Abstract
Studies in human and animal models have shown that cyclooxygenase (COX)-2 is up-regulated in several epithelial carcinomas including colon, breast, and lung. To elucidate the possible involvement of COX-2 in human bladder cancer we examined the expression of COX isoforms in benign tissue and in bladder carcinoma specimens. Paraffin embedded tissues from 75 patients with urothelial carcinomas were immunostained with specific antibodies raised against COX-1 and COX-2. COX-1 expression was detected in smooth muscle cells in both benign and malignant bladders. COX-2 immunoreactivity was absent in benign tissue and in specimens with low-grade urothelial carcinoma (0/23). In contrast, expression of COX-2 was detected in malignant epithelial cells in 38% (17/47) of specimens with high-grade urothelial carcinomas. Expression of COX-2 in high-grade bladder cancer was confirmed by radioactive in situ hybridization using a COX-2-selective riboprobe. Both immunohistochemistry and in situ hybridization showed COX-2 expression in a small subset of malignant cells. COX-2 mRNA was also expressed in three out of seven malignant urothelial cell lines. These data demonstrate elevated expression of COX-2 in a high percentage of high-grade bladder carcinomas, suggesting a possible role of COX-2 in the progression of bladder urothelial carcinoma and supporting its potential as a therapeutic target in human bladder carcinoma.
Urothelial or transitional cell carcinoma (TCC) of the bladder is the fourth most common cancer in men and the eighth most common cancer in women with an annual incidence of 51,000 in the United States alone. 1 Although non-invasive or superficially invasive papillary carcinoma is usually curable, it is prone to recurrence. 2 In contrast, high-grade carcinoma of the urinary epithelium is associated with a poor outcome. 2 Recent studies support an important role for prostaglandins in both the initiation and the progression of cancer derived from epithelial cells. 3 The metabolism of arachidonic acid by cyclooxygenases (COXs) initiates the formation of prostaglandin, converting arachidonic acid to prostaglandin H2 (PGH2). 4 Two isoforms of cyclooxygenase have been identified, both of which are inhibited by nonsteroidal anti-inflammatory drugs (NSAIDs). 5 COX-1 is thought to regulate constitutive processes such as gastric epithelial integrity and platelet aggregation, whereas COX-2 was originally discovered as an early response gene, and is primarily expressed after stimulation with growth factors and inflammatory cytokines. 5,6 COX-2 expression is markedly increased in carcinomas of the gastrointestinal tract, breast, and head and neck. 7-10 Importantly, epidemiological data show that regular NSAID ingestion reduces the risk of fatal colon cancer by 40 to 50%. 11,12 These data suggest that increased COX-2 activity may promote colon cancer. A possible role for COX-2 in human bladder carcinoma is less well defined. Recent animal studies suggest that both nonselective and COX-2-selective NSAIDs reduce the incidence of carcinogen-induced bladder cancers in rodents. 13-15 To investigate the possible involvement of COX-2 in human bladder cancer, we analyzed the expression of COX-1 and COX-2 in tissue from patients with bladder carcinoma and cell lines derived from bladder cancers.
Materials and Methods
Case Selection and Histopathology
Cases were retrieved from the surgical pathology files of the Department of Pathology, Vanderbilt University Medical Center. Seventy-five separate tissue specimens from 69 patients (24 females and 45 males) were analyzed. Cases were selected to achieve a representative mixture of tumor grades and stages and included 29 transurethral resection biopsy specimens and 42 radical cystectomy specimens. All tissues were formalin-fixed and paraffin-embedded using standard conditions. In addition to bladder cancer sections with benign urothelium and urothelial carcinomas, one lymph node with metastatic high-grade urothelial carcinoma, one cystectomy with squamous cell carcinoma, one cystectomy with an intestinal type adenocarcinoma, one renal pelvis urothelial carcinoma, and two ureter urothelial carcinomas were also examined. In addition to review of pathology reports, slides from all cases were re-examined for uniform assignment of grade and stage and other histopathological features. Tumor histological grading was performed according to both the most widely used three-tiered (Grade 1 to 3) WHO scheme for TCC, 16 and the recently recommended WHO/International Society of Urological Pathology revised two-tiered (low- and high-grade) scheme for urothelial carcinoma. 17 Tumor staging was performed according to the American Joint Commission for Cancer-Union Internationale contre le Cancer (AJCC-UICC) classification. 18 Approval by the local ethics committee was obtained.
