LPS injection, all organs had alterations of MMP and TIMP gene expression. However, in the case of the brain, higher LPS doses as compared to the other organs were necessary to induce MMP expression. To some extent it is likely that the blood brain barrier prevents central nervous system entry of LPS or other soluble factors such as cytokines that can induce MMP expression and thus accounts for the considerably restricted expression of these genes after LPS injection. Interestingly however, in the present study low-dose LPS injection was sufficient to induce TIMP-1 mRNA in the brain. This finding suggests that there may be a different regulation for this inhibitor by a factor or factors that enter the brain more easily or induce TIMP-1 mRNA at lower concentrations than do factors that regulate MMPs. Together with the high constitutive expression of TIMP-2 and -3 this finding highlights an exaggeration of TIMP expression in the brain which may represent an important protective mechanism against the potentially harmful consequences of excessive MMP activity.
There was a significant difference in the kinetics of MMP gene expression after LPS treatment and organ-specific patterns of MMP expression were observed. These findings highlight possible differential regulatory mechanisms for the MMPs over time as well as in the different organs. It is well known that in the course of endotoxemia distinct phases can be differentiated in which cytokine production is an early event whereas acute phase proteins such as C-reactive protein or fibrinogen are elevated at later stages. 2 A number of reports have documented that a variety of factors (eg, LPS itself or cytokines such as IL-1; TNF-α; interferon-α, -β, or -γ; or GM-CSF) may act as possible MMP and/or TIMP regulators in endotoxemia. 2,60,61 Moreover, a number of regulatory sites in the upstream regions of the MMP and TIMP genes are known that may be activated by transcriptional factors induced by cytokines. 9,60-63 However, the regulation of MMP and TIMP gene expression is complex and more than one transcription factor may be involved in the activation of several of these genes. 60,61 All in all, the mechanisms responsible for the differential time course and organ expression patterns of the MMP and TIMP genes in LPS-induced endotoxemia are not well understood and remain to be elucidated.
After LPS treatment TIMPs were up-regulated or induced in all organs. The importance of TIMP-1 in MMP inhibition in vivo is underlined by findings in a transgenic (tg) model. Martin and co-workers 64 demonstrated that the tg expression of TIMP-1 under control of the MHC class I promoter inhibited the development of hepatocellular carcinoma induced by tg expression of the SV40 TAg in double-tg mice. Moreover, metastasis to the brain by fibrosarcoma cells was significantly reduced in TIMP-1 transgenic mice. 65 These studies demonstrated that TIMP-1 inhibits MMPs in vivo leading to a significant decrease of tumor progression and metastasis, respectively.
In LPS-resistant mice, LPS injection led to changes only in the spleen, the other organs were not responsive to LPS treatment in terms of MMP and TIMP gene expression. In the spleen, induction of stromelysin 1 and TIMP-1 mRNA was evident. In a recent report a mutation of the gene encoding the Ran/TC4 GTPase was implicated in conferring LPS hyporesponsiveness in C3H/HeJ mice. 66 However, not all cells from these animals seem to be LPS-resistant because alveolar macrophages, but not peritoneal macrophages, secrete TNF on endotoxin stimulation. 67 Moreover, treatment of peritoneal macrophages of C3H/HeJ mice with interferon-γ rendered these cells LPS-sensitive. 68 This latter finding is of particular interest because a recent report demonstrated a defect in IL-12 responsiveness with impaired induction of interferon-γ in endotoxin resistant mice. 69 In addition, it was confirmed that LPS-treated peritoneal macrophages of C3H/HeJ mice do not produce TNF, however, production of gelatinase B was demonstrated. 70 Thus, the induction of stromelysin 1 and TIMP-1 as well as the up-regulation of gelatinase B mRNA we observed in the spleen of C3H/HeJ mice after LPS injection may be accounted for, at least in part, by LPS-responsive cells in the spleen.
