Abstract
Multiple subsets of the bone marrow contain T cell precursors but it remains unclear which is most likely to replenish the adult thymus. Therefore, RAG-1+ early lymphoid progenitors (RAG-1+ ELP), and CD62L/L-selectin+ progenitors (LSP), as well as common lymphoid progenitors (CLP) from C57BL6-Thy1.1-RAG-1/GFP mouse bone marrow were directly compared in transplantation assays. The two cKitHi populations vigorously regenerated the thymus and were superior to CLP in magnitude and frequency of thymic reconstitution. Regeneration was much faster than the 22 days described for transplanted stem cells, and RAG-1+ ELP produced small numbers of lymphocytes within 13 days. As previously reported, LSP were biased to a T cell fate, but this was not the case for RAG-1+ ELP. While RAG-1+ ELP and LSP had reduced myeloid potential, they were both effective progenitors for T lymphocytes and natural killer (NK) cells. The LSP subset overlapped with and included most RAG-1+ ELP and many RAG-1– terminal deoxynucleotidyl transferase (TdT) positive ELP. LSP and RAG-1+ ELP were both present in the peripheral circulation but RAG-1+ ELP had no exact counterpart among immature thymocytes. The most primitive of thymocytes were similar to Lin− cKitHi L-selectin+ TdT+ RAG-1– progenitors present in the marrow, suggesting that this population is normally important for sustaining the adult thymus.
Keywords: lymphoid progenitor, T cells, thymus, bone marrow, differentiation
Introduction
The thymus is remarkable in its ability to produce large numbers of lymphocytes without a resident population of renewing stem cells. Progenitors within the thymus can maintain thymocytopoiesis for only a short period, and must be replenished by marrow cells (1). These progenitors are thought to be rare and could be discharged in periodic fashion into the blood stream (2). The precise nature of those progenitors remains unclear and a number of distinct types of bone marrow cells can produce T lineage cells under experimental circumstances (3). This is especially the case when an OP9-Delta-like 1 stromal cell co-culture system is used to make T lineage lymphocytes (4–6). Multiple markers, reporters and gating parameters have been used to resolve those marrow subsets, and it has been difficult to reach consensus about which ones most efficiently colonize the thymus under normal circumstances. We have now performed side by side comparisons of three categories of bone marrow cells with T lymphopoietic potential.
Developmental relationships between multipotent hematopoietic stem cells (HSC) in the marrow and T-lineage committed cells in the thymus have long been investigated (7–9). HSC are known to be present in the circulation and could enter the thymus (10,11). HSC start producing thymocytes three weeks following either intravenous or intrathymic transplant (12,13); however, cells with stem cell properties have not been found in the thymus (8,11,14,15). This observation and the fact that other marrow fractions can more rapidly seed the thymus under experimental conditions suggest that HSC are not directly responsible for maintaining thymocytopoiesis (12,16). Our focus was on testing marrow cells that rapidly generate large numbers of thymocytes, and on assessing the loss of other differentiation options.
Of the many categories of lymphoid progenitors that have been identified in bone marrow, the most robust thymus repopulating ones have been found among a primitive Lin− Sca-1+ c-KitHi (LSK) multipotent progenitor fraction. Early lymphoid progenitors (ELP) were originally defined as a hormone sensitive, Flt3/Flk-2+ CD27+ subset of the Lin– Sca1+ cKitHi compartment (17). They represent the most primitive cells to express lymphoid-restricted genes such as EBF, terminal deoxynucleotidyl transferase (TdT), RAG-1 or RAG-2 (18). Although ELP are heterogeneous with respect to the combined expression of these genes, RAG-1 represents a useful parameter for sorting viable cells from RAG-1/GFP mice (18). While not firmly lymphoid committed, they are lymphoid specified, and have greatly reduced non-lymphoid differentiation potential when compared to otherwise similar RAG-1− cells. In addition, ELP require much longer intervals to generate CD19+ lymphocytes in culture than Lin− c-KitLo progenitors (18). Of particular importance, RAG-1+ ELP were more effective and persistent T cell precursors than were cKitLo RAG-1+ pro-lymphocytes (18).