Immunostaining
Sections were cut at 7 μm thickness, deparaffinized in xylene, and incubated for 30 minutes in methanol containing 0.3% H2O2 to block endogenous peroxidase activity. Primary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA; goat polyclonal anti-human COX-1: C-20, sc#1752 and COX-2: C-20, sc#1745 antibodies) and Cayman Chemical Company (Ann Arbor, MI; rabbit polyclonal anti-mouse COX-2 160106). All samples were studied with the Santa Cruz antibody (sc#1745) and immunoreactivity was confirmed in some sections with the Cayman antibody (160106). For detection of COX-2-immunoreactive protein, sections were microwaved for 3 minutes in phosphate buffered saline containing 0.1 mol/L sodium citrate, pH 6.0. Polyclonal anti COX-1 and anti COX-2 antibodies were diluted 1: 200 in TBST (50 mmol/L Tris, pH 7.5, 300 mmol/L NaCl, and 0.05% Tween 20) containing 1% bovine serum albumin, 5% normal horse serum, and 1% nonfat dry milk. Immunolabeling was detected using a biotinylated rabbit anti-goat antibody followed by visualization with an avidin-biotin horseradish peroxidase labeling kit (Vectastain ABC kit) and diaminobenzidine staining. The specificity of the antibodies was demonstrated by the following control experiments: (i) omission of the primary antibodies resulted in unstained sections; (ii) in normal human kidney, the COX-1 antibody but not the COX-2 antibody strongly reacted with medullary collecting duct cells, which exclusively express COX-1 protein 19 ; (iii) double immunofluorescence using the goat and rabbit polyclonal anti COX-2 antibodies revealed colocalization; (iv) pre-incubation of the goat polyclonal anti-COX2 antibody with the peptide to which it was generated, blocked immunostaining; and (v) the COX-2 ir-protein colocalized with mRNA expression by in situ hybridization.
In Situ Hybridization
In situ hybridization was performed as previously described. 20 Briefly, before hybridization, tissue sections were deparaffinized, refixed in paraformaldehyde, treated with proteinase K (20 μg/ml), washed with phosphate-buffered saline, refixed in 4% paraformaldehyde, and treated with triethanolamine plus acetic anhydride (0.25% v/v). Finally, sections were dehydrated with 100% ethanol. 35S-labeled antisense and sense riboprobes synthesized from the COX-2-specific 3′ untranslated region of the human COX-2 cDNA (471 bp) were hybridized to the section at 55°C for 18 hours. After hybridization, the sections were washed at 65°C once in 5× SSC plus 10 mmol/L β-mercaptoethanol (BME), once in 50% formamide, 2× SSC, and 100 mmol/L BME for 30 minutes. After additional washes in 10 mmol/L Tris, 5 mmol/L EDTA, 500 mmol/L sodium chloride (TEN) at 37°C, the sections were treated with RNase A (10 μg/ml) at 37°C for 30 minutes, followed by another wash in TEN at 37°C. Sections were then washed twice in 2× SSC, and twice in 0.1× SSC at 65°C. Slides were dehydrated with graded ethanol containing 300 mmol/L ammonium acetate. Slides were then dipped in photoemulsion (Ilford K5, Knutsford, Cheshire, UK) diluted 1:1 with 2% glycerol and exposed for 4 to 5 days at 4°C. After developing in Kodak D-19, slides were counterstained with hematoxylin. Photomicrographs were viewed with a Zeiss Axioskop microscope using either bright- or dark-field optics (Micro Video Instruments, Avon, MA). Pictures were captured with a digital camera (Spot-Cam, Diagnostic Instruments, Sterling Heights, MI) and color composites were generated by using Adobe Photoshop v4.0 on a Power Macintosh.
