Abstract
Little is known about Epstein-Barr virus (EBV) infection of colon mucosa, particularly in inflammatory bowel diseases. Crohn’s disease and ulcerative colitis are thought to differ in T-helper lymphocyte composition and cytokine secretion patterns. Some of the implicated cytokines are growth factors for EBV-infected cells. We examined colon mucosa for differences in the distribution and phenotype of EBV-infected cells. Colon tissues with Crohn’s disease (n = 31) or ulcerative colitis (n = 25) and controls (n = 60) were characterized by in situ hybridization and immunohistology for six EBV gene products as indicators of latent and replicative EBV infection. The cells were additionally phenotyped by combined detection of the EBV transcripts and B- or T-cell antigens. B lymphocytes predominated as the site of latent EBV infection in the colon and were most numerous in ulcerative colitis. In active ulcerative colitis, EBV-positive lymphocytes accumulated under and within the epithelium and displayed evidence for replicative infection. The patterns of mucosal EBV gene expression indicate local impairment of virus-specific T-cell responses in active ulcerative colitis. Detection of EBV may help to discriminate between active ulcerative colitis and other inflammatory bowel diseases. Colon mucosa is a potential site of EBV replication and may be relevant for EBV transmission.
Epstein-Barr virus (EBV) is a ubiquitous human γ-herpesvirus that infects >90% of the population worldwide and is associated with several malignancies. Primary infection occurs early in childhood and is generally asymptomatic. 1 In Western countries primary infection may be delayed to adolescence and, in some individuals, may produce the clinical picture of infectious mononucleosis. 1 After primary infection, EBV establishes an asymptomatic, life-long, latent infection of B lymphocytes with limited expression of viral genes. 1-3 This restricted expression of EBV latent genes in immunocompetent individuals is thought to facilitate the escape of EBV-infected cells from the virus-specific cell-mediated cytotoxicity of the host. In a small proportion of EBV-infected cells a switch from latent to productive infection may be induced. 1 In vivo, such cells have been observed in very small numbers in infectious mononucleosis and in lymphoid tissues in states of immunodeficiency, 2-4 but are usually not observed in tissues of immunocompetent individuals. In vitro, EBV is characterized by its ability to transform resting human B cells into permanently growing lymphoblastoid cell lines. These cell lines produce and respond to growth factors, many of which represent TH2 cytokines such as interleukins (IL)-4, -5, and -10. 5-8
Altered T-lymphocyte response patterns with predominance of TH2 cells have been reported for a variety of conditions such as graft-versus-host disease, antiparasitic, and some antiviral immune reactions. 9-11 The inflammatory infiltrates in both Crohn’s disease (CD) and ulcerative colitis (UC) contain high proportions of T lymphocytes with, however, different cytokine expression patterns. In CD, the pattern is dominated by a TH1 profile with increased production of IL-2, lymphotoxin, and interferon-γ, 12-14 whereas levels of IL-2, IL-12, and interferon-γ are not elevated even in active UC. 14,15 This and the detection of increased IL-5 and EBI3 levels indicate the presence of an TH2 profile in UC. 14-16 Moreover, serum levels of soluble CD30, a potentially EBV-induced activation marker, are increased in UC but not in CD. 17 It is thus conceivable that localized immunological disturbances with predominance of TH2 lymphocytes in UC may be conductive to expansion of EBV-infected cells and may likewise influence the local response to the EBV as mirrored by EBV gene expression.
We studied mucosal biopsy and resection specimens with the diagnoses of CD, UC, collagenous colitis, chronic nonpurulent appendicitis, and diverticulitis as well as colon tissue distant to primary colorectal adenocarcinomas for the presence of EBV-harboring cells to test whether active mucosal lesions of CD and UC differ in their content of EBV-infected cells. These cells were further characterized for the presence of gene products indicative of replicative infection and phenotyped by double- and triple-labeling techniques. Latently EBV-infected cells were detected by in situ hybridization using probes specific for the nonpolyadenylated small nuclear EBV-encoded RNA transcripts, EBER1 and EBER2, which are transcribed at high copy numbers in every known condition of EBV latency facilitating their detection even in paraffin-embedded tissues. Cells switching to productive infection were identified by detection of the BZLF1 protein or of BHLF1 transcripts, the presence of which precedes expression of all structural viral genes. 1
Materials and Methods
Tissues
Formalin-fixed, paraffin-embedded specimens from colon tissues of a total of 116 cases (Table 1) ▶ and tonsillar tissue from four patients with the clinical diagnosis of infectious mononucleosis were drawn from the files of the University Institutes of Pathology in Berlin, Hamburg, and Frankfurt am Main. Tissue blocks were from colon resection, hemicolectomy, or colectomy specimens with the exception of 21 appendectomy specimens and serial biopsies available from two cases of CD, 13 cases of UC, and eight cases of collagenous colitis. The diagnoses of CD or UC were established on the basis of clinical, radiological, and morphological criteria. A combination therapy of prednisolone and sulfasalazine had been administered to most CD and UC patients at the time of surgery or biopsy. All cases of CD displayed moderate to severe inflammatory activity with focally accentuated leukocytic infiltrates of high density, occurrence of aphthous and fissural ulcers, fistulae, segmental transmural fibrosis, and occasional formation of granulomas. UC specimens displayed moderate to severe inflammatory activity primarily restricted to the lamina propria and submucosal layers with ulcers, wide-spread depletion of goblet cells, epithelial regeneration with mild nuclear atypia, and formation of pseudopolyps. The diagnosis of infectious mononucleosis was confirmed by serology and characteristic clinical presentation.
