Abstract
Dilations to endothelium-derived hyperpolarizing factor (EDHF) are significantly attenuated in the middle cerebral artery (MCA) isolated from female compared to male rats. Since gap junctions appear to be involved in the EDHF pathway and cAMP has been shown to enhance gap junction permeability, we tested the hypothesis that elevation of cAMP would enhance EDHF-mediated dilations in female rat MCA. Vascular diameter was measured in perfused MCA segments using videomicroscopy in the presence and absence of IBMX, an inhibitor of cAMP phosphodiesterase.
In the presence of L-NAME and indomethacin, dilation to 10−4M ATP was significantly reduced in females (48±12%) compared to males (92±2%). IBMX, an inhibitor of cAMP phosphodiesterase, had no significant effect on ATP-mediated dilations in both males and females. Basal cAMP levels were comparable in male and female MCAs (1.7 pmol/mg protein). Incubation with IBMX (2X10−4M) significantly elevated cAMP in both male (12.8 pmol/mg protein) and female (11.2 pmol/mg protein) MCAs.
Our results demonstrate that reduced EDHF dilations in female rat MCA cannot be solely attributed to impaired cAMP signaling. Future studies will target other potential sites along the EDHF pathway in order to identify why EDHF dilations are reduced in the female compared to the male rat MCA.
Keywords: cAMP, endothelium-derived hyperpolarizing factor, gender, rat, vascular smooth muscle
1. Introduction
In rat middle cerebral artery (MCA), dilations mediated by endothelium-derived hyperpolarizing factor (EDHF) are significantly attenuated in the female compared to the male (Golding and Kepler, 2001). The underlying reason for this is as yet unknown. While the mechanisms contributing to EDHF dilations in the male rat MCA remain incompletely described, we know that there is an increase in endothelial cell (EC) calcium (Marrelli, 2001), followed by activation of intermediate conductance calcium-sensitive potassium channels, EC hyperpolarization (Marrelli et al., 2003) and subsequent hyperpolarization of the smooth muscle cell (SMC) (You et al., 1999).
In previous studies, we predicted that EC calcium may not reach the required levels in females in order to produce a robust dilation. However, contrary to our hypothesis, we demonstrated that both male and female EC calcium increases to comparable levels during an EDHF response (Golding et al., 2002). These results would suggest that in females, uncoupling of the EDHF mechanism occurs downstream from EC calcium. In this same study, 15mM KCl produced vessel dilation and a decrease in SMC calcium similar to that seen in males, suggesting that when the EC-SMC coupling mechanism is bypassed, the SMC can indeed hyperpolarize and produce vasodilation.
Recently our laboratory has provided both functional and ultrastructural evidence in male rat MCA, implicating myoendothelial gap junctions in communicating the hyperpolarization from the EC to the SMC (Sokoya et al., 2006). Gap peptides (inhibitors of the connexin proteins that comprise gap junctions) nearly completely abolished EDHF dilation and SMC hyperpolarization while EC hyperpolarization was maintained. Rather than being a stagnant bridge between two cells, gap junctions are gated by many factors including pH, voltage and calcium (Spray et al., 2002). Recent studies suggest that cAMP enhances gap junction permeability (Abudara et al., 2000; Burghardt et al., 1995; van Rijen et al., 2000) and EDHF-mediated dilations (Griffith et al., 2002). Since myoendothelial gap junctions appear to be instrumental in the EDHF dilation (Sokoya et al., 2006) and cAMP enhances the electrical conductance of gap junctions (Abudara et al., 2000; Burghardt et al., 1995), we speculated that cAMP signaling may be impaired during EDHF dilations in MCA isolated from females. We tested the hypothesis that elevation of cAMP would enhance EDHF-mediated dilations in female rat MCA.