Cell Lines and RNase Protection Assay
Human bladder cancer cell lines T24, HT1376, RT4, HT1197, HUC-SV1/2, and 5637 were obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured in the recommended media. Cells were harvested at confluence, RNA isolated by a modified chloroform/methanol extraction, and RNase protection assay for COX-2 performed as described previously. 21
Statistical Analysis
Statistical analysis was performed using Fisher’s exact test. P values <0.05 were considered significant.
Results
Immunohistochemistry
The expression pattern of COX-2 immunoreactive protein in bladder carcinomas is summarized in Table 1 ▶ . Patient age ranged from 36 to 96 years; median age was 63.49 ± 12.93 years. There was no correlation between COX-2 expression and either patient gender or age. Results were similar whether tissue was obtained by transurethral bladder biopsy or cystectomy. There was a significant correlation between COX-2 immunoreactivity and tumor grade. COX-2 was detected in no grade I bladder cancers (0/12) and only 1 out of 18 samples with grade 2 cancers exhibited COX-2-immunoreactive protein in malignant epithelial cells. In contrast, 38% of samples with grade 3 tumors exhibited COX-2 immunoreactivity with COX-2 observed predominantly in malignant cells. Results were also analyzed using the recently revised two-tiered scheme for grading urothelial carcinoma. By this analysis, COX-2 immunoreactive protein was not observed in any of the 23 cases with low-grade histology. In contrast, 38% (17/45) of high-grade samples were positive for expression of COX-2 immunoreactive protein in malignant epithelial cells. In addition to the association of COX-2 expression in high-grade bladder urothelial carcinoma, 4 out of 7 TCCs with squamous differentiation expressed COX-2-immunoreactive protein. Statistical analysis confirmed a significant correlation between clinicopathological parameters and expression of COX-2. COX-2 immunoreactivity was significantly associated with either grade III (P < 0.002) or high-grade (P < 0.0003) urothelial carcinoma, respectively (Table 1) ▶ . No significant correlation was observed between COX-2 expression and the individual clinical stages TA to T4. However, when noninvasive tumors (stage TA) were compared to invasive tumors (combined stages T1-T4), a significant correlation of COX-2 expression with tumor invasion was observed (P > 0.0004).
Table 1.
Summary of Results
| Case characteristics | COX-2-neg. | COX-2-pos. | P value |
|---|---|---|---|
| Sex | |||
| Male | 34 | 6 | |
| Female | 18 | 11 | P < 0.0804 |
| Histological Grade | |||
| I | 12 | 0 | |
| II | 18 | 1 | |
| III | 27 | 17 | P < 0.0011 |
| Low grade | 23 | 0 | |
| High grade | 28 | 17 | P < 0.0003 |
| Tumor Type | |||
| TCC | 33 | 15 | |
| Adeno | 1 | 0 | |
| TCC/w/squam. | 0 | 1 | |
| TCC & squam & gland | 1 | 0 | P < 1.0 |
| Stage | |||
| TA | 24 | 0 | |
| T1 | 7 | 6 | |
| T2 | 10 | 3 | |
| T3 | 9 | 9 | |
| T4 | 7 | 0 | P < 1.0 |
| TA-Ta | 24 | 0 | |
| T1–T4 | 33 | 18 | P < 0.0004 |
TCC, transitional cell carcinoma; squam, squamous cell; gland, glandular; Adeno, adenocarcinoma.
In all cases COX-2 epithelial immunostaining was cytoplasmic and positive cells represented only a subset of the malignant epithelial cells (Figure 1A) ▶ . Staining intensity was heterogeneous among the positive cells, ranging from faint reactivity to a dark brown reaction product (Figure 1, A and C) ▶ . Typically, positive cells were observed as clusters of 10 to 30 cells. The overall percentage of COX-2-immunopositive cells within each particular tissue sample was estimated to be 5 to 30% of all cancer cells. COX-2 immunoreactivity was not detected in histologically normal bladder epithelia and was only rarely detected in the smooth muscle between the infiltrating malignant cells (not shown). In contrast, processing of an adjacent section with polyclonal COX-1 antibodies revealed that COX-1 was expressed predominantly in nonvascular smooth muscle cells and was not detected in TCC (Figure 2) ▶ .
