Abstract
Endocytosis is critical to the function and fate of molecules important to Alzheimer’s disease (AD) etiology, including the β protein precursor (βPP), amyloid β (Aβ) peptide, and apolipoprotein E (ApoE). Early endosomes, a major site of Aβ peptide generation, are markedly enlarged within neurons in the Alzheimer brain, suggesting altered endocytic pathway (EP) activity. Here, we show that neuronal EP activation is a specific and very early response in AD. To evaluate endocytic activation, we used markers of internalization (rab5, rabaptin 5) and recycling (rab4), and found that enlargement of rab5-positive early endosomes in the AD brain was associated with elevated levels of rab4 immunoreactive protein and translocation of rabaptin 5 to endosomes, implying that both endocytic uptake and recycling are activated. These abnormalities were evident in pyramidal neurons of the neocortex at preclinical stages of disease when Alzheimer-like neuropathology, such as Aβ deposition, was restricted to the entorhinal region. In Down syndrome, early endosomes were significantly enlarged in some pyramidal neurons as early as 28 weeks of gestation, decades before classical AD neuropathology develops. Markers of EP activity were only minimally influenced by normal aging and other neurodegenerative diseases studied. Inheritance of the ε4 allele of APOE, however, accentuated early endosome enlargement at preclinical stages of AD. By contrast, endosomes were normal in size at advanced stages of familial AD caused by mutations of presenilin 1 or 2, indicating that altered endocytosis is not a consequence of Aβ deposition. These results identify EP activation as the earliest known intraneuronal change to occur in sporadic AD, the most common form of AD. Given the important role of the EP in Aβ peptide generation and ApoE function, early endosomal abnormalities provide a mechanistic link between EP alterations, genetic susceptibility factors, and Aβ generation and suggest differences that may be involved in Aβ generation and β amyloidogenesis in subtypes of AD.
In neurons, the endocytic pathway (EP) internalizes and processes extracellular nutrients and trophic factors; recycles, modifies, and degrades receptors and other integral membrane proteins after neurotransmitter release; and directs information to intracellular biosynthetic pathways. Endocytosis enables neurons to modify or degrade molecules from the cell surface into intracellular compartments by a series of fusion and budding events. This complex of compartments known as the central vacuolar system consists of early and late endosomes and lysosomes that have different capabilities for proteolytic processing. Most resident acid hydrolases in central vacuolar system compartments are processed in the Golgi apparatus and subsequently trafficked to acidic organelles under the regulation of two species (46 kd and 215 kd) of mannose 6-phosphate receptors. The turnover of internalized proteins and lipids was originally thought to be limited to lysosomes, but it is now known that some acid proteases are present in early endosomes and are capable of modifying endocytosed materials.
Early endosomes are the first major sorting station on the endocytic pathway and the site of internalization and initial processing of proteins relevant to AD pathogenesis like the β protein precursor (βPP) and apolipoprotein E (ApoE). Early endosomes are also a major site of amyloid β (Aβ) peptide production in normal cells and mediate the cellular uptake of Aβ and soluble iPP. Numerous studies have implicated both the secretory pathway, specifically the endoplasmic reticulum 1-4 and Golgi apparatus 5-8 and EP 9-12 in βPP processing and the production of Aβ 1–40, Aβ 1–42, or both.
In previous studies of sporadic Alzheimer’s disease (SAD) brain, we found that the volumes of neuronal early endosomes were, on average, threefold larger than normal, which is a morphological change known to be associated with increased EP activity. 13,14 In addition, levels of immunoreactive cathepsins D and B in both their pro- and mature forms were elevated within enlarged endosomes, 15 coinciding in these neurons with increases of cation-dependent mannose 6-phosphate receptors, which mediates the delivery of acid hydrolases, including cathepsins, to early endosomes. One of the cathepsins mistrafficked in these models, cathepsin D, has been shown to have βPP β/γ secretase activity toward model peptides, recombinant βPP and the C-100 fragment of βPP. 16-19 Our previous studies have demonstrated that lysosomal system (LS) activation evidenced by an increase in gene expression and accumulation of lysosomes is an early and distinctive response of neurons in SAD and Down syndrome (DS). 20-22 Neurons exhibiting overt atrophy or neurofibrillary change display robust accumulation of hydrolase-positive lysosomes and lipofuscin granules which are then released into the parenchyma after cell lysis. These compartments containing a battery of enzymatically competent hydrolases, persist in the extracellular space in association with deposits of Aβ in both senile and diffuse plaques. 21-23 By immunocytochemistry, we have found that in cases of familial Alzheimer’s disease (FAD) linked to presenilin (PS) 1 and PS2 mutations, LS activation was greater in pyramidal neurons in cortical laminae III and V than in SAD. 24 Neuronal populations that are less vulnerable in SAD, such as those in cortical lamina II and IV of the prefrontal cortex, showed marked LS up-regulation in PS-FAD. Like SAD, senile plaques in PS-FAD brains displayed intense hydrolase immunoreactivity. 24 Compared with SAD, PS-FAD promotes an earlier and excessive deposition of Aβ 1–42. 25,26 The enhanced LS response seen in PS-FAD is consistent with in vitro studies showing that Aβ 1–42 accumulates in late endosomes and lysosomes. 27
In the present study, we further characterized the EP in SAD using antibody probes to molecules known to regulate specific aspects of the endocytic process. Moreover, we investigated the onset of EP dysfunction in the brains of nondemented individuals exhibiting the earliest Alzheimer-like pathological changes restricted to the entorhinal cortex and hippocampus. The analysis was extended to earlier stages of pathogenesis in brains from fetuses and juveniles with DS (trisomy 21), a form of mental retardation invariably associated with the development of AD neuropathology after age 40. 28 Finally, we analyzed the influence of normal aging, APOE genotype, and PS mutations on endosome morphology in relation to the evolution of AD neuropathology. Our results show that early endosomal abnormalities are the earliest neuropathological alteration yet to be identified in SAD. Their appearance is greatly accelerated by triplication of the distal half of the long arm of chromosome 21 (DS). Inheritance of the APOE ε4 allele, which substantially increases the risk for developing AD and promotes earlier disease onset 29 also promoted earlier appearance of endosome enlargement. Finally, in a variety of neurodegenerative diseases and, significantly, in severely affected individuals with FAD linked to four different mutations of PS1 and the Asn141-Ile mutation of PS2, endosomes were normal in size. These observations indicate that the neuronal endosomal response seen in AD is highly disease-specific and does not seem to be a secondary effect of Aβ deposition. An understanding of the origins and effects of this abnormal endocytic response should provide important insight into pathogenic mechanisms in sporadic AD, the most common and least well understood form of AD.
