Abstract
Dendritic cells (DCs) are sentinel cells of the immune system important in initiating antigen-specific T-cell responses to microbial and transplantation antigens. DCs are particularly found in surface tissues such as skin and mucosa, where the organism is threatened by infectious agents. The human decidua, despite its proposed immunosuppressive function, hosts a variety of immunocompetent CD45 cells such as natural killer cells, macrophages, and T cells. Here we describe the detection, isolation, and characterization of CD45+, CD40+, HLA-DR++, and CD83+ cells from human early pregnancy decidua with typical DC morphology. CD83+ as well as CD1a+ cells were found in close vicinity to endometrial glands, with preference to the basal layer of the decidua. In vitro, decidual CD83+ cells could be enriched to ∼30%, with the remainder of cells encompassing DC-bound CD3+ T cells. Stimulation of allogeneic T cells in a mixed leukocyte reaction by the decidual cell fraction enriched for CD83+ cells, was similar to that obtained with blood monocyte-derived DCs, demonstrating the potent immunostimulatory capacity of these cells. Decidual DCs with morphological, phenotypic, and functional characteristics of immunostimulatory DCs might be important mediators in the regulation of immunological balance between maternal and fetal tissue, leading to successful pregnancy.
The human uterus is generally considered to be an immunologically privileged site that isolates the implanted allogeneic embryo from an aggressive maternal immune response. This is in contrast to the fact that the decidua, like other mucosal surfaces, must be able to respond to different types of foreign antigens, including pathogenic organisms, seminal plasma, and fetal trophoblasts (FTBs). However, during hemochorial placentation, the direct contact between FTBs (which invade uterine mucosa, the decidua) and maternal immunocompetent cells in the decidua 1 suggests that mechanisms of tolerance must exist to avoid rejection of the conceptus.
The human decidua is invested with a significant (up to 75% of all major histocompatibility complex (MHC) class I+ cells are CD45+) and diverse population of leukocytes. 2,3 Nearly one fifth of decidual leukocytes are positive for MHC class II and are thought to be mostly macrophages. 4 The most abundant cell type of decidua are uterine-specific natural killer cells, the large granular lymphocytes (LGLs), 5 whereas T cells are sparse and B cells are virtually absent. 3,5 The decidual leukocyte population has been a center of interest for the understanding of the balance between maternal control of the extent of invasion of FTBs in the uterine wall 2-6 as well as acceptance of the allogeneic fetus in successful pregnancy. 1 Although it is not known how this balance is achieved, it is possible that the antigenic handling and processing in the decidua may differ from that of other regions of the body.
Antigen presenting cells (APCs) are specifically equipped to initiate and maintain immune responses. 7 Among these APCs, bone marrow-derived DCs are the most potent activators of naive T-lymphocyte responses. 8,9 DCs seed surface areas, such as the epidermal layer of the skin and the mucosal membranes as well as the interstitial spaces of solid organs, 10,11 playing a sentinel function for the immune system. In vivo, DCs exhibit a distinct shape, described as veiled or dendritic, 12 express high levels of MHC class II products and are able to migrate selectively through tissues. 10,13 After isolation and a period of cell culture, DCs undergo specific functional changes in migratory properties and co-stimulatory features which may mimic the in vivo maturation process. Cultivated DCs exhibit membrane processes on the surface which bend, retract, and re-extend in a nonpolarized fashion 14 and are the major stimulators of autologous and allogeneic mixed leukocyte reactions (MLRs). 9
Although there is considerable interest in DCs, working with these cells is hampered because of their low frequency in blood 15 and tissue. 16 Additionally, the lack of an antibody specific for the early stages of DCs makes identification and isolation of fresh DCs difficult. In vivo, a proportion of mature DCs may be stained in tissue sections using either the CD83 or CMFR-44 monoclonal antibodies. 17-19 DCs may be conveniently enriched after 1 to 2 days culture from blood or tissue suspensions as a low-density fraction. 14,20,21 Using either direct selection for MHC class II-positive cells or depletion of contaminating lineage marker-positive cells, DCs have been successfully isolated from many tissues including blood, lymph nodes, skin, and lung. 10,13
Recent studies have pointed to a role for DCs in the induction of peripheral tolerance. 22,23 DCs are thus of outstanding interest as immune cells which may have the capacity to act in two roles: as potent APCs and also in the induction of peripheral tolerance. Therefore, DCs could be suitable candidates that mediate the balance of maternal defensive immune responses to foreign antigens but also tolerance to the conceptus in the human decidua.
The data presented here are intended to be the initiation for further studies of DCs in human early pregnancy decidua. To our knowledge, this is the first demonstration of CD83+ dendritic-like cells in human decidua, which show morphological, phenotypic, and functional characteristics of immunostimulatory mature DCs.
Materials and Methods
Tissue Specimens
All investigations were approved by the Ethics Committee of the Medical Faculty of the University of Würzburg, Germany.
Decidual tissue (decidua basalis and parietalis) was obtained from 21 healthy women undergoing legal therapeutic abortion of an intact, normally progressing pregnancy with documented fetal heart activity at 6 to 9 weeks of gestation after the last menstrual period. All specimens contained embryonic components as verified by macroscopic and histological examination. Decidual tissue was taken from each specimen to be snap-frozen in liquid nitrogen for histological examination and immunohistochemical staining. The remainder was kept for no more than 30 minutes in phosphate-buffered saline (PBS) before subsequent cell isolation.
In one case, a hysterectomy sample was obtained of a pregnant uterus at the10th week (last menstrual period) of gestation. The hysterectomy was performed because of cervical cancer in situ (CIN 3) with no involvement of the uterus. Tissue sections through placental and uterine tissue were snap-frozen for a immunohistochemical staining procedure. Ten samples of uterus with endometrium in the secretory phase were obtained after routine hysterectomy because of uterus myomatosus.
Immunohistochemistry
The antibodies applied in this study are listed in Table 1 ▶ .
Table 1.