Figure 1.
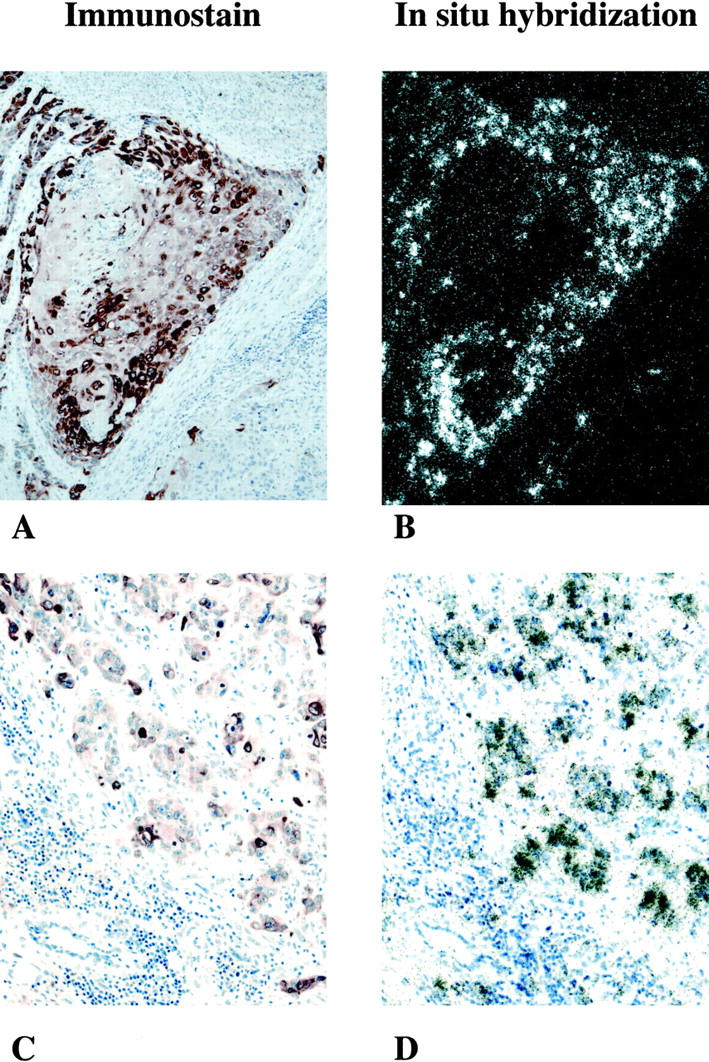
A−D: Expression of COX-2 mRNA and immunoreactive-protein in malignant transitional cells in two patients (A and B, C and D) with grade III/high-grade histology. A and B: 100× bright-field and dark-field illumination of involved human bladder. A: COX-2 immunostaining showing brown reaction product, indicating COX-2-immuoreactive protein seen predominantly over infiltrating malignant transitional cells. No significant immunoreactivity is detected in surrounding stromal tissue. B: In situ hybridization using a COX-2-selective riboprobe. Black grains depict hybridization over submucosal infiltrating malignant transitional cells. Note that distribution of the COX-2 in situ hybridization signal in B is almost superimposable over the COX-2 immunoreactivity shown in A. C and D: 200× bright-field illumination of involved human bladder cancer from a patient with grade III/high-grade TCC. C: COX-2-immunoreactive protein is seen within islands of malignant epithelial cells. Surrounding stromal cells are COX-2-negative. D: Similar to the tissue section A and B; in situ hybridization shows COX-2 mRNA in cells that are COX-2 ir-protein-positive.
Figure 2.
