Abstract
The exocrine pancreas synthesizes and secretes large amounts of digestive proteases as inactive precursor zymogens. Under physiological conditions a variety of cellular defense mechanisms protect the pancreatic acinar cell against a premature and intracellular activation of these zymogens. When these defenses fail, pancreatic autodigestion is initiated and acute pancreatitis can develop. A number of experimental observations suggest that extra- as well as intracellular calcium concentrations play an important part in the initiation of pancreatic protease activation, but the intracellular signaling events that regulate this process are unknown. Using a model system in which we used pancreatic acini (freshly prepared functional units of living acinar cells), we were able to simulate the conditions found during experimental pancreatitis in rodents. By means of a cell permeant fluorescent trypsin substrate we could demonstrate in these acini that premature protease activation is initiated at the apical acinar cell pole and occurs only in the presence of secretagogue concentrations that exceed those required for a maximum secretory response. By combining this technique with fluorescence ratio imaging for the Ca2+-sensitive dye fura-2, we could further show that this protease activation is highly dependent on the spatial as well as the temporal distribution of the corresponding Ca2+ release from stores within the same subcellular compartment and that it is not propagated to neighboring acinar cells.
The exocrine pancreas synthesizes and secretes more protein per cell than any other exocrine organ. Much of its protein secretion consists of digestive proenzymes, called zymogens, that require cleavage of an activation peptide by a protease. Under physiological conditions pancreatic proteases therefore remain inactive during their synthesis, intracellular transport, secretion from acinar cells, and transit through the pancreatic duct. 1 They only become activated after contact with, and cleavage by, the brushborder enzyme enterokinase (enteropeptidase) in the lumen of the small intestine. A century ago it was suggested that pancreatic autodigestion is the underlying pathophysiological mechanism of acute pancreatitis. 2 This autodigestion, however, would require a premature and intracellular activation of pancreatic proteases, and the question of where and the process by which this zymogen activation is initiated has remained the topic of extended research efforts and debate. 3 Recent investigations have suggested that elevated Ca2+ concentrations in the extracellular compartment 4 or within the pancreatic acinar cells 5,6 represent a risk factor for the development of acute pancreatitis and the premature activation of the protease precursor trypsinogen. Because the intracellular signaling events that determine the conditions under which high Ca2+ concentrations lead to intracellular protease activation are unknown, we have studied this process in isolated acini. In a model system that mimics in vivo experimental pancreatitis 7,8 we found that an elevation of overall intracellular Ca2+ levels alone (eg, by means of a ionophore) is not sufficient to induce premature trypsinogen activation in acinar cells, but that this process is initiated in a highly localized fashion and depends on the spatial and temporal distribution of the intracellular Ca2+ release in response to secretagogue.
Materials and Methods
We have previously developed a technique that allows the direct study of the intracellular activation of the serine protease precursor trypsinogen in living pancreatic acini, isolated secretory units of 10–20 exocrine cells. 9 For this method we employ a cell-permeant and cell-specific substrate that, when cleaved by activated trypsin, permits the quantification and subcellular localization of the released fluorochrome rhodamine-110 by either cytofluorometry or direct microscopic imaging. Here we have used this technique for the detection of protease activity in living cells in combination with real-time fura-2 spectrofluorometry to characterize the relationship between intracellular zymogen activation and Ca2+ signaling simultaneously.