Antibodies Used in This Study
| Antigen | Clone | Species/isotype | Major specificities | Supplier |
|---|---|---|---|---|
| CD1a | HI149 | Mouse, IgG1 | Langerhans cells, cortical thymocytes, subsets of DC | Pharmingen |
| CD3 | UCHT1 | Mouse, IgG1 | All T cells | Dianova |
| CD14 | UCHM1 | Mouse, IgG2a | Monocytes, macrophages, granulocytes | Serotec |
| CD40 | B-B20 | Mouse, IgG1κ | Lymphoid dendritic cells, mature peripheral B-cells | Dianova |
| CD45 | MEM28 | Mouse, IgG1 | All leukocytes | Dianova |
| CD56 | NCAM16.2 | Mouse, IgG2b | NK-cells, T cell subsets | Becton Dickinson |
| CD83 | HB15a | Mouse, IgG2b | Mature DC | Immunotech |
| HLA-DR | DDII | Mouse, IgG1 | Monocytes, macrophages, DC, B lymphocytes | Dianova |
| Cytokeratin | KL1 | Mouse, IgG1 | CK 2,5,6,8,10,11,14,15,18,19: epithelial cells, trophoblasts | Immunotech |
Serial frozen sections of decidua and the hysterectomy samples were cut at 5 μm and placed onto 3-amino-propyltriethoxy-silane (Roth, Karlsruhe, Germany) coated slides, air-dried overnight, fixed in acetone for 10 minutes, and rehydrated in Tris-buffered saline (25 mmol/L Tris/HCl, pH 7.4, 137 mmol/L NaCl, 2.7 mmol/L KCl). For double immunohistochemical staining procedures, the sections were incubated with the first monoclonal antibody at appropriate dilutions followed by the horseradish-peroxidase-labeled rabbit-anti-mouse-specific secondary antibody (dilution 1:100; DAKO, Hamburg, Germany). The detection reaction was developed with 3,3′-diaminobenzidine (Sigma, Deisenhofen, Germany). Then the sections were incubated with the second antigen-specific antibody, followed by incubation with the horseradish peroxidase-labeled rabbit-anti-mouse antibody. For the second color (violet) the detection reaction was developed using the Vector VIP peroxidase substrate kit (Vector Laboratories, Burlingame, CA). For triple-fold staining, the same labeling procedure as for the first two antibodies was performed with the third monoclonal antibody and the detection reaction developed with Vector blue substrate kit (Vector Laboratories). Sections were counterstained with hematoxylin or methyl green (Sigma), respectively.
To evaluate the average density value of DCs in human first pregnancy decidua, CD83+ and CD1a+ cells were counted at 250-fold magnification in 150 single fields of 0.13 mm 2 (=20 mm2) for each of two sections per patient by two independent observers. Fields were randomly selected over the entire cryostatic section and completely filled with nonnecrotic cells. Decidua basalis samples with more than five invading FTBs per 25× objective field generally showed too many necrotic cells and thus were excluded from this study. Cells <0.2 mm distant to glandular epithelium were designated as “near to gland” and cells associated with more than 10 lymphocytes as “clustered.”
Single Cell Isolation
For isolation of decidual cells, specimens were dissected free of products of conception and washed twice in PBS. The total decidual tissue (4 to 10 g) was then minced into fragments of ∼1 mm 3 and digested for 20 minutes at 37°C under slight agitation in PBS with 200 U/ml hyaluronidase (Sigma), 1 mg/ml collagenase type IV (Seromed, Berlin, Germany), 0.2 mg/ml DNase I (2500 Kunitz U/mg; Sigma) and 1 mg/ml bovine serum albumin/fraction V (Sigma). The cell suspension obtained was filtered through sterile stainless steel 50-μm wire mesh and washed once in PBS. The mononuclear cell population was then separated by centrifugation over a Histopaque–1077 density gradient (Sigma). For positive selection of CD83+ cells, the mononuclear cell fraction obtained was incubated (5 × 10 6 cells/ml) in RPMI 1640 medium supplemented with 10% inactivated fetal calf serum and 50 μg/ml gentamicin (R10; all supplied by Seromed) in a tissue-culture grade Petri dish at 37°C and 5% CO2 for 16 hours to allow macrophages, fibroblasts, and endothelial cells to adhere to the flask. Nonadherent cells were then collected and resuspended in PBS. The cells were labeled with CD83 (clone: HB15a, 2 μg/10 6 cells in PBS supplemented with 1.6% (w/v) human immunoglobulin; Beriglobin, Centeon, Marburg, Germany) in a total volume of 100 μl for 30 minutes at 4°C, washed once, incubated with bead-conjugated goat-anti-mouse antibody (Miltenyi, Bergisch Gladbach, Germany) in PBS supplemented with 1.6% (w/v) immunoglobulin for 20 minutes at 4°C and separated in a positive-selection column in a magnetic assisted cell sorting (MACS) separator (Vario-MACS, Miltenyi).
The positive CD83-enriched fraction containing decidual DCs was analyzed for the content of CD83+ and HLA-DR+ cells by flow cytometry and used for subsequent analysis in a primary MLR and for preparation of cytospin specimens.
Cytocentrifuge Preparations
For cytospin samples, aliquots of 3 × 10 4 of the isolated decidual cells, enriched for CD83+ cells, were suspended in 100 μl of PBS and cytocentrifuged onto 3-amino-propyltriethoxy-silane-treated slides. They were fixed in acetone for 10 minutes, rehydrated in TBS, and incubated with a goat-anti-mouse Fab fragment to block the attached selection antibodies. Then the slides were incubated with the monoclonal antibody (HLA-DR, CD83, CD1a) at the appropriate dilution. Bound antibodies were detected using the alkaline phosphatase anti-alkaline phosphatase method as described elsewhere, 24 and cells were counterstained with Mayer’s hematoxylin. Detection reactions without primary antibody and with isotype antibodies served as negative controls.
Flow Cytometry
Nonadherent cells after overnight culture and cells isolated by CD83+ selection were analyzed for cell-surface expression of a variety of leukocyte markers by fluorescence-activated cell sorting (FACS) analysis. Flow cytometry was performed with primary antibodies labeled with R-phycoerythrin (CD83) and fluorescein isothiocyanate (HLA-DR, CD45, CD1a, CD40, and CD14) or isotype control (mixture of mouse IgG1, IgG2a, IgG2b) antibodies. Aliquots of 2 × to 5 × 10 5 isolated cells were resuspended in 80 μl of PBS supplemented with 10% human immunoglobulin. Fluorochrome labeled antibodies (10 μl each) were added to the cell suspension and the preparation then incubated for 30 minutes at 4°C. Cell suspensions were washed once, resuspended in 200 μl of PBS, and then analyzed in a FACScan flow cytometer (Becton-Dickinson, Heidelberg, Germany). A total of 20,000 cells per sample were evaluated for specific staining. Results were analyzed using the WinMDI-Program (Version 2.8, 227 Joseph Trotter, The Scripps Research Institute, La Jolla, CA), setting the threshold at 0.5 and smoothing at 1.0 for the contour plots shown.