Materials and Methods
Tissue
Postmortem brain tissue from 15 elderly, nondemented individuals were examined according to The Consortium to Establish a Registry for Alzheimer’s Disease 30 and the criteria proposed by Mirra et al 31 and staged according to Braak and Braak 32 and diagnosed with neuropathological evidence of early-stage Alzheimer’s disease (AD) (neocortex devoid of plaques, Braak stage 0; transentorhinal, entorhinal cortex/hippocampus, sparse plaques, Braak stage I–III). Another 10 age-matched 62- to 80-year-old cases (control group A) were evaluated similarly and found to be neuropathologically normal controls (isocortex, entorhinal cortex/hippocampus devoid of plaques and neurofibrillary tangles). Fixed tissue was obtained from the Bronx Veteran’s Administration Medical Center and Mt. Sinai Medical Centers, the Harvard Brain Tissue Resource Center at McLean Hospital (Belmont, MA), and the Neuropathology Core Facility of the Massachusetts Alzheimer’s Disease Resource Center (Massachusetts General Hospital, Boston, MA). Clinical records indicated that all subjects were cognitively normal and 60% of these cases were neuropsychologically evaluated and assigned the clinical dementia rating score of 0 (cognitively intact). All brains were characterized neuropathologically by hematoxylin and eosin, Bielschowsky silver stain, thioflavin S, Aβ, and τ immunoreactivities. Early-stage AD cases were found to have AD neuropathological changes limited to the entorhinal cortex/hippocampus. Because AD neuropathology begins in the entorhinal cortex/hippocampus and later spreads to neocortical areas, we evaluated the EP in formalin-fixed tissue blocks of the entorhinal cortex/hippocampus containing neuropathological changes suggestive of AD and prefrontal cortex at stages with no neuropathological evidence of AD. 30-32 APOE genotyping was performed in the laboratory of Dr. Bradley Hyman at the Massachusetts General Hospital, Boston, MA, using a modified polymerase chain reaction technique. 33 Tissue from seven cases of early-stage DS ranging in age from 28 weeks of gestation to 12 years and an equal number of young age-matched control cases were procured from the Johns Hopkins University Brain Resource Center, Baltimore, MD. Postmortem tissue from 15 confirmed cases of early-onset FAD linked to various mutations of the PS gene (12 PS1-FAD cases; three PS2-FAD cases) were obtained from a number of individual investigators and tissue resource centers including the Joseph and Kathleen Bryan Alzheimer Disease Research Center, the Duke University Medical School, the Medical College of Pennsylvania and Hahnemann University, and the National Neurological Research Specimen Bank/Veteran’s Administration Medical Center. Although the cognitive status of the PS-FAD cases was unknown, all brains used in this study exhibited evidence of advanced stage AD neuropathology (>30 neuritic plaques per high power field, Braak stage IV–V) in the entorhinal cortex, hippocampus, and frontal cortex). A second group of 22 normal controls (control group B) ranging in age from 1 year to 85 years of age were collected from the Harvard Brain Tissue Resource Center and the Massachusetts Alzheimer’s Disease Resource Center and used solely for the normal aging study. Additional postmortem tissues from individuals with the neuropathological diagnosis of encephalopathy, Pick’s disease, Lewy body disease, Huntington’s disease–grade three, amyotrophic lateral sclerosis (ALS) and progressive supranuclear palsy were procured from the Harvard Brain Tissue Resource Center and Massachusetts Alzheimer’s Disease Resource Center.
Antibodies
Immunocytochemical studies were performed as previously described 34 using polyclonal antibodies raised against a synthetic peptide corresponding to amino acids 193–211 of the C-terminal domain of the human GTP-binding protein, rab5, and amino acids 191–210 of rab4 of human origin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); an affinity-purified polyclonal antibody raised against a synthetic peptide corresponding to an amino acid sequence mapping to the C-terminus of human rabaptin 5 (Santa Cruz Biotechnology Inc.); a monoclonal antibody generated against the N-terminus (amino acids 3–281) of human early endosomal antigen 1 (Transduction Laboratories, Lexington, KY); and a monoclonal antibody generated against a synthetic peptide of human Aβ 17–24 (4G8) (Senetek, Drug Delivery Technologies, Inc., St. Louis, MO). A monoclonal antibody, MC-1, which reacts with two conformationally constrained domains of τ in paired helical filaments (PHF) was a generous gift of Dr. Peter Davies (Department of Pathology, Albert Einstein College of Medicine, Bronx, NY). Details on the generation and immunospecificity of MC-1 have been reported previously. 35 Brain tissue used for immunocytochemical analyses was immersion-fixed in cold 10% phosphate-buffered formalin (0.15 mol/L), pH 7.4.
Immunocytochemistry
Immunoreactivity was demonstrated on 30- to 40-μm-thick vibratome sections as previously described. 34 Negative controls included tissue sections incubated in the absence of primary antisera.
Morphometric Analysis
Vibratome sections of the prefrontal cortex of all early-stage AD, FAD, non-AD neurodegenerative disorders, and control cases were immunostained in tandem under identical conditions with rab5 antiserum. Background staining intensities among all sections were comparable and all neurons were intact. Small, medium, and large pyramidal neurons from lamina III of the prefrontal cortex (Brodmann area 10) were selected at random and the cross-sectional area, number, average, and total early endosomal volume per neuron were analyzed for each neuron as previously described. 15,22
Results
EP Activation Precedes Other Known Neuropathology in SAD
To establish the initial appearance of endosomal alterations in relation to Aβ deposition or neurofibrillary change, we examined cognitively and neuropathologically-normal elderly individuals and two affected populations. The first affected population referred to as “pre-AD” was composed of cognitively-normal elderly individuals who after death were diagnosed with neuropathological evidence early stage/possible AD based on the presence of Alzheimer-like pathology in the transentorhinal and entorhinal cortex and hippocampus (sparse plaques, Braak stage I–III). Cross-sectional neuropathological data analyses using several neuropathological guidelines 30-32 have shown this neuropathological pattern to represent the earliest stages in the evolution toward AD. The second group included individuals with DS who are at very high risk to develop AD 36 but were studied at very young ages when the brains showed no evidence of neuropathology.
In brain sections immunolabeled with anti-rab5, a specific marker for early endosomes, 37 neurons in brains from control subjects displayed endosomes of normal size. 13,14,38 By contrast, in the pre-AD cases, abnormally large endosomes ranging from 400 to 620 nm were prominent in the majority of pyramidal neurons in laminae II of the entorhinal cortex and CA2 and CA3 fields of the hippocampus and in laminae III and V of the prefrontal cortex. Immunolabeling studies on serial adjacent tissue sections of the prefrontal cortex using 4G8 against Aβ 17–24 or MC-1, against a conformation of τ believed to mark the earliest stages of neurofibrillary pathology, or double-immunofluorescence studies with one of these antibodies and rab5 demonstrated that neuronal early endosomal enlargement could be detected in the absence of extracellular Aβ accumulation or neurofibrillary pathology (Figure 1) ▶ . Morphometric analyses performed on 25 neocortical neurons from lamina III of each of the 15 pre-AD cases and 11 controls showed endosomal volume to be nearly twofold larger in the pre-AD cases than normal (control mean, 1.88%, SEM ± 0.066 versus pre-AD mean, 3.68%, SEM ± 0.093; Figure 1 ▶ ) and less than the increase in endosomal volume we observed previously in confirmed moderate-severe SAD cases. 15 The numbers of rab5-positive endosomes in the pre-AD cases were similar to those in control brains (control average = 55, SEM ± 1.87 endosomes/cell; possible AD = 50, SEM ± 1.80 endosomes/cell). Cortical pyramids in lamina V displayed similar alterations (data not shown).