2. Results
EDHF-Mediated Dilations
Maximal vessel diameter as determined in calcium-free PSS was similar between males and females (males, 292±6 um, n=13; females, 296±6 um, n=16). Development of spontaneous tone was also comparable between groups (males, 25±1%, n=13; females, 21±1%, n=16) as was the constriction to L-NAME and indomethacin (males, 32±1%, n=13; females, 36±6%, n=16; calculated with respect to the maximum diameter). Following IBMX treatment there was a reduction in tone (Table 1) however MCA diameter returned to pre-IBMX levels following abluminal exposure to UTP. In control MCAs, DMSO had no significant effect on vessel diameter.
Table 1.
Absolute values of male and female middle cerebral artery diameters after equilibration (Tone) and after addition of L-NAME (3x10−5M), indomethacin (10−5M) and either vehicle (DMSO) or IBMX (2x10−4M).
| Experimental Group | Tone (um) | L-NAME and indomethacin (um) |
|---|---|---|
| Male, DMSO (n=7) | 210±4 | 191±5 |
| Male, IBMX (n=6) | 227±8 | 307±8* |
| Female, DMSO (n=8) | 228±4 | 179±6 |
| Female, IBMX (n=8) | 231±11 | 301±9* |
p<0.05 compared to pre IBMX (one-way ANOVA).
EDHF-mediated dilation in response to luminal delivery of ATP showed a dose-dependent dilation in male MCAs while the dilation in female MCAs was significantly less (Figure 1). The maximum dilation to ATP (10−4M) was 92±2% in males compared to 48±12% in females (p<0.05; 2-way RM ANOVA). IBMX had no significant effect on ATP-mediated EDHF dilations in both males (Figure 2) and females (Figure 3). 2’,5’-dideoxyadenosine, a cell permeable inhibitor of adenylate cyclase, also had no effect on ATP-mediated EDHF dilations in males and females (data not shown).
Figure 1.
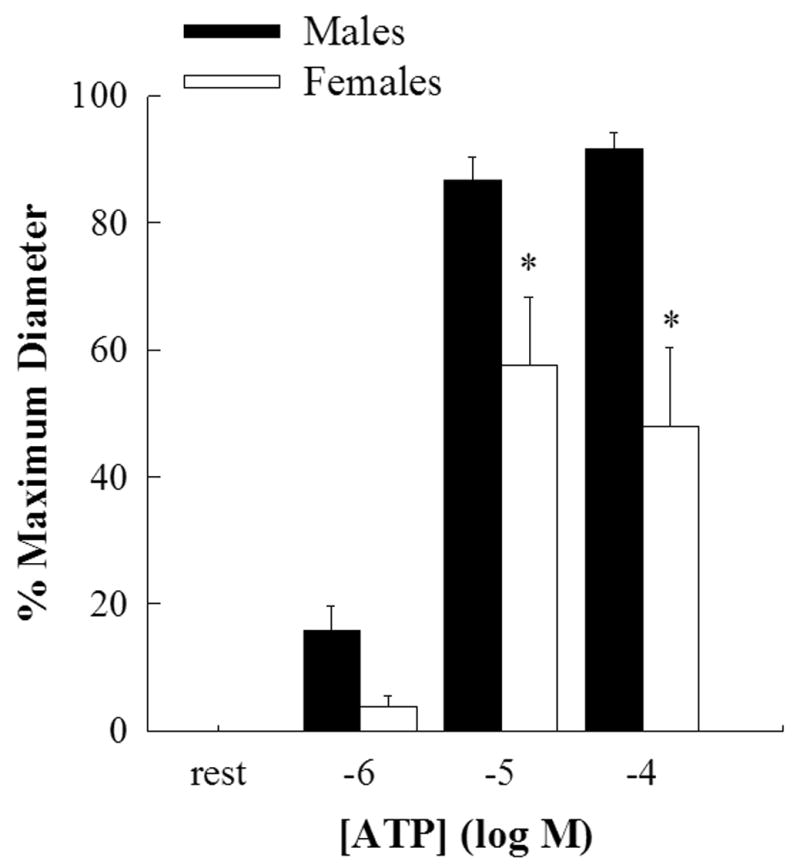
Bar graph showing the concentration response curve for EDHF mediated vasodilation evoked by luminal ATP in the presence of L-NAME and indomethacin. Male (closed bars, n=7) and female (open bars, n=8) middle cerebral arteries were incubated in DMSO for 40 mins. Dilations to 10−5M and 10−4M ATP were significantly reduced in female compared to male MCAs (*p<0.05; 2-way repeated measures ANOVA).