In control animals, PAGE zymography revealed high levels of gelatinolytic activity corresponding to the inactive form of gelatinase B and gelatinase A in liver and spleen and lower levels in kidney and brain. Accordingly, we were not able to detect the cellular source of gelatinolytic activity in control liver and kidney, a finding that corresponded to our observations by in situ hybridization for gelatinase B RNA. In the brain neurons were the source of cerebral gelatinolytic activity. Immunoreactivity as well as RNA transcripts for gelatinase B have been demonstrated in neurons of humans and rats 71,72 indicating that neuronal gelatinase B might be the source of the gelatinolytic activity shown here. Gelatin PAGE zymography revealed that the increased MMP mRNA expression in the various organs after LPS treatment correlated with changes in enzymatic activity. However, the enzymatic activity in brain and kidney was orders of magnitude below that in spleen and liver. Moreover, in the spleen several new gelatinolytic bands became apparent that might indicate activated forms of gelatinase B or they could represent other MMPs (eg, neutrophil collagenase, MMP8). The identity of the diffuse gelatinolytic activity at 200 kd observed by us and other groups 73,74 is not fully understood. In a recent report this diffuse activity has been identified as dimers of gelatinase B, 75 however other reports demonstrated that complexes of gelatinase A and integrins might account for such a gelatinolytic activity. 76 In situ zymography revealed the cellular source of the gelatinolytic activity in all organs, however there was a disparity between the gene expression of gelatinase A and B and the gelatinolytic activity in PAGE zymography and in situ zymography. Several factors might account for these differences: 1) in PAGE zymography TIMP-MMP complexes are dissociated in denaturing sodium dodecyl sulfate loading buffer and accordingly the total MMP activity is detected by this technique. In addition, sodium dodecyl sulfate has been shown to activate the pro form of MMPs and thus the normally inactive forms of gelatinases appear gelatinolytic in PAGE zymography. 77 In contrast, in situ zymography in contrast is not performed with denaturing agents and thus might reflect the actual gelatinolytic activity within the tissue. 2) Low levels of gelatinolytic activity might be present in most cells of the liver and kidney and thus would appear as high background fluorescence. 3) In situ zymography is not specific for gelatinases or even MMPs and the observed gelatinolytic activity might be because of any EDTA-inhibitable enzyme with gelatinolytic capacity. The increased MMP activity after LPS injection was observed despite concurrent up-regulation of TIMP mRNA expression and might indicate that there is insufficient expression of TIMP proteins in relation to MMP proteins. To completely inhibit MMP enzymatic activity a more than 10-fold excess of the inhibitor is necessary. 39 Our RPA data for TIMP expression during endotoxemia show that although TIMP mRNAs are up-regulated, this up-regulation is not excessively higher as compared to the MMP mRNA increases. Moreover, it has been shown that neutrophils are an important source of gelatinase B after stimulation with substances such as phorbol 12-myristate 13-acetate or IL-8. 40,78 Importantly, these cells secrete preformed gelatinase B and thus de novo synthesis that would require mRNA transcription is not necessary. All in all, further studies are needed to investigate the effective MMP activity in different tissues after induction or up-regulation of MMP gene expression.
The increased MMP activity present in the organs might have a significant impact on the tissue damage that occurs in the course of LPS-induced endotoxemia, for example by degrading the basal membrane of capillaries, 58 by releasing ECM-bound cytokines, 29 or by shedding of membrane-bound TNF-α or its receptor. 26,28 In particular, MMP activity has been shown to increase the permeability of vessel walls 20,21,58,79 and thus may contribute to the observed hemorrhage and direct damage to the organs in which they are synthesized in the course of endotoxemia. This notion is supported by our results showing that after a lethal dose of LPS more MMP genes were induced for longer periods of time than after a nonlethal dose. Together with the observed co-localization of hemorrhage and collagenase 3 gene expression in the kidney this finding suggests a causal role of MMPs in the generation of lesions in endotoxemia. Two recent studies demonstrated in LPS-induced models of shock the ameliorative effect of a hydroxamate MMP-inhibitor. 45,80 In the experimental settings, treatment with the MMP inhibitor reduced mortality significantly. This decrease was attributed to inhibition of the cleavage of the 26-kd membrane-bound TNF-α to soluble 17-kd TNF-α by MMPs. Considering the results of the present study, we suggest that the ameliorative effects of MMP inhibitors in endotoxemia are only in part because of an inhibition of the cleavage of membrane-bound TNF-α. The known in vitro and in vivo functions of MMPs, the co-localization of tissue damage and the increased enzymatic activity of MMPs suggest that MMPs are important effectors in the pathogenesis of symptoms in endotoxemia. Therefore, the development and application of more specific inhibitors of MMPs might offer a rational therapeutic approach to ameliorate the harmful consequences of endotoxemia.