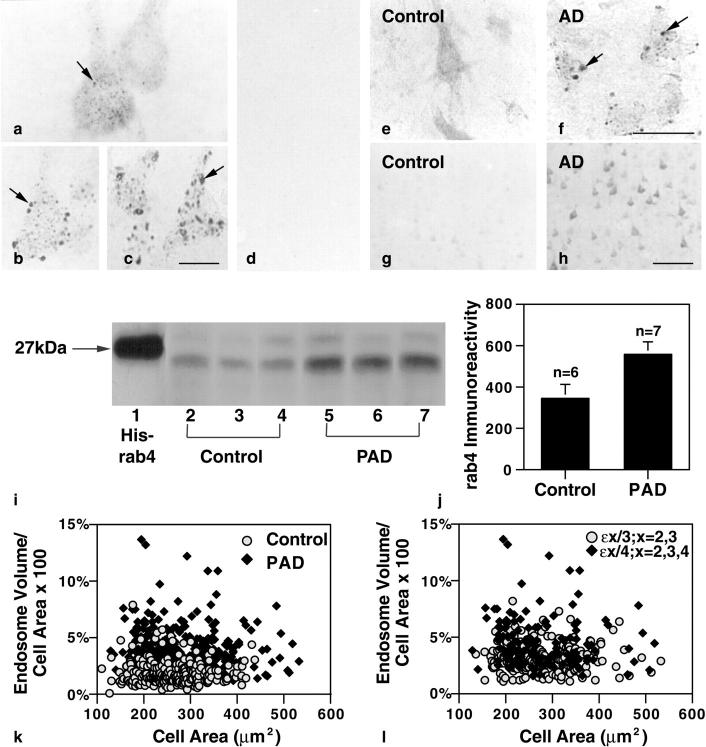
Figure 1.
Early endosomal alterations are the earliest detectable intracellular manifestation of AD and are evident in individuals who have not yet developed clinical symptoms. Pyramidal neurons of the prefrontal cortex of an early-stage sporadic AD case labeled with rab5 (b) and EEA1 (c) show atypically large endosomes (arrows). a: rab5-immunopositive neuronal early endosomes (arrow) from neurons in an age-matched control. Serial adjacent sections immunolabeled with an antibody to Aβ17–24 (d) or MC-1 (not shown) displayed minimal to no evidence of extracellular Aβ deposition or neurofibrillary pathology. Immunolabeling for rabaptin 5, a regulator protein of endocytosis, was detected in the cytosol and associated with small vesicles in control brains (e). By contrast, rabaptin5 immunoreactivity in neurons from pre-AD brains was predominantly located on large endosomes (f, arrows), a change indicative of increased endosomal fusion. Compared with neurons from control brains (g), pyramidal neurons in the pre-AD cortex (h) showed increased levels of rab4 immunoreactivity, which is consistent with an increase in endosome to plasma membrane vesicular recycling. Western blot analysis (i) of brain homogenates (100 μg/lane) prepared from the frontal cortex of three representative neuropathologically-normal controls (n = 6; lanes 2–4) and three representative pre-AD brains (n = 7) (PAD, lanes 5–7) confirmed the immunocytochemical findings and revealed an increase in rab4 immunoreactive protein (Mr ∼23 to 25) in the pre-AD brains. (Lane 1, recombinant His-tagged rab4; CytoSignal Research Products, Irvine CA.) Scan analysis (j) of the rab4 protein levels obtained by Western blot showed approximately twofold higher rab4 levels in pre-AD brains compared with control cases (control mean = 345.1; pre-AD mean = 560.6; P > 0.05. Error bars represent SEM. Morphometric analysis (k) of 25 pyramidal neurons from each of 10 control and 15 early-stage AD brains showed an average twofold larger total endosomal volume per neuron in the AD cases versus control, implying an increase in endocytosis (possible AD mean = 3.68%, SEM ± 0.093; control mean = 1.88%, SEM ± 0.066; P < 0.005). Reassessment (l) of the same early SAD cases, stratified by APOE genotype, showed approximately twofold higher endosomal volume per neuron in lamina III pyramids of the five brains carrying one or both copies of the ε4 allele of APOE versus the 10 brains with ε2 or ε3 alleles (ε4 mean = 4.68, SEM ± 0.206;ε2, ε3 mean = 3.18, ± SEM 0.095, P < 0.005). Scale bars: a–c, 20 μm; e and f, 50 μm; g and h, 200 μm.
To investigate further the functional significance of endosomal enlargement, we studied the cellular distribution of known markers of EP function. Along with rab5, rabaptin 5, and early endosomal antigen 1 (EEA 1), are well-characterized proteins that selectively associate with early endosomes where they regulate docking and endosomal fusion. 39-41 Unlike EEA 1 and rab5, which reside principally on endosomal membranes, rabaptin 5 is present in the cytosol as well as on the endosomal membrane. 39 In its activated GTP-bound form, rab5 recruits rabaptin 5 from the cytosol principally to the endosomal membrane. Using antibodies directed to the functionally distinct markers, rabaptin 5, EEA 1, and rab5, we assessed uptake and fusion in human brain tissue from control and pre-AD cases. The pattern of EEA 1 immunoreactivity paralleled that of rab5 in control and pre-AD brains, confirming the identity of the swollen vacuolar profiles in the pre-AD brains as early endosomes. As expected, rabaptin 5 immunoreactivity in the control cases was present throughout the cytosol and less frequently immunolabeled small vesicles. In pre-AD cases, by contrast, rabaptin 5 immunolabeling in neurons was minimal in the cytosol and most prominent on large endosomes (Figure 1f) ▶ .
Rab4, another GTPase like rab5, plays a complementary role to rab5 by directing the recycling of early endosomes to the cell surface. 42 Serial adjacent tissue sections of frontal cortex from AD brains labeled with anti-rab4 antiserum showed a marked qualitative increase compared to controls in the expression of rab4 in pyramidal neurons which was principally localized within small vesicles of sizes consistent with their being recycling vesicles. The increased level of rab4 detected by immunocytochemistry associated with the AD cases was confirmed by Western blot analysis. Differences in the level of rab4 immunoreactive protein quantitated by scan analysis were nearly twofold higher in the early-stage AD brains versus controls (Figure 1j) ▶ and equivalent to that found in moderate to severe stage cases of AD (data not shown; control mean = 345.1; pre-AD mean = 560.6, P > 0.05).
APOE ε4 Genotype Promotes EP Activation in Early-Stage SAD
Because ApoE function in neurons is dependent on EP activity, we investigated the influence of APOE genotype on endosome size. By stratifying the quantitative data on the AD cases in Figure 1 ▶ according to APOE genotype, we found that the mean total endosomal volume per neuron in brains from the possible AD cases carrying one or two APOE ε4 alleles was nearly 50% larger than the cases carrying APOE ε2 or ε3 (ε2, ε3 group mean = 3.18%, SEM ± 0.095; ε4 group mean = 4.68%, SEM ± 0.206; P < 0.005) (Figure 1) ▶ . That the ε4 allele influences clinical disease onset, but not progression, 43,44 parallels our observation that endosomal abnormalities are accelerated by the ε4 allele at earliest stages of disease but not at moderate to severe stages of clinical disease where we found no detectable differences in neuronal endosomal volume in AD brains from individuals with one or both ε4 alleles as compared to those carrying ε3 or ε2 alleles (mean endosomal volume ε3, ε2 = 4.99, SEM ± 0.10; mean endosomal volume ε4 = 5.29, SEM ± 0.24, P = 0.112).
EP Abnormalities Precede AD Neuropathology by Decades in DS
Individuals with DS invariably develop AD by age 50. To investigate the earliest appearance of endosomal abnormalities, we immunolabeled tissue sections of the prefrontal cortex from seven cases of fetal, infant, and young DS patients ranging in age from 28 weeks of gestation to 12 years with anti-rab5 antiserum. Many pyramidal neurons contained early endosomes of abnormally large sizes that were not seen in neurons from the age-matched young control cases examined (Figure 2) ▶ . Qualitative differences in endosomal size were evident in 21% (SEM ± 2%) of pyramidal neurons (n = 50) per high-power field in the youngest cases (ages 28 weeks to 5 years) and increased to 40% (SEM ± 5%) with age (between 5 and 12 years of age). Although the number of neurons containing enlarged endosomes in the DS brains increased with age, the magnitude of endosomal enlargement in affected neurons in these DS cases was equivalent to that seen in pyramidal neurons from six cases of adult DS ranging in age from 25 to 56 years.