Table 1.
Frequency of EBV-Positive Lamina Propria Cells in IBD and Controls
| Diagnosis | Specimen* | EBV+ cases/no. of cases | EBV+ cells/0.5 cm2 (no. of cases) | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | <1 | 1–5 | 5–20 | 21–50 | >50 | |||
| Ulcerative colitis | R/E | 9/12 (75%) | 3 | 1 | 1 | 1 | 4 | 2 |
| B | 6/13 (46%) | 7 | 3 | 1 | 1 | 1 | ||
| Total | 15/25 (60%) | 10 | 4 | 1 | 2 | 5 | 3 | |
| Crohn’s disease | R | 25/29 (86%) | 4 | 16 | 5 | 4 | ||
| B | 0/2 | 2 | ||||||
| Total | 25/31 (81%) | 6 | 16 | 5 | 4 | |||
| Collagenous colitis | B | 0/8 | 8 | |||||
| Chronic appendicitis | E | 3/21 (14%) | 18 | 3 | ||||
| Chronic diverticulitis | R | 5/12 (42%) | 7 | 2 | 3 | |||
| Adenocarcinoma, | ||||||||
| adjacent mucosa | R | 9/19 (47%) | 10 | 9 | ||||
| tumor stroma | R | 8/19 (42%) | 11 | 5 | 1 | 2 | ||
*Specimen type: R, resection; E, ectomy; B, biopsy specimen.
Immunohistology
Four-μm sections of paraffin-embedded tissue blocks were stained by the immunoalkaline phosphatase method using new fuchsin as chromogen. The monoclonal reagents were CS1-4, a cocktail of four antibodies specific for LMP1, antibody PE2 against EBNA2, antibody BZ.1 specific for BZLF1 (ZEBRA) protein, and the antibody L26 (CD20). CD3 antigen was detected with a polyclonal rabbit antibody. All primary antibodies as well as rabbit-anti-mouse immunoglobulin and immunoalkaline phosphatase-complex were purchased from DAKO (Glostrup, Denmark). CS1-4, PE2, BZ.1, and anti-CD3 antibodies required high pressure cooking for antigen retrieval (3 minutes in 10 mmol/L citrate buffer) to obtain staining in paraffin sections. Tonsillar tissue with infectious mononucleosis served as positive controls for detection of EBV gene products. 3
Probes
Fluorescein isothiocyanate-labeled oligonucleotides specific for BHLF1 transcripts were obtained from DAKO. EBER1- and EBER2-specific pBluescript-based vectors were used for the generation of single-stranded RNA probes. 18,19 EBER1 and EBER2 probes were used in combination to increase sensitivity. For the preparation of human immunoglobulin light chain (IgLC) RNA probes the 550-bp SstI fragment containing the IgLC type κ (IgLCκ) gene constant segment, 20 and the 600-bp BglII/BamHI fragment containing the IgLC type λ (IgLCλ) gene C2 constant segment, 21 respectively, were subcloned into pGEM1 (Promega Biotec, Madison, WI). Phages with the IgLCκ and IgLCλ genomic fragments, were the kind gift of Dr. P. Leder, Cambridge, MA. After linearization with the appropriate restriction enzymes (Gibco BRL, Karlsruhe, Germany), [35S]-labeled anti-sense or sense (control) run-off transcripts were generated using either SP6, T3, or T7 RNA polymerases and [35S]-uridine-5′-(α-thio)-triphosphate (1250 Ci/mmol, New England Nuclear, Dreieich, Germany) or, alternatively, digoxigenin-11-uridine-5′-triphosphate (Boehringer Mannheim, Mannheim, Germany) at a concentration of 0.5 mmol/L. 19 The average specific activity of radioactively labeled probes was 1.3 × 10 9 cpm/μg.