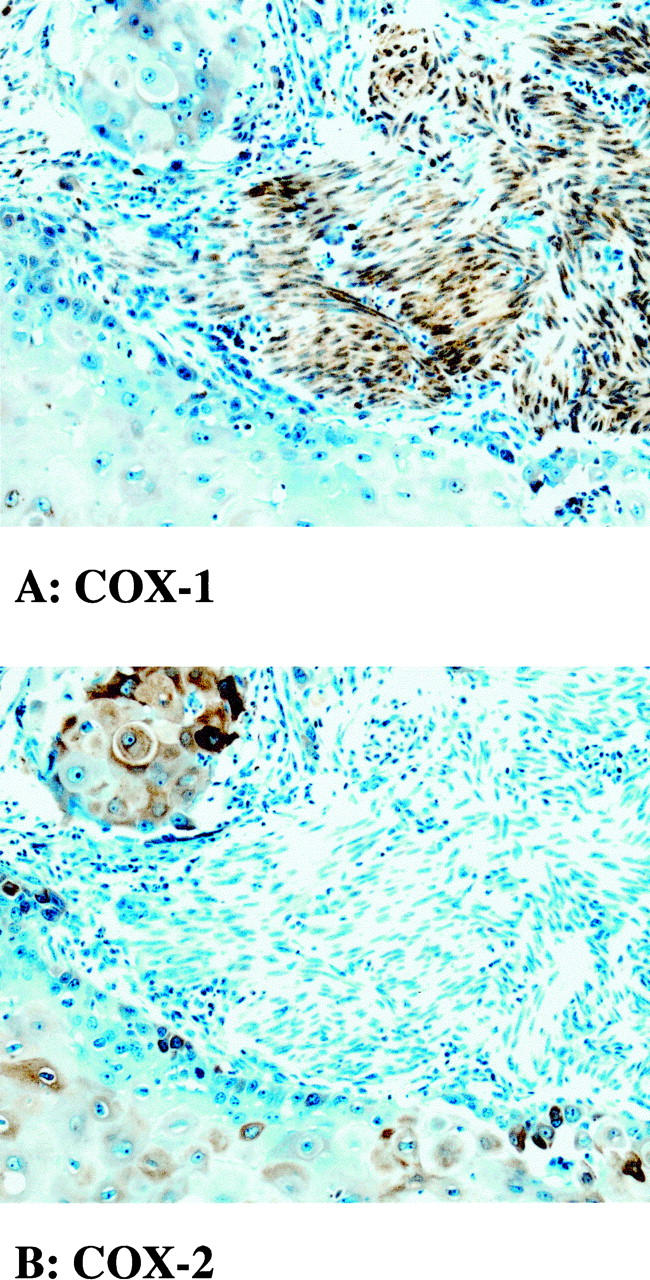
A and B: Expression of COX-1 and COX-2 in high-grade bladder cancer. Adjacent sections were processed for COX-1 (A) and COX-2 immunoreactive protein (B). COX-1 immunoreactivity is expressed predominantly in nonvascular smooth muscle cells. COX-2 immunoreactivity is expressed predominantly in infiltrating malignant transitional cells. Note that expression of COX-2-immunoreactive protein within these cells varies from undetectable to high levels.
In Situ Hybridization
Expression of COX-2 mRNA in cancers from nine patients with grade III or high-grade TCCs exhibiting positive COX-2 immunostaining was examined on serial sections using radioactive in situ hybridization for COX-2. Autoradiography of serial sections showed strong labeling of tumor using the COX-2 mRNA antisense riboprobe. No labeling was observed using the COX-2 mRNA sense riboprobe. In each case, immunohistochemistry (Figure 1A) ▶ and in situ hybridization (Figure 1B) ▶ showed a similar distribution of COX-2. As shown in Figure 1, C and D ▶ , COX-2 mRNA and immunoreactive protein were expressed within clusters of epithelial cells, whereas the surrounding stromal tissue was negative for both COX-2 mRNA and protein. In grade III/high-grade TCC, COX-2 mRNA was also detected in neighboring tissue taken from a section of histologically normal area.
Expression of COX-2 mRNA in Human Urothelial Cell Lines
Expression of COX-2 mRNA in human urothelial cell lines was examined by nuclease protection assays (Figure 3) ▶ . Constitutive expression was observed in three out of five urothelial cancer cell lines, including T24, 5637, and HT11376 cell lines. COX-2 mRNA was not detected in RT4 or HT1197 cells. Nor was COX-2 mRNA detected in SV-40 transformed human urothelial cells (HUC-SV1 and HUC-SV2 cells).
Figure 3.