Acini were freshly prepared from the pancreas of male Wistar rats by collagenase (Sigma, Deisenhofen, Germany) digestion 7,8 ; suspended in HEPES (24.5 mmol/L)-buffered medium (pH 7.5) containing NaCl (96 mmol/L), KCl (6 mmol/L), MgCl2 (1 mmol/L), NaH2PO4 (2.5 mmol/L), CaCl2 (0.5 mmol/L), glucose (11.5 mmol/L), Na-pyruvate (5 mmol/L), Na-glutamate (5 mmol/L), Na-fumarate (5 mmol/L), minimum essential medium (1% v/v), and bovine serum albumin, fraction V (1% w/v); and adjusted to a biovolume concentration of 2 mm3/ml. After an equilibration of 30 minutes the cholecystokinin analog cerulein (Bachem, Heidelberg, Germany) was added at either supramaximal (10 nmol/L) or maximal (0.1 nmol/L) concentrations for up to 60 minutes (as indicated in the respective figure legends) at a temperature of 37°C. Acini were then washed and resuspended in medium without secretagogue but in the presence of the synthetic trypsin substrate (CBZ-Ile-Pro-Arg)2-rhodamine-110 (10 μmol/L) (Molecular Probes, Eugene, OR). To quantify substrate cleavage, acini, together with the substrate, were transferred to 96-well microtiter plates, and the ΔF/Δt ratio was determined by cytofluorometry (CytoFluor 2350; Millipore, Bedford, MA) over 60 minutes as previously reported 9 and at 32°C. For localization experiments a high-resolution residual-light fluorescence imaging system (Till-Photonics, Martinsried, FRG; Ex 485 nm, Em 530 nm) was used, and acini were viewed continuously under the microscope in an open dish incubation chamber (ΔTC3; Bioptechs, Butler, PA) at 37°C. To increase intracellular Ca2+ concentrations in a secretagogue-independent manner we used either the Ca2+ ionophore ionomycin (10–30 μmol/L) or the Ca2+-ATPase 10 inhibitor cyclopiazonic acid (1–50 μmol/L). The latter is known to induce a rapid increase in intracellular Ca2+ concentrations, followed by a nearly complete depletion of intracellular Ca2+ stores. Other means of depleting available intracellular Ca2+ were the incubation in nominally Ca2+-free medium or the addition of the Ca2+ chelator 11 1,2-bis(2-aminophenoxy)ethane-N,N,N,N-tetraacetic acid-acetoxymethyl ester (BAPTA-AM) (10 μmol/L, 100 μmol/L). For simultaneous Ca2+ measurements and activation studies acini were loaded for 30 minutes with the acetoxymethyl ester 12 of the Ca2+-sensitive dye fura-2 (2 μmol/L) (Molecular Probes, Eugene, OR) together with the trypsin substrate. The Ca2+ signal (fura-2 ratio; Ex1 340 nm/Ex2 380 nm, Em 510 nm) and the rhodamine-110 fluorescence signal (Ex 485 nm, Em 530 nm) were recorded in identical regions of interest 5 μm in diameter. For quantitative measurements more than 400 cells per experiment were evaluated.
Results
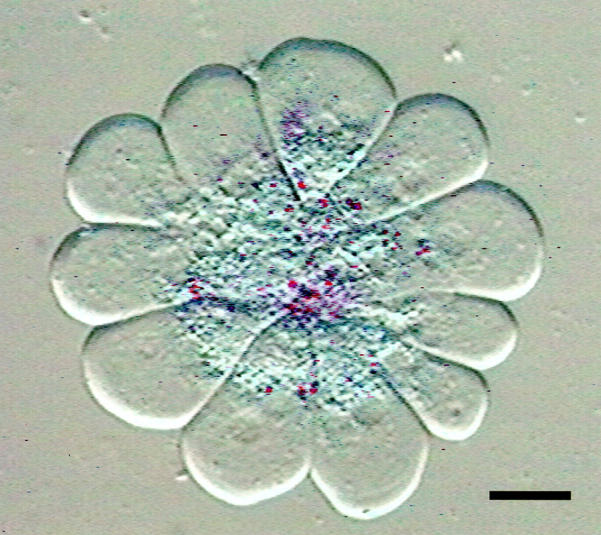
When freshly prepared acini were suspended for 5 minutes in HEPES-buffered medium in the presence of a supramaximal concentration of the secretagogue cerulein a rapid cleavage of the fluorogenic trypsin substrate (CBZ-Ile-Pro-Arg)2-rhodamine-110 resulted. This confirms earlier studies in which a conversion of inactive zymogen to an active protease in response to supramaximal secretagogue stimulation was reported 7 and demonstrates that this activation can be recorded in real time and in living cells. When this substrate cleavage was directly observed by high-resolution residual-light fluorescence microscopy it became visible as bright fluorescent foci within the apical (ie, the secretory vesicle-containing) portion in several cells of the same acinus simultaneously (Figure 1) ▶ .
Figure 1.