Acknowledgments
We thank Dr. N. Boehm from the Department of Pathology, the University of Freiburg, Germany, for his help.
Footnotes
Address reprint requests to Axel Pagenstecher M.D., Dept. of Neuropathology, University of Freiburg, Breisacherstrasse 64, 79106 Freiburg, Germany. E-mail: pagenst@nz11.ukl.uni-freiburg.de.
This study was supported by grants of the Deutsche Forschungsgemeinschaft and the Zentrum für Klinische Forschung I Freiburg (to A. P.) and United States Public Health Service grants MH 50426 and MH 47680 (to I. L. C). A. K. S. was a postdoctoral fellow of the National Multiple Sclerosis Society.
References
- 1.Lynn WA, Cohen J: Adjunctive therapy for septic shock: a review of experimental approaches. Clin Infect Dis 1995, 20:143-158 [DOI] [PubMed] [Google Scholar]
- 2.Vogel SN, Hogan MM: Immunophysiology: The Role of Cells and Cytokines in Immunity and Inflammation. Edited by JJ Oppenheim, EM Shevach. New York, Oxford University Press, 1990, pp 238–258
- 3.Netea MG, Demacker PN, Kullberg BJ, Boerman OC, Verschueren I, Stalenhoef AF, van der Meer JW: Low-density lipoprotein receptor-deficient mice are protected against lethal endotoxemia and severe gram-negative infections. J Clin Invest 1996, 97:1366-1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hailman E, Vasselon T, Kelley M, Busse LA, Hu MC, Lichenstein HS, Detmers PA, Wright SD: Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J Immunol 1996, 156:4384-4390 [PubMed] [Google Scholar]
- 5.Raetz CRH, Ulevitch RJ, Wright SD, Sibley CH, Ding A, Nathan CF: Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J 1991, 5:2652-2660 [DOI] [PubMed] [Google Scholar]
- 6.Muller JM, Ziegler-Heitbrock HW, Baeuerle PA: Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology 1993, 187:233-256 [DOI] [PubMed] [Google Scholar]
- 7.Takahashi M, Ogasawara K, Takeda K, Hashimoto W, Sakihara H, Kumagai K, Anzai R, Satoh M, Seki S: LPS induces NK1.1+ alpha beta T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J Immunol 1996, 156:2436-2442 [PubMed] [Google Scholar]
- 8.Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P: A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature 1990, 348:699-704 [DOI] [PubMed] [Google Scholar]
- 9.Masure S, Nys G, Fiten P, Van Damme J, Opdenakker G: Mouse gelatinase B. cDNA cloning, regulation of expression and glycosylation in WEHI-3 macrophages and gene organisation. Eur J Biochem 1993, 218:129-141 [DOI] [PubMed] [Google Scholar]
- 10.Okada A, Bellocq JP, Rouyer N, Chenard MP, Rio MC, Chambon P, Basset P: Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci USA 1995, 92:2730-2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro SD, Kobayashi DK, Ley TJ: Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem 1993, 268:23824-23829 [PubMed] [Google Scholar]
- 12.Malik N, Greenfield BW, Wahl AF, Kiener PA: Activation of human monocytes through CD40 induces matrix metalloproteinases. J Immunol 1996, 156:3952-3960 [PubMed] [Google Scholar]
- 13.Goetzl EJ, Banda MJ, Leppert D: Matrix metalloproteinases in immunity. J Immunol 1996, 156:1-4 [PubMed] [Google Scholar]
- 14.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M: A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994, 370:61-65 [DOI] [PubMed] [Google Scholar]
- 15.Gottschall PE, Yu X: Cytokines regulate gelatinase A and B (matrix metalloproteinase 2 and 9) activity in cultured rat astrocytes. J Neurooncol 1995, 24:75-81 [DOI] [PubMed] [Google Scholar]
- 16.Gottschall PE, Yu X, Bing B: Increased production of gelatinase B (matrix metalloproteinase-9) and interleukin-6 by activated rat microglia in culture. J Neurosci Res 1995, 42:335-342 [DOI] [PubMed] [Google Scholar]