RNase protection showing COX-2 mRNA expression (471-bp protected band) in three human bladder cancer cell lines: T24, 5637, and HT-1376. A protected fragment was not detected in RT-4 or HT 1197 cell lines or in two transformed human ureter epithelial cell lines (SV-HUC-1/HUC-2).
Discussion
Recent studies have demonstrated COX-2 expression is aberrantly increased in several types of epithelial cancers including stomach, colon, lung, breast, and pancreas. 7-10,22,23 The significance of COX-2 expression in these cancers has been most thoroughly studied in colon cancer, where increased COX-2 expression has been correlated with reduced patient survival. 10 Furthermore, ingestion of COX-2-inhibiting NSAIDs is associated with reduced risk of de novo colon cancer, suggesting COX-2 plays a role in promoting colon cancer. 12 The present study demonstrates that COX-2 expression is also increased in human bladder TCC and that this expression correlates with tumor grade and invasion.
Serial sections from several specimens demonstrate by in situ hybridization that COX-2 mRNA and COX-2 immunostaining colocalized. This independent confirmation of COX-2 distribution at both mRNA and protein levels is significant, because cross-reactivity of anti COX-2-antibodies with COX-1 has been reported. 24 Furthermore, immunostaining with an anti-COX-1 antibody yielded a distinctly different expression pattern (ie, in smooth muscle) also supporting the specificity of the COX-2 immunostain. The expression of COX-2 immunoreactivity in human TCC was predominantly in subpopulations of infiltrating malignant cells. The heterogeneity of cellular COX-2 expression in TCC suggests significant differences in cells comprising these tumors. Interestingly, heterogeneity of COX-2 expression was also observed in cultured TCC cell lines, with only three of seven exhibiting detectable COX-2.
The significance of COX-2 expression in human bladder cancers is uncertain. Administration of both nonselective COX-1/2-inhibiting NSAIDs and a COX-2-selective inhibitor reduced the incidence of bladder cancer in chemical carcinogenesis models in rodents. 13-15 These findings suggest that COX-2 activity may promote carcinogenesis. Studies in colon cancer also support a role for COX-2 as a tumor promoter. COX-2 knockout mice bearing both mutations in the familial adenomatous polyposis coli (APC) gene product and COX-2−/− exhibit a profound reduction in spontaneous polyp formation as compared to their COX-2+/+ littermates. 25 In vitro studies suggest COX-2 overexpression may favor the growth of malignant epithelial cells by reducing apoptosis, increasing cell adhesion, 26 and promoting angiogenesis. 27 Finally, growth of COX-2 expressing human colon cancer xenografts in nude mice is markedly reduced by a COX-2 inhibitor. 28 Whether COX-2 expression also promotes transitional cell cancer in humans remains to be clarified. The expression of COX-2 in bladder cancer cell lines documented herein suggests that these cells may provide useful tools for investigating the contribution of COX-2 to bladder cancer growth in experimental models.
During the preparation of this manuscript Mohammed et al reported the presence of COX-2 in human bladder TCCs. 29 Those studies examined 40 cases and demonstrated COX-2 immunostaining in a high percentage of invasive carcinomas. They documented COX-2 expression exclusively by immunohistochemistry and did not report a correlation with tumor stage or grade. We have now confirmed the expression of COX-2 in TCCs documenting its expression by using both in situ hybridization and immunohistochemistry.
Furthermore, the present findings suggest that COX-2 expression correlates with tumor stage and grade. We failed to detect COX-2 in any of the 23 low-grade carcinomas studied by immunostain. We also observed a significant difference in the COX-2 expression bladder TCCs without invasion of the lamina propria (TA) versus invasive cancers (T1–4). Although Mohammed et al did not observe COX-2 in normal bladders, they did detect COX-2 immunoreactivity in 7 of 9 low-grade tumors. 29 The differences between these two reports could be due in part to differences in sensitivity of immunostaining technique, given the higher percentage of COX-2-expressing tumor cells noted by those investigators. Nonetheless, the percentage of COX-2-positive cells observed in our studies was similar by both immunostaining and in situ hybridization, and by the present technique allows discrimination between low- and high-grade cancers.
