Abstract
Matrix metalloproteinases are matrix degrading enzymes implicated in many biological processes, including development and inflammation. Gelatinase B (gelB; also known as MMP-9) is expressed in the kidney and is hypothesized to be involved in basement membrane remodeling and in preventing pathogenic accumulation of extracellular matrix in the kidney. Inhibition of gelB activity in metanephric organ culture disrupts branching morphogenesis of the ureteric bud, suggesting that gelB plays a role in kidney development in vivo. We studied kidneys of gelB-deficient mice to search for developmental, histological, molecular, ultrastructural, and functional defects. Surprisingly, no differences between gelB−/− and control kidneys were detected, and renal function was normal in gelB mutants. In addition, gelB−/− embryonic kidneys developed normally in organ culture. Gelatinase B-deficient mice were bred with Col4a3−/− mice, a model for Alport syndrome, to determine whether gelB influences the progression of glomerulonephritis. This is an important question, as it has been hypothesized that proteases are involved in damaging Alport glomerular basement membrane. However, the presence or absence of gelB did not affect the rate of progression of renal disease. Thus, gelB does not have a discernible role in the normal kidney and gelB is not involved in the progression of glomerulonephritis in a mouse model of Alport syndrome.
Matrix metalloproteinases (MMPs) are a large family of zinc-requiring enzymes that degrade extracellular matrices. They are expressed in a variety of tissue locations pertinent to development, reproduction, health, and disease. Accordingly, MMPs are thought to be involved in implantation, embryogenesis, normal matrix remodeling, wound healing, tumor metastasis, angiogenesis, inflammation, atherosclerosis, and emphysema. 1-6
Gelatinases are MMPs first identified based on their ability to cleave denatured collagen (gelatin). They include gelatinase A (gelA), also known as MMP-2 and 72-kd gelatinase, and gelatinase B (gelB), also known as MMP-9 and 92-kd gelatinase. In addition to their gelatinolytic activities, gelA and gelB possess the ability to cleave type IV collagen and entactin, which are major components of all basement membranes, as well as other extracellular matrix molecules, including elastin and proteoglycans. 6 Thus, gelA and gelB could be involved in both normal and aberrant degradation or remodeling of basement membranes in vivo.
Gelatinase B has garnered special attention for its potential involvement in kidney development and disease. Several groups have documented expression and localization of gelB in the developing, mature, and diseased kidney. 7-9 However, the expression patterns reported for gelB during kidney development are conflicting: in the mouse, gelB protein was detected in metanephric mesenchyme, 8 whereas in the rat, gelB was associated with endothelial and mesangial cells. 9 Gelatinase B is also expressed by a variety of kidney-derived cells in vitro. 10-12 Together, these studies suggest that gelB could be involved in the branching morphogenesis fundamental to renal development, in the vascularization of glomeruli, and in the aberrant accumulation or breakdown of extracellular matrix that is associated with diverse renal diseases.
The hypothesis that gelB (as well as other MMPs) could play an important role in the kidney is an attractive one. The kidney is a basement membrane-rich organ that relies on its basement membranes, especially the glomerular basement membrane (GBM), for proper function. The mature GBM, a major component of the kidney’s ultrafiltration barrier between the vasculature and the urinary space, is the product of complex developmental transitions in expression and deposition of basement membrane proteins. 13-17 These transitions are characterized by the deposition of new components and the elimination of old ones from the developing GBM, a remodeling process that could require MMPs.
Both gelB blocking antibodies and the tissue inhibitor of metalloproteinase-1 (TIMP-1), a natural inhibitor of gelB, inhibit ureteric bud branching in cultured embryonic mouse kidneys. 8 TIMP-2, a natural inhibitor of gelA, exhibits similar activity. 18 It was concluded that gelB is required for branching morphogenesis of the ureteric bud in vitro. 8 Insofar as metanephric organ culture is a model for normal renal development, 19 these results suggest a crucial role for gelB in kidney development in vivo. In support of this, ilomastat, an inhibitor of multiple MMPs, also reduces ureteric bud branching in vitro. 18
We have taken advantage of gelB-deficient mice to investigate the necessity of gelB for kidney development, structure, and function in vivo. These mice harbor a targeted null mutation in Mmp9, the gene that encodes gelB, and produce no active gelB. Gelatinase B−/− mice are viable and fertile but exhibit transient aberrant skeletal growth plate vascularization and ossification. 20 Although the long-term viability of gelB−/− mice suggests that there are likely no severe defects in kidney structure or function, we thought it important to look for both subtle and obvious kidney defects in a systematic fashion. In addition, we considered the possibility that gelB plays an important role in the progression of Alport syndrome, a hereditary disease of collagen IV that affects the GBM and leads to glomerulonephritis and renal failure. 21-25 It has been hypothesized that Alport GBM may be abnormally susceptible to endoproteolysis by proteases normally found in the kidney. 26 Because gelB is one such protease, we determined the effects of its absence on the progression of renal disease in a mouse model of Alport syndrome.
Materials and Methods
Mating and Genotyping of Mice
Production of gelB−/− and Col4a3−/− mice has been described. 20,27 For timed matings, noon on the day a vaginal plug was found was considered ∼E0.5. To produce gelB−/−; Col4a3−/− and gelB+/−; Col4a3−/− mice, gelB+/− or−/−; Col4a3+/− females were mated with gelB−/− or+/−; Col4a3−/− males, respectively. Mice were genotyped by Southern blot (for gelB) and by PCR (for Col4a3) using DNA from tail biopsies. All procedures were performed in accordance with Institutional Animal Care and Use Committee regulations.
Organ Cultures
For zymography, E12.5 kidney pairs were removed from each embryo and placed into 40 μl of medium. 28 Organs were incubated at 37°C for 48 hours. The medium was harvested and 15 μl were subjected to zymography as described. 29 DNA was prepared from remaining portions of the embryos for genotyping. For developmental analyses, E12.5 to E13.5 kidneys were cultured on a Nuclepore filter (Whatman-Nuclepore, Tewksbury, MA) for several days as previously described. 28 Organs were photographed daily to document their development.