Digital fluorescence micrograph superimposed on the differential interference contrast (DIC) image of a living pancreatic acinus 10 minutes after stimulation with a supramaximum concentration of cerulein (10 nmol/L) and 30 minutes after subsequent addition of the substrate. At this time interval the bright focal fluorescence (pseudocolor red for better contrast) that corresponds to the site of cleavage of the trypsin substrate (CBZ-Ile-Pro-Arg)2-rhodamine-110 remains strictly confined to the secretory vesicle-containing compartment in the apical portion of the acinar cells. Note that the acinus was placed under a coverslip to allow a better optical resolution of intracellular structures. Scale bar, 10 μm.
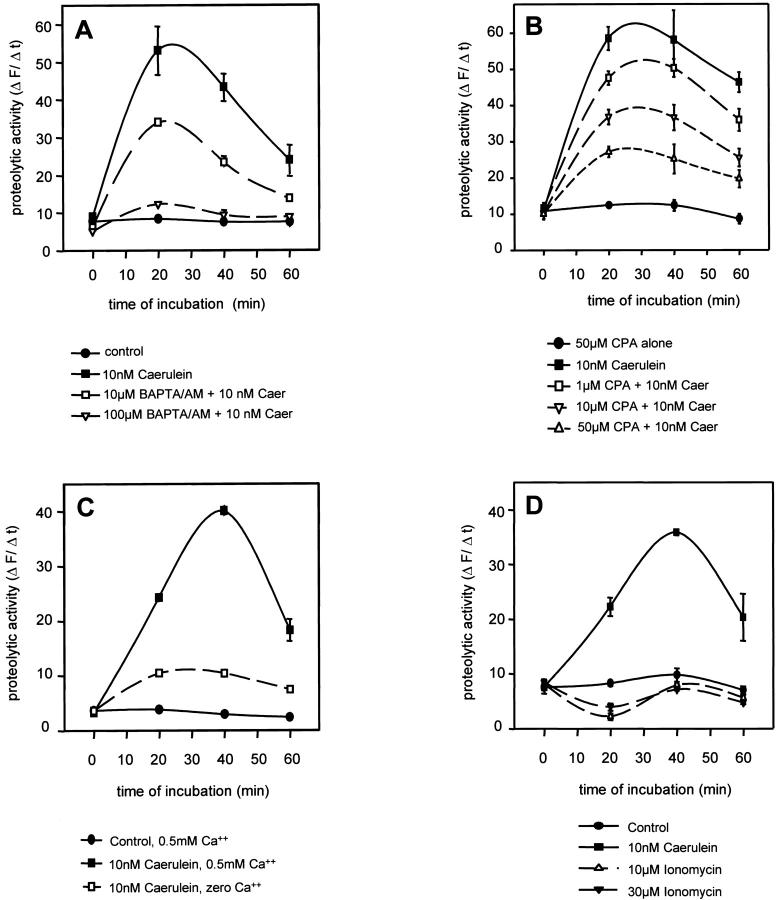
A 5-minute pretreatment of the acini with the cell-permeant Ca2+ chelator BAPTA-AM led to a concentration-dependent reduction of intracellular substrate cleavage induced by supramaximal cerulein concentrations (Figure 2A) ▶ . This observation already indicates that the presence of Ca2+ within the pancreatic acini is required for premature intracellular trypsinogen activation to occur. A 5-minute pretreatment of acini with the Ca2+-ATPase inhibitor cyclopiazonic acid, which induces a rapid overall Ca2+ release within the acinar cells and subsequently depletes the intracellular Ca2+ pool by inhibiting its reuptake into endoplasmic reticulum-associated stores, also reduced the intracellular activation of trypsinogen in a concentration-dependent manner, by up to 70% at 50 μmol/L (Figure 2B) ▶ . The addition of cyclopiazonic acid alone, which is known to induce the initial Ca2+ elevation, was not followed by premature trypsinogen activation. This result again confirms that the presence of Ca2+ is required for intracellular trypsinogen activation, but also suggests that a mere increase in acinar cell Ca2+ concentrations is not, in itself, sufficient to trigger this event. Correspondingly, a reduction of either the cerulein concentration to 0.1 nmol/L (not shown) or of the extracellular Ca2+ concentration to zero (Figure 2C) ▶ reduced or abolished the intracellular cleavage of the fluorescent substrate by active trypsin. The rapid increase of intracellular Ca2+ concentrations induced by the Ca2+ ionophore ionomycin (10 or 30 μmol/L) was not followed by a subsequent trypsinogen activation. Both concentrations, however, when given 5 minutes before the addition of cerulein, reduced secretagogue-induced zymogen activation in living pancreatic acini (not shown).