In Situ Hybridization
The hybridization with either [35S]-labeled, digoxigenin-labeled, or combinations of [35S]-labeled and digoxigenin-labeled probes on paraffin sections and autoradiography were carried out as described. 18,19 Digoxigenin-labeled probes were detected by immunohistology with a digoxigenin-specific alkaline phosphatase-conjugated antibody Fab fragment (Boehringer Mannheim). In situ hybridization with BHLF1-specific oligonucleotides was performed as recommended by the manufacturer (DAKO). For combined detection of IgLC and EBER, immunohistological detection of digoxigenin-labeled EBER probes was followed by autoradiography for up to 28 days of exposure.
Sequential Immunohistochemistry and in Situ Hybridization
Antibodies were used in freshly prepared RPMI 1640 medium (Gibco BRL), pH 7.5, containing 10 mg/ml bovine serum albumin, 1.0 mg/ml yeast tRNA, and 5000 U/ml heparin ammonium salt (Sigma, Munich, Germany) to inhibit RNase activity. To estimate the RNA loss during immunostaining procedures, adjacent tissue sections were subjected to in situ hybridization not preceded by immunostaining. The autoradiographic exposure times for slides subjected to the double-labeling procedure were adjusted accordingly. In situ hybridization using sense probes for EBER or IgLC genes alone showed only weak nonspecific background. Prolonged exposure times up to 3 months ensured that negative results were not because of premature termination of the exposure. Cells presenting with grain counts four times above background signal as defined by hybridization with sense-strand control probes were considered specifically labeled. 18,19
For triple labeling, immunohistology was performed with a peroxidase-labeled antibody L26 (CD20, EPOS; DAKO) before hybridization with a combination of digoxigenin-labeled EBER probes and 35S-labeled IgLCκ and IgLCλ probes. Slides were treated with 0.3% H2O2 for 3 minutes, rinsed, incubated with the antibody for 45 minutes, and washed. Immobilized antibody was detected with diaminobenzidine (DAB) chromogen before hybridization.
To avoid loss of hybridization signal when applying the polyclonal CD3-specific antibody, the procedure was reversed. Digoxigenin-labeled EBER probes were hybridized and detected with peroxidase-labeled antibody Fab fragments (Boehringer Mannheim) after blocking with 0.3% H2O2. Detection with DAB chromogen was followed by a 3-minute high pressure cooking procedure and routine anti-CD3 immunohistology using the immunoalkaline phosphatase technique.
Statistics
Statistical evaluation was performed using the Craddock-Flood chi-square test. 22
Results
Distribution and Prevalence of EBV-Infected Cells in Colon Mucosa
EBER-positive cells were detected in colon tissue specimens of 57 out of 116 (49%) patients. All of the EBER-positive cells displayed the morphology of lymphocytes. Labeling of epithelial cells was not noted in any instance. EBER-positive cells were absent from all of the serial biopsies of eight patients with collagenous colitis. The number of EBV-infected cells did not exceed five cells per 0.5 cm 2 in chronic appendicitis, diverticulitis, and uninvolved mucosa from adenocarcinoma resectates, but was slightly increased relative to uninvolved mucosa in the stroma of 3 out of 19 adenocarcinomas (Table 1) ▶ . In CD, EBER-positive cells were detected in 25 of 31 (81%) cases, but the average number of cells per section area was only slightly elevated compared to mucosa from appendicitis and diverticulitis cases. EBER-positive cells were randomly distributed within the lamina propria of diverticulitis, appendicitis, and CD cases. In CD, a proportion of EBER-positive cells was found around lymphoid follicles in the lamina propria (Figure 1) ▶ and deep within the bowel wall. Fifteen of 25 (60%) of the UC specimens displayed EBV-positive cells, and several of the positive cases displayed a significantly increased load of EBER-positive cells as compared to CD (P < 0.002) and controls (P < 0.001) (Figure 2) ▶ . In contrast to CD and controls, EBER-positive cells were clustered in sub- and intraepithelial location in UC cases in areas with moderate to high inflammatory activity (Figure 2) ▶ . Comparison of figures obtained with radioactive and nonradioactive detection of EBER transcripts revealed no differences in sensitivity between these methods. In general, intramucosal EBER-positive cells showed the morphology of small lymphocytes. In three UC cases with high inflammatory activity, occasional mitotic figures and slightly enlarged nuclei were noted in a small proportion of EBER-positive lymphocytes.
Figure 1.