RNA Preparation and Analyses
RNA was isolated from pooled embryonic and individual adult kidneys as described. 30 Twenty μg of wild-type E15.5 tissue RNA or 50 μg of adult tissue RNA were treated with 1.5 μg or 2.5 μg of RNase-free DNase I (Promega, Madison, WI), respectively, in a buffer containing 40 mmol/L Tris, pH 7.9, 10 mmol/L CaCl2, 6 mmol/L MgCl2, and 10 mmol/L NaCl at 37°C for 20 minutes. The RNA was extracted once with phenol/chloroform (1:1) then with chloroform, and then ethanol precipitated. Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed on 1 μg RNA with the GeneAmp RNA PCR Kit (PE Applied Biosystems, Forest City, CA). Mock reactions were done in the absence of RT. One half of each reaction was amplified with gelA or gelB primers, whereas the other half was amplified with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers. Gelatinase A primer sequences were 5′-CTTTGCAGGAGACAAGTTCTGG-3′ and 5′-TTAAGGTGGTGCAGGTATCTGG-3′ (701-bp product); gelB primer sequences were 5′-CCATGAGTCCCTGGCAG-3′ and 5′-ATGACAATGTCCGCTTCG-3′ (505-bp product); and the GAPDH primer sequences were 5′-CCATGTTTGTGATGGGTGTGAACC-3′ and 5′-TGTGAGGGAGATGCTCAGTGTTGG-3′ (712-bp product). The gelA cycling conditions were as follows: 94°C for 1 minute; five cycles of 94°C for 20 seconds, 75°C for 1 minute; five cycles of 94°C for 20 seconds, 70°C for 1 minute; and 34 cycles of 94°C for 20 seconds, 62°C for 30 seconds, 72°C for 1 minute. Gelatinase B cycling conditions were as follows: 94°C for 1 minute; five cycles of 94°C for 20 seconds, 65°C for 1 minute; five cycles of 94°C for 20 seconds, 60°C for 1 minute; and 40 cycles of 94°C for 20 seconds, 50°C for 30 seconds, 72°C for 1 minute. GAPDH cycling conditions consisted of an initial denaturation at 94°C for 1 minute, then 30 cycles of 94°C for 20 seconds and 65°C for 1 minute.
Histological Analyses
For conventional histology, tissues were fixed in 4% buffered formaldehyde, dehydrated, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) and periodic-acid Schiff reagents. For electron microscopy, tissues were fixed in 2% glutaraldehyde/2% paraformaldehyde/0.15 mol/L cacodylate buffer and processed, embedded in plastic, sectioned, and stained by standard methods in the Electron Microscopy Facility of the Department of Cell Biology and Physiology, Washington University School of Medicine. For immunofluorescence, tissues were frozen fresh and sectioned at ∼7 μm on a cryostat. Antibodies were diluted in 1% (w/v) bovine serum albumin in phosphate-buffered saline (PBS) and incubated on sections for 1 to 2 hours. After rinsing off unbound primary antibody with PBS, secondary antibodies were applied for 1 to 2 hours. Sections were rinsed again and then mounted in glycerol-para-phenylenediamine and observed with epifluorescent illumination.
Primary antibodies were as follows: rabbit anti-mouse collagen α1(IV)/α2(IV) was purchased from Collaborative Biomedical Products/Becton Dickinson Labware (Franklin Lakes, NJ). Rabbit anti-mouse collagen α4(IV) and laminin α5 have been described. 14,15 Monoclonal rat anti-mouse laminin-1 antibodies 5A2 and 8B3 31 were produced and generously provided by Dale Abrahamson, University of Kansas Medical Center, Kansas City, KS. These antibodies recognize laminin β1 and α1, respectively. 32,33 Fluorescein isothiocyanate and Cy3-labeled secondary antibodies were purchased from ICN/Cappel (Costa Mesa, CA) and Jackson ImmunoResearch Laboratories (West Grove, PA).
Physiological Analyses
To obtain urine and blood at the time of sacrifice, mice were first mildly anesthetized by inhalation of Metofane (Mallinckrodt Veterinary, Mundelein, IL) and then deeply anesthetized by an intraperitoneal injection of ketamine/xylazine. Urine was obtained directly from the bladder by aspiration and blood was obtained by cardiac puncture from the right ventricle with a heparin-treated syringe and 27-gauge needle. Serum was obtained by microcentrifugation of the blood at 7,000 rpm for 1 minute. Blood creatinine and urea nitrogen concentrations and urinary creatinine and protein concentrations were measured with a Cobas Mira Plus analyzer (Roche, Somerville, NJ).
Results
Expression of Gelatinase B in Embryonic Kidney
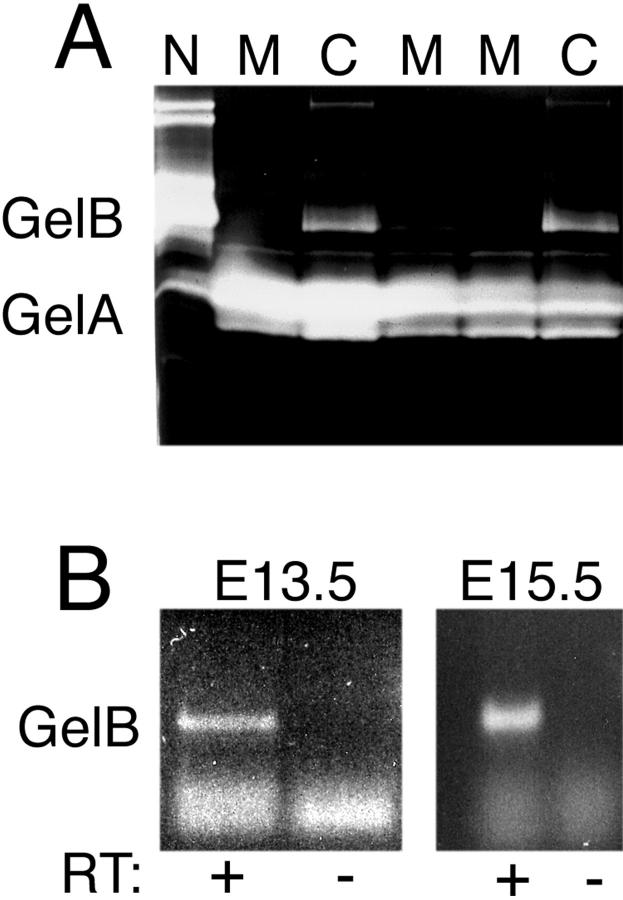
Before proceeding with a detailed study of the effects of gelB deficiency on kidney development and function, we wished to confirm that in the mouse background we were using, embryonic kidneys produced gelB. We also wanted to ensure that gelB activity was absent in kidneys from embryos that were genotypically gelB−/− (as determined by Southern analysis). To address both these issues, we arranged timed matings of gelB−/− females with gelB+/− males and isolated embryos at E12.5. Both kidneys of each embryo were removed and cultured together in 40 μl of medium for 48 hours. The medium was harvested, and a 15-μl aliquot was subjected to gelatin zymography to detect gelatinolytic activity. Neutrophil extract, which is rich in gelB proenzyme, was used as a positive control. Medium conditioned by all gelB+/− kidneys produced lysis bands corresponding to gelB (∼102 kd) and gelA (∼60 to 64 kd triplet), but medium conditioned by gelB−/− kidneys produced only the gelA triplet (Figure 1A) ▶ . The intensity of the gelA bands did not vary significantly between gelB+/− and −/− lanes, suggesting that there is no compensatory up-regulation of gelA in the absence of gelB. The identification of the triplet as gelA is based on the fact that the bands are apparent in the gelB−/− lanes and thus cannot result from gelB activity.