Motility and Morphology of Isolated DCs
To determine the morphology and motility of isolated cells, 1 × 10 5 cells/well per sample were seeded in a 96-well flat-bottom tissue culture plate in 100 μl of R10-medium per well. Morphology and motility were monitored daily for 7 days with an inverted microscope (Leica, Bensheim, Germany) with a video camera and documented for 3 minutes per day. One picture per 30 seconds was taken on an attached camera.
Primary MLRs
For control purposes, DCs were generated from blood monocytes after standard procedures. 25 In brief, peripheral blood mononuclear cells derived from buffy coats of healthy volunteer donors were prepared by isolation over a Histopaque–1077 density gradient, resuspended in R10 and incubated at 5 × 10 6 cells/ml in a tissue-grade Petri dish to allow monocytes to adhere to the bottom. The nonadherent cells were removed after 1 hour. The adherent fraction was then cultured in R10 supplemented with GM-CSF (1000 U/ml) and IL-4 (800 U/ml) for 7 days. One half the volume of medium was replaced with fresh R10/GM-CSF/IL-4 at days 3 and 5, and at day 7 one half of the volume of medium was replaced by fresh R10 supplemented with IL-1β (1000 U/ml), TNF-α (1000 U/ml), IL-6 (1000 U/ml), and PGE2 (10−8 mol/L). At day 10 of culture, nonadherent cells with typical appearance of DCs were collected, resuspended in 90% fetal calf serum/10% DMSO, and aliquots stored in liquid nitrogen. Comparison of fresh and thawed DCs showed no difference in their capacity as stimulator cells in the MLRs.
For the MLRs, graded doses of decidual or blood monocyte DCs were cultured with 10 5 allogeneic T lymphocytes (prepared by 0.8% NH4Cl lysis of neuraminidase-treated sheep red blood cell rosettes of peripheral blood mononuclear cells) in a final volume of 200 μl of R10 in round-bottom 96-well plates (Falcon/Becton Dickinson, Franklin Lakes, NJ). For the MLR graphs, decidual and blood DC numbers were normalized to show the actual number of CD83+ cells per well. After 120 hours of culture at 37°C in 5% CO2, cultures were pulsed with 1 μCi of [3H]-thymidine (Amersham International, Arlington Heights, IL) per well for the final 16 hours before harvesting and liquid scintillation counting. All assays were performed in triplicate.
Statistical Analysis
Statistically significant differences between the anatomical localization of CD83+ and CD1a+ cells in immunohistochemistry was analyzed using the two-tailed Student’s t-test. Two sections per patient were assessed. There was no statistically significant difference between the distribution of the same cell type as determined by two independent assessors (P > 0.9). The mean values of MLR triplicates from each experiment were compared for statistical significance using the two-tailed Wilcoxon’s test.
Results
In Situ Detection of CD83+ and CD1a+ Cells in Decidual Tissue
To identify DCs in early human pregnancy decidua, patient tissue sections were stained with CD83 or CD1a and cytokeratin monoclonal antibodies. A summary of the results of immunostaining is shown in Table 2 ▶ . The cytokeratin staining allowed the identification of endometrial glands and cytotrophoblasts and thus the anatomical localization of the CD83+ and CD1a+ cells in the decidua. For both antibodies, the variation between each patient sample was statistically not significant (P > 0.1). The numbers of CD1a+ or CD83+ cells did not change significantly relative to the week of gestation. Likewise there was no statistically significant difference in CD1a+ or CD83+ cells between decidua parietalis and basalis. The CD83+ cells, as well as the CD1a+ cells, were of a veiled shape typical for DCs, with long dendrites extending to the surrounding tissue (Figure 1, a and b) ▶ . The mean density of CD83+ was 4.97 ± 1.88 SD per mm 2 (n = 21). In those tissue sections with clear visible lymphoid aggregates (n = 18), 57% (2.55 ± 1.7 SD) of all CD83+ cells were found within these cell clusters, directly associated with CD3+ T cells (Figure 1, d and f) ▶ . In those tissue sections with clear visible lymphatic vessels, as identified by their appearance as flattened channels which were delimitated by a thin and irregular endothelial wall (n = 10), 12.7% ± 6.9 SD of the total CD83+ cells were located within those vessels (Figure 1j) ▶ . Most CD83+ cells not associated in T-cell clusters or located in lymphatic vessels could be observed in close vicinity to endometrial glands (1.22 ± 0.61 SD, P < 0,005; Figure 1, b and d ▶ ). Only a minority of CD83+ cells was scattered throughout the stroma (0.64 ± 0.54 SD). The hysterectomy sample allowed us to determine the anatomical localization of CD83+ cells in the uterine wall which were localized with preference to the basal layer of decidua (Figure 2 ▶ , scheme of uterine wall). This location was found in the endometrium in cases of nonpregnant uterus samples as well (data not shown).
Table 2.
Distribution of CD83+ and CD1a+ Cells per mm2 in Decidual Tissue
| Distribution of DC | CD83+ (n = 21) | CD1a+ (n = 10) | Single cells | CD83+ | CD1a+ |
|---|---|---|---|---|---|
| Single cell | 2.42 ± 1.06 | 1.62 ± 0.93 | Near to gland | 1.22 ± 0.61 | 0.91 ± 0.59 |
| Cluster | 2.55 ± 1.70 | 0.55 ± 0.47 | Stroma | 0.64 ± 0.54 | 0.61 ± 0.33 |
| Total | 4.97 ± 1.88 | 2.17 ± 1.23 | Lymphatic vessel | 0.56 ± 0.13 | 0.10 ± 0.07 |
Figure 1.