Figure 2.
Cleavage of the cell-permeant fluorogenic trypsin substrate (CBZ-Ile-Pro-Arg)2-rhodamine-110 and the corresponding release of rhodamine-110 fluorescence were quantified by cytofluorometry of living cells (Ex 485 nm, Em 530 nm) as described in Materials and Methods. A: Five-minute preincubation with the Ca2+ chelator BAPTA-AM reduced the subsequent, secretagogue-induced (10 nmol/L cerulein) activation of intracellular trypsinogen in a concentration-dependent manner. B: Incubation of acini with the Ca2+-ATPase inhibitor cyclopiazonic acid (CPA 50 μmol/L). When given alone CPA induces a rapid increase in intracellular Ca2+ but does not increase intracellular trypsin activity. When acini were preincubated with CPA for 5 minutes to ultimately deplete intracellular Ca2+ stores and supramaximum cerulein (10 nmol/L) was added thereafter, the secretagogue-induced trypsin activation decreased in a CPA-concentration-dependent manner. C: The same inhibitory effect could be obtained by incubating acini in nominally Ca2+-free medium. D: The addition of the Ca2+ ionophore ionomycin, which rapidly increases intracellular Ca2+ concentrations, had no effect, as seen with CPA, on intracellular trypsinogen activation.
Because the secretagogue and the ionophore or cyclopiazonic acid-induced elevations of intracellular Ca2+ concentrations are not known to differ in magnitude, we localized the corresponding Ca2+ signal by fura-2 fluorescence microscopy. The addition of cerulein to isolated acini induced a fluorescent signal that was initiated at the apical (ie, the secretory granule-containing) pole of acinar cells (Figure 3A) ▶ and was propagated from there to the remaining cytoplasm. This observation is in accordance with earlier studies. 13 The addition of either ionomycin or cyclopiazonic acid, on the other hand, induced a fluorescent signal that was initiated at the basolateral compartment of the acinar cells (Figure 3B) ▶ and was propagated from there to the remaining cytoplasm and to the apical cell pole. These observations could indicate that only the release of Ca2+ from localized stores in specific subcellular regions is followed by intracellular zymogen activation.
Figure 3.
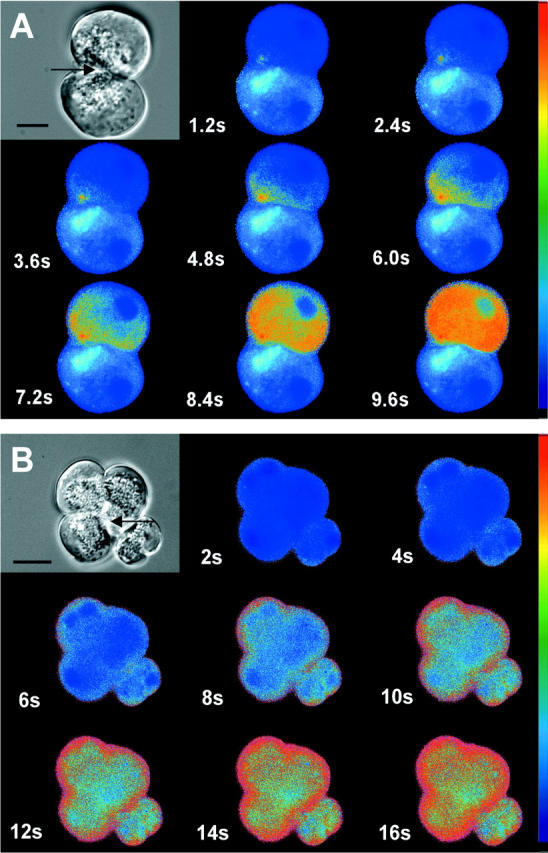
Acini were loaded with the Ca2+-sensitive dye fura-2 (2 μmol/L) for 30 minutes and exposed to either supramaximum concentrations of cerulein (10 nmol/L, A) or to the Ca2+ ionophore ionomycin (30 μmol/L, B). Intracellular Ca2+ concentrations were recorded as fura-2 fluorescence (Ex1 340 nm/Ex2 380 nm, Em 510 nm) and visualized by fluorescence microscopy. Note that under secretagogue stimulation the fluorescent signal was initiated at the apical pole of the acinar cell (arrows), whereas the ionophore treatment induced an increase in Ca2+ fluorescence that began at the basolateral aspect of the acinus. Top left panels represent transmission images of the respective acini. Scale bars, 10 μm.