Figure 1.
Expression of gelA and gelB by embryonic kidney. A: Zymographic analysis of expression of gelA and gelB by gelB+/− (C) and gelB−/− (M) cultured embryonic kidneys. Mutants did not express gelB. A gelB +/+ neutrophil extract (N) was rich in gelB. B: RT-PCR assay for gelB mRNA in E13.5 and E15.5 control kidneys. Both were positive for the appropriate-sized RT-dependent product. The lowest bands represent primer background.
Although we could detect ample gelB activity in media conditioned by normal embryonic kidneys (in agreement with Lelongt et al 8 ), we could not detect activity in lysates from freshly isolated E12.5 to E15.5 kidneys (data not shown). To further assess expression of gelB, we pooled kidneys from litters of normal E13.5 and E15.5 embryos and prepared total RNA. The RNA was treated with DNase and subjected to RT-PCR with gelB-specific primers, and we detected a band of the appropriate size (505 bp) at both ages (Figure 1B) ▶ . Thus, gelB is expressed by the embryonic kidney in vivo, and, taken together with the protein data presented here and previously, 8,9 it is plausible that gelB could be involved in kidney development in vivo. This would be consistent with the notion that MMPs play roles in branching morphogenesis.
Kidney and Basement Membrane Structure in Adult Gelatinase B−/− Mice
The production of gelB−/− mice by targeted mutagenesis presented the perfect opportunity to directly assess the function of gelB in the kidney. Mice lacking gelB exhibit transient delays in ossification of long bones, 20 but they exhibit no other obvious abnormalities. Because gelB is expressed in developing kidney and has been implicated as being involved in kidney development in vitro (Figure 1 ▶ and Refs. 8 and 9 ), we looked for structural defects in adult kidneys of mice lacking gelB. The gross appearance of whole kidneys from gelB−/− mice did not differ significantly from those of their gelB+/− littermates (data not shown), and histological analysis of renal architecture using H&E-stained paraffin sections failed to reveal any differences between mutant and control kidneys (Figure 2 ▶ and data not shown). Furthermore, ultrastructural analysis of the GBM at several ages did not reveal any defects in the gelB mutants (Figure 2 ▶ and data not shown), suggesting that gelB is not involved either in forming or maintaining a proper GBM.
Figure 2.
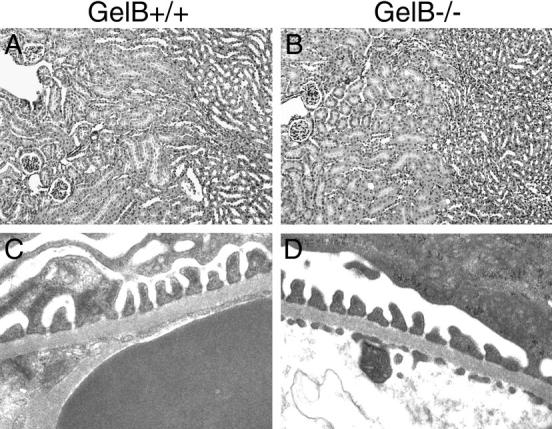
Histological analyses of gelB+/− and gelB−/− kidneys. No significant differences between gelB+/− (control) and gelB−/− (mutant) kidneys at 5 months of age were detected in H&E-stained paraffin sections (A and B) or by electron microscopy (C and D).
Because gelB is thought to be involved in matrix degradation and remodeling, we examined the distribution of several extracellular matrix proteins in the basement membranes of control and mutant adult kidneys. Basement membranes in the mature kidney are heterogeneous, especially in terms of the laminin and collagen IV isoforms that are found associated with the various functionally and anatomically distinct nephron segments. 34 For example, the laminin α1 chain is found primarily in tubular basement membranes associated with proximal tubules and loops of Henle, but it is absent from the GBM and other tubular segments. In contrast, laminin α5 is essentially ubiquitous in renal basement membranes. 15,16 Likewise, the collagen α3-α5(IV) chains are found in the GBM and in a subset of tubular basement membranes, whereas the collagen α1 and α2(IV) chains are found in all tubular basement membranes, but are absent from the GBM in mice. 14 In mice lacking gelB, we could detect no alterations from the norm in the distribution of collagen α1 and α2(IV), collagen α4(IV), laminin α1, or laminin α5 (Figure 3 ▶ and data not shown).
Figure 3.
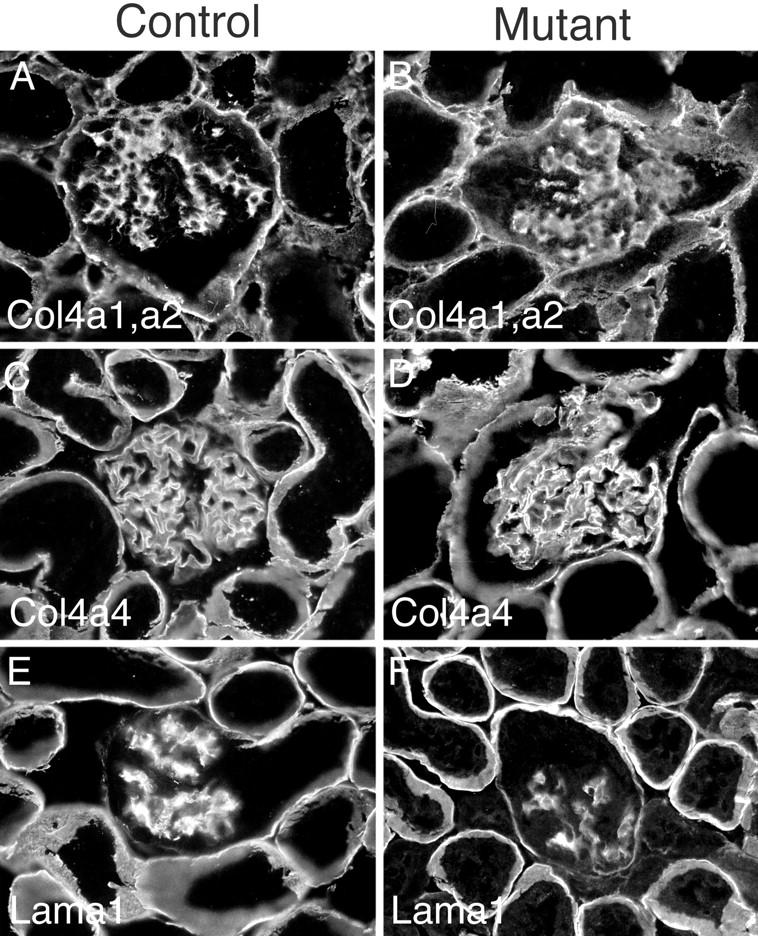
Immunohistochemical analysis of control and mutant adult kidney basement membranes. No significant differences were detected in deposition of several basement membrane components, including: collagen α1 and α2(IV) (A and B); collagen α4(IV) (C and D); laminin α1 (E and F).