- 17.Stetler-Stevenson WG: Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol 1996, 148:1345-1350 [PMC free article] [PubMed] [Google Scholar]
- 18.Stetler-Stevenson WG: Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest 1999, 103:1237-1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar R, Dong Z, Fidler IJ: Differential regulation of metalloelastase activity in murine peritoneal macrophages by granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor. J Immunol 1996, 157:5104-5111 [PubMed] [Google Scholar]
- 20.Rosenberg GA, Estrada EY, Dencoff JE, Stetler-Stevenson WG: Tumor necrosis factor-alpha-induced gelatinase B causes delayed opening of the blood-brain barrier: an expanded therapeutic window. Brain Res 1995, 703:151-155 [DOI] [PubMed] [Google Scholar]
- 21.Xia M, Leppert D, Hauser SL, Sreedharan SP, Nelson PJ, Krensky AM, Goetzl EJ: Stimulus specificity of matrix metalloproteinase dependence of human T cell migration through a model basement membrane. J Immunol 1996, 156:160-167 [PubMed] [Google Scholar]
- 22.Opdenakker G, Masure S, Grillet B, Van DJ: Cytokine-mediated regulation of human leukocyte gelatinases and role in arthritis. Lymphokine Cytokine Res 1991, 10:317–324 [PubMed]
- 23.Marbaix E, Kokorine I, Henriet P, Donnez J, Courtoy PJ, Eeckhout Y: The expression of interstitial collagenase in human endometrium is controlled by progesterone and by oestradiol and is related to menstruation. Biochem J 1995, 305:1027-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paemen L, Jansen PM, Proost P, Van DJ, Opdenakker G, Hack E, Taylor FB: Induction of gelatinase B and MCP-2 in baboons during sublethal and lethal bacteraemia. Cytokine 1997, 9:412–415 [DOI] [PubMed]
- 25.Ito A, Mukaiyama A, Itoh Y, Nagase H, Thogersen IB, Enghild JJ, Sasaguri Y, Mori Y: Degradation of interleukin 1 beta by matrix metalloproteinases. J Biol Chem 1996, 271:14657-14660 [DOI] [PubMed] [Google Scholar]
- 26.Chandler S, Cossins J, Lury J, Wells G: Macrophage metalloelastase degrades matrix and myelin proteins and processes a tumour necrosis factor-alpha fusion protein. Biochem Biophys Res Commun 1996, 228:421-429 [DOI] [PubMed] [Google Scholar]
- 27.Schönbeck U, Mach F, Libby P: Generation of biologically active IL-1β by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1β processing. J Immunol 1998, 161:3340-3346 [PubMed] [Google Scholar]
- 28.Crowe PD, Walter BN, Mohler KM, Otten-Evans C, Black RA, Ware CF: A metalloprotease inhibitor blocks shedding of the 80-kD TNF receptor and TNF processing in T lymphocytes. J Exp Med 1995, 181:1205-1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilat D, Cahalon L, Hershkoviz R, Lider O: Interplay of T cells and cytokines in the context of enzymatically modified extracellular matrix. Immunol Today 1996, 17:16-20 [DOI] [PubMed] [Google Scholar]
- 30.Gijbels KSM, Carton H, Opdenakker G: Gelatinase in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological disorders. J Neuroimmunol 1992, 41:29-34 [DOI] [PubMed] [Google Scholar]
- 31.Pagenstecher A, Stalder AK, Kincaid CL, Shapiro SD, Campbell IL: Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol 1998, 152:729-741 [PMC free article] [PubMed] [Google Scholar]
- 32.Gijbels K, Proost P, Masure S, Carton H, Billiau A, Opdenakker G: Gelatinase B is present in the cerebrospinal fluid during experimental autoimmune encephalomyelitis and cleaves myelin basic protein. J Neurosci Res 1993, 36:432-440 [DOI] [PubMed] [Google Scholar]