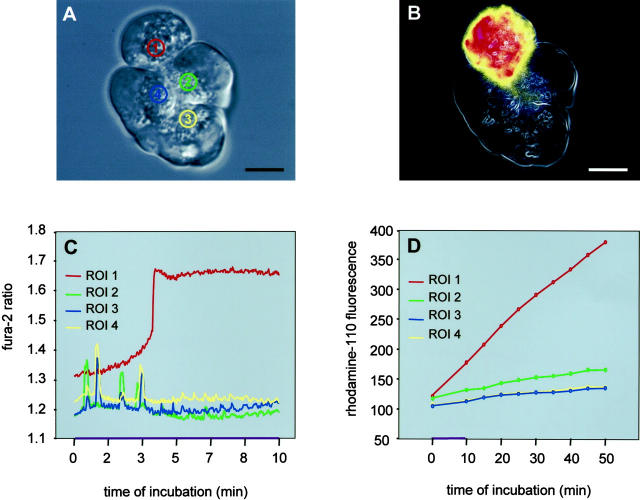
Having thus established that premature intracellular protease activation not only is highly dependent on the presence and availability of high intracellular Ca2+ concentrations but also begins at the subcellular site of the initial Ca2+ release, we selected acini composed of four to eight individual cells in which the Ca2+ signal and the trypsinogen activation signal were imaged simultaneously. After a 30-minute preloading of acini with the calcium-sensitive dye fura-2 together with the trypsin substrate, the Ca2+ signal and the rhodamine-110 fluorescence signal have been recorded in the same apical regions of interest no larger than 5 μm in diameter (Figure 4) ▶ . In the presence of supramaximal concentrations of cerulein (10 nmol/L), 69.2% of all cells studied (n = 550) responded with a single, sustained Ca2+ rise in the apical region of interest, while 20% responded with repetitive Ca2+ oscillations and 10.8% showed no response. Conversely, after exposure to physiological cerulein concentrations (0.1 nmol/L), 79.1% of all cells studied (n = 620) responded with Ca2+ oscillations, 3.2% showed a sustained Ca2+ rise and 17.7% were nonresponders. Different modes of Ca2+ response were frequently (>50%) found in individual cells of the same acinus. Interestingly, only in cells with a rapid and sustained Ca2+ rise (Figure 4C) ▶ in the narrowly confined apical region was a trypsinogen activation subsequently detected (Figure 4D) ▶ . The wave of substrate fluorescence and thus the intracellular cascade of premature protease activation characteristically progressed from the apical pole of the acinar cell (Figure 1) ▶ to the basolateral cytosol of affected cells (Figure 4B) ▶ . This wave of detectable trypsin activity was not propagated from one cell to any of its neighboring cells within the same acinus.
Figure 4.
Time course of the calcium release (C) and of the corresponding and subsequent protease activation (D) in the four regions of interest denoted in the differential interference contrast image of the acinus in A. After supramaximum cerulein stimulation (10 nmol/L) microfluorometric measurements in all four regions of interest were carried out for fura-2 fluorescence (Ex1 340 nm/Ex2 380 nm, Em 510 nm) and protease activation [(CBZ-Ile-Pro-Arg)2-rhodamine-110, 10 μM; Ex 485 nm, Em 530 nm] simultaneously. These parallel measurements indicate that only in the region of interest 1, where Ca2+ is released in a peak-plateau-like manner—and not in the regions of interest 2–4, in which Ca2+ is released in an oscillatory pattern—a subsequent trypsinogen activation can be observed. In B the same acinus is shown as a pseudocolor fluorescence image 25 minutes after exposure to supramaximum cerulein, and at this time interval the rhodamine-110 fluorescence has spread from the apical region of interest 1 to the entire cytosol of the affected cell. The neighboring cells containing the regions of interest 2–4 show no substrate cleavage. Scale bar, 10 μm.