Figure 2.
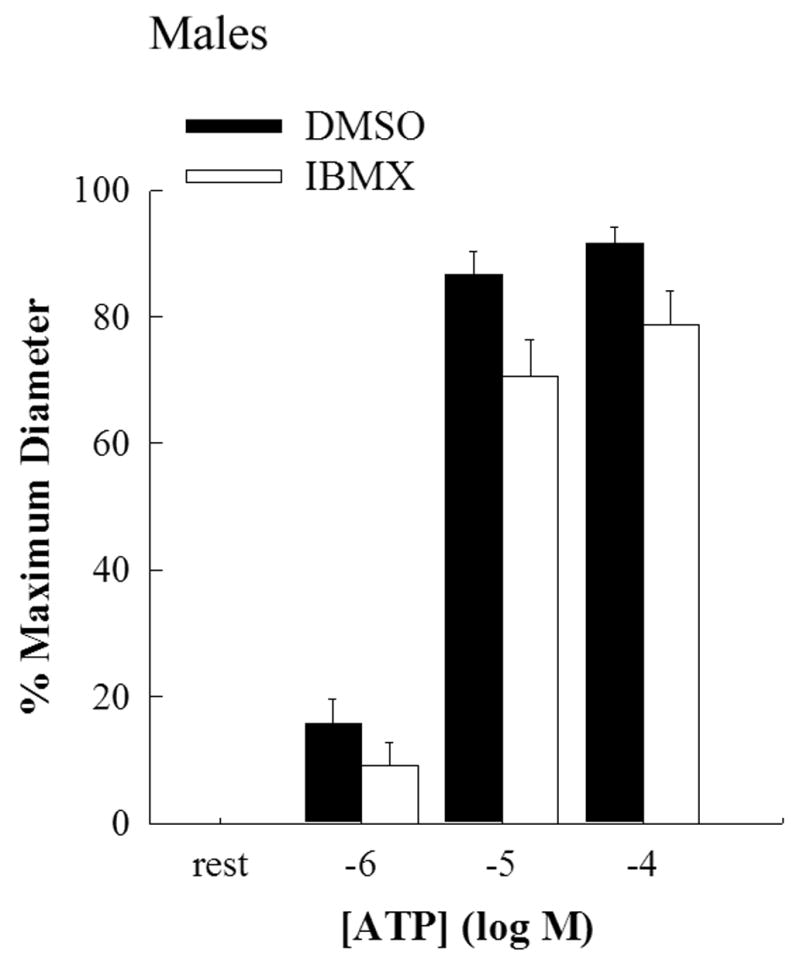
Bar graph showing the concentration response curve for EDHF mediated vasodilation evoked by luminal ATP in the presence of L-NAME and indomethacin in male middle cerebral arteries. Incubation with IBMX (2X10−4M; open bars; n=6) for 40 mins had no significant effect on EDHF mediated dilation compared to incubation with DMSO (closed bars; n=7).
Figure 3.