Consistent with these findings, Jacobsen and colleagues found effective lymphoid progenitors among LSK with the highest density of Flt3/Flk-2+ (19). When compared to HSC, this fraction had greatly reduced erythroid differentiation potential. Kondo and colleagues found that down regulation of VCAM-1 on LSK coincided with progression of HSC through the multipotent progenitor stage to become ELP (20). Cloning and transplantation assays revealed that this transition was accompanied by substantial loss of non-lymphoid differentiation potential.
Long and short term repopulating stem cells in a particular congenic strain of mice express the CD90.1/Thy1.1 marker (12,21–23). Comparison of progressively smaller bone marrow subsets showed that thymic repopulating capacity was enriched in the Sca1+ cKit+ Thy1.1– bone marrow compartment (12). Testing with a panel of antibodies revealed that the L-selectin adhesion molecule further defined primitive subsets with lymphoid potential. Intravenous transfer of marrow subsets into sub-lethally irradiated mice showed that Lin– Sca1+ cKitHi Thy1.1– L-selectin+ (termed L-selectin+ progenitors, LSP) are effective thymus repopulating cells. LSP defined in this way represent approximately 17% of the LSK fraction (16) (and our unpublished observations). Moreover, they are distinctly better T than B cell progenitors (16).
CLP were originally isolated as Lin– Sca1+ cKitLo Thy1.1– IL7Rα + cells with potential for T, B and NK cells (23). However, primitive cells are present in the thymus of Ikaros knockout mice that have no CLP, and several studies have shown that primitive thymocytes express little IL7Rα (14,24). CLP removed from bone marrow and transferred intrathymically matured faster than cells present in the thymus (23,24).
Benz and Bleul recently used a chemokine receptor reporter strain to isolate bone marrow cells with T lineage potential (25,26). While some GFP marked marrow cells were among the LSK subset, most of them were c-KitLo and expressed IL-7Rα. That is, they had overlapping properties with CLP.
Von Boehmer and colleagues identified more differentiated c-Kit− CD45R/B220+ CD19− IL-7Rα + progenitors in pre-Tα reporter transgenics (27,28). Although not directly compared to LSK subsets, these common lymphoid progenitor 2 (CLP2) cells homed to the thymus on transplantation and had B lineage potential.
Clues to the nature of thymus-replenishing cells have also been sought by characterizing primitive progenitors resident within this organ. The most primitive thymocytes lack markers associated with all hematopoietic lineages (Lin–), including CD4 and CD8 (double negative, DN) (29–32). Furthermore, a consensus has developed that these early thymic progenitors (ETP) express high levels of c-Kit (9,24,33,34). The primitive cKitHi ETP are uniformly L-selectin+(16,35), and regenerating thymocytes in irradiated mice are initially Thy1– (13). As noted above, ETP express little IL-7Rα , but all are Sca-1+ and ones that retain some B potential are Flt3/Flk-2+ (3,14,15,24,33,36). Thymocytes with high levels of CCR9 reporter activity are not T lineage restricted and also produce B, dendritic and myeloid cells(25,26). The CD44+ CD25– DN1 category can also be divided into five subsets on the basis of CD117 (cKit), and CD24 (HSA) expression (14,24,33). Two c-KitHi subsets, designated DN1a and DN1b were potent T progenitors, but had little if any B lineage potential (14). Therefore, thymus colonizing cells would likely be Lin– CD24– CD25– Thy1– Sca1+ cKitHi Flt3/Flk-2+ CD44+ L-selectin+ unless they quickly change their characteristics on entry to the thymus. Once arriving, effective T precursors must be capable of rapid and robust expansion to accommodate the rate of lymphocyte production intrinsic to that organ (1,13,37,38).
We have now crossed C57BL6 (B6)-RAG-1/GFP mice to B6-Thy1.1 animals so that ELP and LSP, as well as CLP could be isolated and compared. An experimental model was used where transplanted cells home and compete with host progenitors for limited niches in thymuses of sub-lethally irradiated mice. While a range of marrow cells ability to produce T cells, our findings support reports that most thymic engraftment potential is concentrated among the LSK fraction of bone marrow. While progenitors previously designated LSP are have some T versus B lineage biased, they include RAG-1+ ELP that are effective at restoring both cell types. However, none of the primitive ETP category of thymocytes express RAG-1 and ELP may only replenish the thymus under circumstances of special need.
