Abstract
PECAM-1 is a 130-kd member of the Ig superfamily present on endothelial cells, platelets, polymorphonuclear leukocytes, monocytes, and lymphocytes. Its expression begins early in development and persists through adulthood. PECAM-1 functions as an adhesion and signaling molecule between adjacent endothelial cells and between endothelial cells and circulating blood elements. Antibodies directed against PECAM-1 have been shown to affect angiogenesis, endothelial cell migration, and polymorphonuclear leukocyte transmigration. Furthermore, its dimerization is associated with the modulation of integrin affinity. Antibody inhibition studies suggest that PECAM-1 plays a role in modulating thrombosis; however, recent in vitro aggregation studies performed on platelets harvested from PECAM-1-deficient mice revealed no abnormalities. In this report we demonstrate prolonged in vivo bleeding times in PECAM-1-deficient mice. This abnormality was not corrected when wild-type hematopoietic precursors were engrafted into marrow-ablated PECAM-1-deficient mice. Furthermore, normal bleeding times were observed when marrow-ablated wild-type mice were engrafted with hematopoietic precursors harvested from PECAM-1-deficient mice. These studies are consistent with a role for PECAM-1 in modulating thrombosis in the vasculature, which is potentially mediated by endothelial cell PECAM-1 expression.
The expression of PECAM-1 (CD31), a 130-kd member of the Ig superfamily, begins early in development at the stage of hemangioblast formation and persists through adulthood. 1-3 It is expressed on endothelial cells, platelets, polymorphonuclear leukocytes, monocytes, and T and B lymphocytes and is thought to serve several functions, including that of an adhesion molecule mediating cell-cell adhesion between adjacent endothelial cells and between endothelial cells and polymorphonuclear leukocytes, platelets, monocytes, and lymphocytes. 1-5 It has been shown to be a modulator of in vitro and in vivo angiogenesis, endothelial cell migration, and polymorphonuclear leukocyte transmigration. PECAM-1 localization and phosphorylation state are known to be affected by integrin engagement, hypoxia, hyperglycemia, and osmolarity. 4-13
In addition to its adhesive functions, PECAM-1 has been shown to be a participant in signaling pathways. 3,8,11,14 After engagement of PECAM-1, integrin affinity changes have been noted on platelets and lymphocytes, 15-19 and changes in intracellular calcium localization have been documented. 20 PECAM-1-mediated signaling is thought to occur, in part, via its cytoplasmic ITIM (immunoregulatory tyrosine inhibitory motif) domain. This domain is known to mediate binding of signaling and adapter molecules having one or tandem SH2 domains, when the tyrosine residues residing in the PECAM-1 ITIM domain are phosphorylated. 3,11,12 Specifically, the phosphatase SHP-2 has been shown to bind to tyrosine-phosphorylated PECAM-1. 19,21 Recently we have found that in addition to its interactions with SHP-2, PECAM-1 can serve as a reservoir for and a modulator of β-catenin, binding tyrosine-phosphorylated β-catenin, and, if PECAM-1 is tyrosine phosphorylated, bringing β-catenin into close proximity to SHP-2, facilitating dephosphorylation of the bound β-catenin. 22
These observations prompted the generation of PECAM-1-deficient mice. The animals were found to be viable and exhibited an abnormal transit of polymorphonuclear leukocytes across vascular basement membranes as their only demonstrable phenotype. 23 Other recent investigations using vital microscopy techniques have revealed delays in leukocyte transmigration in PECAM-1-deficient mice. 24
In this manuscript we report further analyses of the PECAM-1-deficient mice. Specifically, we have demonstrated a prolongation in bleeding time in PECAM-1-deficient mice. Furthermore, we have shown that this phenotype persists when the PECAM-1-deficient mice are engrafted with wild-type hematopoietic precursors.
Materials and Methods
PECAM-1-deficient mice were generated as described. 23 The colony was bred at the Yale University Animal Care Facility (New Haven, CT) and the Blood Research Institute (Milwaukee, WI), in accordance with established protocols. Phenotype was assessed by flow cytometric analysis (FACS) of peripheral blood elements as described. 23
Platelet, RBC, and WBC counts were performed on blood collected by retroorbital bleeding with a heparinized capillary tube. Two hundred microliters of blood was immediately transferred to Eppendorf tubes containing K3 EDTA (2.0 mg/ml). Samples were diluted in saline and counted on a Baker System 9110+CP Hematology Analyzer (Biochem Immunosystems, Allentown, PA) or sent to a commercial laboratory (ANTECH Diagnostics, Farmingdale, NY) for analysis.
Bleeding times were assessed using an adaptation of the method used by Kung et al. 25 Deep anesthesia was induced with metofane gas. The mice were then secured into a tabletop holder, with their tails taped downward and perpendicular to their bodies. After being pulled through a 1.5-mm-diameter template, the tails were transected with a scalpel blade and bled onto a Whatman filter paper. The filter was dabbed to the wound every 30 seconds without disrupting the forming clot. Any blood dripping during the 30-second intervals was allowed to drop freely onto the filter. The experiment was continued until bleeding stopped completely (wild type and heterozygous). The bleeding of PECAM-1-deficient animals was stopped by cauterization at 20 minutes to prevent hypovolemic shock.