Having thus determined that 1) both the Ca2+ release from intracellular stores as well as the zymogen activation in response to supramaximal secretagogue concentrations are initiated within an identical, narrowly confined region of the acinar cell apex, and that 2) both events are propagated within affected acinar cells but not necessarily to neighboring cells, we tried to further define the sustained Ca2+ elevation that is required for a subsequent protease activation.
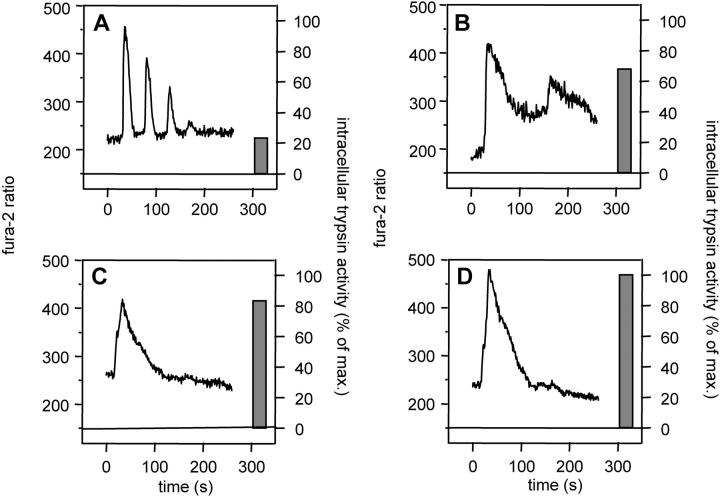
When we correlated individual Ca2+ recordings (fura-2 ratio) with the extent of substrate cleavage in identical regions of interest, a clear relationship between the patterns of Ca2+ signaling and that of protease activation emerged and could be confirmed in >75% of cells. Individual acinar cells that responded to 10 nmol/L cerulein with either a sustained Ca2+ rise (>100 seconds) or with prolonged oscillations (>150-second duration), but not cells that responded with short, repetitive Ca2+ oscillations (40–50 seconds), consistently underwent significant intracellular trypsinogen activation (examples in Figure 5 ▶ ). When the duration of the Ca2+ rise was less than 30 seconds, no activation resulted, even in the presence of large amounts of Ca2+ released (high increases in fura-2 ratio), whereas much lower Ca2+ concentrations at the apical pole were apparently sufficient to induce significant protease activation when the Ca2+ peak lasted longer than 80 seconds. Again, neither the addition of the ionophore ionomycin nor that of the Ca2+-ATPase inhibitor cyclopiazonic acid to the medium, which both resulted in an intracellular fura-2 fluorescence that was not initiated at the apical cell pole, was followed by intracellular trypsinogen activation.
Figure 5.
Representative patterns of calcium release (curves at left of panels) and the corresponding protease activation (bars at right of panels) in apical regions of interest from individual acinar cells. Note that all different Ca2+ patterns were observed in response to supramaximal concentrations of cerulein (10 nmol/L). Short repetitive Ca2+ oscillations (A) were not followed by significant trypsinogen activation, whereas prolonged oscillations (B) or a sustained Ca2+ release (C and D) was followed by extensive intracellular protease activation irrespective of the magnitude of the fura-2 ratio. Note that intracellular trypsin activity is shown as a percentage of the maximum activatable trypsin activity in living cells in response to cerulein (10 nmol/L).