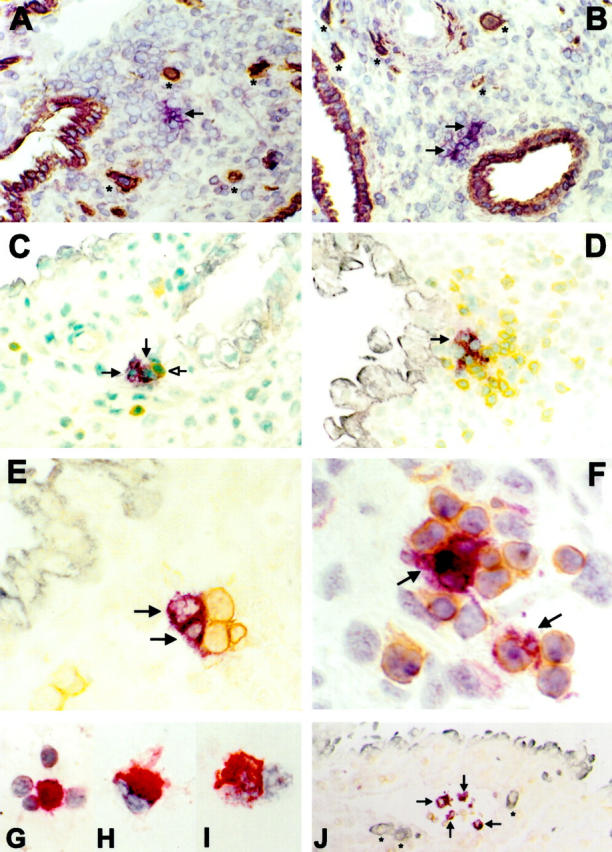
Immunohistochemistry of CD83+ and CD1a+ cells in early human pregnancy. Violet; VIP, brown; DAB, gray-blue; Vector blue, red; New fuchsin. A, C, and E: CD1a+ cells (violet, arrows); B, D, F, and J: CD83+ cells (violet, arrows). A: CD1a+ cells in decidua basalis exhibit a typical veiled appearance of DCs with long dendrites extending into the surrounding tissue. Brown: cytokeratin staining of invading FTBs (*) and endometrial glands (original magnification, ×160). B: Most CD83+ cells are located close to cytokeratin-positive endometrial glands (brown; original magnification, ×160). C: Typical location of CD1a+ cells in close vicinity to endometrial glands (gray-blue). CD1a+ cells are associated with a single CD3+ cell (brown, open head arrow; original magnification, ×250). D: CD83+ cells are often associated with CD3+ cells (brown) in clusters near an endometrial gland (gray-blue; original magnification, ×250). E: Association of CD1a+ cells with CD3+ cells (brown; original magnification, ×400). F: Association of CD83+ cells with CD3+ cells (brown; original magnification, ×630). J: CD83+ cells in a lymphatic vessel together with CD45+ lymphocytes (brown). * indicates invading FTBs indicating the tissue as decidua basalis (blue; original magnification, ×63). Cytocentrifuge preparations of enriched CD83+ cells demonstrate them to be often associated with lymphocytes (G; original magnification, ×630), to exhibit typical veiled morphology (H; ×1000) and to express HLA-DR (I; original magnification, ×1000).
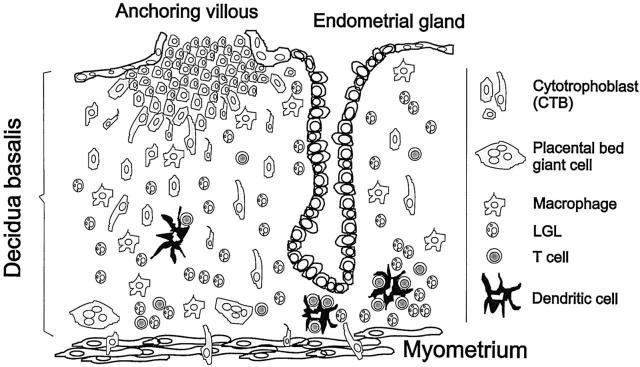
Figure 2.
Scheme of the implantation site. The placental anchoring villous is at the top of the diagram with the FTBs invading the decidua basalis beneath the anchoring villous. Most DCs are located in the basal layer of decidua (as characterized by the presence of the placental bed giant cells) directly at the myometrium at the bottom of the scheme. Most of the DCs are associated with T cells and some LGL.
Immunohistochemistry with CD1a revealed significantly (P < 0.005) fewer CD1a+ cells as compared to CD83+ cells (average, 2.17 ± 1.23 SD CD1a+ cells/mm2; n = 10). In contrast to the CD83+ cells, only a minority of CD1a+ cells was associated with lymphocytes in clusters (0.55 ± 0.47 SD, P < 0.003; Figure 1c ▶ ) and most of those clusters contained less CD3+ cells than clusters with CD83+ cells (Figure 1, e and f) ▶ . In those tissue sections with clear visible lymphoid vessels (n = 10), 5% ± 0.6 SD of CD1a+ cells could be observed in those vessels. However, similar to CD83+ cells, non-T-cell clustered CD1a+ cells were predominantly associated with endometrial glands (0.91 ± 0.59, P < 0.05; Figure 1a ▶ ). This intimate gland contact of CD83+ as well as CD1a+ cells indicate that, in early human pregnancy, the endometrial glands may be subject to a form of active immune response.
Enrichment of CD83+ Cells from Decidua
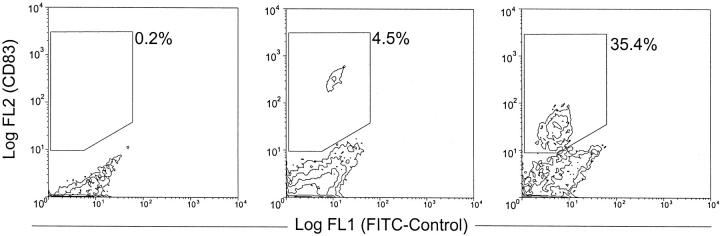
For phenotypic and functional studies on this DC-like decidual subset, the cells were enriched by immunomagnetic separation techniques. Mononuclear cells were obtained from enzymatically digested decidual tissue yielding an average of 3.6 ± 3.0 SD × 10 6 cells per gram of decidua (n = 16) by density gradient centrifugation over Histopaque. This decidual mononuclear cell fraction contained a population of 0.3% ± 0.2 SD CD83+ cells. After overnight culture, the nonadherent cell fraction contained 4.5% ± 3.2 SD CD83+ cells and was further enriched for DCs by CD83+ selection. The cell fraction (mean, 2 ± 2 SD × 10 5 cells/g tissue; n = 16) obtained by magnetic separation comprised 28.1% ± 4.3 SD CD83+ cells (corresponding to a 100-fold enrichment from fresh decidual mononuclear cells; Figure 3 ▶ ). The weaker staining of isolated CD83+ cells in comparison to those before isolation may be because of the competitive binding of the CD83 antibody bound to the anti-mouse immunoglobulin MACS-beads used for the magnetic separation. Determination of cell numbers by flow cytometric analysis and counting of positively stained cells on cytospins yielded comparable results (data not shown).