Hematopoietic precursor engraftment of PECAM-1-deficient and wild-type mice was performed as described. 26 Recipient mice were irradiated (500 Rads twice, separated by 2 hours) using a cesium 127 irradiator. Donor animals were euthanized by cervical dislocation. Femur, tibia, and iliac crest were aseptically removed and cleaned free of muscle, and the ends were cut off with a scalpel blade. The marrow was flushed into a 50-ml tube, using a 22-gauge needle attached to a 5-ml syringe containing 10% fetal bovine serum in 1× phosphate-buffered saline. Cells from multiple animals were pooled, filtered through a 40-μm cell strainer (Beckton Dickinson, Franklin New Jersey), washed, and counted. Marrow precursor cells were diluted to 2.5 × 106/200 μl and injected retroorbitally into each irradiated recipient deficient or wild-type mouse. After 30 days recipients were tested for platelet number and bleeding time.
Statistics (averages, standard deviations, Student’s t-test) were performed on a Power Macintosh 9600/300 computer using Excel 4.0. Statistical differences were assessed by one-way analysis of variance and the Student-Newman-Keuls method for post hoc analysis, with SigmaStat software (Jandel Corporation, San Rafael, CA).
Results
PECAM-1 Expression Is Reduced in Heterozygous Mice and Is Not Detectable in Homozygous Null Mice
As previously described, 19 PECAM-1 expression in circulating blood elements is not detectable in PECAM-1 homozygous null mice (−/−, KO), whereas it is present in reduced amounts in heterozygotes (+/−, Het) compared to wild-type (+/+, WT) littermates. The presence or absence of PECAM-1 expression was confirmed by immunofluorescence of tissue sections (data not shown).
PECAM-1 Null Mice Exhibit Prolonged Bleeding Times
During our harvests of blood for FACS analysis of phenotype we noticed a difference (prolongation) in bleeding times of some of the mice. When the FACS data were correlated with this observation we determined that the animals exhibiting prolonged bleeding times were the PECAM-1-deficient (−/−) mice. This finding prompted us to perform standardized bleeding times on all of our litters. Bleeding times were performed in a blinded fashion at two sites by separate investigators over a 2-year period. At the Yale University School of Medicine the mice wre bled at 3 weeks of age. In all of the mice studied to date (60 mice of varying ages spanning a 2-year period), all of the homozygous null (−/−, KO) mice exhibited a prolonged bleeding time of more than 20 minutes (the test was arbitrarily stopped at this time point to avoid possible death due to hypovolemic shock). The heterozygous animals(+/−, Het) exhibited bleeding times similar to those of wild-type (+/+, WT) littermates (11 ± 1.5 minutes versus 12 ± 1.4 minutes) (Figure 1) ▶ . Repeat bleedings of the same mice yielded similar results. At the Blood Research Institute at the Blood Center of Southeastern Wisconsin, mice were bled at 8–9 weeks of age. Similar to the findings noted at Yale University, the homozygous null (−/−, KO) mice exhibited prolonged bleeding times (16.6 ± 3.2 minutes) compared to wild-type littermates (12.1 ± 6.9 minutes) (n = 15, P = 0.03). In addition, when the area of blood deposited on the filter paper was analyzed, a significant increase in area was noted for the homozygous null (−/−, KO) mice (1097.2 ± 390.7 mm2) compared to wild-type littermates (689.1 ± 382.3 mm2) (n = 15, P = 0.01). Differences in the bleeding times obtained at Yale University and the Blood Research Center are most likely the result of subtle differences in technique of particular individuals. It should be noted, however, that at both sites the knockout animals exhibited prolonged bleeding times compared to wild-type mice.
Figure 1.
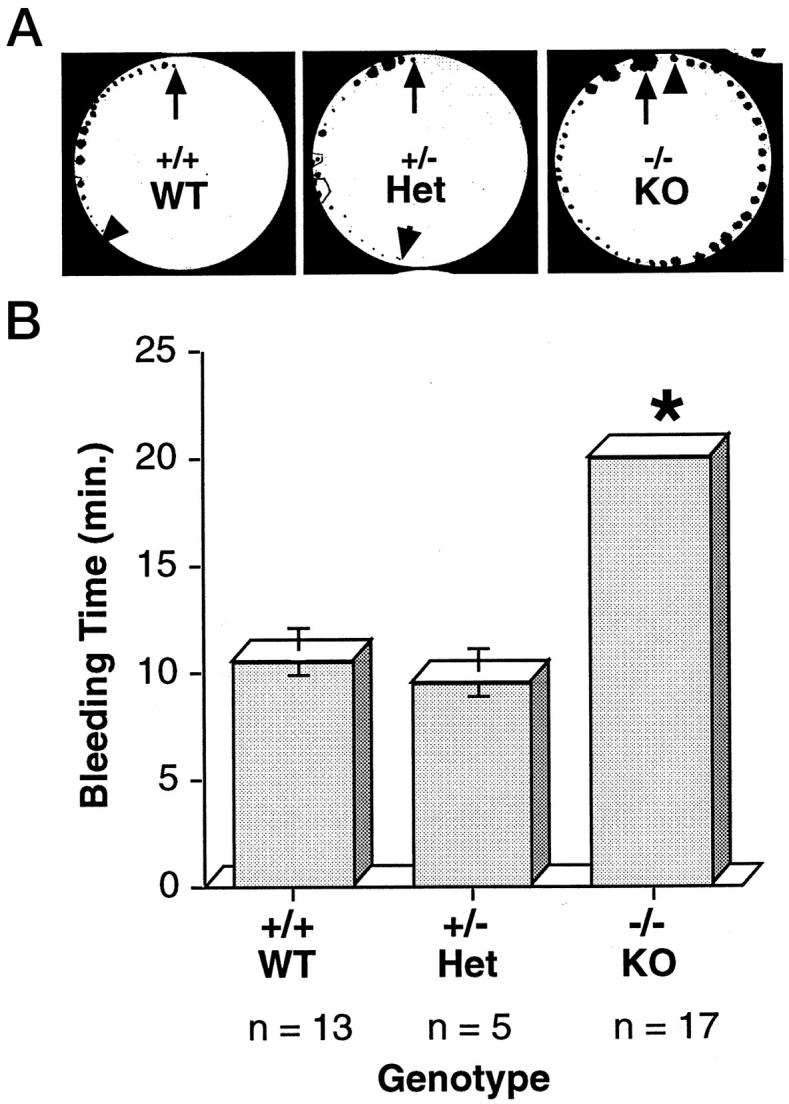
PECAM-1-deficient mice exhibit prolonged bleeding times. Bleeding times were determined with a standardized filter paper test (see Materials and Methods). A: Representative bleeding times of wild-type (+/+, WT), heterozygous (+/−, Het), and PECAM-1-deficient (−/−, KO) mice. The arrows at the 12 o’clock position denote the start of the assay. Each dot represents a 30-second time point. The arrowheads denote the cessation of bleeding. B: Average bleeding times obtained from a typical experiment consisting of 13 (+/+), 5 (+/−), and 17 (−/−) mice. The vertical bars represent standard deviations. * P value of <0.05 comparing KO and both WT and Het mice.