Figure 2.
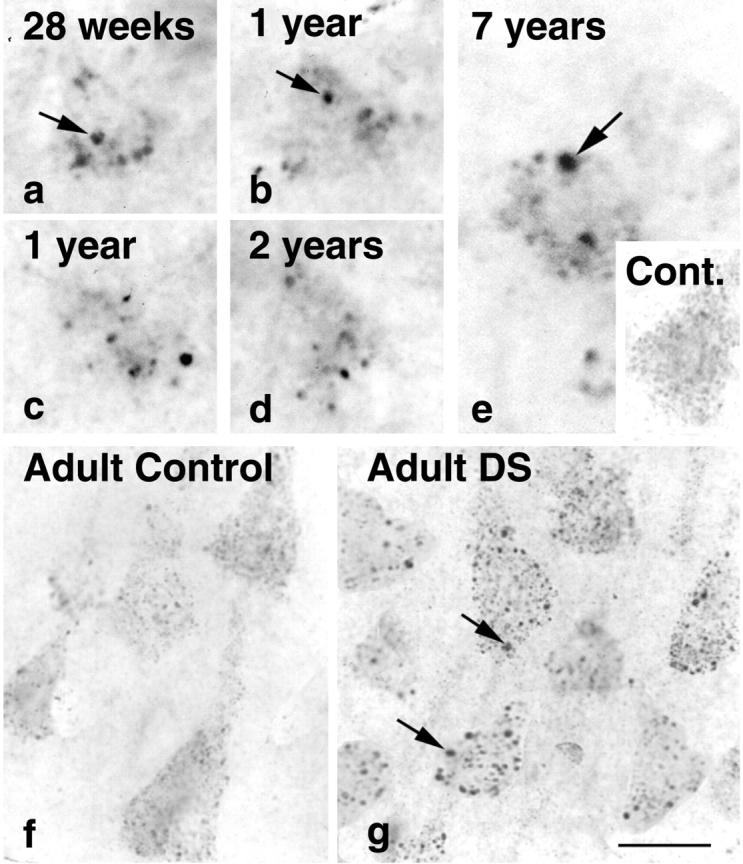
Early endosomal enlargement is evident in single neurons from the prefrontal cortices of a fetus (a), infants of 1 and 2 years (b–d), and a young 7-year-old child (e) with DS immunolabeled with rab5 (a–e). The numbers of neurons containing enlarged endosomes increased with the age of the individual. The magnitude of endosomal enlargement in neurons detected with rab5 in young DS individuals did not differ significantly from that observed in cases of adult DS (g). e, inset, and f show representative neurons from young and aged control brains, respectively. Scale bars: a–g, 20 μm
EP Abnormalities Are Not Observed in Other Neurodegenerative Disorders or Normal Aging
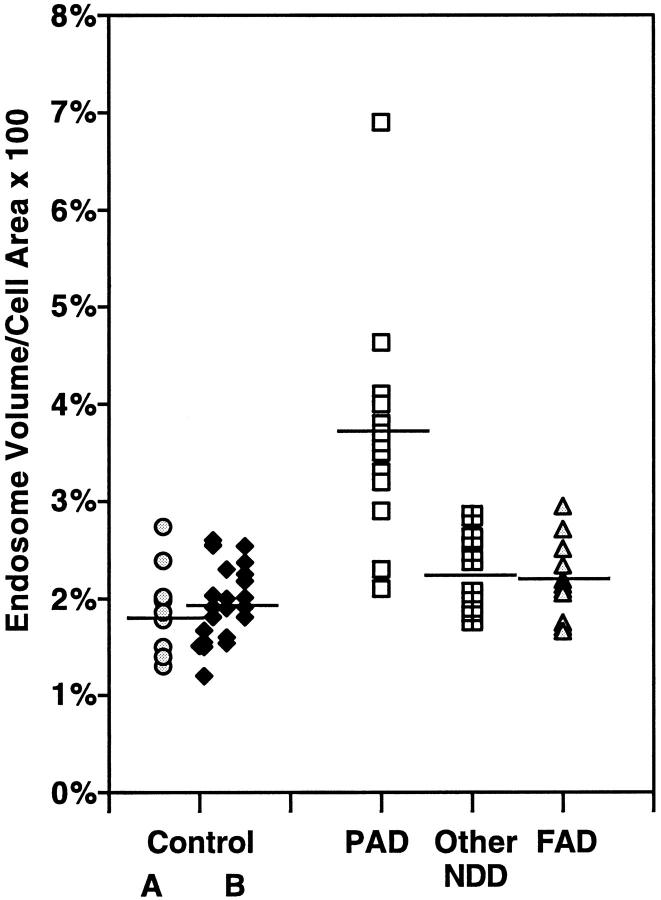
We next determined the specificity of endosomal enlargement to SAD and DS by examining severely affected neuronal populations from several non-AD neurodegenerative diseases including cases of encephalitis, Pick’s disease, Lewy body dementia, Huntington’s disease, progressive supranuclear palsy, and amyotrophic lateral sclerosis. We found that within the various affected neuronal populations of the non-AD neurodegenerative group, early endosomal size was similar to that seen in neurologically normal controls (average mean endosomal volume: control = 1.88%; SEM ± 0.066; non-AD neurodegenerative diseases = 2.26%, SEM ± 0.067, P = 0.137) (Figure 3) ▶ .
Figure 3.
Morphometric analyses of neuronal endosomal volume in neurodegenerative diseases and normal individuals of varying age. The values are expressed as the percentage of cell area occupied by rab5-positive early endosomes averaged for 25 pyramidal neurons in each brain indicated by a data point on the graph. Results show endosomal enlargement is specific to non-PS forms of AD (elderly, nondemented control group A, mean = 1.88%, SEM ± 0.066; pre-AD/PAD mean = 3.68%, SEM ± 0.093; other non-AD neurodegenerative disorders, mean = 2.26%, SEM ± 0.067; PS-FAD mean = 2.22, SEM ± 0.062). Control group B represents a second pool of 22 neurologically normal individuals of ascending age comprising four groups: 1 to 15 years, n = 5, mean = 1.45%, SEM ± 0.095; 16 to 35 years, n = 5, mean = 2.19%, SEM ± 0.062; 36 to 55 years, n = 5, mean = 1.86%, SEM ± 0.077; and >55 years, n = 7, mean = 2.14%, SEM ± 0.094.
Given the importance of aging as a risk factor for AD, we evaluated aging-related effects on neuronal endocytosis by examining 22 additional neurologically-normal control brains ranging in age from <1 year to 85 years. The brains were divided into four subgroups −1 to 15 years, 16 to 35 years, 36 to 55 years, and >55 years. Rab5-positive early endosomes in neocortical pyramids from brains of each age were equivalent in size, number per neuron, and volume/neuron (Figure 3) ▶ .