Materials and Methods
Mice
C57BL6 (B6; CD45.2 alloantigen), B6-Thy1.1, BALB/c, and B6-SJL (CD45.1 alloantigen) mice (Jackson, Bar Harbor, ME) were bred and maintained in the Laboratory Animal Resource Center of the Oklahoma Medical Research Foundation. B6-Thy1.1 were crossed with B6-RAG-1/GFP knock-in (39) mice to produce animals expressing Thy1.1, RAG-1/GFP, and the CD45.2 alloantigen.
Isolation of Cell Populations and Flow Cytometry
Cell manipulations were performed in Hanks’ balanced salt solution with 5% fetal calf serum. Marrow cells were isolated from the long bones of donor mice, and erythrocytes were lysed by briefly resuspending in NH4Cl–/K+ hypotonic solution. Progenitors were further enriched by labeling marrow with RB6-8C5 (Ly6G+C), M1/70 (CD11b), TER-119, RM2-5 (CD2), 17A2 (CD3), 53–7.3 (CD5), 53–6.7 (CD8), 1D3 (CD19), RA3-6B2 (B220), and then immunomagnetically depleting cells (DynaTech, Oslo). Because of the possibility of early or passive expression of CD4, this was not used as a lineage depletion parameter. Lineage negative cells were stained with IL7Rα Phycoerythrin (PE; SB/14), Thy1.1 Biotin (Bio; OX-7; secondary Streptavidin PE-TexasRed; Caltag, Burlingame, CA), Sca-1 PE-Cy5 (D7, eBioscience, San Diego, CA), cKit Allophycocyanin (APC; 2B8), and L-selectin APC-Cy7 (MEL-14, eBioscience), then separated using a FACSAria Flow Cytometer (BD Biosciences, San Jose, CA) into specific populations. Dead cells were excluded by propidium iodide staining (Molecular Probes, Eugene, OR). Cells harvested were stained with antibodies to determine the phenotypes of engrafted cells. Antibodies included 145-2C11 PE (CD3), GK1.5 PE (CD4), 53-6.7 APC, HL3 PE (CD11c), 1D3 APC, 1A8 PE (Ly-6G), PK136 APC (NK1.1), RA3-6B2 APC, AL-21 Bio (Ly6C), and 104 FITC (CD45.2). Analysis was done on a FACSCalibur (BD Biosciences). For TdT expression, progenitor populations were fixed with 5% acetic acid in ethanol, then incubated with rabbit anti-TdT IgG (SuperTechs, Inc., Rockville, MD). Cells were illuminated with PE-conjugated goat anti-rabbit IgG (Caltag, Burlingame, CA). All antibodies came from BD Pharmingen, (San Diego, CA) unless otherwise stated. Purification of each lymphocyte subset was done according to published parameters (16,18,23) and was confirmed in each experiment by post-sorting analyses (see Fig. 1).
Fig. 1. Isolation of three categories of lymphoid progenitors.
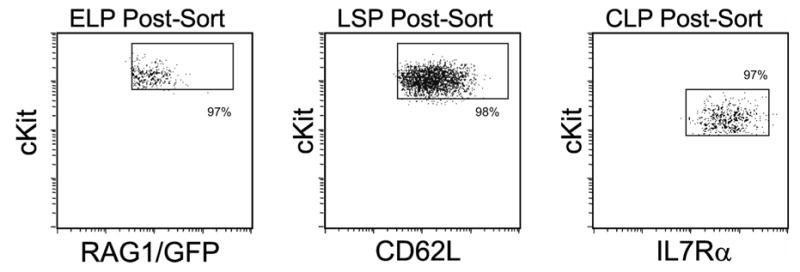
As detailed in the Materials & Methods, bone marrow suspensions were depleted of lineage positive cells and then sorted as early lymphoid progenitors (ELP; Lin– Sca1+ cKitHi RAG-1/GFP+), L-selectin+ progenitors (LSP; Lin– Sca1+ cKitHi Thy1.1– L-selectin+) and common lymphoid progenitors (CLP; Lin– Sca1Lo cKitLo Thy1.1– IL7R+). All parameters matched the originally described characteristics of these progenitors, and typical post-sort purities are shown. In some experiments, cells were subjected to two rounds of sorting and biological activities were indistinguishable.