Discussion
In pancreatic acinar cells large amounts of the divalent cation calcium are found in zymogen granules. One of the possible functions of Ca2+ in this subcellular compartment is the stabilization of zymogens and thus the protection against autoactivation and the autodegradation of proteolytic enzymes. A number of elegant in vitro studies have shown that the presence of Ca2+ significantly delays trypsin-induced trypsinogen activation as well as trypsin-induced trypsin degradation in pancreatic homogenates or purified enzyme preparations. 14,15 Although these mechanisms may be of clinical relevance in a situation where trypsinogen has been secreted from the pancreas but cannot flow freely from the pancreatic duct (eg, in the event of an obstructing gallstone or tumor) 16 they may not be of relevance for the signaling events inside the acinar cell. Here Ca2+ is a critical intracellular second messenger for the regulated exocytosis of digestive enzymes, and its release from intracellular stores in response to secretagogue stimulation has been reported to occur near the apical pole of acinar cells. 13 Pathological changes in cellular Ca2+ homeostasis have been found to be associated with the onset of diseases of the pancreas. One clinical example of this association are the endocrine disorders that lead to hypercalcemia, and hypercalcemia is known to be a risk factor for the development of pancreatitis. 4 Another example is the observation that patients who develop pancreatitis after extracorporal blood circulation for major cardiac surgery develop the disease because of an exposure to supraphysiological concentrations of calcium. 17 In animal experiments hypercalcemia was shown to either decrease the threshold level for the onset of pancreatitis or to induce morphological alterations equivalent to pancreatitis. 18 In studies that have investigated the initial phase of experimental pancreatitis, a progressive disruption of the intracellular Ca2+ signaling was reported, 19 and it has been proposed that an elevation of acinar cell cytosolic free ionized calcium represents the most probable common denominator for the onset of various clinical varieties of acute and chronic pancreatitis. 5 Recent studies, in which calcium chelators were found to prevent pancreatic enzyme activation, 6 appear to confirm this hypothesis.
The question, however, of which Ca2+-related signaling events promote or prevent a premature and intracellular activation of proteolytic enzymes remained unanswered. In a model system that re-creates in isolated pancreatic acini a situation that corresponds to secretagogue-induced pancreatitis in rodents, 7,8 we found that trypsinogen activation begins in a strictly confined apical region of the acinar cell and spreads from there throughout the entire cytosol of the affected cell. Surprisingly this process was not propagated to neighboring acinar cell via gap junctions in analogy to other, previously reported signaling events. 20 This confinement of the protease activation cascade to individual acinar cells can be regarded as a protective cellular device that can limit the amount of autodigestion in the pancreas once premature protease activation has begun. When we studied individual Ca2+ signals together with trypsinogen activation within the same narrowly confined (5-μm) apical compartment of acinar cells, a clear correlation between the type of Ca2+ release and the corresponding trypsin fluorescence emerged. Only in apical regions in which a prolonged (>100 seconds) Ca2+ release was recorded could a subsequent trypsinogen activation be detected. Neither a brief Ca2+ peak or oscillation (regardless of the intensity of the signal) nor a rapid or prolonged increase in intracellular Ca2+ concentrations in regions other than the apical compartment (ie, as induced by ionomycin of cyclopiazonic acid) was followed by subsequent protease activation. This indicates that premature protease activation is highly dependent on the duration of the Ca2+ signal and the localization of the initial Ca2+ release and not on the absolute concentration of Ca2+ ions in the acinar cell cytosol.
In experiments, on the other hand, in which we depleted the intracellular Ca2+ pool it was immaterial whether we used calcium ATPase inhibition, the withdrawal of extracellular Ca2+, or the complex formation with Ca2+ chelators to achieve a reduction in intracellular Ca2+ concentrations, because under all of the above conditions the intracellular protease activation in response to supramaximum hormone stimulation was greatly reduced or abolished.
These experiments show, for the first time, that a premature and intracellular activation of trypsinogen in living pancreatic acinar cells is highly dependent on the spatial and temporal distribution of the Ca2+ release from intracellular stores, that both events begin in a strictly confined apical compartment, and that pancreatic acini possess protective mechanisms that prevent the propagation of premature protease activation to neighboring cells. Increases in cytosolic Ca2+ concentrations alone do not lead to premature intracellular activation of digestive zymogens and may therefore be insufficient to induce pancreatitis.
Acknowledgments
We thank U. Naumann and S. Rackow for their expert technical assistance.
Footnotes
Address reprint requests to either Dr. Burkhard Krüger, Division of Medical Biology, Institute of Pathology, University of Rostock, Schillingallee 70, 18057 Rostock, Germany. E-mail: burkhard.krueger@med.uni-rostock.de; or Dr. Markus M. Lerch,
Supported by grants from the Deutsche Forschungsgemeinschaft and the IZKF-Münster (B. K. and M. M. L.).
Drs. Krüger and Lerch were equal contributors to this article.