Figure 3.
FACS analysis of the enrichment procedure of CD83+ cells from human decidua. Left: decidual mononuclear cells contain 0.2% CD83+ cells. Middle: after overnight culture of the decidual mononuclear cells, the nonadherent fraction contains 4.5% of CD83+ cells. Right: after positive selection, the CD83+ cells are enriched to 35%. Win-MDI-Program, threshold: 0.5, smoothing: 1.
CD83+ Cells from Decidua Exhibit Phenotype and Morphology of DCs
Flow cytometric analysis of the nonadherent cell population after overnight culture demonstrated that all CD83+ cells expressed CD45, CD40, and HLA-DR. Expression of CD14 at very low levels and no co-expression of CD56 by CD83+ cells indicates the cells to be distinct from macrophages and LGLs (Figure 4a) ▶ . Within the CD83− decidual cells, a population of CD45+ cells was weakly positive for HLA-DR, CD40, and CD14 and therefore may represent macrophages. Furthermore a distinct population of CD56+ LGLs could be seen.
Figure 4.
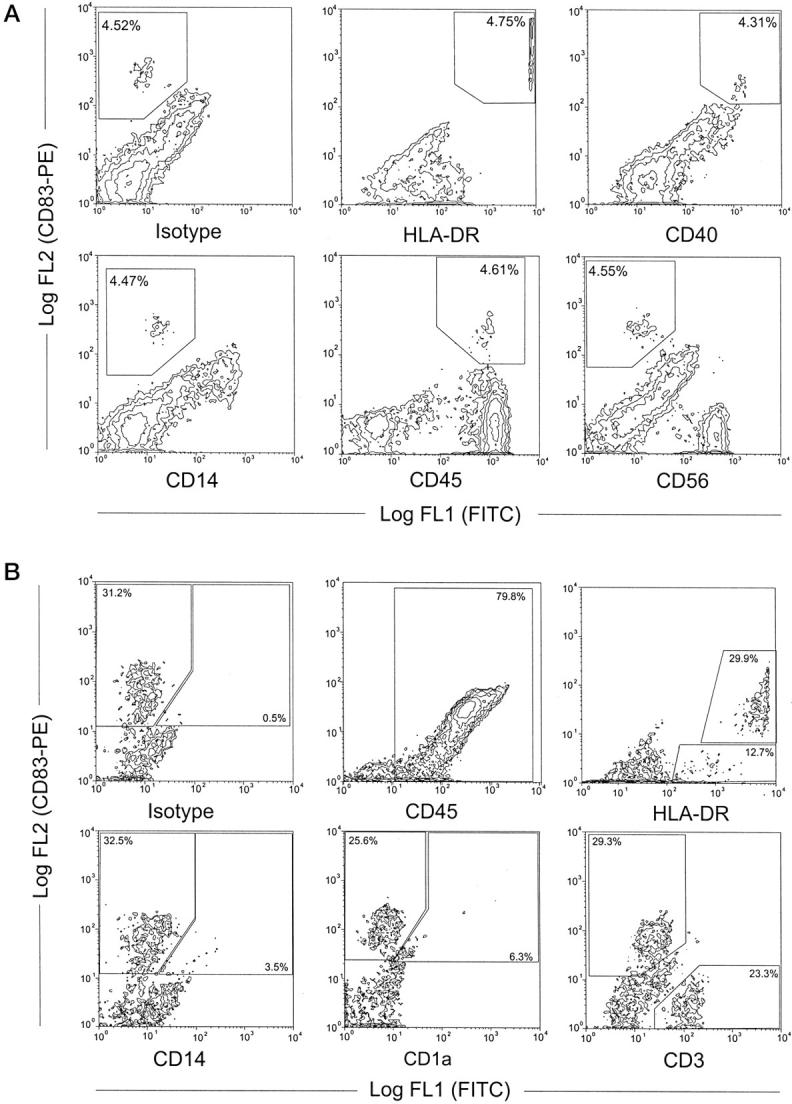
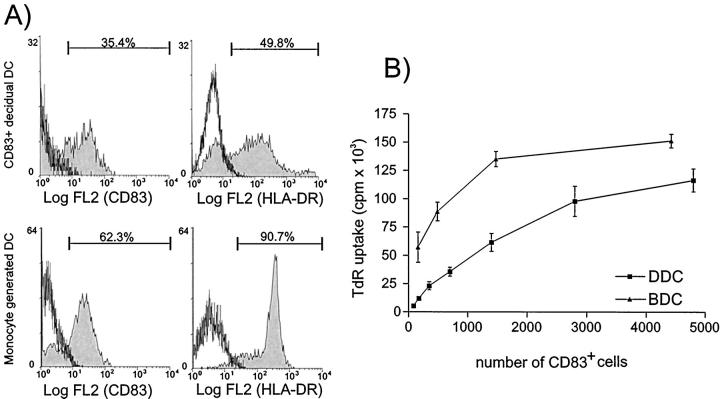
A: Double-staining FACS analysis of the phenotype of decidual cells (nonadherent fraction after overnight culture). Approximately 4.5% of the cells are positive for CD83 (upper left) and these co-stain with HLA-DR, CD40, CD45 and, at a very low level, with CD14, but not with CD56. A population of CD83− cells weakly positive for HLA-DR, CD40, and CD14 may represent macrophages, the CD56+ cells (lower right) represent the LGLs typical for human decidua. B: Flow cytometry of immunomagnetically-enriched decidual DC demonstrated 79.8% of the cells to be CD45+, 32.5% to be CD83+, and all CD83+ cells to be highly positive for HLA-DR. A small population of CD83+ cells co-stains with CD14 (3.5%) or CD1a (6.3%). A population of CD83− but weak HLA-DR+ cells (12.7%) may represent immature DC. The main population of the CD83− cells are CD3+ T cells (23.3% of all cells).