- 33.Clements JM, Cossins JA, Wells GM, Corkill DJ, Helfrich K, Wood LM, Pigott R, Stabler G, Ward GA, Gearing AJ, Miller KM: Matrix metalloproteinase expression during experimental autoimmune encephalomyelitis and effects of a combined matrix metalloproteinase and tumour necrosis factor-alpha inhibitor. J Neuroimmunol 1997, 74:85-94 [DOI] [PubMed] [Google Scholar]
- 34.Murphy G, Willenbrock F, Crabbe T, O’Shea M, Ward R, Atkinson S, O’Connell J, Docherty A: Regulation of matrix metalloproteinase activity. Ann NY Acad Sci 1994, 732:31-41 [DOI] [PubMed] [Google Scholar]
- 35.Leco KJ, Apte SS, Taniguchi GT, Hawkes SP, Khokha R, Schultz GA, Edwards DR: Murine tissue inhibitor of metalloproteinases-4 (Timp-4): cDNA isolation and expression in adult mouse tissues. FEBS Lett 1997, 401:213-217 [DOI] [PubMed] [Google Scholar]
- 36.Leco KJ, Khokha R, Pavloff N, Hawkes SP, Edwards DR: Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem 1994, 269:9352-9360 [PubMed] [Google Scholar]
- 37.Kordula T, Guttgemann I, Rose-John S, Roeb E, Osthues A, Tschesche H, Koj A, Heinrich PC, Graeve L: Synthesis of tissue inhibitor of metalloproteinase-1 (TIMP-1) in human hepatoma cells (HepG2). Up-regulation by interleukin-6 and transforming growth factor beta 1. FEBS Lett 1992, 313:143–147 [DOI] [PubMed]
- 38.Leco KJ, Hayden LJ, Sharma RR, Rocheleau H, Greenberg AH, Edwards DR: Differential regulation of TIMP-1 and TIMP-2 mRNA expression in normal and Ha-ras-transformed murine fibroblasts. Gene 1992, 117:209-217 [DOI] [PubMed] [Google Scholar]
- 39.Stetler-Stevenson WG, Krutzsch HC, Liotta LA: Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem 1989, 264:17374–17378 [PubMed]
- 40.Masure S, Proost P, Van DJ, Opdenakker G: Purification and identification of 91-kDa neutrophil gelatinase. Release by the activating peptide interleukin-8. Eur J Biochem 1991, 198:391–398 [DOI] [PubMed]
- 41.Scheller M, Zimmermann B, Bernimoulin JP, Scholz P: Induction of metalloproteinase activity, cartilage matrix degradation and inhibition of endochondral mineralization in vitro by E. coli lipopolysaccharide is mediated by interleukin 1 alpha. Cytokine 1995, 7:331-337 [DOI] [PubMed] [Google Scholar]
- 42.Yao PM, Buhler JM, d’Ortho MP, Lebargy F, Delclaux C, Harf A, Lafuma C: Expression of matrix metalloproteinase gelatinases A and B by cultured epithelial cells from human bronchial explants. J Biol Chem 1996, 271:15580-15589 [DOI] [PubMed] [Google Scholar]
- 43.Colton CA, Keri JE, Chen WT, Monsky WL: Protease production by cultured microglia: substrate gel analysis and immobilized matrix degradation. J Neurosci Res 1993, 35:297-304 [DOI] [PubMed] [Google Scholar]
- 44.Welgus HG, Campbell EJ, Cury JD, Eisen AZ, Senior RM, Wilhelm SM, Goldberg GI: Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest 1990, 86:1496-1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solorzano CC, Ksontini R, Pruitt JH, Auffenberg T, Tannahill C, Galardy RE, Schultz GP, MacKay SLD, Copeland EM, III, Moldawer LL: A matrix metalloproteinase inhibitor prevents processing of tumor necrosis factor alpha (TNF alpha) and abrogates endotoxin-induced lethality. Shock 1997, 7:427-431 [DOI] [PubMed] [Google Scholar]
- 46.Pagenstecher A, Stalder AK, Campbell IL: RNase protection assays for the simultaneous and semiquantitative analysis of multiple murine matrix metalloproteinase (MMP) and MMP inhibitor mRNAs. J Immunol Methods 1997, 206:1-9 [DOI] [PubMed] [Google Scholar]
- 47.Dudov KP, Perry RP: The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell 1984, 37:457-464 [DOI] [PubMed] [Google Scholar]
- 48.Badley JE, Bishop GA, St. John T, Frelinger JA: A simple, rapid method of purification of polyA+ RNA. Biotechniques 1988, 6:114-116 [PubMed] [Google Scholar]