Intravenous Progenitor Transfers
Recipient mice received 6.5 Gy radiation from a 137Cs source (Mark I gamma irradiator; J.L. Shepard and Associates, Glendale, CA). Mice were anesthetized with isofluorane (Isosol, Vedco, St Joseph, MO) and sorted populations were infused i.v. Host mice were 6–12 week old B6-CD45.1; donor cells were from 4–10 week-old B6-RAG-1/GFP-Thy1.1 mice expressing the CD45.2 alloantigen. Thymic lobes, spleen, and marrow from one femur were collected separately from recipient mice, and donor-derived cells were assayed by flow cytometry. Limiting dilution analyses of thymic progenitor frequencies were determined by transplanting graded numbers of progenitors to sublethally irradiated mice. At least eight thymic lobes were analyzed at each dose and percentages of thymic lobes containing greater than 105 donor-derived thymocytes at day 17 were determined. Data were pooled from two independent sorting experiments and used to construct lines of best fit (40). Correlation coefficients (r) exceeded 0.9 for each plot.
Peripheral Blood and DN Thymus Preparations
Leukocytes were collected from peripheral blood as previously described (41). Lineage-committed cells were identified through incubation with biotin-conjugated lineage antibodies, and cell markers were stained as outlined for marrow. A similar strategy was used to label primitive thymocytes, except CD2 and CD5 were removed from the lineage cocktail, and TCR-β (H57-597) and TCR-γ /δ (GL3) were added. DN subsets were identified using CD44 (IM7) and CD25 (PC61).
Fetal Thymic Organ Culture
Fetal thymic organ culture was done as described (42). BALB/c fetuses were harvested at E15 and thymic lobes were cultured in RPMI 1640, 10% FCS with 0.36 mg/ml 2’-deoxyguanosine for 7 days. Lobes were then transferred to V-bottom culture plates, one lobe per well with 100 progenitors and 200 μL of culture media. On day 7, media was replaced with X-VIVO15 (BioWhittaker, Cambrex, East Rutherford, NJ) supplemented with 1% BSA (StemCell Technologies, Vancouver, Canada), 100 ng/ml stem cell factor and 1 ng/ml IL-7 (R&D Systems, Minneapolis, MN). Plates were sealed in gas sample bags (MiDan Company, Chino, CA) with 70% O2, 25% N2 and 5% CO2 gas mixture and cultured at 37°C with media changed every 5 days. After 14 days, lobes were disrupted for cell suspensions, stained with antibodies for surface markers and analyzed.
Results
A model for direct comparison of lymphoid progenitors
Several subsets of bone marrow contain T cell precursors, but it remains unclear which is likely to maintain thymocytopoiesis (16,18,23,28). We constructed a model for comparison of common lymphoid progenitors (CLP; Lin– Sca1Lo cKitLo Thy1.1– IL7R+), early lymphoid progenitors (ELP; Lin– Sca1+ cKitHi RAG-1/GFP+), and L-selectin+ progenitors (LSP; Lin– Sca1+ cKitHi Thy1.1– L-selectin+). C57BL6 (B6)-PL mice (Thy1.1 allele of CD90) were crossed with B6-RAG-1GFP/GFP mice so markers would be available to isolate all progenitor populations in parallel (Fig. 1). One thousand sorted progenitors were transplanted i.v. into sublethally irradiated (6.5 Gy) CD45 congenic recipients and evaluated for their reconstitution of lineages. Particular attention was paid to the kinetics of thymic colonization, degree of restriction to the T lineage, and numbers of thymocytes produced.
Figure 2A shows representative CD4 vs CD8 profiles of donor thymocyte recovery over time. All progenitors contributed to thymocyte recovery in advance of the 21 days required by hematopoietic stem cells (12). However, unseparated bone marrow and ELP yielded thymocytes earlier than the other populations.
Fig. 2. Lineage potentials of defined progenitor populations.