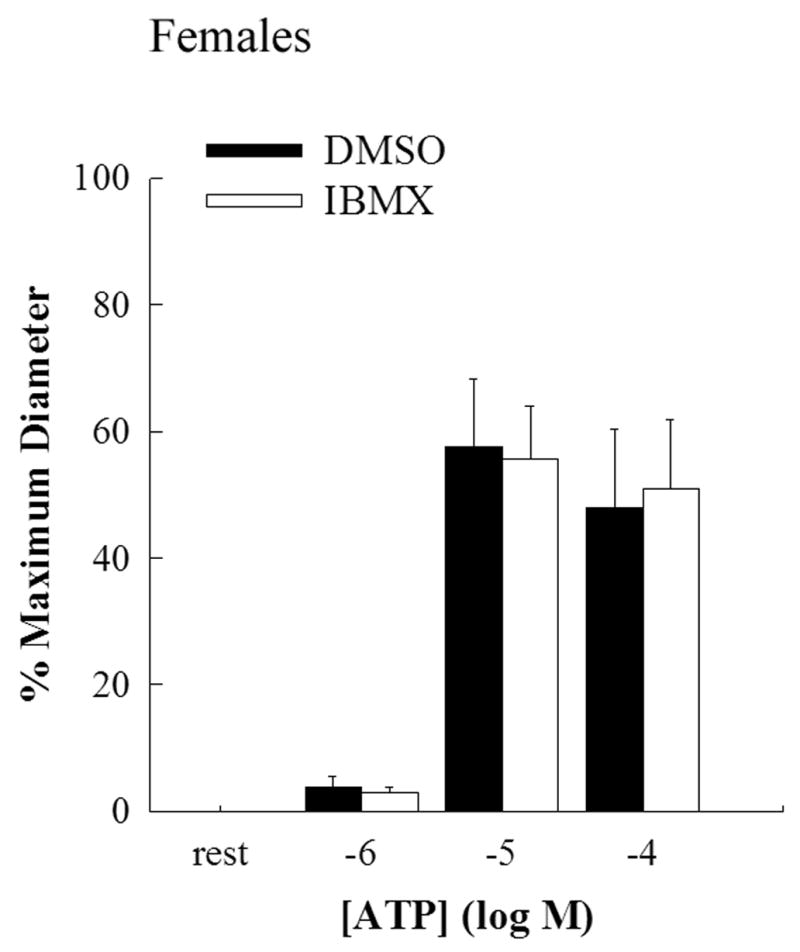
Bar graph showing the concentration response curve for EDHF mediated vasodilation evoked by luminal ATP in the presence of L-NAME and indomethacin in female middle cerebral arteries. Incubation with IBMX (2X10−4M; open bars; n=8) for 40 mins had no significant effect on EDHF mediated dilation compared to incubation with DMSO (closed bars; n=8).
cAMP Accumulation
cAMP levels were measured in pooled MCAs from male and female rats. Basal cAMP levels were comparable in males and females (1.7 pmol/mg protein in both groups). Following a 40 min incubation with IBMX (2x10−4M), cAMP was significantly elevated to similar levels in males (12.8 pmol/mg protein) and females (11.2 pmol/mg protein). This reflects a 7.5 fold and 6.6 fold increase in cAMP accumulation in the presence of IBMX in males and females, respectively.
3. Discussion
In the present study we demonstrated that elevations in cAMP did not restore EDHF-mediated dilations in the female rat MCA. We have also confirmed previous findings showing that EDHF-mediated dilations are reduced in the female compared to the male rat MCA. Our data showing reduced EDHF dilations in the female rat MCA is consistent with previous reports both in vitro (Golding et al., 2002; Golding and Kepler, 2001) and in vivo (Xu et al., 2001).