EP Activation Distinguishes Subtypes of AD
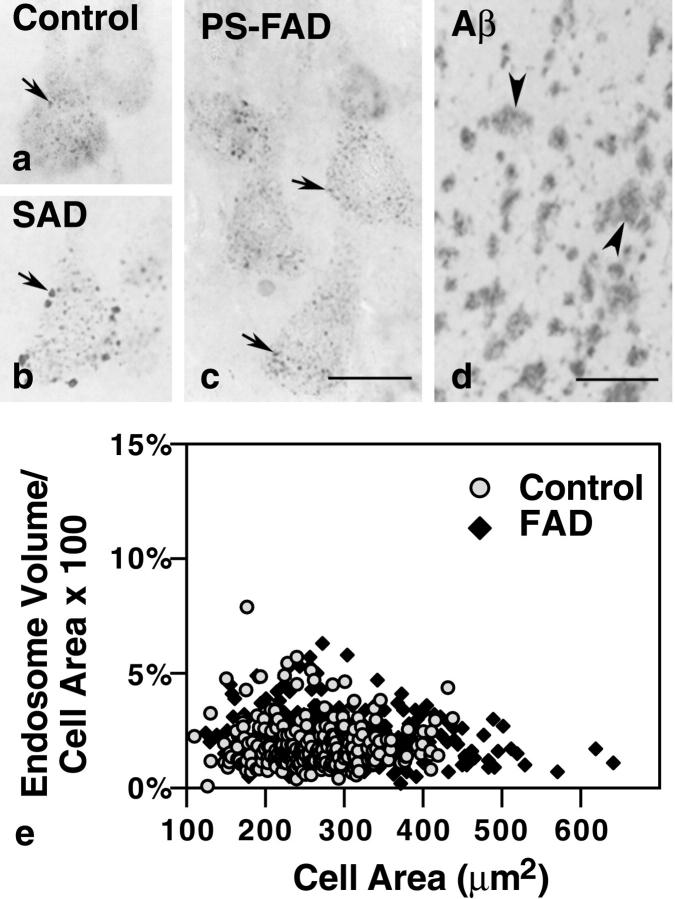
We find that, in all forms of AD, the neuronal expression of lysosomal proteases (cathepsins) is strongly up-regulated and lysosomes markedly accumulate. 20-22 Because it might be anticipated that certain cellular alterations arising at the earliest stages of AD pathogenesis may develop in some forms of AD and not in others, we assessed whether the same structural abnormalities of early endosomes seen in SAD and accelerated in development in late-onset FAD-APOE ε4 and DS, were also promoted by mutations of PS causing present early-onset FAD. Serial adjacent tissue sections from the prefrontal cortex of 12 patients with moderate to severe FAD caused by any of four different mutations in PS, immunolabeled with rab5 and 4G8, displayed no apparent early endosomal abnormalities in neurons despite abundant Aβ plaques (Figure 4) ▶ . Morphometric analysis showed that the average endosomal volume per neuron in the PS-FAD cases was similar to that of normal controls (control mean = 1.88%, SEM ± 0.066; PS-FAD mean = 2.22%, SEM ± 0.061; P = 0.097) (Figure 4) ▶
Figure 4.
Neuronal endosomes in individuals with PS mutations (c) are comparable in size to those in age-matched controls (a) despite high levels of parenchymal Aβ deposition in the PS-FAD brains (d, arrowheads). By contrast, most pyramidal neurons in sporadic Alzheimer brain display large endosomes (b, arrow). e: Morphometric analysis of early endosomal size from each of 10 control and 15 PS-FAD brains. Expressed as percent cell area occupied by rab5-positive early endosomes, the results show no significant difference in endosomal volume in the PS-FAD brains versus control (PS-FAD mean = 2.22%, SEM ± 0.06; control mean = 1.88%, SEM ± 0.07). Scale bars: a–c, 20 μm; d, 200 μm.
Discussion
Our studies show that the EP of cortical pyramidal neurons responds in an Alzheimer-selective pattern years or decades before the classical neuropathological lesions appear in SAD and DS, respectively. This response cannot be attributed exclusively to either Aβ overproduction or deposition because it begins in DS before Aβ is detectable and does not develop in PS1-FAD even though Aβ is overproduced and Aβ deposition is robust. Neuronal abnormalities considered to mark early-stage AD have previously been described but none has been shown to precede the extracellular deposition of Aβ, unlike the EP abnormalities reported here. This striking and early involvement of the EP is particularly significant given that the early endosome is unique in being a point of convergence within the cell for many key etiological factors in AD, including βPP, Aβ peptide, ApoE, lipoprotein receptor-related protein, and Fe65. Although inferences about the dynamics of the EP are difficult to make from postmortem studies, the observed changes in the established marker proteins for endosomal compartments support the view that EP activity is increased. First, overexpression of rab5 has been shown in cultured cells to be one way to increase endocytosis and expand the size of early endosomes. We found neuronal early endosomes in AD brain to be increased in size and volume and similar morphologically to those seen in rab5-transfected cells. 14,38,45 Second, the overexpression of rab4 stimulates endosome recycling to the plasma membrane and promotes formation of rab4-positive recycling vesicles. 42,46 We observed markedly elevated rab4 immunoreactivity within small vesicles in lamina III pyramids from the pre-AD brains, which we believe represents increased numbers of recycling vesicles. Morphometric analysis will be required, however, for confirmation. This observation implies activity in the recycling pathway is more active, which is a response complementary to the stimulated internalization reflected by elevated levels of rab5 immunoreactivity. Third, rabaptin 5 and EEA1 are downstream effector proteins of vesicle membrane fusion and docking associated with rab5 which play a critical role in endocytosis. 39-41 Overexpression of rabaptin 5 in transfected cells results in the characteristic enlargement of early endosomes and increased numbers of transferrin receptor-positive vesicular compartments 14,38,45 —changes morphologically consistent with increased endocytosis. 14,38,45 We find that, like the transfected cells, immunocytochemical labeling of sections from possible AD brains with antibodies to rabaptin 5 and EEA1 detected early endosomes that were morphologically identical to swollen, rab5-positive endosomal profiles.
The activation of the EP is a disease-selective response of neurons in populations susceptible to AD. Our inability to detect this response in other neurodegenerative diseases establishes that it is not a generalized response to neuronal injury or degeneration. We observed, however, that its onset is substantially influenced by genetic effects relevant to AD pathogenesis. The appearance of early endosome abnormalities in some neurons before birth in DS implies that dosage of particular genes on chromosome 21 greatly accelerates the development of the EP activation. Triplication of the βPP gene, by itself, may be an insufficient stimulus because βPP overexpression in mice does not detectably alter endocytosis whereas, in the partial trisomy 16 mouse (Ts65Dn), endosome enlargement is comparable to that in its human counterpart, trisomy 21 (unpublished data; AM Cataldo et al, manuscript in preparation). We also found that the APOE ε4 allele accentuates the EP response in early AD. At later stages of disease when the EP response is fully developed, no APOE allele influence is apparent suggesting that, like its effect on clinical disease, APOE genotype accelerates the onset but not the final magnitude of the EP response. These influences of APOE alleles support the pathogenic relevance of EP abnormalities to AD pathogenesis and are consistent with evidence that ApoE binding to its neuronal receptor activates endocytosis 47,48 and that ApoE interacts with βPP in the endosomal compartment. 48
The neuronal EP activation observed in this study is the only known feature of cellular AD neuropathology that differentiates subtypes of AD. Because AD is heterogeneous in etiology, it could well be anticipated that cellular responses that are close to the primary initiating events of AD would not only appear early but also may not be shared by all AD subtypes. The different alterations of calcium homeostasis or Aβ generating pathways associated with various FAD mutations in fibroblasts and transgenic mouse models of FAD may be biochemical evidence of these distinctive early pathways. 49-52 The absence of an EP response in PS-FAD is paralleled by recent findings that, unlike SAD and DS, PS-FAD does not seem to be influenced by APOE genotype. 53,54 The latter finding is consistent with the view that PS, which resides principally in the endoplasmic reticulum 55 promotes Aβ generation and disease progression mainly via nonendocytic routes 55 that might not be expected to be influenced by ApoE. Together, these findings imply that activation of endocytosis and its accentuation by APOE ε4 genotype constitute a mechanism particularly relevant to the most common, sporadic form of AD, in which initial stages of pathogenesis are unknown.