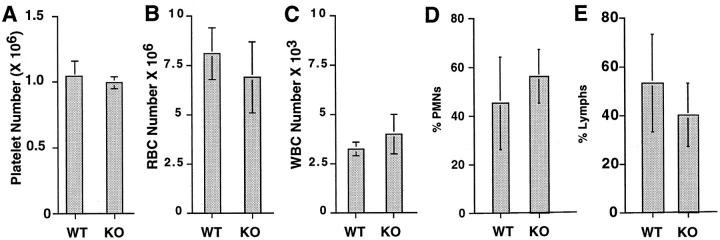
Platelet counts performed on blood from wild-type (+/+), heterozygotes (+/−), and homozygotes (−/−) revealed similar platelet numbers (1.03 × 10 6 ± 1.21 × 105, 1.01 × 10 6 ± 3.53 × 104, and 9.93 × 10 5 ± 4.72 × 104, respectively) in all of the mice tested (see Figures 2A and 4B ▶ ▶ ). Transmission electron microscopy of circulating platelets harvested from wild type (+/+), heterozygotes (+/−), and homozygotes (−/−) revealed no detectable morphological differences (data not shown). Similarly, RBC and WBC counts performed on wild-type and homozygous null mice revealed similar cell numbers. RBC counts were 8.1 × 10 6 ± 1.3 × 10 5 for WT (+/+) mice versus 6.9 × 10 6 ± 1.8 × 10 5 for KO (−/−) mice (Figure 2B) ▶ , and WBC counts were 3.25 × 10 3 ± 3.5 × 10 2 for WT mice versus 4.0 × 10 3 ± 1.0 × 10 2 for KO mice (Figure 2C) ▶ . Further analysis of the WBC fraction revealed no statistically significant differences in polymorphonuclear leukocyte or lymphocyte fractions (Figure 2, D and E) ▶ .
Figure 2.
PECAM-1-deficient mice exhibit similar platelet, RBC, and WBC numbers and differing percentages of PMNs and lymphocytes similar to those of wild-type mice. Blood from wild-type (WT) and knockout (KO) mice was withdrawn, and platelet, RBC, and WBC counts and differential counts were performed. Platelet (n = 8) (A), RBC (n = 4) (B), and WBC (n = 3) (C) levels and differential blood cell counts (D, % PMN; E, % lymphocytes) were determined to be similar for WT and KO mice (P > 0.1).
Figure 4.