After immunomagnetic enrichment of decidual CD83+ HLA-DR++ DC contaminating CD83− cells comprised approximately 52.4% ± 6.3 SD CD3+ T cells and 19.8% ± 6.5 SD CD45− decidual stromal cells or fibroblasts (Figure 4b) ▶ . A minor population of ∼12% CD83−/HLA-DRlow cells presumably represent immature DCs. Only a few cells, both CD83− and CD83+, weakly stained positive for CD14 and a small population of CD83+ DCs co-stained with CD1a (5.2% ± 2.3 SD).
Cytospin specimens of isolated decidual cells enriched for DCs revealed that CD83+ or HLA-DR+ cells exhibited a typical DC morphology (Figure 1, h and i) ▶ . CD83+ cells were often clustered to multiple, small lymphocytes (Figure 1g) ▶ . The CD83+-enriched fraction of cultured decidual cells included a subset of cells with shape, morphology, and motility of DCs. Those cells were nonadherent, of approximately 10 μm in diameter, and with an irregularly shaped cell body. They were characterized by a varying number of long dendrites on their surface which continually extended, retracted, and bent in many directions giving a characteristic veiled appearance (Figure 5) ▶ . Decidual DCs were motile for up to 2 days in cell culture, but had lost viability by day 3 (data not shown).
Figure 5.
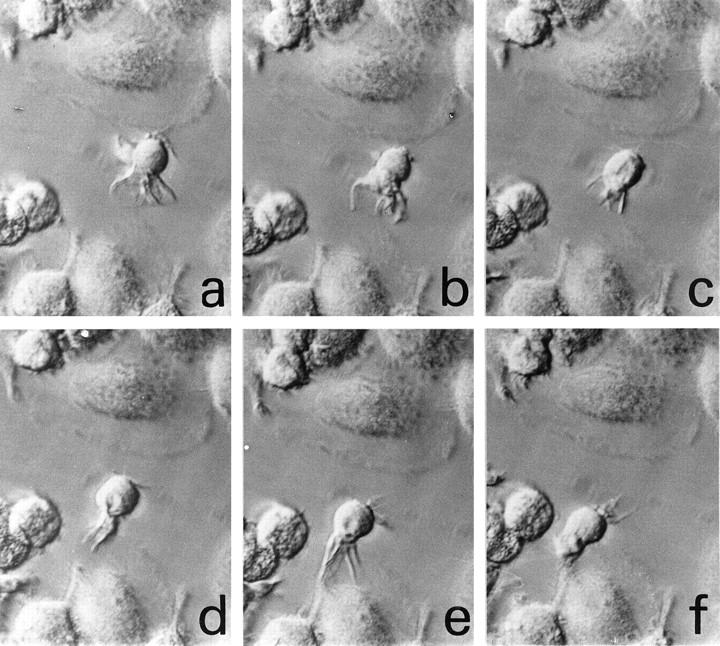
Morphology and motility of DCs isolated from human decidua over plastic. Monitoring over a time course of one picture per 30 seconds (a–f) demonstrates the motility of cells with characteristic extending and retracting dendrites from the surface of the cells (interference contrast, ×640).
CD83+ Cells from Decidua Are Potent Immunostimulatory DCs
We next compared the ability of decidual CD83+ enriched DCs (n = 6) and blood monocyte-derived DCs (n = 5) to stimulate the proliferation of allogeneic T cells. Blood-monocyte derived DCs (BDCs) were analyzed by flow cytometry to confirm their maturation state. They express CD83 in 59.2% ± 12.0 SD, HLA-DR in 85.48% ± 8.9 SD (Figure 6a) ▶ , CD40 in 80.95% ± 9.4 SD, and CD80 in 25.5% ± 2.1 SD (data not shown). Numbers of decidual DCs and BDCs used in the MLRs were normalized in the presented graphs for the number of CD83+ cells. As shown in Figure 6 ▶ , approximately twofold higher numbers of decidual DCs were required to stimulate a similar level of allogeneic T-cell proliferation in the MLRs as compared to fully matured BDCs.
Figure 6.
A: Phenotype of cells used for the mixed lymphocyte reaction. Top: CD83-enriched cells from human decidua. Bottom: DCs generated from blood monocytes. Cells were labeled as indicated and the percent of positive staining cells is shown. Gates were set using the isotype-matched negative controls to be <0.2% positive. B: Mixed lymphocyte reaction (MLR) of DCs. Graded numbers of CD83+ cells were used as stimulator cells in an allogeneic MLR together with 1 × 10 5 purified T-lymphocyte responders. Results are shown as the mean [3H]-thymidine uptake (TdR) ± SEM from all six CD83+ decidual DC samples (DDC; squares) and the five blood monocyte-derived DC samples (BDC; triangles). [3H]-thymidine uptake of all decidual DC and BDC populations as well as the responder T cells alone was <500 cpm and is not shown.
Discussion
The data presented here clearly demonstrate that human early pregnancy decidua harbors immunostimulatory CD83+ DCs, similar to those described for other mucosal surfaces. 11 Although an exclusive lineage-specific marker of human DCs is still lacking, CD83 has been proven to be a suitable and selective cell-surface marker for mature DCs. 17-19 Recently described mature DC markers such as CMRF-44 26 and DC-Lamp, 27 will be of interest for further studies for the identification and characterization of mature DCs in human decidua.
We have identified mature CD83+ DCs in decidual tissue by immunohistochemistry. The identification of CD1a+ cells in decidual sections with the same distribution pattern to CD83+ DCs, but in lower numbers, could possibly point to a heterogeneity of the DC population in this tissue. As described for skin 19 and lymphoid tissue, 28 decidua may harbor all three subsets of CD1a−/CD83+, CD1a+/CD83−, and CD1a+/CD83+ DCs. Whether the CD1a−/CD83+ cells within in tissue reflect mature DCs, or immature DCs expressing CD83 intracellularly, cannot be determined by immunohistochemistry. A typical feature of mature tissue DCs is their interaction with T cells 19 and indeed, the decidual CD83+ DCs were often seen in close association with CD3+ T lymphocytes.
Localization of CD83+ and CD1a+ DCs in close vicinity to endometrial glands, preferentially to the basal layer of decidua (stratum basale), is similar to their distribution in nonpregnant endometrium (data not shown), where leukocytes were also concentrated in the stratum basale. 29 This seems to be because of the fact that the upper layers of endometrium are repeatedly shed during menstruation leaving behind the stratum basale with its immunocompetent cells. 29 This may help to avoid diminution in the immunological capacity of endometrium during the shedding phase, a distribution which continues in early pregnancy decidua. In 1984, Bulmer and Sunderland 3 described the very occasional CD1a+ cell immediately beneath the endometrial gland endothelium in a few decidual tissue sections supporting our observations of the anatomical localization of decidual DCs.