A second explanation for the absence of an endosomal response in the PS-FAD cases comes from a recent study by Van Uden et al 56 showing a link between mutant PS expression and down-regulation of the LDL receptor-related protein, which modulates the cellular uptake of AD-associated proteins such as βPP and ApoE. Other factors that could explain the difference in endocytic response between AD subtypes would be the greater rate of neurodegeneration in PS-FAD versus SAD 57 which could limit the window within which endosomal disturbances could be detected. Evidence against this notion is supported by our studies showing the absence of endosomal abnormalities in primary fibroblasts from PS-FAD subjects and in neurons from PS/βPP transgenic mice (unpublished observations).
Altered endocytosis is likely to have a major influence on pathogenic events in AD. Although EP activation in AD may begin as a compensatory response, chronic activation produces functional, morphological, and compositional effects on the EP that could increase neuronal vulnerability. Endocytic activation may, by itself, lead to sequestration and inappropriate degradation of vital plasma membrane proteins, growth factors, or receptors in the early endosome. Such an effect on neuronal glucose transporters may be one mechanism for compromised glucose uptake in AD 58,59 and has direct implications for reduced cell viability. The EP is also the portal of entry for oxidizable substrates, such as Aβ and apolipoproteins which could selectively damage lysosome integrity or impair its functions. Endocytic activation is likely to contribute to the robust up-regulation of the LS as seen in AD brain, which has been linked to increased neuronal vulnerability. 60-62 LS activation progressively worsens with advancing disease and accounts, at least in part, for cell dysfunction leading to cell loss. 63-66 The pathogenic significance endosomal abnormalities in AD is further indicated by the recent identification of a β-secretase (BACE) which was found to be enriched in endosomes and to have an optimal pH requirement consistent with activity in early endosomes. 67 Therefore, it is noteworthy that, in AD, we find hydrolase trafficking to early endosomes to be enhanced. 15 Recent evidence using transfected cell lines that mimic the enhanced trafficking of hydrolases to early endosomes seen in human brain, secrete two- to threefold higher levels of Aβ40 and Aβ 42, respectively. 68 Aβ production, Aβ clearance, or both could be affected by EP abnormalities and, based on current knowledge of endosome biology, be subject to influences by ApoE. In light of the importance of ApoE in Aβ deposition, early endosomes, the only known site where ApoE, βPP, and Aβ are known to co-exist, represents an attractive site in SAD and DS brain to initiate Aβ formation—a process favored by acidic conditions. 18,69-73 This notion is further strengthened by our recent findings showing that in cases of early-stage AD and DS, the appearance of endosomal abnormalities coincides with increased soluble Aβ levels and the presence of intraneuronal Aβ immunoreactivity which co-localizes predominantly to enlarged endosomes (Cataldo et al, 2000, in preparation). However, we cannot exclude the possibility that elevated Aβ levels could contribute to or cause endosomal alterations that represent an attempt by cells to clear Aβ. Further studies of new cell and animal models to investigate these cellular pathways, the roles of which are poorly understood in AD, are expected to help clarify the cellular pathogenesis of AD and to identify targets for therapeutic intervention.
Acknowledgments
We thank Lucy Morales for secretarial assistance in preparing the manuscript for publication.
Footnotes
Address reprint requests to Anne M. Cataldo, Ph.D., Nathan Kline Institute, 140 Old Orangeburg Road, Orangeburg, NY 10962. E-mail: cataldo@nki.rfmh.org.
Supported in part by National Institutes of Health grants AG 10916 (to R. A. N.) and AG14762 (to A. M. C.).
References
- 1.Chyung ASC, Greenberg BD, Cook DG, Doms RW, Lee VM: Novel beta-secretase cleavage of beta-amyloid precursor protein in the endoplasmic reticulum/intermediate compartment of NT2N cells. J Cell Biol 1997, 138:671-680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook DG, Forman MS, Sung JC, Leight S, Kolson DL, Iwatsubo T, Lee VM, Doms RW: Alzheimer’s A beta(1–42) is generated in the endoplasmic reticulum/intermediate compartment of NT2N cells. Nat Med 1997, 3:1021-1023 [DOI] [PubMed] [Google Scholar]
- 3.Hartmann H, Busciglio J, Baumann KH, Staufenbiel M, Yankner BA: Developmental regulation of presenilin-1 processing in the brain suggests a role in neuronal differentiation. J Biol Chem 1997, 272:14505-14508 [DOI] [PubMed] [Google Scholar]
- 4.Xia W, Zhang J, Ostaszewski BL, Kimberly WT, Seubert P, Koo EH, Shen J, Selkoe DJ: Presenilin 1 regulates the processing of beta-amyloid precursor protein C-terminal fragments and the generation of amyloid beta-protein in endoplasmic reticulum and Golgi. Biochemistry 1998, 37:16465-16471 [DOI] [PubMed] [Google Scholar]
- 5.Xu H, Sweeney D, Wang R, Thinakaran G, Lo AC, Sisodia SS, Greengard P, Gandy S: Generation of Alzheimer beta-amyloid protein in the trans-Golgi network in the apparent absence of vesicle formation. Proc Natl Acad Sci USA 1997, 94:3748-3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomita S, Kirino Y, Suzuki T: Cleavage of Alzheimer’s amyloid precursor protein (APP) by secretases occurs after O-glycosylation of APP in the protein secretory pathway. Identification of intracellular compartments in which APP cleavage occurs without using toxic agents that interfere with protein metabolism. J Biol Chem 1998, 273:6277-6284 [DOI] [PubMed] [Google Scholar]
- 7.Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, Hardy J, Levey AI, Gandy SE, Jenkins NA, Copeland NG, Price DL, Sisodia SS: Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 1996, 17:181-190 [DOI] [PubMed] [Google Scholar]
- 8.Peraus GC, Masters CL, Beyreuther K: Late compartments of amyloid precursor protein transport in SY5Y cells are involved in beta-amyloid secretion. J Neurosci 1997, 17:7714-7724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ: Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature 1992, 357:500-503 [DOI] [PubMed] [Google Scholar]
- 10.Koo EH, Squazzo SL: Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem 1994, 269:17386-17389 [PubMed] [Google Scholar]
- 11.Soriano S, Chyung AS, Chen X, Stokin GB, Lee VM, Koo EH: Expression of beta-amyloid precursor protein-CD3gamma chimeras to demonstrate the selective generation of amyloid beta(1–40) and amyloid beta(1–42) peptides within secretory and endocytic compartments. J Biol Chem 1999, 274:32295-32300 [DOI] [PubMed] [Google Scholar]