There is evidence to suggest that gap junctions may play a role in the impaired EDHF dilations in the female rat MCA. First, the upregulation of EDHF effects in ovariectomized females involves gap junctions (Xu et al., 2002) and second, gap junctions appear to be instrumental in the EDHF dilation in males (Sokoya et al., 2006). Recent studies suggest that cAMP facilitates EDHF dilations by enhancing gap junctional communication in the rabbit iliac artery (Griffith et al., 2002) and the rat mesenteric artery (Matsumoto et al., 2004). However the present studies suggest that this is not the case in the rat MCA since incubation with IBMX had no effect on EDHF dilations in the male or female rat MCA (Figures 2 and 3). In order to elevate cAMP levels, vessels were incubated with IBMX, an inhibitor of cAMP phosphodiesterase, the enzyme responsible for the breakdown of cAMP to 5’-AMP. IBMX is also known to inhibit cGMP phosphodiesterases (Elks and Manganiello, 1984) however we can rule out a potential effect of cGMP hydrolysis inhibition since we have previously shown that EDHF-mediated dilations in the male rat MCA does not involve cGMP using the guanylate cyclase inhibitor, ODQ (You et al., 1999). In our study, incubation with 200uM IBMX resulted in a significant increase in cAMP concentration in both male and female MCAs. This increase in cAMP was similar to that reported in rabbit iliac arteries where enhancement of dye transfer between endothelium and smooth muscle was reported (Griffith et al., 2002). Therefore, one would predict that cAMP levels in our preparation would have been sufficient to enhance gap junction conductivity. Although we did not directly assess gap junction conductivity, previous studies have shown that gap junction channels comprised of Cx40 are modulated by cAMP (van Rijen et al., 2000) and our studies have shown that Cx40 comprises gap junction channels in the rat MCA (Sokoya et al., 2006). Myoendothelial gap junctions have been shown to comprise both Cx37 and Cx40 in the rat basilar artery (Haddock et al., 2006) and if this was the case in the rat MCA, the heteromeric nature of the gap junction channels may adversely affect their modulation by cAMP.
To our knowledge, no other study has investigated the effect of sex on cAMP signaling during EDHF-mediated vasodilations. Interestingly, the documented effect of estradiol on cAMP is widely varying. Acute exposure to estradiol had been shown to inhibit cAMP production in ventricular myocytes (Li et al., 2000) and to enhance cAMP in human coronary artery rings (Mugge et al., 1993).
In conclusion, the results of our study demonstrate that the reduced EDHF dilations in the female rat MCA cannot be solely attributed to impaired cAMP signaling. Future studies will target other potential sites along the EDHF pathway in order to identify why EDHF dilations are reduced in the female compared to the male rat MCA.
4. Experimental Procedure
Experiments were carried out in accordance with NIH guidelines for the care and use of laboratory animals and were approved by the Animal Protocol Review Committee at Baylor College of Medicine. Rats were housed under a 12 h light/12 h dark cycle with unrestricted access to food and water. Experiments were performed on age-matched (70–90 days old) male (n=17) and female (n=18) Long-Evans rats.
Harvesting and Mounting Cerebral Vessels
Animals were placed in an anesthetic chamber, allowed to spontaneously breathe isoflurane and then decapitated. The brain was removed from the cranium and placed in cold physiological salt solution (PSS). The middle cerebral artery (MCA) was excised, cleaned of surrounding connective tissue and cannulated with micropipettes in a vessel chamber. PSS was circulated abluminally through a heat-exchanger in order to maintain the bath temperature at 37°C. Monitoring of intraluminal pressure was performed via inline transducers, which were connected to two strain gauge panel meters (Omega, Stamford, CT). Once mounted, vessels were tested for leaks and those that did not maintain a steady pressure were discarded. The vessel chamber was mounted on the stage of an inverted microscope. Transmural pressure was set at 85 mmHg with a flow of 100 μl/min through the lumen, and the vessels allowed to equilibrate for 1 h. During this time they developed spontaneous tone by constricting from their fully dilated diameter at initial pressurization. After the development of tone, the experiment was initiated (see EDHF-Mediated Dilations). Changes in vessel diameter were observed with an inverted microscope (Nikon) equipped with a video camera and monitor. Outer diameters were measured directly on-line from the video monitor (final magnification 600x). Dynamic changes in vessel diameter were digitized (post-hoc) using image-analysis software (Optimas, Bothell, WA), allowing an acquisition frequency of 1.1 Hz.
EDHF-Mediated Dilations
Following the development of spontaneous tone, the luminal and abluminal compartments were exposed to L-NAME (3x10−5M) and indomethacin (10−5M) for 30 mins. Vessels were randomly selected to be exposed to either IBMX (2x10−4M) or vehicle (DMSO) in the luminal and abluminal baths for 40 mins. In those vessels incubated with IBMX, UTP (3X10−5M) was added to the abluminal compartment in order to constrict the vessel to pre-IBMX levels.