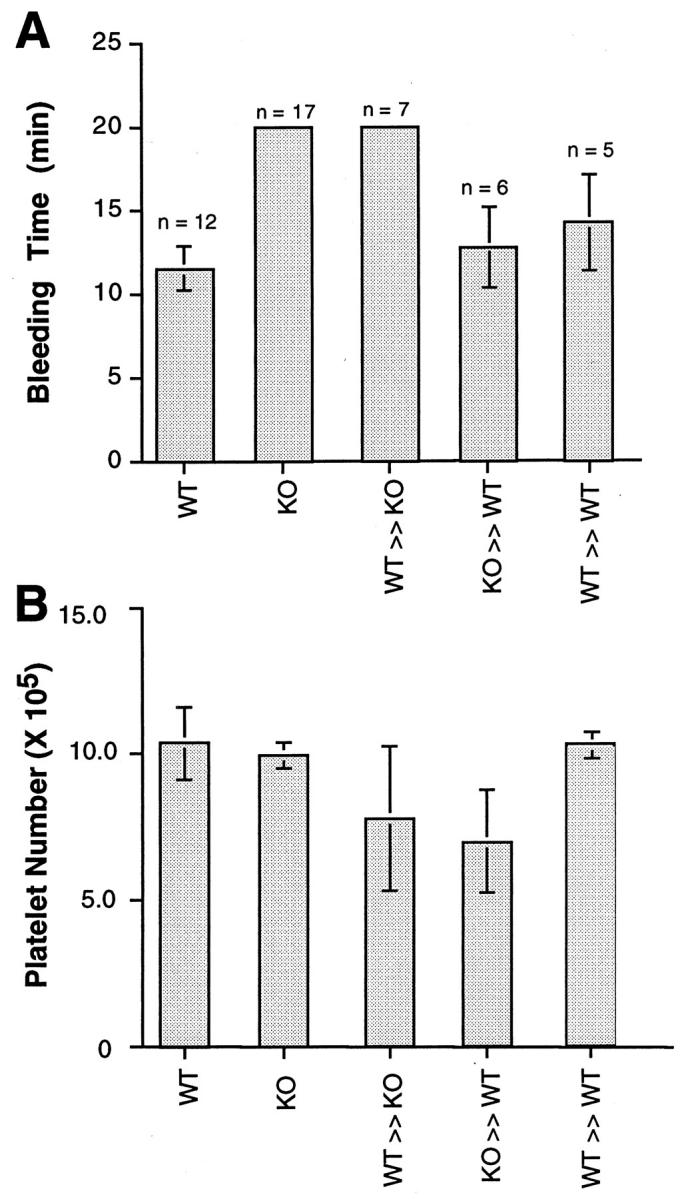
Bleeding times correlate with the recipient after marrow ablation and subsequent engraftment. Groups of WT and KO mice underwent marrow ablation and engraftment with donor bone marrow. Thirty days after engraftment bleeding times and platelet numbers were assessed. A: WT (n = 12) and KO (n = 17) mice exhibited the expected bleeding times (see Figure 2 ▶ ). KO mice engrafted with WT bone marrow (WT ≫ KO, n = 7) exhibited prolonged bleeding times (>20 minutes), whereas WT mice engrafted with either KO (KO ≫ WT, n = 6) or WT (WT ≫ WT, n = 5) bone marrow exhibited bleeding times indistinguishable from WT mice. The vertical lines denote standard deviations. When compared, bleeding times of WT, KO ≫ WT, and WT ≫ WT mice were not statistically different from each other but were statistically different (P < 0.05) from KO and WT ≫ KO mice. Bleeding times for KO and WT ≫ KO mice were not statistically different from each other. B: Platelet counts in the same groups of WT, KO, WT ≫ KO, KO ≫ WT, and WT ≫ WT mice. All mice had appreciable levels of platelets, and there was no correlation between the modest differences in platelet numbers and bleeding times.
Engraftment of Wild-Type Hematopoietic Precursors into Homozygous PECAM-1 Null Mice Does Not Correct the Prolonged Bleeding Time
In light of recent findings suggesting that modulation of platelet PECAM-1 interactions can influence platelet function, 16,27-30 we embarked on a stem cell therapy approach to attempt a correction of the prolonged bleeding time and to gain a better understanding of the role of PECAM-1 roles in thrombosis. As illustrated in Figure 3, A and B ▶ , engraftment of wild-type bone marrow stem cells in marrow-ablated PECAM-1-deficient mice resulted in reconstitution of the bone marrow with PECAM-1-positive hematopoietic precursors and circulating blood elements. Platelet counts obtained from these engrafted mice were essentially indistinguishable from those of wild-type mice (1.03 × 10 6 ± 1.21 × 10 5 versus 8.2 × 10 5 ± 2.25 × 105, respectively) (Figure 4B) ▶ . Surprisingly, bleeding times in these mice remained prolonged (more than 20 minutes) (Figure 4A) ▶ , despite the presence of PECAM-1-positive platelets (Figure 3B) ▶ .
Figure 3.
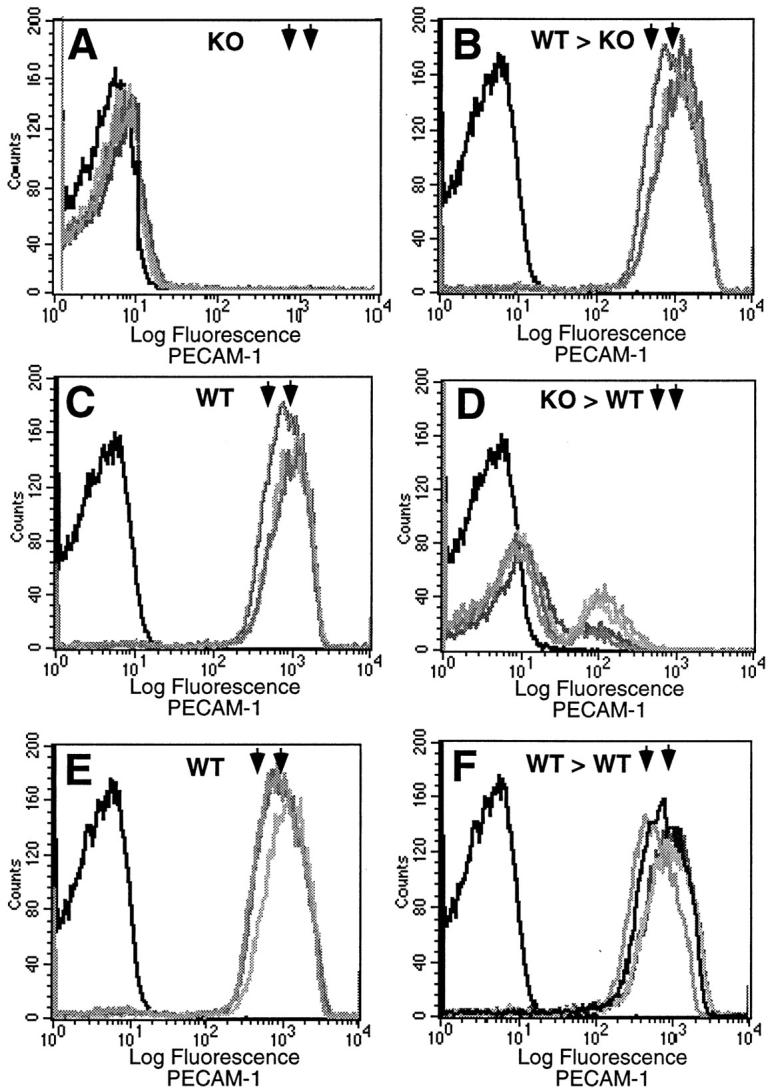
FACS analysis of wild-type (WT) and PECAM-1-deficient (KO) mice before and 30 days after marrow ablation and engraftment with donor bone marrow. A and B: PECAM-1 expression on circulating blood elements of five KO mice before (A) and after (B) engraftment with WT marrow (WT > KO). Note that after ablation and engraftment there is expression of PECAM-1 at wild-type levels (rightmost arrowheads). C and D: PECAM-1 expression on circulating blood elements of five WT mice before (C) and after (D) engraftment with KO marrow (KO > WT). Note that after ablation and engraftment there is a substantial reduction in the expression of PECAM-1, well below heterozygous levels (leftmost arrowheads). E and F: PECAM-1 expression on circulating blood elements of five WT mice before (E) and after (F) engraftment with WT marrow (WT > WT). Note that after ablation and engraftment there is expression of PECAM-1 at wild-type levels (rightmost arrowheads), essentially at preablation levels. This pair serves as a control. The dark leftmost tracing in all of the panels represents the baseline fluorescence without primary antibody. Rightmost arrowheads, wild-type levels; leftmost arrowheads, heterozygous levels.