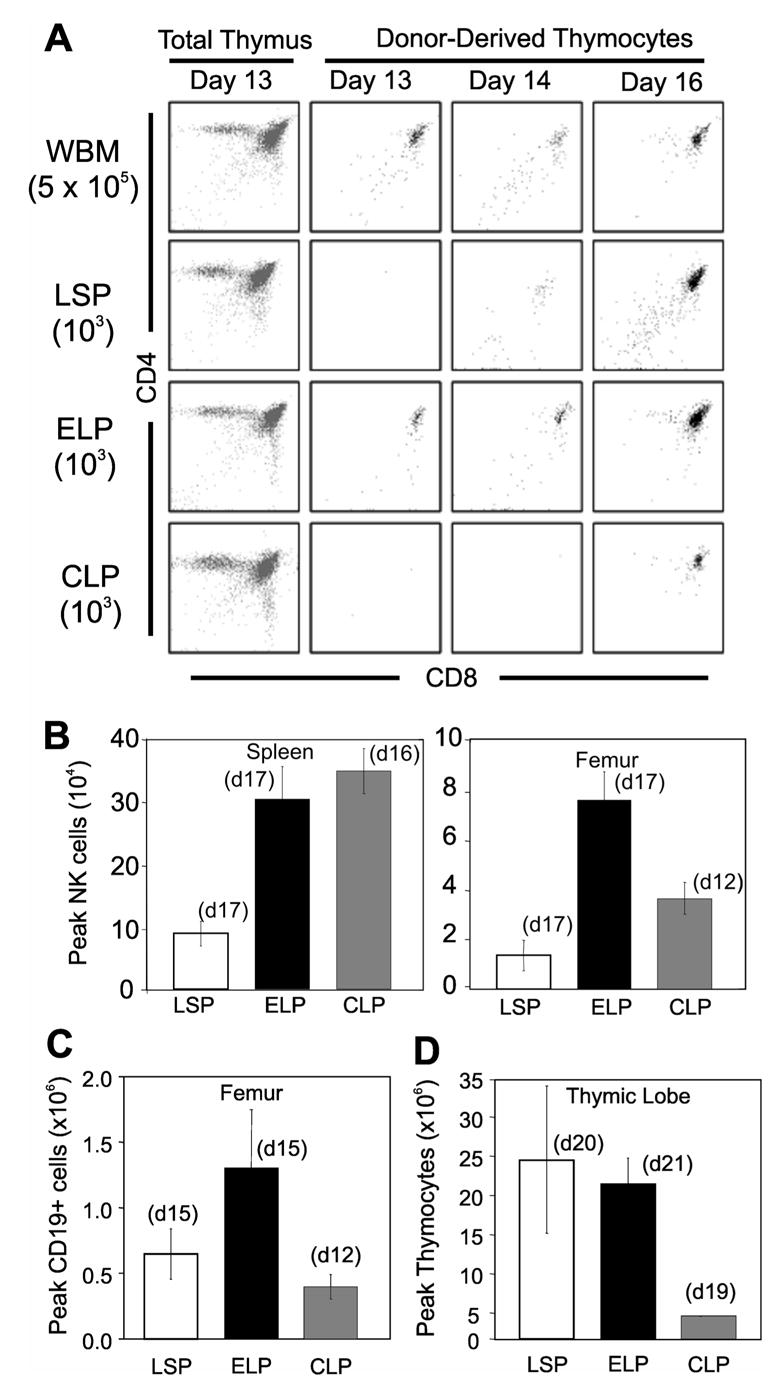
(A) Representative plots of thymic engraftment from i.v. transplants of either 5x105 whole bone marrow leukocytes (WBM) or 103 sorted progenitors. The far left panels show how total thymocytes recover by 13 days post transplant. The remaining dot plots are gated on donor type cells and show how transplanted progenitors generate thymocytes over time. Similar results were seen in experiments that utilized >80 thymic lobes for each transplanted population.Peak contributions of donor cells to NK (B), B cell (C), and thymocyte (D) lineages were evaluated in the indicated tissues. Days at which peak engraftment was achieved per population transplanted are noted in parentheses above each bar. Averages were taken from 5 sorting experiments for LSP, 5 for ELP, and 3 for CLP. Error bars indicate SEM.
The degree of lineage restriction and magnitude of thymic engraftment were evaluated in transplanted mice (Fig. 2B–D). ELP and CLP generated more splenic NK1.1+ natural-killer (NK) cells than LSP, a trend also in the marrow (Fig. 2B). The kinetics of CD19+ B cell production by CLP was three days in advance of LSP and ELP (Fig. 2C), however, ELP produced more B cells in the marrow. Compared to transplants of whole bone marrow, the progenitor populations generated only small numbers of myeloid cells (18,43) (and data not shown). Myeloid production peaked on day 16, and was rarely observed thereafter. LSP and ELP expanded at least 20,000 fold in the thymus, while CLP expanded five fold less (Fig. 2D).