EDHF-mediated dilations were assessed by performing a concentration response curve (CRC) to luminal application of ATP (10−6 to 10−4M). Experiments were terminated by replacing PSS with calcium-free PSS containing 1 mM EGTA in order to obtain the maximum diameter of the vessel.
cAMP Radioimmunoassay
MCAs were incubated for 40 mins at 37°C in either IBMX (2x10−4M) or DMSO, immediately frozen in liquid N2 and stored at −80°C. Pilot studies demonstrated that basal cAMP concentration in a single MCA was below the lower limit of detection of the radioimmunoassay. We therefore combined 10 MCAs from 5 rats in each treatment group. Pooled MCAs were homogenized in 6% trichloroacetic acid, centrifuged and the pellet was frozen for the subsequent measurement of protein (modified Lowry assay). The supernatant was neutralized with water-saturated diethyl ether and cAMP was measured using a cAMP [125I] Biotrak Assay System (Amersham Biosciences). [cAMP] was expressed as pmol/mg protein.
Reagents and Buffers
All chemicals were purchased from Sigma (St Louis, MO, USA). PSS buffer contained (in mM): 119 NaCl, 19.2 NaHCO3, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4, 1.6 CaCl2, 5.5 glucose and 0.026 EDTA. Stock solutions of ATP (10-2M), UTP (10-2M) and L-NAME (3x10−2M) were prepared in distilled water, a stock solution of indomethacin (10−2M) was prepared in a solution of Na2CO3 and distilled water (1:1 by weight) and a stock solution of IBMX (6x10−2M) was prepared in DMSO. All stock solutions were aliquotted and frozen.
Data Analysis and Calculations
Data are presented as mean±sem. Diameter measurements were averaged over a 5 minute period immediately following luminal exposure to ATP. Changes in vascular diameter are presented as a percentage of the maximum diameter of MCAs, as described previously (Golding and Kepler, 2001).
Statistical comparisons of the concentration-response curves to ATP were performed using a two-way analysis of variance with repeated measures and all pairwise multiple comparison procedures were made using the Holm-Sidak method. Differences were considered significant at error probabilities less than 0.05 (p<0.05).
Acknowledgments
This work was supported by the National American Heart Association Scientist Development Grant 0130250N and the National Institutes of Health HL72954 (both awarded to EMS).
Abbreviations
- EC
endothelial cell
- EDHF
endothelium-derived hyperpolarizing factor
- MCA
middle cerebral artery
- PSS
physiological salt solution
- SMC
smooth muscle cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abudara V, Eyzaguirre C, Saez JC. Short- and long-term regulation of rat carotid body gap junctions by cAMP. Identification of connexin43, a gap junction subunit. Adv Exp Med Biol. 2000;475:359–69. doi: 10.1007/0-306-46825-5_33. [DOI] [PubMed] [Google Scholar]
- Burghardt RC, Barhoumi R, Sewall TC, Bowen JA. Cyclic AMP induces rapid increases in gap junction permeability and changes in the cellular distribution of connexin43. J Membr Biol. 1995;148:243–53. doi: 10.1007/BF00235042. [DOI] [PubMed] [Google Scholar]
- Elks ML, Manganiello VC. Selective effects of phosphodiesterase inhibitors on different phosphodiesterases, adenosine 3',5'-monophosphate metabolism, and lipolysis in 3T3-L1 adipocytes. Endocrinology. 1984;115:1262–8. doi: 10.1210/endo-115-4-1262. [DOI] [PubMed] [Google Scholar]
- Golding EM, Ferens DM, Marrelli SP. Altered calcium dynamics do not account for attenuation of endothelium-derived hyperpolarizing factor-mediated dilations in the female middle cerebral artery. Stroke. 2002;33:2972–7. doi: 10.1161/01.str.0000035907.82204.39. [DOI] [PubMed] [Google Scholar]