The observed immunostaining of high-grade tumors including carcinoma in situ reported by Mohammed et al corresponds with our observations of increased COX-2 expression in high-grade tumors and suggests that increased COX-2 could be an early event in at least some biologically and cytologically high-grade bladder tumor processes. Conversely, the restriction of COX-2 expression to a subset of tumor cells in high-grade tumors, as well as its decreased expression in low-grade carcinomas, suggests that COX-2 up-regulation could also occur at later stages of tumor progression from low- to high-grade tumors. These considerations may have relevance to the potential for treating bladder carcinoma with COX-2-selective inhibitors. A population-based study of patients ingesting aspirin failed to reveal a reduced incidence of bladder cancer in humans, despite its beneficial effect on colon cancer. 12,30 Because aspirin is a relatively poor COX-2 inhibitor, 31 the possibility that more potent COX-2 inhibitors might provide an effective antitumor therapy for high-grade bladder cancers remains open.
In summary, we have shown that COX-2 is expressed in human TCCs and, for the first time, report that its expression correlates with tumor grade. The availability of selective COX-2 inhibitors, which have been successfully used in animal models of cancer, 3 and the poor prognosis of high-grade human urothelial carcinoma 2 warrant further investigation to determine the role of COX-2 in human urothelial carcinoma.
Acknowledgments
We thank Kim Newsom-Johansson for providing tissue sections.
Footnotes
Address reprint requests to M. D. Breyer, M.D., F-427 ACRE Bldg., Veterans Administration Medical Center, Nashville, TN 37212. E-mail: Matthew.Breyer@mcmail.vanderbilt.edu.
These studies were supported by funds from the National Institute of Diabetes and Digestive and Kidney Diseases George M. O’Brien Kidney Center (P50-DK-39261 to M. D. B). M. Kömhoff is a recipient of a research grant (KO1855/1–1) from the Deutsche Forschungsgemeinschaft.
References
- 1.Boring CC, Squires TS, Tong T, Montgomery S: Cancer statistics, 1994. CA Cancer J Clin 1994, 44:7-26 [DOI] [PubMed] [Google Scholar]
- 2.Hudson MA, Catalona WJ: Urothelial tumors of the bladder, upper tracts and prostate. Gillenwater JY Grayhack JT Howards SS Duckett JW eds. Adult and Pediatric Urology. 1996, :pp 643-694 Mosby, St. Louis MO [Google Scholar]
- 3.Subbaramaiah K, Zakim D, Weksler BB, Dannenberg AJ: Inhibition of cyclooxygenase: a novel approach to cancer prevention. Proc Soc Exp Biol Med 1997, 216:201-210 [DOI] [PubMed] [Google Scholar]
- 4.Smith W: Prostanoid biosynthesis and mechanism of action. Am J Physiol 1992, 263:F181-F191 [DOI] [PubMed] [Google Scholar]
- 5.Herschman HR: Function and regulation of prostaglandin synthase 2. Adv Exp Med Biol 469:3–8 [DOI] [PubMed]
- 6.Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR: TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem 1991, 266:12866-12872 [PubMed] [Google Scholar]
- 7.Ristimäki A, Honkanen N, Jankala H, Sipponen P, Harkonen M: Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res 1997, 57:1276-1280 [PubMed] [Google Scholar]
- 8.Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, Masferrer JL, Dannenberg AJ: Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res 1999, 59:991-994 [PubMed] [Google Scholar]
- 9.Hwang D, Scollard D, Byrne J, Levine E: Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst 1998, 90:455-460 [DOI] [PubMed] [Google Scholar]
- 10.Sheehan KM, Sheahan K, O’Donoghue DP, MacSweeney F, Conroy RM, Fitzgerald DJ, Murray FE: The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA 1999, 282:1254-1257 [DOI] [PubMed] [Google Scholar]
- 11.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ: Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993, 328:1313-1316 [DOI] [PubMed] [Google Scholar]