Kidney Function in Gelatinase B−/− Mice
Despite the fact that we could detect no gross structural or molecular differences between gelB+/− and −/− kidneys, the possibility existed that there were functional defects in the gelB−/− kidneys. To look for defects in kidney function, we collected blood and urine from gelB+/− and −/− mice at various ages. Serum creatinine and urea nitrogen levels were not elevated in the gelB−/− mice (data not shown), indicating that there was no reduction in the glomerular filtration rate. Another possibility was that a defect existed in the perm-selectivity of the GBM. Accordingly, we tested urine from mutants for protein and found that concentrations of total protein with respect to creatinine did not vary from the normal range (data not shown). Thus, we could find no defects in renal structure or function in the absence of gelB.
Kidney Development in Gelatinase B−/− Mice
Gelatinase B blocking antibodies and TIMP-1 inhibit ureteric bud branching and kidney growth in organ culture at an early stage of kidney development. 8 We considered the possibility that the lack of gelB in our mutant mice might cause a similar early defect, but that a compensatory mechanism rescues the defect at a later stage, thus resulting in an essentially normal adult kidney. (Indeed, the ossification defect described for these mice is only apparent during a restricted developmental period. 20 ) To investigate this possibility, we examined the histological structure of gelB−/− kidneys at E13.5, only 2 days after the onset of kidney development. We observed no differences between gelB−/− and control littermate kidneys by H&E staining of paraffin sections. Both exhibited branched ureters and many developing nephrons at various stages of maturity and the kidneys were similar in size (Figure 4, A and B) ▶ . Thus, in vivo there are no histological defects in gelB−/− kidneys at an early stage of development.
Figure 4.
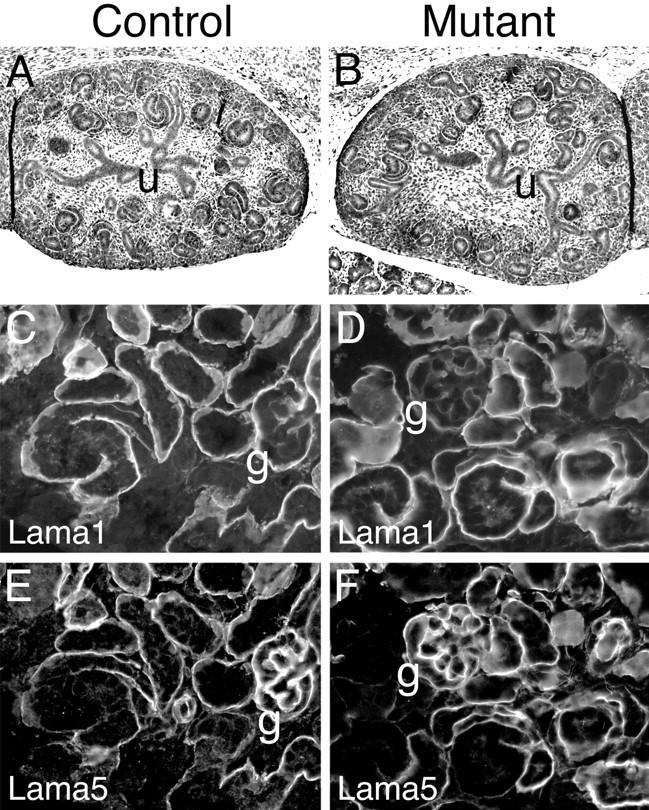
Analysis of development in control and mutant kidneys. A and B: H&E stained paraffin sections of E13.5 kidneys. Robust ureteric bud (u) branching was apparent in control and mutant kidneys, and many primitive nephron structures were present. C–F: Immunohistochemical analysis of postnatal day 2 kidney basement membranes. Both control and mutant kidneys were undergoing appropriate GBM and other developmental transitions in deposition of laminin α1 (C and D) and laminin α5 (E and F). For example, note the low levels of α1 and high levels of α5 in the maturing GBM (g).
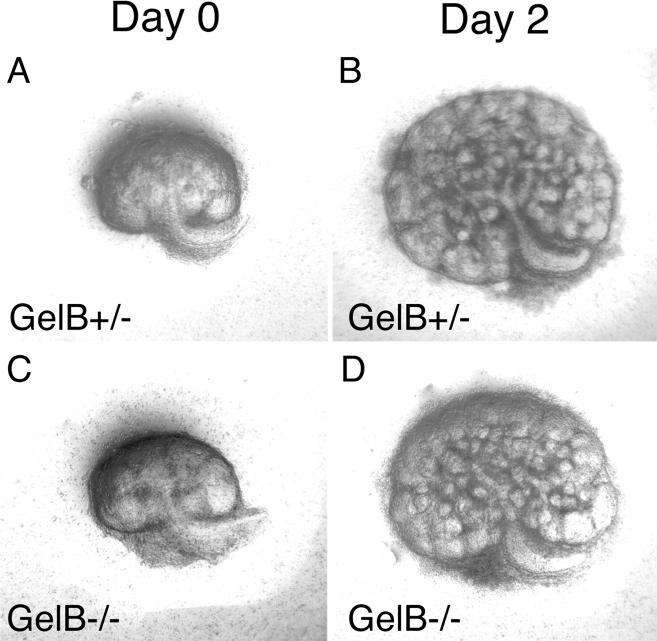
We next considered the possibility that in vivo there are constitutive mechanisms that compensate for the absence of gelB that do not operate in vitro. This would explain why blocking gelB activity in vitro inhibits metanephric growth and development, but eliminating the protein by mutation in vivo had no apparent effect. To address this, we cultured both gelB−/− and control E12.5 and E13.5 kidneys in vitro for several days. We observed no significant differences between the control and mutant metanephroi in either extent of ureteric bud branching or overall growth (Figure 5, A–D) ▶ .
Figure 5.
Kidney development in organ culture. GelB+/− and −/− metanephroi were removed at E12.5, cultured on a filter for several days, and photographed daily. Representative photos after 0 and 2 days in culture are shown. No differences in the extent of growth or branching were observed.
During nephrogenesis, there are complex developmental transitions characterized by temporally regulated deposition and elimination of several basement membrane components. 17 Importantly, many of these transitions occur in the nascent GBM, and gelB has been shown to be expressed by cells adjacent to this structure. 9 To determine whether the absence of gelB affected these transitions in developing nephrons, we immunostained sections of kidneys from postnatal day 2 control and mutant mice. Because the stepwise induction of new nephrons in the mouse continues until ∼3 weeks after birth, a section of perinatal kidney contains nephrons at all stages of development. We found no alterations in the developmental transitions that occur in renal basement membranes with respect to the laminin α1, α5, and β1 chains and the collagen IV α1 and α2 chains. For example, in both control and mutant kidneys, laminin α1 was present in the developing GBM in the S-shaped structure but was replaced by α5 at the capillary loop stage and in maturing GBM (Figure 4, C–F) ▶ . Similarly, the laminin β1 and collagen α1 and α2(IV) chains were present in the nascent GBM but were slowly eliminated from the GBM beginning at the capillary loop stage; in addition, they were deposited in the glomerular mesangium and in all tubular basement membranes (data not shown).