- 12.Stokin GB, Chyung ASC, Perez RG, Lee VMY, Koo E: The Pathway of Alpha-Beta 42 Production in APP with Codon 717 Mutation. 1998. Sixth International Conference on Alzheimer’s Disease, Amsterdam
- 13.Gorvel JP, Chavrier P, Zerial M, Gruenberg J: rab5 controls early endosome fusion in vitro. Cell 1991, 64:915-925 [DOI] [PubMed] [Google Scholar]
- 14.Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M: The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 1992, 70:715-728 [DOI] [PubMed] [Google Scholar]
- 15.Cataldo AM, Barnett JL, Pieroni C, Nixon RA: Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer’s disease: neuropathologic evidence for a mechanism of increased beta-amyloidogenesis. J Neurosci 1997, 17:6142-6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chevallier N, Vizzavona J, Marambaud P, Baur CP, Spillantini M, Fulcrand P, Martinez J, Goedert M, Vincent JP, Checler F: Cathepsin D displays in vitro beta-secretase-like specificity. Brain Res 1997, 750:11-19 [DOI] [PubMed] [Google Scholar]
- 17.Dreyer RN, Bausch KM, Fracasso P, Hammond LJ, Wunderlich D, Wirak DO, Davis G, Brini CM, Buckholz TM, Konig G, Kamarck ME, Tamburini PP: Processing of the pre-beta-amyloid protein by cathepsin D is enhanced by a familial Alzheimer’s disease mutation. Eur J Biochem 1994, 224:265-271 [DOI] [PubMed] [Google Scholar]
- 18.Evin G, Cappai R, Li QX, Culvenor JG, Small DH, Beyreuther K, Masters CL: Candidate gamma-secretases in the generation of the carboxyl terminus of the Alzheimer’s disease beta A4 amyloid: possible involvement of cathepsin D. Biochemistry 1995, 34:14185-14192 [DOI] [PubMed] [Google Scholar]
- 19.Mackay EA, Ehrhard A, Moniatte M, Guenet C, Tardif C, Tarnus C, Sorokine O, Heintzelmann B, Nay C, Remy JM, Higaki J, Van Dorsselaer A, Wagner J, Danzin C, Mamont P: A possible role for ca-thepsins D, E, and B in the processing of beta-amyloid precursor protein in Alzheimer’s disease. Eur J Biochem 1997, 244:414-425 [DOI] [PubMed] [Google Scholar]
- 20.Nixon RA, Cataldo AM, Paskevich PA, Hamilton DJ, Wheelock TR, Kanaley-Andrews L: The lysosomal system in neurons. Involvement at multiple stages of Alzheimer’s disease pathogenesis. Ann N Y Acad Sci 1992, 674:65-88 [DOI] [PubMed] [Google Scholar]
- 21.Cataldo AM, Hamilton DJ, Nixon RA: Lysosomal abnormalities in degenerating neurons link neuronal compromise to senile plaque development in Alzheimer disease. Brain Res 1994, 640:68-80 [DOI] [PubMed] [Google Scholar]
- 22.Cataldo AM, Hamilton DJ, Barnett JL, Paskevich PA, Nixon RA: Properties of the endosomal-lysosomal system in the human central nervous system: disturbances mark most neurons in populations at risk to degenerate in Alzheimer’s disease. J Neurosci 1996, 16:186-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cataldo AM, Barnett JL, Mann DA, Nixon RA: Colocalization of lysosomal hydrolase and β-amyloid diffuse plaques of Alzheimer’s disease and Down’s syndrome brains. J Neuropath Exp Neurol 1996, 55:704-715 [DOI] [PubMed] [Google Scholar]
- 24.Nixon RA, Cataldo AM, Mathews PM, Mohan P: Abnormalities of the endosomal-lysosomal system in Alzheimer’s disease as potential therapeutic targets. Neurobiol Aging 1998, 19:S136 [Google Scholar]
- 25.Borchelt DR, Thinarkaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS: Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron 1996, 17:1005-1013 [DOI] [PubMed] [Google Scholar]
- 26.Mann DM, Iwatsubo T, Cairns NJ, Lantos PL, Nochlin D, Sumi SM, Bird TD, Poorkaj P, Hardy J, Hutton M, Prihar G, Crook R, Rossor MN, Haltia M: Amyloid beta protein (Abeta) deposition in chromosome 14-linked Alzheimer’s disease: predominance of Abeta42(43). Ann Neurol 1996, 40:149-156 [DOI] [PubMed] [Google Scholar]
- 27.Yang AJ, Chandswangbhuvava D, Margol L, Glabe CG: Loss of endosomal/lysosomal membrane impermeability is an early event in amyloid Abeta1–42 pathogenesis. J Neurosci Res 1998, 52:691-698 [DOI] [PubMed] [Google Scholar]
- 28.Lai F, Kammann E, Rebeck GW, Anderson A, Chen Y, Nixon RA: APOE genotype and gender effects on Alzheimer disease in 100 adults with Down syndrome. Neurology 1999, 53:331-336 [DOI] [PubMed] [Google Scholar]
- 29.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA: Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families [see comments]. Science 1993, 261:921-923 [DOI] [PubMed] [Google Scholar]
- 30.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991, 41:479-486 [DOI] [PubMed] [Google Scholar]
- 31.Mirra SS, Hart MN, Terry RD: Making the diagnosis of Alzheimer’s disease. Arch Path Lab Med 1993, 117:132-144 [PubMed] [Google Scholar]
- 32.Braak H, Braak E: Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 1991, 82:239-259 [DOI] [PubMed] [Google Scholar]
- 33.Hyman BT, West HL, Rebeck GW, Lai F, Mann DA: Neuropathological changes in Down syndrome hippocampal formation: effect of age and apolipoprotein E genotype. Arch Neurol 1995, 52:373-378 [DOI] [PubMed] [Google Scholar]
- 34.Cataldo AM, Thayer CY, Bird ED, Wheelock TR, Nixon RA: Lysosomal proteinase antigens are prominently localized within senile plaques of Alzheimer’s disease: evidence for a neuronal origin. Brain Res 1990, 513:181-192 [DOI] [PubMed] [Google Scholar]
- 35.Jicha GA, Bowser R, Kazam IG, Davies P: Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J Neurosci Res 1997, 48:128-132 [DOI] [PubMed] [Google Scholar]
- 36.Lai MM, Cavanagh D: The molecular biology of coronaviruses. Adv Virus Res 1997, 48:1-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M: Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 1990, 62:317-329 [DOI] [PubMed] [Google Scholar]
- 38.de Hoop MJ, Huber LA, Stenmark H, Williamson E, Zerial M, Parton RG, Dotti CG: The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron 1994, 13:11-22 [DOI] [PubMed] [Google Scholar]
- 39.Stenmark H, Vitale G, Ullrich O, Zerial M: Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell 1995, 83:423-432 [DOI] [PubMed] [Google Scholar]
- 40.Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H: EEA1 links PI(3)K function to Rab5 regulation of endosome fusion [see comments]. Nature 1998, 394:494-498 [DOI] [PubMed] [Google Scholar]
- 41.Gournier H, Stenmark H, Rybin V, Lippe R, Zerial M: Two distinct effectors of the small GTPase Rab5 cooperate in endocytic membrane fusion. Embo J 1998, 17:1930-1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Sluijs P, Hull M, Webster P, Male P, Goud B, Mellman I: The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 1992, 70:729-740 [DOI] [PubMed] [Google Scholar]
- 43.Slooter AJ, Houwing-Duistermaat JJ, van Harskamp F, Cruts M, Van Broeckhoven C, Breteler MM, Hofman A, Stijnen T, van Duijn CM: Apolipoprotein E genotype and progression of Alzheimer’s disease: the Rotterdam Study. J Neurol 1999, 246:304-308 [DOI] [PubMed] [Google Scholar]