- 12.Thun MJ, Namboodiri MM, Heath CW Jr: Aspirin use and reduced risk of fatal colon cancer. (see comments) N Engl J Med 1991, 325:1593–1596 [DOI] [PubMed]
- 13.Holmang S, Cano M, Grenabo L, Hedelin H, Johansson SL: Effect of indomethacin on N-[4-(5-nitro-2-furyl)-2-thiazolyl]formamide-induced urinary tract carcinogenesis. Carcinogenesis 1995, 16:1493-1498 [DOI] [PubMed] [Google Scholar]
- 14.Rao K, Detrisan C, Steele V, Hawk E, Kelloff G, McCormick D: Differential activity of aspirin, ketoprofen and sulindac as cancer chemopreventive agents in the mouse urinary bladder. Carcinogenesis 1996, 17:1435-1438 [DOI] [PubMed] [Google Scholar]
- 15.Okajima E, Denda A, Ozono S, Takahama M, Akai H, Sasaki Y, Kitayama W, Wakabayashi K, Konishi Y: Chemopreventive effects of nimesulide, a selective cyclooxygenase-2 inhibitor, on the development of rat urinary bladder carcinomas initiated by N-butyl-N-(4-hydroxybutyl)nitrosamine. Cancer Res 1998, 58:3028-3031 [PubMed] [Google Scholar]
- 16.Mostofi FK, Sobin HL, Torlini H: Histologic Typing of Urinary Bladder Tumors. 1973. World Health Organization, Geneva
- 17.Epstein JI, Amin MB, Reuter VR, Mosofi FK: The World Health Organization/International Society of Urology Pathology Consensus Classification of Urothelial (Transitional Cell) Neoplasms of the Urinary Bladder. Am J Surg Pathol 1998, 22:1435-1448 [DOI] [PubMed] [Google Scholar]
- 18.International Union Against Cancer: TNM Classification of Malignant Tumors, ed 4. Geneva, Springer-Verlag, 1987
- 19.Komhoff M, Grone HJ, Klein T, Seyberth HW, Nusing RM: Localization of cyclooxygenase-1 and -2 in adult and fetal human kidney: implication for renal function. Am J Physiol 1997, 272:F460-F468 [DOI] [PubMed] [Google Scholar]
- 20.Breyer MD, Jacobson HR, Davis LS, Breyer RM: In situ hybridization and localization of mRNA for the rabbit prostaglandin EP3 receptor. Kidney Int 1993, 44:1372-1378 [DOI] [PubMed] [Google Scholar]
- 21.Guan Y, Zhang Y, Breyer RM, Davis LS, Breyer MD: Expression of peroxisome proliferator-activated receptor γ (PPARγ) in human transitional bladder cancer and its role inducing cell death. Neoplasia 1999, 1:330-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A: Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res 1998, 58:4997-5001 [PubMed] [Google Scholar]
- 23.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ, III: Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res 1999, 59:987-990 [PubMed] [Google Scholar]
- 24.McKanna JA, Zhang MZ, Wang JL, Cheng H, Harris RC: Constitutive expression of cyclooxygenase-2 in rat vas deferens. Am J Physiol 1998, 275:R227-R233 [DOI] [PubMed] [Google Scholar]
- 25.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM: Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 1996, 87:803-809 [DOI] [PubMed] [Google Scholar]
- 26.Tsujii M, DuBois RN: Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 1995, 83:493-501 [DOI] [PubMed] [Google Scholar]
- 27.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN: Cyclooxygenase regulates angiogenesis induced by colon cancer cells (published erratum appears in Cell 1998, 94: following 271). Cell 1998, 93:705-716 [DOI] [PubMed] [Google Scholar]
- 28.Sheng J, Shao J, Kirkland S, Isackson P, Coffey R, Morrow J, Beauchamp R, DuBois R: Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest 1997, 99:2254-2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammed SI, Knapp DW, Bostwick DG, Foster RS, Khan KN, Masferrer JL, Woerner BM, Snyder PW, Koki AT: Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res 1999, 59:5647-5650 [PubMed] [Google Scholar]
- 30.Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW, Jr: Aspirin use and risk of fatal cancer (see comments). Cancer Res 1993, 53:1322-1327 [PubMed] [Google Scholar]
- 31.Vane J, Bakhle Y, Botting R: Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 1998, 38:97-120 [DOI] [PubMed] [Google Scholar]