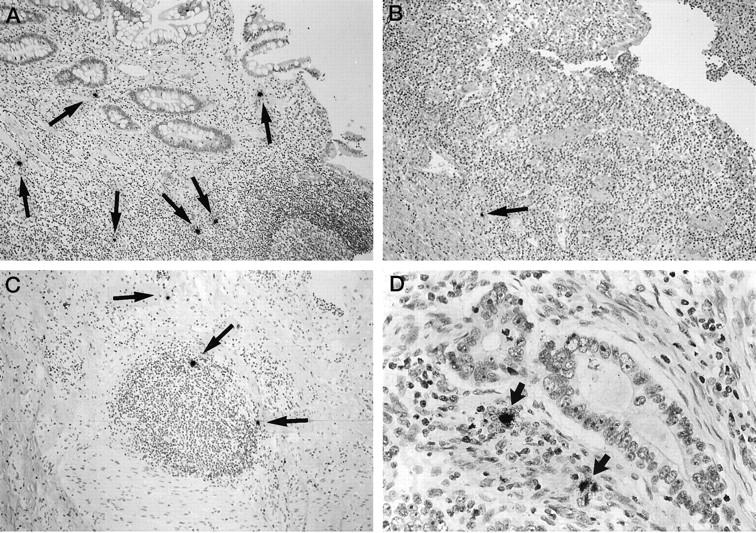
Detection of EBER-positive cells (arrows) in the lamina propria (A), in the neighborhood to a fissural ulcer (B), and in the periphery of a lymphoid follicle (C) in specimens with Crohn’s disease and in the stroma of an adenocarcinoma of the colon (D) by in situ hybridization using [35S]-labeled anti-sense RNA probes. The relative density of EBER-positive cells per section area is low. Autoradiographic exposure time, 4 days; original magnifications, ×50 (A–C) and ×75 (D).
Figure 2.
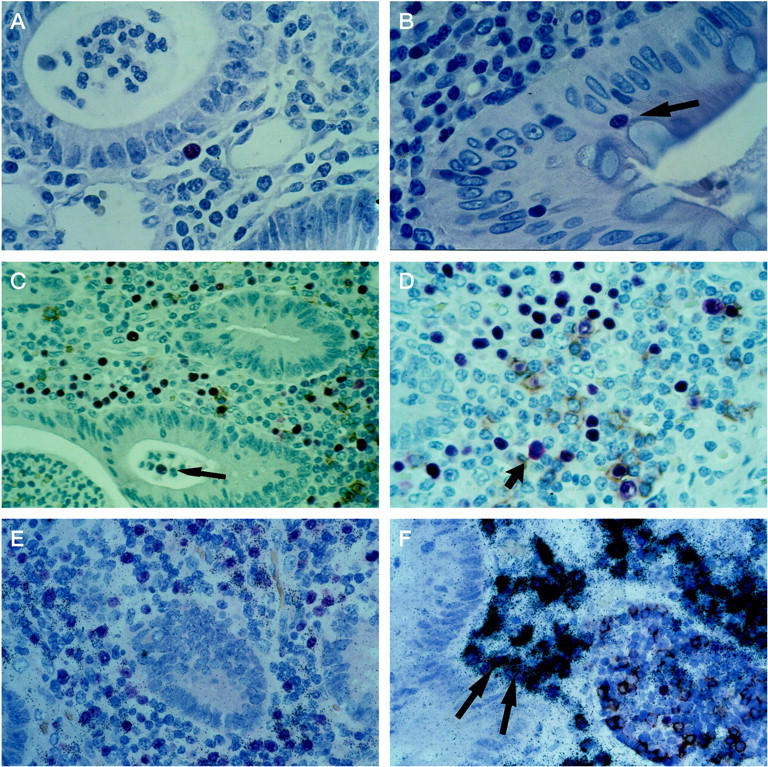
EBV gene expression and phenotype of EBV-infected cells in cases of ulcerative colitis. A small proportion of subepithelial and intraepithelial infected lymphocytes express the early lytic cycle gene products BZLF1 protein (A) and BHLF1 transcript (B) detected by immunohistology with the antibody BZ.1 or an oligonucleotide probe, respectively. The colon mucosa displays flattened epithelium with reduced numbers of goblet cells and a leukocyte infiltrate containing polymorphonuclear cells in the crypt lumen. A proportion of EBER-positive cells (red signal) display CD20 expression detectable by immunohistology (brown cytoplasmic and membrane signal) indicating the B-lymphoid nature of these infected cells (C and D). Note the subepithelial distribution of many EBER-positive lymphocytes and the presence of one infected cell (arrow) in the crypt lumen (C) as well as a mitotic figure in an EBER-positive cell (arrow; D). When detection of EBER and IgLC transcripts is combined, the majority of EBV-infected cells can be identified as B-lymphocytes with polytypic IgLC expression pattern: ∼50% of EBER-positive cells are labeled by the IgLC-κ probe (E), although a small proportion of EBER-positive cells does not display IgLC expression even after extended autoradiographic exposure time (F). Two EBER- and IgLC-positive cells are marked by arrows (F). Autoradiographic exposure time, 14 days (E) and 24 days (F). Original magnifications, ×120 (A and B), ×60 (D), ×75 (C, E, F).