References
- 1.Geokas MC, Largman C, Durie PR, Brodrick JW, Ray SB, O’Rourke M, Vollmer J: Immunoreactive forms of cationic trypsin in plasma and ascitic fluid of dogs in experimental pancreatitis. Am J Pathol 1981, 105:31-39 [PMC free article] [PubMed] [Google Scholar]
- 2.Chiari H: Über die Selbstverdauung des menschlichen Pankreas. Z Heilk 1896, 17:69-96 [Google Scholar]
- 3.Whitcomb DC: Early trypsinogen activation in acute pancreatitis. Gastroenterology 1999, 116:770-772 [PubMed] [Google Scholar]
- 4.Mithofer K, Fernandez-del Castillo C, Frick TW, Lewandrowski KB, Rattner DW, Warshaw AL: Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat. Gastroenterology 1995, 109:239–246 [DOI] [PubMed]
- 5.Ward JB, Peterson OH, Jenkins SA, Sutton R: Is an elevated concentration of acinar cytosolic free ionised calcium the trigger for acute pancreatitis? Lancet 1995, 346:1016-1019 [DOI] [PubMed] [Google Scholar]
- 6.Saluja AK, Bhagat L, Lee HS, Bhatia M, Frossard JL, Steer: Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am J Physiol 1999, 276:G835-G842 [DOI] [PubMed] [Google Scholar]
- 7.Leach SD, Modlin IM, Scheele GA, Gorelick FS: Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of cholecystokinin. J Clin Invest 1991, 87:362-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou W, Levine BA, Olson MS: Platelet-activating factor: a mediator of pancreatic inflammation during cerulein hyperstimulation. Am J Pathol 1993, 142:1504-1512 [PMC free article] [PubMed] [Google Scholar]
- 9.Krüger B, Lerch MM, Tessenow T: Direct detection of premature protease activation in living pancreatic acinar cells. Lab Invest 1998, 78:763-764 [PubMed] [Google Scholar]
- 10.Gilon P, Arredouani A, Gailly P, Gromada J, Henquin JC: Uptake and release of Ca2+ by the endoplasmic reticulum contribute to the oscillations of the cytosolic Ca2+ concentration triggered by Ca2+ influx in the electrically excitable pancreatic B-cell. J Biol Chem 1999, 274:20197-20205 [DOI] [PubMed] [Google Scholar]
- 11.Smith JB, Selak MA, Dangelmaier C, Daniel JL: Cytosolic calcium as a second messenger for collagen-induced platelet responses. Biochem J 1992, 288:925-929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grynkiewicz G, Poenie M, Tsien RY: A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985, 260:3440-3450 [PubMed] [Google Scholar]
- 13.Kasai H, Augustine GJ: Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature 1990, 348:735-738 [DOI] [PubMed] [Google Scholar]
- 14.Kassell B, Kay J: Zymogens of proteolytic enzymes. Science 1973, 180:1022-1027 [DOI] [PubMed] [Google Scholar]
- 15.Colomb E, Figarella C: Comparative studies on the mechanism of activation of the two human trypsinogens. Biochim Biophys Acta 1979, 571:343-351 [DOI] [PubMed] [Google Scholar]
- 16.Allan BJ, Tournut R, White TT: Intraductal activation of pancreatic zymogens behind a carcinoma of the pancreas. Gastroenterology 1973, 65:412-418 [PubMed] [Google Scholar]
- 17.Fernandez-del Castillo C, Harringer W, Warshaw AL, Vlahakes GJ, Koski G, Zaslavsky AM, Rattner DW: Risk factors for pancreatic cellular injury after cardiopulmonary bypass. N Engl J Med 1991, 325:382–387 [DOI] [PubMed]
- 18.Frick TW, Fernandez-del Castillo C, Bimmler D, Warshaw AL: Elevated calcium and activation of trypsinogen in rat pancreatic acini. Gut 1997, 41:339–343 [DOI] [PMC free article] [PubMed]
- 19.Ward JB, Sutton R, Jenkins SA, Petersen OH: Progressive disruption of acinar cell calcium signaling is an early feature of cerulein-induced pancreatitis in mice. Gastroenterology 1996, 111:481-491 [DOI] [PubMed] [Google Scholar]
- 20.Yule DI, Stuenkel E, Williams JA: Intercellular calcium waves in rat pancreatic acini: mechanism of transmission. Am J Physiol 1996, 271:C1285-C1294 [DOI] [PubMed] [Google Scholar]