Gelatinase B and the Progression of Renal Disease
Considering the results of Lelongt et al, 8 we were surprised to find that gelB was not essential for proper kidney development, structure, or function. Given that these gelB−/− mice are resistant to experimentally-induced bullous pemphigoid, 35 a skin blistering disease which involves cleavage of a component of the dermal-epidermal junction extracellular matrix, we next considered the possibility that gelB could be involved in pathological disorders of the kidney. Such disorders are, in many cases, caused by abnormalities in the deposition or turnover of extracellular matrix molecules or by intrinsic abnormalities in matrix proteins. For example: 1) diabetic nephropathy is characterized by a thickened GBM and an expansion of the mesangial matrix thought to result from both increased deposition of matrix proteins and decreased turnover 36,37 ; and 2) the hereditary nephritis known as Alport syndrome is caused by mutations in the α3, α4, or α5 type IV collagen chain genes and results in thinning, thickening, and splitting of the GBM. 38
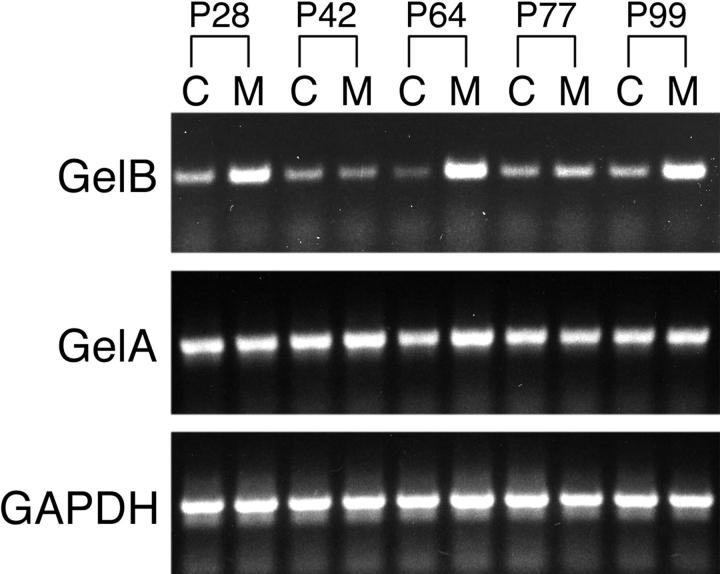
A recent study suggests that Alport GBM is more susceptible than normal GBM to degradation by proteases either resident in the kidney or conveyed by the circulation. 26 To ascertain whether gelB may play a role in the progression of Alport syndrome, we studied a knockout mouse line bearing a targeted collagen α3(IV) chain gene (Col4a3) mutation which serves as a model for autosomal recessive Alport syndrome. 27 First, we used semiquantitative RT-PCR to determine the relative levels of gelB mRNA in control and Col4a3−/− kidneys at several ages: 28, 42, 64, 77, and 99 days of age. We also assayed the levels of gelA, which exhibits proteolytic activities similar to gelB, and GAPDH, a housekeeping gene, for comparison. We found an increase in gelB levels in Col4a3−/− kidneys at four of the five ages tested, whereas gelA and GAPDH levels were similar for all samples (Figure 6) ▶ . Thus, increased levels of a specific metalloproteinase mRNA were detected in kidneys undergoing the deterioration associated with Alport syndrome.
Figure 6.
RT-PCR analysis of gelB, gelA, and GAPDH expression in Col4a3+/− and Col4a3−/− kidneys at various postnatal (P) ages. Higher steady-state levels of gelB mRNA in Col4a3−/− kidneys (M) compared to Col4a3+/− kidneys (C) was apparent at all ages except P42. Levels of gelA mRNA were not obviously higher at most ages in mutant kidneys.
To investigate whether gelB is involved in the deterioration of Col4a3−/− GBM, we generated mice that were both gelB−/− and Col4a3−/− by breeding the two lines together. As controls, we used mice of genotype gelB+/−; Col4a3−/−. Mice at a range of ages were deeply anesthetized, and blood and urine were collected. We used renal function tests as an indirect indicator of the degree of damage to the GBM (Figure 7) ▶ . We found that the gelB genotype did not correlate with the progression of renal disease in Col4a3−/− mice. Although the progression of renal disease did vary considerably among the mice, most likely because of the mixed genetic background, statistical analysis supported the conclusion that there is no statistical difference between the two groups (P > 0.1 by multivariate regression analysis). In addition, some kidneys were processed for histological examination. Ultrastructural analyses of kidneys from age-matched littermates did not reveal differential GBM damage that correlated with their gelB genotypes (Figure 8) ▶ . Thus, formation of the characteristic Alport GBM lesion does not require gelB. Taken together, these results demonstrate that gelB does not play a significant role in the progression of glomerulonephritis in the Col4a3−/− mice and, by analogy, suggests that gelB is unlikely to play a significant role in the progression of Alport syndrome in humans.
Figure 7.
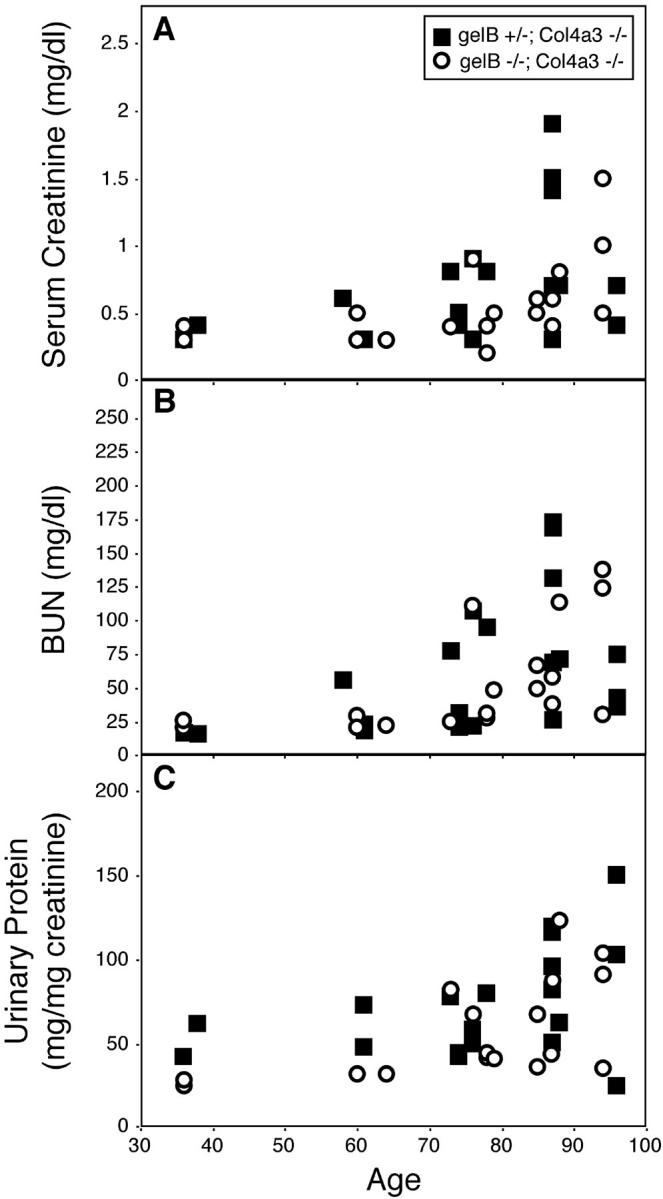
Physiological analysis of renal function at various ages in gelB+/−; Col4a3−/− and gelB−/−; Col4a3−/− mice. Serum creatinine (A), blood urea nitrogen (B) and urinary protein/creatinine (C) concentrations were measured at the time of sacrifice and plotted versus age. There was no significant effect of the gelB mutation on the progression of renal disease (P > 0.1). Symbols are: (▪) gelB+/−, Col4a3−/− mice and (○) gelB−/−, Col4a3−/− mice.