- 49.Stalder AK, Campbell IL: Simultaneous analysis of multiple cytokine receptor mRNAs by RNase protection assay in LPS-induced endotoxemia. Lymphokine Cytokine Res 1994, 13:107-112 [PubMed] [Google Scholar]
- 50.Simmons DM, Arriza JL, Swanson LW: A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabeled single-stranded RNA probes. J Histotechnol 1989, 12:169-181 [Google Scholar]
- 51.Oh LYS, Larsen PH, Krekoski CA, Edwards DR, Donovan F, Werb Z, Yong VW: Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci 1999, 19:8464-8475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saarialho-Kere UK, Welgus HG, Parks WC: Distinct mechanisms regulate interstitial collagenase and 92-kDa gelatinase expression in human monocytic-like cells exposed to bacterial endotoxin. J Biol Chem 1993, 268:17354-17361 [PubMed] [Google Scholar]
- 53.Takino T, Sato H, Shinagawa A, Seiki M: Identification of the second membrane-type matrix metalloproteinase (MT-MMP-2) gene from a human placenta cDNA library. MT-MMPs form a unique membrane-type subclass in the MMP family. J Biol Chem 1995, 270:23013-23020 [DOI] [PubMed] [Google Scholar]
- 54.Yamada T, Yoshiyama Y, Sato H, Seiki M: White matter microglia produce membrane-type matrix metalloprotease, an activator of gelatinase A, in human brain tissues. Acta Neuropathologica 1995, 90:421-424 [DOI] [PubMed] [Google Scholar]
- 55.Flenniken AM, Campbell CE, Williams BR: Regulation of TIMP gene expression in cell culture and during mouse embryogenesis. Matrix 1992, 1:275-280 [PubMed] [Google Scholar]
- 56.Knauper V, Will H, Lopez-Otin C, Smith B, Atkinson SJ, Stanton H, Hembry RM, Murphy G: Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem 1996, 271:17124-17131 [DOI] [PubMed] [Google Scholar]
- 57.Fridman R, Toth M, Pena D, Mobashery S: Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2). Cancer Res 1995, 55:2548-2555 [PubMed] [Google Scholar]
- 58.Baricos WH, Murphy G, Zhou YW, Nguyen HH, Shah SV: Degradation of glomerular basement membrane by purified mammalian metalloproteinases. Biochem J 1988, 254:609-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davies M, Martin J, Thomas GJ, Lovett DH: Proteinases and glomerular matrix turnover. Kidney Int 1992, 41:671-678 [DOI] [PubMed] [Google Scholar]
- 60.Yokoo T, Kitamura M: Dual regulation of IL-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-kappa B and AP-1. Am J Physiol 1996, 270:F123-F130 [DOI] [PubMed] [Google Scholar]
- 61.Logan SK, Garabedian MJ, Campbell CE, Werb Z: Synergistic transcriptional activation of the tissue inhibitor of metalloproteinases-1 promoter via functional interaction of AP-1 and Ets-1 transcription factors. J Biol Chem 1996, 271:774-782 [DOI] [PubMed] [Google Scholar]
- 62.Sato H, Kita M, Seiki M: v-Src activates the expression of 92-kDa type IV collagenase gene through the AP-1 site and the GT box homologous to retinoblastoma control elements. A mechanism regulating gene expression independent of that by inflammatory cytokines. J Biol Chem 1993, 268:23460-23468 [PubMed] [Google Scholar]
- 63.Pierce RA, Sandefur S, Doyle GA, Welgus HG: Monocytic cell type-specific transcriptional induction of collagenase. J Clin Invest 1996, 97:1890-1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin DC, Rüther U, Sanchez-Sweatman OH, Orr FW, Khokha R: Inhibition of SV 40 T antigen-induced hepatocellular carcinoma in TIMP-1 transgenic mice. Oncogene 1996, 13:569-576 [PubMed] [Google Scholar]
- 65.Kruger A, Sanchez SO, Martin DC, Fata JE, Ho AT, Orr FW, Ruther U, Khokha R: Host TIMP-1 overexpression confers resistance to experimental brain metastasis of a fibrosarcoma cell line. Oncogene 1998, 16:2419-2423 [DOI] [PubMed] [Google Scholar]