As illustrated in Figure 3, C and D ▶ , engraftment of PECAM-1-deficient bone marrow stem cells in marrow-ablated wild-type mice resulted in reconstitution of the bone marrow with PECAM-1-negative hematopoietic precursors and circulating blood elements. Platelet counts obtained from these engrafted mice were also similar to those of wild-type mice (1.03 × 10 6 ± 1.21 × 10 5 versus 8.31 × 10 5 ± 1.77 × 104, respectively) (Figure 4B) ▶ . Bleeding times in these animals remained normal (11 ± 1.6 minutes) (Figure 4A) ▶ , despite the presence of PECAM-1-negative platelets (Figure 3D) ▶ . Figure 3, E and F ▶ , illustrates engraftment of WT bone marrow stem cells in marrow-ablated wild-type mice, which resulted in reconstitution of the bone marrow with PECAM-1-positive hematopoietic precursors and circulating blood elements.
Taken together, these data suggest that the prolonged bleeding in PECAM-1-deficient animals is due to a vascular rather than a platelet defect.
Discussion
PECAM-1 (CD31) has been implicated as a modulator of a variety of vascular endothelial, polymorphonuclear leukocyte, monocyte, lymphocyte, and platelet functions. 2,3,8,11,14,27 Until recently the conclusions drawn regarding the role of PECAM-1 in many of these diverse processes were based on overexpression of dominant negative and dominant positive constructs and antibody blocking and activation studies. 28-31 The generation of PECAM-1-deficient mice now provides the means to study the a priori significance of the molecule and its physiological relevance from implantation through adulthood. It was expected that knocking out PECAM-1 would produce obvious and catastrophic changes affecting the developing animal. However, likely because of redundancy, PECAM-1-deficient mice are viable, with seemingly subtle functional irregularities. To date the most obvious of these has been a delay at the basal lamina after polymorphonuclear leukocytes migrate through the endothelium, after an inflammatory stimulus. 23
Our observation of prolonged bleeding times in PECAM-1-deficient mice and data implicating PECAM-1 as a participant in platelet/platelet and platelet/endothelial associations 16,28-33 prompted us to study these interactions in the PECAM-1-deficient mice. In our initial attempt to characterize this deficiency, we assessed platelet numbers and morphological characteristics and noted no major differences between PECAM-1-deficient and wild-type mice. In this study, our approach has been to quantify differences in bleeding time. Wild-type mice reconstituted with PECAM-1-deficient marrow, PECAM-1-deficient mice reconstituted with wild-type marrow, and wild-type mice reconstituted with wild-type marrow hematopoietic precursors were generated and used to further characterize the PECAM-1-deficient phenotype.
The techniques used to measure tail bleeding time vary and can often contribute to disparate findings between laboratories. 34 We achieved the most consistent results by using a variation of the methods described by Kung et al, 25 combined with principles outlined by the template method developed by Mielke, 35 which uses a pressure cuff and standardized incisions when bleeding times in humans are taken. We anesthetized the animals and normalized the tail transection diameters, using a 1.5-mm template. The tail was positioned downward and perpendicular to the animal’s body, corresponding the application of a pressure cuff in humans 34 and contributing less to inconsistent clotting kinetics due to collapsing capillaries. 36 To minimize investigator bias and unrecognized differences in technique, bleeding times were performed in a blinded fashion at two distinct sites by different investigators over a long time period. In all instances the homozygous null mice were found to exhibit significantly prolonged bleeding times.