Figure 8.
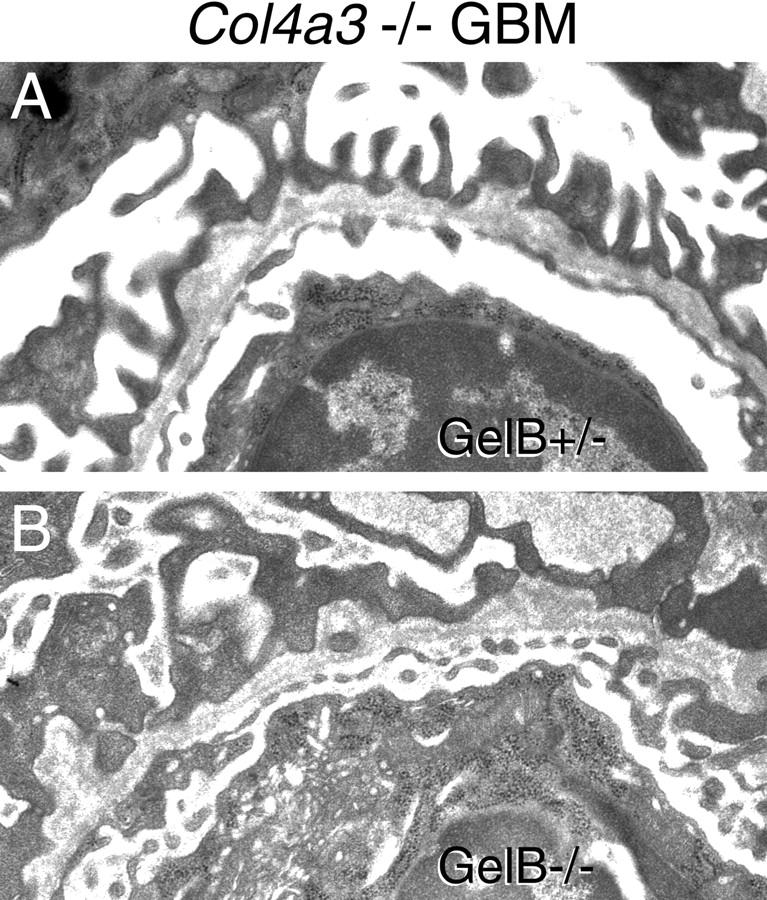
Ultrastructural analysis of 8-week-old GBM from gelB+/−; Col4a3−/− (A) and gelB−/−; Col4a3−/− (B) kidney. Both show the characteristic splitting and thickening seen in Alport syndrome.
Discussion
MMPs have been hypothesized to play roles in tissue morphogenesis, angiogenesis, implantation, metastasis, wound healing, and in the abnormal accumulation or degradation of extracellular matrices in diverse disease states. 6 In some cases, specific MMPs have been implicated in distinct processes by the results of in vitro studies. However, with some exceptions, little in vivo evidence that supports these hypotheses has been obtained.
Here, we used mice with a targeted mutation in the gelB gene (Mmp9) to explore roles for gelB in the developing kidney in vivo that had been hypothesized based on in vitro studies. Kidney development involves branching morphogenesis, mesenchyme to epithelium transitions, and complex developmental switches in the deposition of extracellular matrix molecules. 13-16,19,39 Thus, the hypothesis that gelB (or other MMPs) might be involved in any or all of these processes is an attractive one. Previous studies in mice showed that gelB was expressed in embryonic kidney mesenchyme at a very early stage and that inhibiting its activity in metanephric organ cultures, either with function-blocking antibodies to gelB or with exogenously added TIMP-1 or TIMP-2, specifically inhibited branching of the ureteric bud, 8,18 a process required for proper kidney morphogenesis. Although we confirmed expression of gelB in normal embryonic kidneys at both the RNA and protein levels (Figure 1) ▶ , we found no developmental, structural, or functional defects in the kidneys of mice lacking gelB at any age (Figures 2 to 4) ▶ ▶ ▶ . A developmental study in the rat did not detect gelB in uninduced mesenchyme, but instead showed that gelB RNA and protein were associated with endothelial and mesangial cells in developing glomeruli. 9 In contrast, gelA was associated with the epithelial components of both the developing nephron and the ureteric bud. Taken together, these results do not support a role for gelB in branching morphogenesis. In addition, we have found that gelB is expressed in normal embryonic lung, but no lung defects were observed in gelB−/− mice. 47
What might be the origin of the discrepancies between the in vitro results of Lelongt et al 8 and those we obtained from the knockout mice? One possibility is that gelB activity is required for kidney development in vitro but not in vivo, perhaps because another enzyme is able to compensate in vivo. We tested this by culturing metanephroi from gelB−/− embryos in vitro, and we found that kidney development proceeded normally.
Given these findings, it is difficult to reconcile the in vitro and in vivo results without considering the possibility that anti-gelB antibodies and exogenously added TIMP-1 affect organogenesis independent of their ability to block gelB activity. In support of this, both TIMP-1 and TIMP-2 have been shown to stimulate growth of metanephric mesenchyme in vitro independent of their ability to inhibit MMP activity. 18 In the studies of Lelongt et al, 8 the antibodies and TIMP-1 which bind specifically to gelB may somehow, either directly or indirectly, disrupt cell-cell or cell-matrix interactions that are necessary for branching morphogenesis. In any event, our results demonstrate that gelB enzyme activity is not required for branching morphogenesis or for mesenchyme-epithelium interactions in the kidney.