- Golding EM, Kepler TE. Role of estrogen in modulating EDHF-mediated dilations in the female rat middle cerebral artery. Am J Physiol Heart Circ Physiol. 2001;280:H2417–23. doi: 10.1152/ajpheart.2001.280.6.H2417. [DOI] [PubMed] [Google Scholar]
- Griffith TM, Chaytor AT, Taylor HJ, Giddings BD, Edwards DH. cAMP facilitates EDHF-type relaxations in conduit arteries by enhancing electrotonic conduction via gap junctions. Proc Natl Acad Sci U S A. 2002;99:6392–7. doi: 10.1073/pnas.092089799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, Hill CE. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol Heart Circ Physiol. 2006;291:H2047–56. doi: 10.1152/ajpheart.00484.2006. [DOI] [PubMed] [Google Scholar]
- Li HY, Bian JS, Kwan YW, Wong TM. Enhanced responses to 17beta-estradiol in rat hearts treated with isoproterenol: involvement of a cyclic AMP-dependent pathway. J Pharmacol Exp Ther. 2000;293:592–8. [PubMed] [Google Scholar]
- Marrelli SP. Mechanisms of endothelial P2Y(1)- and P2Y(2)-mediated vasodilatation involve differential [Ca2+]i responses. Am J Physiol Heart Circ Physiol. 2001;281:H1759–66. doi: 10.1152/ajpheart.2001.281.4.H1759. [DOI] [PubMed] [Google Scholar]
- Marrelli SP, Eckmann MS, Hunte MS. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am J Physiol Heart Circ Physiol. 2003;285:H1590–9. doi: 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Wakabayashi K, Kobayashi T, Kamata K. Diabetes-related changes in cAMP-dependent protein kinase activity and decrease in relaxation response in rat mesenteric artery. Am J Physiol Heart Circ Physiol. 2004;287:H1064–71. doi: 10.1152/ajpheart.00069.2004. [DOI] [PubMed] [Google Scholar]
- Mugge A, Riedel M, Barton M, Kuhn M, Lichtlen PR. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc Res. 1993;27:1939–42. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- Sokoya EM, Burns AR, Setiawan CT, Coleman HA, Parkington HC, Tare M. Evidence for the involvement of myoendothelial gap junctions in EDHF-mediated relaxation in the rat middle cerebral artery. Am J Physiol Heart Circ Physiol. 2006;291:H385–93. doi: 10.1152/ajpheart.01047.2005. [DOI] [PubMed] [Google Scholar]
- Spray DC, Suadicani SO, Srinivas M, Gutstein DE, Fishman GI. Gap junctions in the cardiovascular system. In: Page E, Fozzard HA, Solaro RJ, editors. Handbook of Physiology -The Heart. I. Oxford University Press; 2002. [Google Scholar]
- van Rijen HV, van Veen TA, Hermans MM, Jongsma HJ. Human connexin40 gap junction channels are modulated by cAMP. Cardiovasc Res. 2000;45:941–51. doi: 10.1016/s0008-6363(99)00373-9. [DOI] [PubMed] [Google Scholar]
- Xu HL, Santizo RA, Baughman VL, Pelligrino DA. ADP-induced pial arteriolar dilation in ovariectomized rats involves gap junctional communication. Am J Physiol Heart Circ Physiol. 2002;283:H1082–91. doi: 10.1152/ajpheart.00031.2002. [DOI] [PubMed] [Google Scholar]
- Xu HL, Santizo RA, Koenig HM, Pelligrino DA. Chronic estrogen depletion alters adenosine diphosphate-induced pial arteriolar dilation in female rats. Am J Physiol Heart Circ Physiol. 2001;281:H2105–12. doi: 10.1152/ajpheart.2001.281.5.H2105. [DOI] [PubMed] [Google Scholar]
- You J, Johnson TD, Marrelli SP, Mombouli JV, Bryan RM., Jr P2u receptor-mediated release of endothelium-derived relaxing factor/nitric oxide and endothelium-derived hyperpolarizing factor from cerebrovascular endothelium in rats. Stroke. 1999;30:1125–33. doi: 10.1161/01.str.30.5.1125. [DOI] [PubMed] [Google Scholar]