The antigen presenting capacity of human decidual cells has been noted in previous publications. 30-34 Oksenberg et al 30 reported that first-trimester decidua-derived cells can act as accessory cells for mitogen-induced lymphoproliferation and for the presentation of soluble and particulate antigens to primed T cells. They suggested that dendritic-like cells are the main decidual APCs, but the precise identity of these cells remained unknown. In 1988, Dorman and Searle 31 reported the alloantigen presenting capacity of cells in human decidual tissue and speculated that the decidual APCs may belong to the MHC class II+ DC lineage. However, those studies were performed on the adherent or complete fraction of isolated decidual cells. Therefore these earlier studies may demonstrate the antigen presenting capacity of adherent decidual macrophages, an assumption subsequently confirmed by Mizuno et al. 32 In contrast to those previous publications, we have used the nonadherent cell fraction of cultured decidual cells to study DCs, because human DCs isolated from blood or tissue are known to be nonadherent. 10,13,21 In addition these studies used cells cultured for several weeks before analysis, 33,34 whereas we performed our cell isolation and analyses after only 1 night of culture.
The 10-fold increase in the number of CD83+ cells after the overnight culture step may reflect, in addition to the depletion of adherent contaminating cell types, the complete maturation of immature CD83− decidual DCs. 35 However, this short culture time makes a culture-dependent differentiation process of monocytes into activated APCs, which could express CD83, 35,36 unlikely. Additionally, as demonstrated in double-fluorescence analysis, the lack of CD14 expression argues against the possibility that these decidual CD83+ cells were activated monocytes. Only a few CD83−/HLA-DR+ cells were seen in the CD83-enriched cell fraction, indicating that this enrichment procedure seems to be suitable to remove nearly all non-DC that could express HLA-DR. The small population of CD83+/CD14low decidual DCs detected by FACS may represent a subset of DCs with a phenotype as reported for skin-derived populations 37
Enrichment of DCs beyond 30% purity was made difficult because many T cells were tightly clustered to DCs (see Figure 1g ▶ ), as previously described for DC migrating from skin explants. 38 T cells remained clustered to DCs during the magnetic sorting procedure, even at high concentrations of EDTA in the buffers used (unpublished observations). However after labeling and FACS-acquisition, a fraction of DC T-cell clusters were disrupted resulting in distinct CD3+ and CD83+ populations. Therefore future use of cell sorting by FACS technology may lead to higher purity of decidual DCs. The small amount of available cells, a fact we share with other studies handling DCs from nonlymphoid tissue, 39,40 minimizes the possibility of successive enrichment steps to improve the percentage of CD83+ cells.
Despite their impurity, the isolated decidual DC fractions were able to stimulate high levels of allogeneic T cell proliferation, although decidual DCs were not quite as potent allogeneic T cell stimulators as fully cytokine-matured BDCs. Because of the overnight cultivation step associated with the isolation of decidual DCs, this potent immunostimulatory property may not reflect the true in vivo situation. In addition, other APC populations may have been lost during the immunoselection of CD83+ cells. Therefore we cannot rule out that in vivo, in addition to the immunostimulatory DCs described here, other APC populations (perhaps with T cell tolerating capacity) may also exist in decidua.
In summary, among the many CD45+ leukocytes contained in decidual tissue, we have identified a subpopulation of CD40+/CD45+/CD83+/HLA-DR++, cells with morphological and functional features of typical mature DCs. The role of these potent APCs for immunoregulation of human pregnancy awaits further studies.
Acknowledgments
We thank Christian Linden for his technical assistance.
Footnotes
Address reprint requests to Ulrike Kämmerer, Ph.D., Department of Obstetrics and Gynaecology, Josef-Schneider-Strasse 4, D-97080 Würzburg, Germany. E-mail: frak057@mail.uni-wuerzburg.de.
Supported by grant Di-390/3–2 from the Deutsche Forschungsgemeinschaft (to J. D.) and grants Interdisziplinäres Zentrum für Klinische Forschung (IZKF)-A1 (to A. D. M.) and IZKF-A2 (to M. S.) from the University of Würzburg.
References
- 1.Billingham RE: Transplantation immunity and the maternal fetal relation. New Engl J Med 1964, 270:667-672 [DOI] [PubMed] [Google Scholar]
- 2.Starkey PM, Sargent IL, Redman CWG: Cell populations in human early pregnancy decidua: characterization and isolation of large granular lymphocytes by flow cytometry. Immunology 1988, 65:129-134 [PMC free article] [PubMed] [Google Scholar]
- 3.Bulmer JN, Sunderland CA: Immunohistological characterization of lymphoid cell populations in the early human placental bed. Immunology 1984, 52:349-357 [PMC free article] [PubMed] [Google Scholar]
- 4.Mincheva-Nilsson L, Baranov V, Yeung MM, Hammarstrom S, Hammarstrom ML: Immunomorphologic studies of human decidua-associated lymphoid cells in normal early pregnancy. J Immunol 1994, 152:2020-2032 [PubMed] [Google Scholar]
- 5.King A, Wellings V, Gardner L, Loke YW: Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum Immunol 1989, 24:195-205 [DOI] [PubMed] [Google Scholar]
- 6.Pijnenborg R, Dixon G, Robertson WB, Brosens I: Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980, 1:3-19 [DOI] [PubMed] [Google Scholar]
- 7.Knight SC, Stagg AJ: Antigen-presenting cell types. Curr Opin Immunol 1993, 5:374-382 [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM, Witmer MD: Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci USA 1978, 75:5132-5136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuntz-Crow M, Kunkel HG: Human dendritic cells: major stimulators of the autologous and allogeneic mixed leukocyte reactions. Clin Exp Immunol 1982, 49:338-346 [PMC free article] [PubMed] [Google Scholar]