PECAM-1 on the surface of platelets has been shown to act as a costimulatory agonist receptor capable of modulating integrin function in human platelets during adhesion and aggregation. 16 PECAM-1 expression is not limited to the surface of platelets, as 20–30% is contained in the α-granules. 37 Furthermore, thrombin-activated degranulated platelets show increased PECAM-1 expression at the surface. Blocking studies using a monoclonal antibody against PECAM-1 inhibited ADP-, collagen-, and epinephrine-induced platelet aggregation. However, pooled PECAM-1-deficient and wild-type platelets aggregate at nearly the same rate after ADP stimulation. 23 This apparent disparity may be explained by the following: 1) Recent studies have shown that hemodynamic forces as well as substrate characteristics influence platelet adhesion pathways, further addressing the need for more physiologically relevant assays. 38,39 2) Increased expression or surface redistribution of PECAM-1 expression concomitantly on platelets and endothelial cells may, in a thrombogenic microenvironment, augment adhesion and potentiate signal transduction through platelet/platelet as well as through heterotypic interactions with endothelial cells. 40,41 Furthermore, Rosenblum et al showed in vivo that two different antibodies directed against PECAM-1 delayed platelet aggregation after light/dye energy transferring, nondenuding injury to endothelium. 28-30 However, platelets harvested from mice after systemic infusion with the same antibodies and activated with arachidonate and ADP did not exhibit delayed aggregation. 29
Taken together, these data implicate a potential role for PECAM-1 on the endothelium in mediating signaling cascades and adhesive functions during thrombosis. Bombeli et al have demonstrated that adhesion molecules on endothelial cells can act to tether secreted or soluble plasma proteins, facilitating activated platelet adhesion and further aggregation. 42 Our finding that bleeding time characteristics remain with the recipient animal after bone marrow engraftment is consistent with a potential role for PECAM-1 on the endothelium, mediating signaling cascades and/or adhesive functions during thrombosis. Elucidation of the role(s) of PECAM-1 in modulating thrombotic phenomena will require further investigation. 39
We expected to correct the bleeding time anomaly in PECAM-1-deficient mice through reconstitution with wild-type bone marrow stem cells and to induce abnormal bleeding times in wild-type mice through reconstitution with PECAM-1-deficient bone marrow stem cells. However, although platelet numbers remained approximately equivalent, the bleeding time of each recipient prevailed. These results suggest that PECAM-1 on the endothelium and not necessarily on platelets is important for normal thrombotic phenomena in the periphery. Previous studies have shown that PECAM-1 on endothelial cells plays a role in the transmigration of hematopoietic progenitor cells through the vasculature and that PECAM-1 can undergo heterophilic interactions with other molecules. 43-47 The roles that endothelial PECAM-PECAM and endothelial PECAM-heterophilic interactions play in these processes and in modulating the bleeding time in the periphery remain to be determined.
Taken together, our data and published reports 4,7- 9,14,15,43-45,47-52 suggest a role of PECAM-1 on endothelial cells in signal transduction, heterophilic/heterotypic interactions, and modulation of thrombosis in the peripheral vasculature. Further studies are needed to further elucidate the mechanism(s) involved in these processes.
Footnotes
Address reprint requests to Dr. Joseph A. Madri, Department of Pathology, Yale University School of Medicine, 310 Cedar Street, P.O. Box 208023, New Haven, CT 06520-8023. E-mail: joseph.madri@yale.edu.
Supported in part by U.S. Public Health Service grants R37-HL-28373, RO1-HL-51018, and PO1-DK-38979 to J. A. M. and RO1-HL-40926 to J. P. N.
References
- 1.Newman PJ: The role of PECAM-1 in vascular cell biology. Ann NY Acad Sci 1994, 714:165-174 [DOI] [PubMed] [Google Scholar]
- 2.Newman PJ: The biology of PECAM-1. J Clin Invest 1997, 100:S25-S29 [PubMed] [Google Scholar]
- 3.Newman PJ: Switched at birth: a new family for PECAM-1. J Clin Invest 1999, 103:5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chosay JG, Fisher MA, Farhood A, Ready KA, Dunn CJ, Jaeschke H: Role of PECAM-1 (CD31) in neutrophil transmigration in murine models of liver and peritoneal inflammation. Am J Physiol 1998, 37:G776-G782 [DOI] [PubMed] [Google Scholar]
- 5.Vaporciyan AA, DeLisser HM, Yan HC, Mendiguren II, Thom SR, Jones ML, Ward PA, Albelda SM: Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science 1993, 262:1580-1582 [DOI] [PubMed] [Google Scholar]
- 6.Bogen S, Pak J, Garifallou M, Deng X, Muller WA: Monoclonal antibody to murine PECAM-1 (CD31) blocks acute inflammation in vivo. J Exp Med 1994, 179:1059-1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elias CG, Spellberg JP, Karantamir B, Lin CH, Wang YJ, McKenna PJ, Muller WA, Zukowski MM, Andrew DP: Ligation of Cd31/Pecam-1 modulates the function of lymphocytes, monocytes and neutrophils. Eur J Immunol 1998, 28:1948-1958 [DOI] [PubMed] [Google Scholar]