Gelatinase B and Alport Syndrome
Despite the fact that we found no discernible roles for gelB in normal kidney development, structure, or function, the possibility existed that gelB could influence the progression of renal diseases that involve the extracellular matrix. Gelatinase B could have a protective role, in which case it would prevent the pathogenic accumulation of extracellular matrix proteins. On the other hand, it could have a detrimental role, in which case it would inappropriately degrade properly functioning extracellular matrices in certain circumstances and cause disease. A precedent for such a detrimental role exists: knockout mice lacking MMP-12 (macrophage elastase) do not develop pulmonary emphysema when subjected to cigarette smoke. 40 We searched for a similar role for gelB by crossing the gelB−/− mice with mice homozygous for a targeted mutation in the Col4a3 gene.
Col4a3−/− mice are a model for Alport syndrome (hereditary glomerulonephritis). In Col4a3 mutant glomeruli, the mature GBM collagen IV isoforms (α3 to α5) do not assemble; instead, the embryonic isoforms (α1 and α2) accumulate and are found in the adult GBM. This leads to a delayed onset deterioration of the GBM, decreased glomerular filtration, and end-stage renal disease. 27 It has been hypothesized that MMPs play a role in the deterioration of Alport GBM, 26 and we found that gelB but not gelA transcripts were increased in Col4a3−/− kidneys at several ages (Figure 6) ▶ .
Gelatinase B−/−; Col4a3−/− double-mutant mice did not exhibit any alterations in disease progression, either physiologically, histopathologically, or ultrastructurally, when compared to gelB + Col4a3−/− mice (Figures 7 and 8 ▶ ▶ and data not shown). Thus, gelB does not play a disease-promoting role in this particular case. Of course, we cannot rule out a role for gelB in other renal diseases. Indeed, altered gelB expression has been hypothesized to be involved in animal models of both membranous nephropathy 7 and glomerulosclerosis. 41 It will be interesting to examine these and other diseases, such as diabetic nephropathy, in the context of the gelB mutation.
Is There Compensation for the Absence of Gelatinase B?
Because gelA exhibits activities similar to those of gelB, it is possible that this enzyme and perhaps other MMPs compensate for the absence of gelB in the knockout mice. Such compensation would tend to mask the normal functions of gelB. Thus, although we cannot formally rule out a role for gelB in the kidney, we can predict that any role there must be redundant, easily compensated, or unnecessary. If there is compensation, then it must be tissue-specific, because compensation does not occur in the growth plate of bones in gelB−/− mice, where there is an abnormal pattern of vascularization and ossification. 20 Also, gelB−/− mice are resistant to experimentally-induced bullous pemphigoid 35 and to necrotizing tail lesions, 42 suggesting that gelA does not compensate in these experimental diseases. Mice lacking gelA do not exhibit any obvious defects in branching morphogenesis; they develop normally and are fertile, though they do have an ∼15% slower growth rate than control littermates. 43 Although levels of gelB do not seem to be elevated in these mice, the possibility that gelB activity compensates for the absence of gelA has not been ruled out. 43 Compensation and redundancy have complicated the determination of functions for genes in many different knockouts. 4,44-46 Mice lacking both gelA and gelB could be very informative, because both the redundant and the distinct activities of these enzymes would be eliminated. This might reveal otherwise masked functions for these evolutionarily related proteins in diverse developmental and physiological processes.
Acknowledgments
We thank Dale Abrahamson for his generous gift of antibodies and Erika Maus, Jacqueline Mudd, Cong Li, and Sue King for technical assistance.
Footnotes
Address reprint requests to Dr. Jeffrey Miner, Renal Division Box 8126, Washington University School of Medicine, 660 S. Euclid Ave., St. Louis, MO 63110. E-mail: minerj@pcg.wustl.edu.
Supported by grants from the National Institutes of Health (R01-DK53196 and P50-DK45181 to J. H. M. and R01-HL47328 to R. M. S.); the Alan A. and Edith L. Wolff Charitable Trust (to R. M. S.); a research fellowship from the Japan Society for the Promotion of Science for Young Scientists (to T. B.); and the Parker B. Francis Fellowship Program (to J. M. S.).
References
- 1.Matrisian LM: Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet 1990, 6:121-125 [DOI] [PubMed] [Google Scholar]
- 2.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA: Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 1993, 4:197-250 [DOI] [PubMed] [Google Scholar]
- 3.Stetler-Stevenson WG, Aznavoorian S, Liotta LA: Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 1993, 9:541-573 [DOI] [PubMed] [Google Scholar]
- 4.Shapiro SD: Mighty mice: transgenic technology “knocks out” questions of matrix metalloproteinase function. Matrix Biol 1997, 15:527-533 [DOI] [PubMed] [Google Scholar]
- 5.Vu TH, Werb Z: Gelatinase B: structure, regulation, and function. Parks WC Mecham RP eds. Matrix Metalloproteinases. 1998, :pp 115-148 Academic Press, San Diego [Google Scholar]
- 6.Parks WC, Mecham RP (Eds): Matrix Metalloproteinases. San Diego, Academic Press, 1998
- 7.McMillan JI, Riordan JW, Couser WG, Pollock AS, Lovett DH: Characterization of a glomerular epithelial cell metalloproteinase as matrix metalloproteinase-9 with enhanced expression in a model of membranous nephropathy. J Clin Invest 1996, 97:1094-1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lelongt B, Trugnan G, Murphy G, Ronco PM: Matrix metalloproteinases MMP2 and MMP9 are produced in early stages of kidney morphogenesis but only MMP9 is required for renal organogenesis in vitro. J Cell Biol 1997, 136:1363-1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanney DC, Feng L, Pollock AS, Lovett DH: Regulated expression of matrix metalloproteinases and TIMP in nephrogenesis. Dev Dyn 1998, 213:121-129 [DOI] [PubMed] [Google Scholar]
- 10.Rankin CA, Suzuki K, Itoh Y, Ziemer DM, Grantham JJ, Calvet JP, Nagase H: Matrix metalloproteinases and TIMPS in cultured C57BL/6J-cpk kidney tubules. Kidney Int 1996, 50:835-844 [DOI] [PubMed] [Google Scholar]
- 11.Yokoo T, Kitamura M: Dual regulation of IL-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-kappa B and AP-1. Am J Physiol 1996, 270:F123-F130 [DOI] [PubMed] [Google Scholar]
- 12.Martin J, Steadman R, Knowlden J, Williams J, Davies M: Differential regulation of matrix metalloproteinases and their inhibitors in human glomerular epithelial cells in vitro. J Am Soc Nephrol 1998, 9:1629-1637 [DOI] [PubMed] [Google Scholar]