Phenotype and EBV Gene Expression
LMP1- and EBNA2-expressing cells were not detectable in the colon specimens whereas these antigens were found in sections of infectious mononucleosis tonsils (data not shown). In two of four UC cases with severe inflammatory activity and an increased density of EBER-positive cells of >21 and >50 per 0.5 cm 2 section area, respectively, labeling for BZLF1 protein and BHLF1 transcripts revealed nuclear staining in a small proportion of the sub- and intraepithelial lymphocytes, indicating entry into the lytic cycle of EBV infection (Figure 2) ▶ . In contrast, all of three CD cases and both of two carcinoma cases with elevated burden of EBV-infected lymphocytes displayed no signs of replicative infection when tested in serial sections for BZLF1 and BHLF1 expression (data not shown). CD3 antigen was detected in large proportions of intramucosal lymphocytes. EBER- and CD3-specific signals were not co-localized, however. In contrast, 25 to 30% of EBER-positive cells stained for the B-cell antigen CD20. By combined in situ hybridization using radioactively-labeled IgLC probes and digoxigenated EBER probes, >90% of EBER-positive cells were identified as B cells with a slight excess of IgLC-κ- over IgLC-λ-positive cells.
Discussion
Studying the distribution and phenotype of EBV-infected cells in colon tissues, we found differences between CD, UC, and controls that may provide further insight into the biology of inflammatory bowel disease (IBD) and EBV infection. In conditions morphologically diagnosed as chronic, nonpurulent appendicitis and diverticulitis of the sigmoid, in biopsies with collagenous colitis as well as in colon mucosa adjacent to invasive adenocarcinoma, only few EBER-positive small lymphocytes were found in scattered distribution in approximately one third of the cases. A similar distribution was observed in CD specimens with, however, slightly elevated occurrence of EBV-positive cells as 81% of the CD cases displayed occasional EBER-positive cells. This seems to indicate an uncharacteristic expansion of the pool of circulating latently virus-infected cells accompanying the hyperplasia of mucosal lymphatic tissue in CD and may also reflect individual variation in absolute numbers of EBV-positive lymphocytes. The situation is reminiscent of the distribution of EBV-infected cells in hyperplastic lymph nodes and tonsils, proportions of which regularly display an increased frequency of EBER-positive cells in reactive lesions even under conditions unrelated to EBV infection such as toxoplasmosis. 18 The findings are well in line with a polymerase chain reaction study of viral genomes in CD and UC which found an increased prevalence of EBV in UC over CD in five of six and six of 10 cases, respectively, 23 as well as with a more recent study of 16 IBD cases displaying increased numbers of EBER1 positivity in nonepithelial cells in each 60% of UC and CD cases. 24
In active UC, we observed a distribution of EBV-infected cells and a pattern of EBV gene expression different from CD and controls. Although some of the biopsies did not display any EBER-positive cells, cases with moderate to high inflammatory activity contained a moderately or, in six of our 23 cases, clearly increased load of EBV-infected cells. A similar density of EBV-infected cells was previously observed in HIV-associated lymphadenopathy and infectious mononucleosis. 17 Moreover, among the cases with highest EBV content, two of four UC specimens tested for BZLF1 and BHLF1 expression provided evidence not only for latent but also for replicative EBV infection of lymphocytes. In contrast, all of the CD and carcinoma cases with increased prevalence of EBV-infected lymphocytes showed no signs of EBV replication. The relative frequency of such EBV-producing cells was similar to tonsils with infectious mononucleosis. 3,4,17 As previously observed in infectious mononucleosis, BZLF1- and BHLF1-positive cells in UC were small and occurred predominantly in sub- or intraepithelial locations. 3,4,17 In one instance, EBV-infected cells were found within the crypt lumen. At variance with infectious mononucleosis tonsils and suggesting a restricted expression of latent genes (ie, latency type I 1 ) in UC, expression of the EBV latent gene products, EBNA2 and LMP1, was not detectable in our study specimens, even in UC cases displaying EBER-positive cells in mitosis. More than 90% of the EBV-infected cells could be confidently phenotyped as B lymphocytes by virtue of their IgLC RNA expression. The pattern of IgLC expression also indicated a polyclonal composition of that cell population. A far smaller proportion of infected cells was identified by CD20 immunostaining. This discrepancy has previously been attributed to a down-regulation of CD20 in EBV-infected cells. 18,25 EBER- and CD3-positive cells, ie, EBV-infected T cells, could not be identified though the presence of occasional EBV-harboring T cells cannot be excluded. The phenotypic characteristics of mucosal EBV-infected cells also mirror closely the situation observed in lymph nodes and tonsils. 3,18
The apparently increased tolerance toward EBV antigens in those UC cases with high numbers of EBV-positive cells does not seem to represent an iatrogenic artifact, because the immunosuppressive therapy was not significantly different from the other IBD cases. The high load with EBV-infected cells indicates a microenvironment particularly favoring the polyclonal expansion of EBV-infected cells and permitting their entry into the lytic cycle of viral infection. Immediate early and early lytic-cycle antigens are known to be targets of EBV-induced cytotoxic T- cell responses. 26 The pattern of EBV infection may therefore be related to an altered reactivity to EBV, and perhaps to infection with other viruses as well.