- 8.Pinter EM, Barreuther M, Lu T, Imhof BA, Madri JA: Platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) tyrosine phosphorylation state changes during vasculogenesis in the murine conceptus. Am J Pathol 1997, 150:1523-1530 [PMC free article] [PubMed] [Google Scholar]
- 9.Pinter E, Mahooti S, Wang Y, Imhof BA, Madri JA: Hyperglycemia-induced vasculopathy in the murine vitelline vasculature: correlation with PECAM-1/CD31 tyrosine phosphorylation state. Am J Pathol 1999, 154:1367-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalra VK, ShenY, Sultana C, Rattan V: Hypoxia induces PECAM-1 phosphorylation and transendothelial migration of monocytes. Am J Physiol 1996, 271:H2025–H2034 [DOI] [PubMed]
- 11.Lu TT, Yan LG, Madri JA: Integrin engagement mediates tyrosine dephosphorylation on platelet-endothelial cell adhesion molecule 1. Proc Natl Acad Sci USA 1996, 93:11808-11813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu TT, Barreuther M, Davis S, Madri JA: Platelet endothelial cell adhesion molecule-1 is phosphorylatable by c-Src, binds Src-Src homology 2 domain, and exhibits immunoreceptor tyrosine-based activation motif-like properties. J Biol Chem 1997, 272:14442-14446 [DOI] [PubMed] [Google Scholar]
- 13.DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, Albelda SM: Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol 1997, 151:671-677 [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CS, Wang T, Madri JA: Platelet endothelial cell adhesion molecule-1 expression modulates endothelial cell migration in vitro. Lab Invest 1998, 78:583-590 [PubMed] [Google Scholar]
- 15.Bogen SA, Baldwin HS, Watkins SC, Albelda SM, Abbas AK: Association of murine CD31 with transmigrating lymphocytes following antigenic stimulation. Am J Pathol 141:843–854 [PMC free article] [PubMed]
- 16.Varon D, Jackson DE, Shenkman B, Dardik R, Tamarin I, Savion N, Newman PJ: Platelet/endothelial cell adhesion molecule-1 serves as a costimulatory agonist receptor that modulates integrin-dependent adhesion and aggregation of human platelets. Blood 1998, 91:500-507 [PubMed] [Google Scholar]
- 17.Rainger GE, Buckley C, Simmons DL, Nash GB: Cross-talk between cell adhesion molecules regulates the migration velocity of neutrophils. Curr Biol 1977, 7:316-325 [DOI] [PubMed] [Google Scholar]
- 18.Pellegatta F, Chierchia SL, Zocchi MR: Functional association of platelet endothelial cell adhesion molecule-1 and phosphoinositide 3-kinase in human neutrophils. J Biol Chem 1998, 273:27768-27771 [DOI] [PubMed] [Google Scholar]
- 19.Sagawa K, Kimura T, Swieter M, Siraganian RP: The protein-tyrosine phosphatase Shp-2 associates with tyrosine-phosphorylated adhesion molecule PECAM-1 (CD31). J Biol Chem 1977, 272:31086-31091 [DOI] [PubMed] [Google Scholar]
- 20.Gurubhagavatula I, Amrani Y, Pratico D, Ruberg FL, Albelda SM, Panettieri RA: Engagement of human PECAM-1 (CD31) on human endothelial cells increases intracellular calcium ion concentration and stimulates prostacyclin release. J Clin Invest 1998, 101:212-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson DE, Ward CM, Wang R, Newman PJ: The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation: evidence for a mechanistic link between PECAM-1- and integrin-mediated cellular signaling. J Biol Chem 1977, 272:6986-6993 [DOI] [PubMed] [Google Scholar]
- 22.Ilan N, Mahooti S, Rimm DL, Madri JA: PECAM-1 (CD31) functions as a reservoir for and a modulator of tyrosine phosphorylated beta catenin. J Cell Sci 1999, 112:3005-3014 [DOI] [PubMed] [Google Scholar]
- 23.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la Pompa J, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW: Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol 1999, 162:3022–3030 [PubMed]
- 24.Thompson RD, Larbi KY, Harrison V, Duncan GS, Mak TW, Nourshargh S: IL-1B-induced leukocyte responses in cremasteric venules of mice deficient in PECAM-1. FASEB J 1999, 13:A670 [Google Scholar]
- 25.Kung SH, Hagstrom JN, Cass D, Tai SJ, Lin HF, Stafford DW, High KA: Human factor IX corrects the bleeding diathesis of mice with hemophilia B. Blood 1998, 91:784-790 [PubMed] [Google Scholar]
- 26.Becker PS, Nilsson SK, Li Z, Berrios VM, Dooner MS, Cooper CL, Hsieh CC, Quesenberry PJ: Adhesion receptor expression by hematopoietic cell lines and murine progenitors: modulation by cytokines and cell cycle status. Exp Hematol 1999, 27:533-541 [DOI] [PubMed] [Google Scholar]
- 27.Schimmenti LA, Yan HC, Madri JA, Albelda SM: Platelet endothelial cell adhesion molecule, PECAM-1, modulates cell migration. J Cell Physiol 1992, 153:417-428 [DOI] [PubMed] [Google Scholar]
- 28.Rosenblum WI, Murata S, Nelson GH, Werner PK, Ranken R, Harmon RC: Anti-CD31 delays platelet adhesion/aggregation at sites of endothelial injury in mouse cerebral arterioles. Am J Pathol 1994, 145:33-36 [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenblum WI, Nelson GH, Wormley B, Werner P, Wang J, Shih CC: Role of platelet-endothelial cell adhesion molecule (PECAM) in platelet adhesion/aggregation over injured but not denuded endothelium in vivo and ex vivo. Stroke 1996, 27:709-711 [DOI] [PubMed] [Google Scholar]