- 10.Hart DNJ: Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 1997, 90:3245-3287 [PubMed] [Google Scholar]
- 11.Wakabayashi T, Onoda H, Kirita Y, Uchida H: Distribution of the dendritic cell in human organs and the incidence of GVHD. Transplant Proc 1997, 29:737-738 [DOI] [PubMed] [Google Scholar]
- 12.Knight SC: Veiled cells: dendritic cells of the peripheral lymph. Immunobiology 1984, 168:349-361 [DOI] [PubMed] [Google Scholar]
- 13.Steinman RM: The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 1991, 9:271-296 [DOI] [PubMed] [Google Scholar]
- 14.Freudenthal PS, Steinman RM: The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci USA 1990, 87:7698-7702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fearnley DB, Whyte LF, Carnoutsos SA, Cook AH, Hart DNJ: Monitoring human blood dendritic cell numbers in normal individuals and in stem cell transplantation. Blood 1999, 93:728-736 [PubMed] [Google Scholar]
- 16.Romani N, Schuler G: The immunologic properties of epidermal Langerhans cells as a part of the dendritic cell system. Springer Semin Immunopathol 1992, 13:265-279 [DOI] [PubMed] [Google Scholar]
- 17.Troy A, Davidson P, Atkinson C, Hart D: Phenotypic characterisation of the dendritic cell infiltrate in prostate cancer. J Urol 1998, 160:214-219 [PubMed] [Google Scholar]
- 18.Zhou L-J, Tedder TF: Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol 1995, 154:3821-3835 [PubMed] [Google Scholar]
- 19.McLellan AD, Heiser A, Sorg RV, Fearnley DB, Hart DNJ: Dermal dendritic cells associated with T lymphocytes in normal human skin display an activated phenotype. J Invest Dermatol 1998, 111:841-849 [DOI] [PubMed] [Google Scholar]
- 20.McLellan AD, Starling GC, Hart DNJ: Isolation of human blood dendritic cells by discontinuous Nycodenz gradient centrifugation. J Immunol Methods 1995, 184:81-89 [DOI] [PubMed] [Google Scholar]
- 21.Knight SC, Farrant J, Bryant A, Edwards AJ, Burman S, Lever A, Clarke J, Webster AD: Non-adherent, low-density cells from human peripheral blood contain dendritic cells and monocytes, both with veiled morphology. Immunology 1986, 57:595-603 [PMC free article] [PubMed] [Google Scholar]
- 22.Zal T, Volkmann A, Stockinger B: Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J Exp Med 1994, 180:2089-2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinbrink K, Wölfl M, Jonuleit H, Knop J, Enk AH: Induction of tolerance by IL-10-treated dendritic cells. J Immunol 1997, 159:4772-4780 [PubMed] [Google Scholar]
- 24.Cordell JL, Falini B, Erber WN, Ghosh AK, Abdulaziz Z, MacDonald S, Pulford KA, Stein H, Mason DY: Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APPAP complexes). J Histochem Cytochem 1984, 32:219-229 [DOI] [PubMed] [Google Scholar]
- 25.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G: Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods 1996, 196:137-151 [DOI] [PubMed] [Google Scholar]
- 26.Hock BD, Starling GC, Daniel PB, Hart DN: Characterization of CMRF-44, a novel monoclonal antibody to an activation antigen expressed by the allostimulatory cells within the peripheral blood, including dendritic cells. Immunology 1994, 83:573-581 [PMC free article] [PubMed] [Google Scholar]
- 27.De Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin J-J, Ait-Yahia S, Patel S, Mattei M-G, Banchereau J, Zurawski S, Davoust J, Caux C, Lebeque S: A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity 1998, 9:325-336 [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K, Asagoe K, Zaishun J, Yanai H, Yoshino T, Hayashi K, Akagi T: Heterogeneity of dendritic cells in human superficial lymph node. Am J Pathol 1998, 153:745-755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamat BR, Isaacson PG: The immunocytochemical distribution of leukocytic subpopulations in human endometrium. Am J Pathol 1987, 127:66-73 [PMC free article] [PubMed] [Google Scholar]
- 30.Oksenberg JR, Mor-Yosef S, Persitz E, Schenker Y, Mozes E, Brautbar C: Antigen-presenting cells in human decidual tissue. AJRIM 1986, 11:82-88 [DOI] [PubMed] [Google Scholar]
- 31.Dorman PJ, Searle RF: Alloantigen presenting capacity of human decidual tissue. J Reprod Immunol 1988, 13:101-112 [DOI] [PubMed] [Google Scholar]
- 32.Mizuno M, Aoki K, Kimbara T: Functions of macrophages in human decidual tissue in early pregnancy. AJRI 1994, 31:180-188 [DOI] [PubMed] [Google Scholar]
- 33.Olivares EG, Montes MJ, Oliver C, Galindo JA, Ruiz C: Cultured human decidual stromal cells express B7–1 (CD80) and B7–2 (CD86) and stimulate allogeneic T cells. Biol Reprod 1997, 57:609-615 [DOI] [PubMed] [Google Scholar]
- 34.Montes MJ, Aleman P, Garcia-Tortosa C, Borja C, Ruiz C, Garcia-Olivares E: Cultured human decidual stromal cells express antigens associated with hematopoietic cells. J Reprod Immunol 1996, 30:53-66 [DOI] [PubMed] [Google Scholar]
- 35.Palucka KA, Taquet N, Sanches-Chapuis F, Gluckman JC: Dendritic cells as the terminal stage of monocyte differentiation. J Immunol 1998, 160:4587-4595 [PubMed] [Google Scholar]
- 36.Zhou L-J, Tedder TF: CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA 1996, 93:2588-2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nestle FO, Zheng XG, Thompson CB, Turka LA, Nicklolff BJ: Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol 1993, 151:6535-6545 [PubMed] [Google Scholar]
- 38.Pope M, Betjes MG, Hirmand H, Hoffman L, Steinman RM: Both dendritic cells and memory T lymphocytes emigrate from organ cultures of human skin and form distinctive dendritic-T-cell conjugates. J Invest Dermatol 1995, 104:11-17 [DOI] [PubMed] [Google Scholar]
- 39.Koch F, Kampgen E, Schuler G, Romani N: Effective enrichment of murine epidermal Langerhans cells by a modified (mismatched) panning technique. I Invest Dermatol 1992, 99:803-807 [DOI] [PubMed] [Google Scholar]
- 40.Holt PG, Oliver J, McMenamin C, Schon-Hegrad MA: Studies on the surface phenotype and functions of dendritic cells in parenchymal lung tissue of the rat. Immunology 1992, 75:582-587 [PMC free article] [PubMed] [Google Scholar]