- 13.Abrahamson DR, St. John PL: Loss of laminin epitopes during glomerular basement membrane assembly in developing mouse kidneys. J Histochem Cytochem 1992, 40:1943-1953 [DOI] [PubMed] [Google Scholar]
- 14.Miner JH, Sanes JR: Collagen IV α3, α4, and α5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J Cell Biol 1994, 127:879-891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR: The laminin α chains: expression, developmental transitions, and chromosomal locations of α1–5, identification of heterotrimeric laminins 8–11, and cloning of a novel α3 isoform. J Cell Biol 1997, 137:685-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorokin LM, Pausch F, Durbeej M, Ekblom P: Differential expression of five laminin α (1–5) chains in developing and adult mouse kidney. Dev Dyn 1997, 210:446-462 [DOI] [PubMed] [Google Scholar]
- 17.Miner JH: Developmental biology of glomerular basement membrane components. Curr Opin Nephrol Hypertens 1998, 7:13-19 [DOI] [PubMed] [Google Scholar]
- 18.Barasch J, Yang J, Qiao J, Tempst P, Erdjument-Bromage H, Leung W, Oliver JA: Tissue inhibitor of metalloproteinase-2 stimulates mesenchymal growth and regulates epithelial branching during morphogenesis of the rat metanephros. J Clin Invest 1999, 103:1299-1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saxen L: Organogenesis of the kidney. 1987. Cambridge University Press, Cambridge UK
- 20.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z: MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998, 93:411-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashtan CE, Michael AF: Alport syndrome: from bedside to genome to bedside. Am J Kidney Dis 1993, 22:627-640 [DOI] [PubMed] [Google Scholar]
- 22.Tryggvason K, Zhou J, Hostikka SL, Shows TB: Molecular genetics of Alport syndrome. Kidney Int 1993, 43:38-44 [DOI] [PubMed] [Google Scholar]
- 23.Gubler M-C, Antignac C, Deschenes G, Knebelmann B, Hors-Cayla MC, Grunfeld J-P, Broyer M, Habib R: Genetic, clinical, and morphologic heterogeneity in Alport’s syndrome. Adv Nephrol 1993, 22:15-35 [PubMed] [Google Scholar]
- 24.Antignac C: Molecular genetics of basement membranes: the paradigm of Alport syndrome. Kidney Int 1995, 47(Suppl. 49):S29-S33 [PubMed] [Google Scholar]
- 25.Kashtan CE: Alport syndrome. Kidney Int 1997, 51(Suppl. 58):S69-S71 [PubMed] [Google Scholar]
- 26.Kalluri R, Shield CF, III, Todd P, Hudson BG, Neilson EG: Isoform switching of type IV collagen is developmentally arrested in X-linked Alport syndrome leading to increased susceptibility of renal basement membranes to endoproteolysis. J Clin Invest 1997, 99:2470-2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miner JH, Sanes JR: Molecular and functional defects in kidneys of mice lacking collagen α3(IV): implications for Alport syndrome. J Cell Biol 1996, 135:1403-1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers SA, Ryan G, Hammerman MR: Insulin-like growth factors I and II are produced in the metanephros and are required for growth and development in vitro. J Cell Biol 1991, 113:1447-1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner CA, Adler RR, Rappolee DA, Pedersen RA, Werb Z: Genes for extracellular-matrix-degrading metalloproteinases and their inhibitor, TIMP, are expressed during early mammalian development. Genes Dev 1989, 3:848-859 [DOI] [PubMed] [Google Scholar]
- 30.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 31.Abrahamson DR, Irwin MH, St. John PL, Perry EW, Accavitti MA, Heck LW, Couchman JR: Selective immunoreactivities of kidney basement membranes to monoclonal antibodies against laminin: localization of the end of the long arm and the short arms to discrete microdomains. J Cell Biol 1989, 109:3477-3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin PT, Ettinger AJ, Sanes JR: A synaptic localization domain in the synaptic cleft protein, s-laminin/laminin β2. Science 1995, 269:413-416 [DOI] [PubMed] [Google Scholar]
- 33.Miner JH, Cunningham J, Sanes JR: Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin α5 chain. J Cell Biol 1998, 143:1713-1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miner JH: Renal basement membrane components. Kidney Int 1999, 56:2016-2024 [DOI] [PubMed] [Google Scholar]
- 35.Liu Z, Shipley JM, Vu TH, Zhou X, Diaz LA, Werb Z, Senior RM: Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J Exp Med 1998, 188:475-482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziyadeh FN: The extracellular matrix in diabetic nephropathy. Am J Kidney Dis 1993, 22:736-744 [DOI] [PubMed] [Google Scholar]
- 37.Adler S: Structure-function relationships associated with extracellular matrix alterations in diabetic glomerulopathy. J Am Soc Nephrol 1994, 5:1165-1172 [DOI] [PubMed] [Google Scholar]
- 38.Kashtan CE, Sibley RK, Michael AF, Vernier RL: Hereditary nephritis: Alport syndrome and thin glomerular basement membrane disease. Tisher CC Brenner BM eds. Renal Pathology: with Clinical and Functional Correlations. 1994, :pp 1239-1266 J. B. Lippincott Company, Philadelphia [Google Scholar]
- 39.Sorokin L, Ekblom P: Development of tubular and glomerular cells of the kidney. Kidney Int 1992, 41:657-664 [DOI] [PubMed] [Google Scholar]
- 40.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD: Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997, 277:2002-2004 [DOI] [PubMed] [Google Scholar]
- 41.Jacot TA, Striker GE, Stetler-Stevenson M, Striker LJ: Mesangial cells from transgenic mice with progressive glomerulosclerosis exhibit stable, phenotypic changes including undetectable MMP-9 and increased type IV collagen. Lab Invest 1996, 75:791-799 [PubMed] [Google Scholar]
- 42.Dubois B, Masure S, Hurtenbach U, Paemen L, Heremans H, van Den Oord J, Sciot R, Meinhardt T, Hammerling G, Opdenakker G, Arnold B: Resistance of young gelatinase B-deficient mice to experimental autoimmune encephalomyelitis and necrotizing tail lesions. J Clin Invest 1999, 104:1507-1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S: Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem 1997, 272:22389-22392 [DOI] [PubMed] [Google Scholar]
- 44.Melton DW: Gene targeting in the mouse. Bioessays 1994, 16:633-638 [DOI] [PubMed] [Google Scholar]
- 45.Burn J, Goodship J: Developmental genetics of the heart. Curr Opin Genet Dev 1996, 6:322-325 [DOI] [PubMed] [Google Scholar]
- 46.Keverne EB: An evaluation of what the mouse knockout experiments are telling us about mammalian behaviour. Bioessays 1997, 19:1091-1098 [DOI] [PubMed] [Google Scholar]
- 47.Betsuyaku T, Miner JH, Shipley JM, Andrews KL, Senior RM: Gelatinase B expression during the pseudoglandular stage of lung development. Am J Respir Crit Care Med 1999, 159:A663(abstr.) [Google Scholar]