- 30.Rosenblum WI: Platelet adhesion and aggregation without endothelial denudation or exposure of basal lamina and/or collagen. J Vasc Res 1997, 34:409-417 [DOI] [PubMed] [Google Scholar]
- 31.Wu XW, Lian EC: Binding properties and inhibition of platelet aggregation by a monoclonal antibody to CD31 (PECAM-1). Arterioscler Thromb Vasc Biol 1997, 17:3154-3158 [DOI] [PubMed] [Google Scholar]
- 32.Hagiwara T, Nagasawa T, Nagahisa H, Takizawa M, Osada M, Abe T: Expression of adhesion molecules on cytoplasmic processes of human megakaryocytes. Exp Hematol 1996, 24:690-695 [PubMed] [Google Scholar]
- 33.Watt SM, Gschmeissner SE, Bates PA: PECAM-1: its expression and function as a cell adhesion molecule on hemopoietic and endothelial cells. Leuk Lymphoma 1995, 17:229-244 [DOI] [PubMed] [Google Scholar]
- 34.Dejana E, Quintana A, Callioni A, de Gaetano G: Bleeding time in laboratory animals. III. Do tail bleeding times in rats only measure a platelet defect? (the aspirin puzzle). Thromb Res 1979, 15:199-207 [DOI] [PubMed] [Google Scholar]
- 35.Mielke CH, Kaneshiro MM, Jr, Maher IA, Weiner JM, Rapaport SI: The standardized normal ivy bleeding time and its prolongation by aspirin. Blood 1969, 34:204-215 [PubMed] [Google Scholar]
- 36.McKenzie SB: Textbook of Hematology. 1996:p xvi Williams and Wilkins, Baltimore
- 37.Cramer EM, Berger G, Berndt MC: Platelet alpha-granule and plasma membrane share two new components: CD9 and PECAM-1. Blood 1994, 84:1722-1730 [PubMed] [Google Scholar]
- 38.Palmer DS, Aye MT, Ganz PR, Halpenny M, Hashemi S: Adenosine nucleotides and serotonin stimulate von Willebrand factor release from cultured human endothelial cells. Thromb Haemost 1994, 72:132-139 [PubMed] [Google Scholar]
- 39.Savage B, Almus-Jacobs F, Ruggeri ZM: Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell 1998, 94:657-666 [DOI] [PubMed] [Google Scholar]
- 40.Quarmby S, Kumar P, Wang J, Macro JA, Hutchinson JJ, Hunter RD, Kumar S: Irradiation induces upregulation of CD31 in human endothelial cells. Arterioscler Thromb Vasc Biol 1999, 19:588-597 [DOI] [PubMed] [Google Scholar]
- 41.Piedboeuf B, Gamache M, Frenette J, Horowitz S, Baldwin HS, Petrov P: Increased endothelial cell expression of platelet-endothelial cell adhesion molecule-1 during hyperoxic lung injury. Am J Respir 1998, 19:543-553 [DOI] [PubMed] [Google Scholar]
- 42.Bombeli T, Schwartz BR, Harlan JM: Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPIbalpha. J Exp Med 1998, 187:329-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller WA, Berman ME, Newman PJ, DeLisser HM, Albelda SM: A heterophilic adhesion mechanism for platelet/endothelial cell adhesion molecule 1 (CD31). J Exp Med 1992, 175:1401-1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prager E, Sunder-Plassmann R, Hansmann C, Koch C, Holter W, Knapp W, Stockinger H: Interaction of CD31 with a heterophilic counterreceptor involved in downregulation of human T cell responses. J Exp Med 1996, 184:41-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA: CD31/PECAM-1 is a ligand for alpha v beta 3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol 1995, 130:451-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeLisser HM, Newman PJ, Albelda SM: Platelet endothelial cell adhesion molecule (CD31). Curr Top Microbiol Immunol 113, 184:37–45 [DOI] [PubMed]
- 47.Buckley CD, Doyonnas R, Newton JP, Blystone SDE, Brown EJ, Watt SM, Simmons DL: Identification of alpha v beta 3 as a heterotypic ligand for CD31/PECAM-1. J Cell Sci 1996, 109:437-445 [DOI] [PubMed] [Google Scholar]
- 48.Rainger GE, Buckley C, Simmons DL, Nash GB: Neutrophils rolling on immobilised platelets migrate into homotypic aggregates after activation. Thromb Haemost 1998, 79:1177-1183 [PubMed] [Google Scholar]
- 49.Treutiger CJ, Heddini A, Fernandez V, Muller WA, Wahlgren M: PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat Med 1997, 3:1405-1408 [DOI] [PubMed] [Google Scholar]
- 50.Wakelin MW, Sanz MJ, Dewar A, Albelda SM, Larkin SW, Boughton-Smith N, Williams TJ, Nourshargh S: An anti-platelet-endothelial cell adhesion molecule-1 antibody inhibits leukocyte extravasation from mesenteric microvessels in vivo by blocking the passage through the basement membrane. J Exp Med 1996, 184:229-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yong KL, Watts M, Thomas NS, Sullivan A, Ings S, Linch DC: Transmigration of Cd34(+) cells across specialized and nonspecialized endothelium requires prior activation by growth factors and is mediated by PECAM-1 (CD31). Blood 1998, 91:1196-1205 [PubMed] [Google Scholar]
- 52.Zehnder JL, Shatsky M, Leung LL, Butcher EC, McGregor JL, Levitt LJ: Involvement of CD31 in lymphocyte-mediated immune responses: importance of the membrane-proximal immunoglobulin domain and identification of an inhibiting CD31 peptide. Blood 1995, 85:1282-1288 [PubMed] [Google Scholar]