Recent research provided evidence for altered immune responsiveness in the intestinal lamina propria of IBD patients. Specifically, an immune reaction dominated by TH1 cytokine-producing T cells was identified in CD, whereas a TH2 pattern and elevated serum levels of soluble CD30 protein were found to characterize UC. 12-16 TH2 responses are known to result in diminished antihelminthic, antiviral, and cytotoxic reactivity. 9-11 The onset of overt immunodeficiency in HIV-infected patients, eg, is associated with an overproduction of TH2 cytokines and elevated serum levels of soluble CD30 protein, 27 and may be paralleled by an expansion of EBV-infected cells. 18 Some of the TH2 cytokines are growth factors for EBV-immortalized lymphoblastoid cell lines. 5-8 EBV may contribute to this pattern of cytokine production, eg, through expression of the BNLF1 gene product, a viral IL-10 homologue. 28,29 Whereas viral IL-10 effects may be restricted to the very microenvironment of BNLF1 expressing cells, perturbation of mucosal immune functions may be because of EBV antigen or superantigen driven expansion of distinct T cell clones. 30 UC patients display an enhanced humoral immune responsiveness to a recombinant protein, p542, cross-reacting with the EBNA1 protein. 31 This observation may reflect an impaired suppression of EBV-infected B cells leading to constant EBNA1 challenge, and related to the development of the autoimmune phenomena observed in UC patients.
The impairment of cellular immunity may be related to dysplastic changes and the subsequent development of overtly invasive malignancy. Although no EBV-positive epithelial cells were identified in any of our cases, it is conceivable that EBV-associated adenocarcinomas are not restricted to the gastric mucosa where a minor proportion of the carcinomas of intestinal type may be EBV-positive. 32 Moreover, intestinal high-grade non-Hodgkin lymphomas of B-cell type in UC patients 33 may also be thought to be associated with EBV. Anecdotal evidence points to the involvement of the intestinal lymphoid tissue in infectious mononucleosis with distribution of lymphoblasts as seen in infectious mononucleosis. 34 Shedding of EBV into the saliva is considered the major route of viral transmission, and viral infection of oropharyngeal squamous epithelium has been implicated as a major site of viral replication. 35 Several of the more recent studies, however, failed to detect infection of oropharyngeal epithelium, but pointed to the involvement of intraepithelial virus-infected B-lymphoid cells. 3,36 Our observations are well in accord with that latter view and indicate an involvement of the colon mucosa in EBV infection. Because of the similarity in the distribution relative to the epithelium and in the viral lytic gene expression patterns between UC cases and infectious mononucleosis, it may be of interest to study the fecal virus content in patients with primary EBV infection. The potential importance of the lower gastrointestinal tract for EBV transmission is underlined by the fact that, in areas with poor hygienic conditions, seroconversion occurs very early in childhood rather than in adolescence as observed in Western countries. 1
In conclusion, latently and productively EBV-infected cells are detectable in many, but not all cases of IBD, and are more frequently detectable in active UC than in CD or controls. EBV is unlikely to have a role as an etiological agent in IBD. However, intramucosal expansion of EBV-infected cells may be an indicator for a local impairment of antiviral immunity. After the onset of the disease triggered by as yet unknown factors, 37 EBV infection may influence the make-up of the inflammatory infiltrate in UC and may contribute to self-perpetuation of the disease as well as to the development of autoimmune disease. The characterization of EBV-reactive T cells may therefore shed light on the pathogenesis of the immune dysfunctions associated with UC, and detection of subepithelial and intraepithelial clusters of EBV-infected cells may help to discriminate between active UC and other IBD. Moreover, colon mucosa is a potential site of EBV replication and may be relevant for EBV transmission.
Acknowledgments
We thank Mrs. U. Tank and Mrs. C. von Ostau for their excellent technical assistance and Mr. H. Koppelmeyer for phototechnical assistance.
Footnotes
Address reprint requests to Hermann Herbst, M.D., Gerhard-Domagk-Institute of Pathology, University of Muenster, Domagkstr. 19, 48129 Muenster, Germany. E-mail: herbsth@uni-muenster.de.