- 44.Ohm TG, Scharnagl H, Marz W, Bohl J: Apolipoprotein E isoforms and the development of low and high Braak stages of Alzheimer’s disease-related lesions. Acta Neuropathol (Berl) 1999, 98:273-280 [DOI] [PubMed] [Google Scholar]
- 45.Bucci C, Wandinger-Ness A, Lutcke A, Chiariello M, Bruni CB, Zerial M: Rab5a is a common component of the apical and basolateral endocytic machinery in polarized epithelial cells. Proc Natl Acad Sci USA 1994, 91:5061-5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daro E, van der Sluijs P, Galli T, Mellman I: Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc Natl Acad Sci USA 1996, 93:9559-9564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams KR, Saunders AM, Roses AD, Armati PJ: Uptake and internalization of exogenous apolipoprotein E3 by cultured human central nervous system neurons. Neurobiol Dis 1998, 5:271-279 [DOI] [PubMed] [Google Scholar]
- 48.Trommsdorff M, Borg JP, Margolis B, Herz J: Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem 1998, 273:33556-33560 [DOI] [PubMed] [Google Scholar]
- 49.Guo Q, Sopher BL, Furukawa K, Pham DG, Robinson N, Martin GM, Mattson MP: Alzheimer’s presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J Neurosci 1997, 17:4212-4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Begley JG, Duan W, Chan S, Duff K, Mattson MP: Altered calcium homeostasis and mitochondrial dysfunction in cortical synaptic compartments of presenilin-1 mutant mice. J Neurochem 1999, 72:1030-1039 [DOI] [PubMed] [Google Scholar]
- 51.Buxbaum JD, Choi EK, Luo Y, Lilliehook C, Crowley AC, Merriam DE, Wasco W: Calsenilin: a calcium-binding protein that interacts with the presenilins and regulates the levels of a presenilin fragment [see comments]. Nat Med 1998, 4:1177-1181 [DOI] [PubMed] [Google Scholar]
- 52.Gibson GE, Vestling M, Zhang H, Szolosi S, Alkon D, Lannfelt L, Gandy S, Cowburn RF: Abnormalities in Alzheimer’s disease fibroblasts bearing the APP670/671 mutation. Neurobiol Aging 1997, 18:573-580 [DOI] [PubMed] [Google Scholar]
- 53.van Broeckhoven C: Presenilins and Alzheimer disease. Nat Genet 1995, 11:230-232 [DOI] [PubMed] [Google Scholar]
- 54.Houlden H, Crook R, Backhovens H, Prihar G, Baker M, Hutton M, Rossor M, Martin JJ, Van Broeckhoven C, Hardy J: ApoE genotype is a risk factor in nonpresenilin early-onset Alzheimer’s disease families. Am J Med Genet 1998, 81:117-121 [DOI] [PubMed] [Google Scholar]
- 55.Mattson MP, Guo Q: Cell and molecular neurobiology of presenilins: a role for the endoplasmic reticulum in the pathogenesis of Alzheimer’s disease? J Neurosci Res 1997, 50:505-513 [DOI] [PubMed] [Google Scholar]
- 56.Van Uden E, Carlson G, St. George-Hyslop P, Westaway D, Orlando R, Mallory M, Rockenstein E, Masliah E: Aberrant presenilin-1 expression downregulates LDL receptor-related protein (LRP): is LRP central to Alzheimer’s disease pathogenesis? Mol Cell Neurosci 1999, 14:129-140 [DOI] [PubMed] [Google Scholar]
- 57.Gomez-Isla T, Growden WB, McNamara MJ, Nochlin D, Bird TD, Arango JC, Lopera F, Kosik K, Lantos PL, Cairns NJ, Hyman BT: The impact of different presenilin 1 and presenilin 2 mutations on amyloid deposition, neurofibrillary changes and neuronal loss in the familial Alzheimer’s disease brain: evidence for other phenotype-modifying factors. Brain 1999, 122:1709-1719 [DOI] [PubMed] [Google Scholar]
- 58.Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P: Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer’s disease [see comments]. Ann Neurol 1994, 35:546-551 [DOI] [PubMed] [Google Scholar]
- 59.Vannucci SJ, Reinhart R, Maher F, Bondy CA, Lee WH, Vannucci RC, Simpson IA: Alterations in GLUT1 and GLUT3 glucose transporter gene expression following unilateral hypoxia-ischemia in the immature rat brain. Brain Res Dev Brain Res 1998, 107:255-264 [DOI] [PubMed] [Google Scholar]
- 60.Frautschy SA, Horn DL, Sigel JJ, Harris-White ME, Mendoza JJ, Yang F, Saido TC, Cole GM: Protease inhibitor coinfusion with amyloid beta-protein results in enhanced deposition and toxicity in rat brain. J Neurosci 1998, 18:8311-8321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maher F, Simpson IA: The GLUT3 glucose transporter is the predominant isoform in primary cultured neurons: assessment by biosynthetic and photoaffinity labelling. Biochem J 1994, 301:379-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nixon RA, Cataldo AM: The lysosomal system in neuronal cell death: a review. Ann N Y Acad Sci 1993, 679:87-109 [DOI] [PubMed] [Google Scholar]
- 63.Brunk UT, Dalen H, Roberg K, Hellquist HB: Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic Biol Med 1997, 23:616-626 [DOI] [PubMed] [Google Scholar]
- 64.Fossel ET, Zanella CL, Fletcher JG, Hui KK: Cell death induced by peroxidized low-density lipoprotein: endopepsis. Cancer Res 1994, 54:1240-1248 [PubMed] [Google Scholar]
- 65.Horner HC, Packan DR, Sapolsky RM: Glucocorticoids inhibit glucose transport in cultured hippocampal neurons and glia. Neuroendocrinology 1990, 52:57-64 [DOI] [PubMed] [Google Scholar]
- 66.Roberg K, Ollinger K: Oxidative stress causes relocation of the lysosomal enzyme cathepsin D with ensuing apoptosis in neonatal rat cardiomyocytes. Am J Pathol 1998, 152:1151-1156 [PMC free article] [PubMed] [Google Scholar]
- 67.Vassar R, Bennett BD, Babu-Khan S, Khan S, Mendiaz EA, Denis P, Teplow D, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis J-C, Collins F, Treanor J, Rogers G, Citron M: β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286:735-741 [DOI] [PubMed] [Google Scholar]
- 68.Nixon RA, Mathews PM, Mohan P, Beard M, Guerra CB, Duff K, Yang A, Cataldo AM: Endosomal-Lysosomal System Abnormalities Differentiate Subtypes of Alzheimer’s Disease: Lysosomal System Activation in Trangenic Mouse Models of Familial AD. 1999. New Mexico, Keystone Conference, Taos
- 69.Sinha S, Lieberburg I: Cellular mechanisms of beta-amyloid production and secretion. Proc Natl Acad Sci USA 1999, 96:11049-11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siman R: Proteolytic mechanism for the neurodegeneration of Alzheimer’s disease. Ann N Y Acad Sci 1992, 674:193-202 [DOI] [PubMed] [Google Scholar]
- 71.Siman R, Mistretta S, Durkin JT, Savage MJ, Loh T, Trusko S, Scott RW: Processing of the beta-amyloid precursor. Multiple proteases generate and degrade potentially amyloidogenic fragments. J Biol Chem 1993, 268:16602-16609 [PubMed] [Google Scholar]
- 72.Schrader-Fischer G, Paganetti PA: Effect of alkalizing agents on the processing of the beta-amyloid precursor protein. Brain Res 1996, 716:91-100 [DOI] [PubMed] [Google Scholar]
- 73.Selkoe DJ, Yamazaki T, Citron M, Podlisny MB, Koo EH, Teplow DB, Haass C: The role of APP processing and trafficking pathways in the formation of amyloid beta-protein. Ann N Y Acad Sci 1996, 777:57-64 [DOI] [PubMed] [Google Scholar]