- 66.Wong PMC, Kang A, Chen H, Yuan Q, Fan P, Sultzer BM, Kan YW, Chung S-W: Lpsd/Ran of endotoxin-resistant C3H/HeJ mice is defective in mediating lipopolysaccharide endotoxin responses. Proc Natl Acad Sci USA 1999, 96:11543-11548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ryan LK, Vermeulen MW: Alveolar macrophages from C3H/HeJ mice show sensitivity to endotoxin. Am J Respir Cell Mol Biol 1995, 12:540-546 [DOI] [PubMed] [Google Scholar]
- 68.Beutler B, Tkacenko V, Krochin N, Milsark IW, Liedke C, Cerami A: Effect of gamma-interferon on cachectin expression by mononuclear phagocytes: reversal of the lpsd (endotoxin resistance) phenotype. J Exp Med 1986, 164:1791-1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balkhy HH, Heinzel FP: Endotoxin fails to induce IFN-gamma in endotoxin-tolerant mice: deficiencies in both IL-12 heterodimer production and IL-12 responsiveness. J Immunol 1999, 162:3633-3638 [PubMed] [Google Scholar]
- 70.Jin F, Nathan CF, Ding A: Paradoxical preservation of a lipopolysaccharide response in C3H/HeJ macrophages: induction of matrix metalloproteinase-9. J Immunol 1999, 162:3596-3600 [PubMed] [Google Scholar]
- 71.Backstrom JR, Lim GP, Cullen MJ, Tokes ZA: Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1–40). J Neurosci 1996, 16:7910-7919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaillant C, Didier-Bazes M, Hutter A, Belin M-F, Thomasset N: Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J Neurosci 1999, 19:4994-5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnatty RN, Taub DD, Reeder SP, Turcovski-Corrales SM, Cottam DW, Stephenson TJ, Rees RC: Cytokine and chemokine regulation of proMMP-9 and TIMP-1 production by human peripheral blood lymphocytes. J Immunol 1997, 158:2327-2333 [PubMed] [Google Scholar]
- 74.Lyons JG, Birkedal-Hansen B, Pierson MC, Whitelock JM, Birkedal-Hansen H: Interleukin-1 beta and transforming growth factor-alpha/epidermal growth factor induce expression of M(r) 95,000 type IV collagenase/gelatinase and interstitial fibroblast-type collagenase by rat mucosal keratinocytes. J Biol Chem 1993, 268:19143-19151 [PubMed] [Google Scholar]
- 75.Dubois B, Masure S, Hurtenbach U, Paemen L, Heremans H, van Den Oord J, Sciot R, Meinhardt T, Hammerling G, Opdenakker G, Arnold B: Resistance of young gelatinase B-deficient mice to experimental autoimmune encephalomyelitis and necrotizing tail lesions (in process citation). J Clin Invest 1999, 104:1507-1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA: Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell 1996, 85:683-693 [DOI] [PubMed] [Google Scholar]
- 77.Nagase H: Activation mechanisms of matrix metalloproteinases. Biol Chem 1997, 378:151-160 [PubMed] [Google Scholar]
- 78.Pruijt JF, Fibbe WE, Laterveer L, Pieters RA, Lindley IJ, Paemen L, Masure S, Willemze R, Opdenakker G: Prevention of interleukin-8-induced mobilization of hematopoietic progenitor cells in rhesus monkeys by inhibitory antibodies against the metalloproteinase gelatinase B (MMP-9). Proc Natl Acad Sci USA 1999, 96:10863-10868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bejarano PA, Noelken ME, Suzuki K, Hudson BG, Nagase H: Degradation of basement membranes by human matrix metalloproteinase 3 (stromelysin). Biochem J 1988, 256:413-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Solorzano CC, Ksontini R, Pruitt JH, Hess PJ, Edwards PD, Kaibara A, Abouhamze A, Auffenberg T, Galardy RE, Vauthey JN, Copeland EM, Edwards CK, Lauwers GY, Clare-Salzler M, MacKay SL, Moldawer LL, Lazarus DD: Involvement of 26-kDa cell-associated TNF-alpha in experimental hepatitis and exacerbation of liver injury with a matrix metalloproteinase inhibitor. J Immunol 1997, 158:414-419 [PubMed] [Google Scholar]