Supported in part by grants from Deutsche Krebshilfe—Mildred-Scheel-Stiftung and the Werner-Otto-Stiftung. In partial fulfilment of M.D. thesis requirements (of T. S.).
References
- 1.Rickinson AB, Kieff E: Epstein-Barr virus. Fields Virology, vol. 2, ed 2. Edited by BN Fields, DM Knipe, PM Howley. Philadelphia, Lippincott-Raven, 1996, pp 2397–2446
- 2.Anagnostopoulos I, Hummel M, Kreschel C, Stein H: Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood 1995, 85:744-750 [PubMed] [Google Scholar]
- 3.Niedobitek G, Agathangellou A, Herbst H, Whitehead L, Wright DH, Young LS: Epstein-Barr virus (EBV) infection in infectious mononucleosis: virus latency, replication and phenotype of EBV-infected cells. J Pathol 1997, 182:151-159 [DOI] [PubMed] [Google Scholar]
- 4.Herbst H, Foss HD, Samol J, Araujo I, Klotzbach H, Krause H, Agathanggelou A, Niedobitek G, Stein H: Frequent expression of interleukin-10 by Epstein-Barr virus-harboring tumor cells of Hodgkin’s disease. Blood 1996, 87:2918-2929 [PubMed] [Google Scholar]
- 5.Paul WE: Interleukin-4: a prototypic immunoregulatory lymphokine. Blood 1991, 77:1859-1870 [PubMed] [Google Scholar]
- 6.Mosmann TR: Properties and functions of interleukin-10. Adv Immunol 1994, 56:1-26 [PubMed] [Google Scholar]
- 7.Tosato G, Tanner J, Jones KD, Revel M, Pike SE: Identification of interleukin-6 as an autocrine growth factor for Epstein-Barr virus-immortalized B cells. J Virol 1990, 64:3033-3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumann MA, Paul CC: Interleukin-5 is an autocrine growth factor for Epstein-Barr virus transformed B lymphocytes. Blood 1992, 79:1763-1767 [PubMed] [Google Scholar]
- 9.Sher A, Gazzinelli RT, Oswald IP, Clerici M, Kullberg M, Pearce EJ, Berzofsky JA, Mosmann TR, James SL, Morse HC: Role of T cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev 1992, 127:183-204 [DOI] [PubMed] [Google Scholar]
- 10.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O’Garra A: IL-10 acts on the antigen presenting cell to inhibit cytokine production by Th1 cells. J Immunol 1991, 146:3444-3451 [PubMed] [Google Scholar]
- 11.Clerici M, Shearer GM: A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol Today 1993, 14:107-111 [DOI] [PubMed] [Google Scholar]
- 12.Desreumaux P, Brandt E, Gambiez L, Emilie D, Geboes K, Klein O, Ectors N, Cortot A, Capron M, Colombel JF: Distinct cytokine patterns in early and chronic lesions of Crohn’s disease. Gastroenterology 1997, 113:118-126 [DOI] [PubMed] [Google Scholar]
- 13.Parronchi P, Romagnani P, Annunziato F, Sampognaro S, Becchio A, Giannarini L, Maggi E, Pupilli C, Tonelli F, Romagnani S: Type-1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn’s disease. Am J Pathol 1996, 150:823-832 [PMC free article] [PubMed] [Google Scholar]
- 14.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W: Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-γ, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol 1996, 157:1261–1270 [PubMed]
- 15.Mullin GE, Lazenby AJ, Harris ML, Bayless TM, James SP: Increased interleukin-2 messenger RNA in the intestinal mucosal lesions of Crohn’s disease but not ulcerative colitis. Gastroenterology 1992, 102:1620-1627 [DOI] [PubMed] [Google Scholar]
- 16.Christ AD, Stevens AC, Koeppen H, Walsh S, Omata F, Devergne O, Birkenbach M, Blumberg RS: An interleukin 12-related cytokine is upregulated in ulcerative colitis but not in Crohn’ s disease. Gastroenterology 1998, 115:307-313 [DOI] [PubMed] [Google Scholar]
- 17.Giacomelli R, Passacantando A, Parazanese I, Vernia P, Klidara N, Cucinelli F, Lattanzio R, Santori E, Cipriani R, Tonietti G: Serum levels of soluble CD30 are increased in ulcerative colitis (UC) but not in Crohn’s disease (CD). Clin Exp Immunol 1998, 111:523-535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niedobitek G, Herbst H, Young LS, Brooks L, Masucci MG, Crocker J, Rickinson AB, Stein H: Patterns of Epstein-Barr virus infection in non-neoplastic lymphoid tissue. Blood 1992, 79:2520-2526 [PubMed] [Google Scholar]
- 19.Herbst H, Steinbrecher E, Niedobitek G, Young LS, Brooks L, Muller-Lantzsch N, Stein H: Distribution and phenotype of Epstein-Barr virus-harboring cells in Hodgkin’s disease. Blood 1992, 80:484-491 [PubMed] [Google Scholar]
- 20.Hieter PA, Max EE, Seidman JG, Maizel JV, Leder P: Cloned human and mouse kappa immunoglobulin constant and J region genes conserve homology in functional segments. Cell 1980, 22:197-207 [DOI] [PubMed] [Google Scholar]
- 21.Hieter PA, Hollis GF, Korsmeyer SJ, Waldmann TA, Leder P: Clustered arrangement of immunoglobulin lambda constant regions in man. Nature 1981, 294:536-540 [DOI] [PubMed] [Google Scholar]
- 22.Craddock JM, Flood CR: The distribution of chi-squared statistic in small contingency tables. Appl Statistics 1970, 19:173-181 [Google Scholar]
- 23.Wakefield AJ, Fox JD, Sawyerr AM, Taylor JE, Sweenie CH, Smith M, Emery VC, Hudson M, Tedder RS, Pounder RE: Detection of herpesvirus DNA in the large intestine of patients with ulcerative colitis and Crohn’s disease using the nested primer polymerase chain reaction. J Med Virol 1992, 38:183-190 [DOI] [PubMed] [Google Scholar]
- 24.Yanai H, Shimizu N, Nagasaki S, Mitani N, Okita K: Epstein-Barr virus infection of the colon with inflammatory bowel disease. Am J Gastroenterol 1999, 94:1582-1586 [DOI] [PubMed] [Google Scholar]
- 25.Deamant FD, Albujar PF, Chen YY, Weiss LM: Epstein-Barr virus in nonneoplastic lymph nodes. Mod Pathol 1993, 6:729-732 [PubMed] [Google Scholar]
- 26.Steven NM, Annels NE, Kumar A, Leese AM, Kurilla MG, Rickinson AB: Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cells response. J Exp Med 1997, 185:1605-1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Prete G, Maggi E, Pizzolo G, Romagnani S: CD30, Th2 cytokines and HIV infection: a complex and fascinating link. Immunol Today 1995, 16:76-80 [DOI] [PubMed] [Google Scholar]
- 28.Vieira P, De Waal-Malefyt R, Dang MN, Johnson KE, Kastelein R, Fiorentino DF, de Vries JE, Roncarolo MG, Mosmann TR, Moore KW: Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein Barr virus open reading frame BCRFI. Proc Natl Acad Sci USA 1991, 88:1172-1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burdin N, Peronne C, Banchereau J, Rousset F: Epstein Barr virus transformation induces B lymphocytes to produce human interleukin 10. J Exp Med 1993, 177:295-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutkowski N, Palkama T, Ciurli C, Sekaly RP, Thorley-Lawson DA, Huber BT: An Epstein-Barr virus-associated superantigen. J Exp Med 1996, 184:971-980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaughan JH, Nguyen MD, Valbracht JR, Patrick K, Rhodes GH: Epstein-Barr virus-induced autoimmune responses. II. Immunoglobulin G autoantibodies to mimicking and nonmimicking epitopes. Presence in autoimmune disease. J Clin Invest 1995, 95:1316-1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osato T, Imai S: Epstein-Barr virus and gastric carcinoma. Semin Cancer Biol 1996, 7:175-182 [DOI] [PubMed] [Google Scholar]
- 33.Shepherd NA, Hall PA, Williams GT, Codling BW, Jones EL, Levison DA, Morson BC: Primary malignant lymphoma of the large intestine complicating chronic inflammatory bowel disease. Histopathology 1989, 15:325-337 [DOI] [PubMed] [Google Scholar]
- 34.O’Brien A, O’Briain DS: Infectious mononucleosis: appendiceal lymphoid tissue involvement parallels characteristic lymph node changes. Arch Pathol Lab Med 1985, 109:680-682 [PubMed] [Google Scholar]
- 35.Sixbey JW, Nedrud JG, Raab-Traub N, Hanes RA, Pagano JS: Epstein-Barr virus replication in oropharyngeal epithelial cells. N Engl J Med 1984, 310:1225-1230 [DOI] [PubMed] [Google Scholar]
- 36.Karajannis MA, Hummel M, Anagnostopuolos I, Stein H: Strict lymphotropism of Epstein-Barr virus during acute infectious mononucleosis in nonimmunocompromised individuals. Blood 1997, 89:2856-2862 [PubMed] [Google Scholar]
- 37.Shanahan F: Pathogenesis of ulcerative colitis. Lancet 1993, 342:407-411 [DOI] [PubMed] [Google Scholar]