These findings indicated that all three subsets were relatively restricted to lymphoid lineages but differed in the kinetics of thymic recovery. LSP were effective sources of thymocytes while ELP were enriched for T, B and NK progenitors. CLP peaked early for B lineage differentiation in the marrow and were effective NK producers.
ELP and LSP, but not CLP, produce robust thymic engraftment
The thymus contains primitive cells that repopulate the organ within 9 days of i.v. transplantation (1,12). A marrow progenitor might have similar kinetics but be capable of more sustained expansion. Therefore, our analysis focused on the speed and durability of T lineage regeneration from marrow subsets (Fig. 3). The kinetics of engraftment were similar in LSP and ELP grafted animals, while CLP had limited expansion potential (Fig. 3A, left panel). An early peak was observed in animals transplanted with whole bone marrow, followed by robust replenishment (Fig. 3A, right panel). This pattern was matched in mice with ELP, while LSP transplanted mice had no early wave, but expansion proceeded from day 15. T-lineage cells were generated in CLP grafted mice from day 16 (Fig. 3A, right panel). To determine if the marrow contained rare progenitors comparable to primitive thymocytes, large numbers (107) of unfractionated cells were transplanted, but no repopulation was observed before 13 days (Fig 3A, right panel).
Fig. 3. Kinetics and frequencies of thymocyte engraftment.

(A- left panel) Kinetic summary of early thymocytes developing per engrafted lobe from transplants of sorted LSP (squares), ELP (diamonds) or CLP (circles) as described for panel A. Each symbol represents average engraftment from 16–64 lobes analyzed at that time point. (Right panel) The X axis in panel B is expanded to show details of thymic engraftment at early time points. Short-term engraftment from transplantation of 5x105 WBM is given for comparison (triangles). (B) Percentages of lobes containing at least 105 donor thymocytes in the same experiments are shown. (C) Limiting dilution analysis of thymic progenitor frequencies in the LSP and ELP populations. The symbols represent percentages of thymic lobes (minimum n = 8 per dose) containing greater than 105 donor-derived thymocytes on day 17. Lines are best fit of data combined from two independent sorting experiments. Active progenitor frequencies as estimated by limiting dilution analysis for each population are indicated on the x-axis.
ELP and LSP produced nearly identical numbers of donor thymocytes and we consider thymic lobes to be engrafted when they contain >105 donor cells (Fig. 3A, left panel). ELP generated two waves of engraftment within the first three weeks post transplant. Peak activity for ELP in this interval occurred on day 17 (~70%), nearly twofold and seven-fold greater than with LSP or CLP, respectively (Fig. 3B). However, LSP engraftment increased with time until the percentages of positive lobes for LSP and ELP recipients were identical. CLP engraftment increased with time but was consistently less than LSP or ELP.
Inspection of these curves suggested that the three subsets contained different frequencies of engraftable thymic progenitors (Fig. 3B), a point addressed with limiting dilution experiments. We calculate that there was at least one progenitor capable of producing detectable engraftment within 17 days of transplant per ~1,000 cells in the ELP fraction, as compared to one per ~2,000 LSP (Fig. 3C). Low frequencies of engraftment with CLP precluded a similar analysis.
These observations demonstrate that lymphoid progenitors generate an early wave of T lymphopoiesis, followed by a second, more robust engraftment. While the ELP fraction was more enriched with respect to thymus repopulating cells than LSP, both could restore thymic lobes to normal size within three weeks post transplant. The CLP fraction contributed to T lymphopoiesis but with less efficiency than the other subsets.
The LSP subset contains a majority of the RAG-1+ ELP
As shown above, LSP and ELP have similar thymocyte potential following engraftment. We conducted six-color flow cytometry to determine the correlation between these populations (Fig. 4). This revealed that the majority of RAG-1+ ELP (Fig. 4A, right panel) reside in the Thy1.1Neg subset (>80%, left of hashed line). Further, evaluation of Thy1.1 and L-selectin expression on ELPs showed that ~60% are within the previously designated LSP category (Fig. 4A, left panel). Gating LSPs for RAG-1/GFP expression showed that only 8% of LSP express RAG-1 (Fig. 4A, right panel). Therefore, many RAG-1+ ELP overlap with, but represent a small fraction of LSP.
Fig. 4. Overlap between ELP and LSP populations.
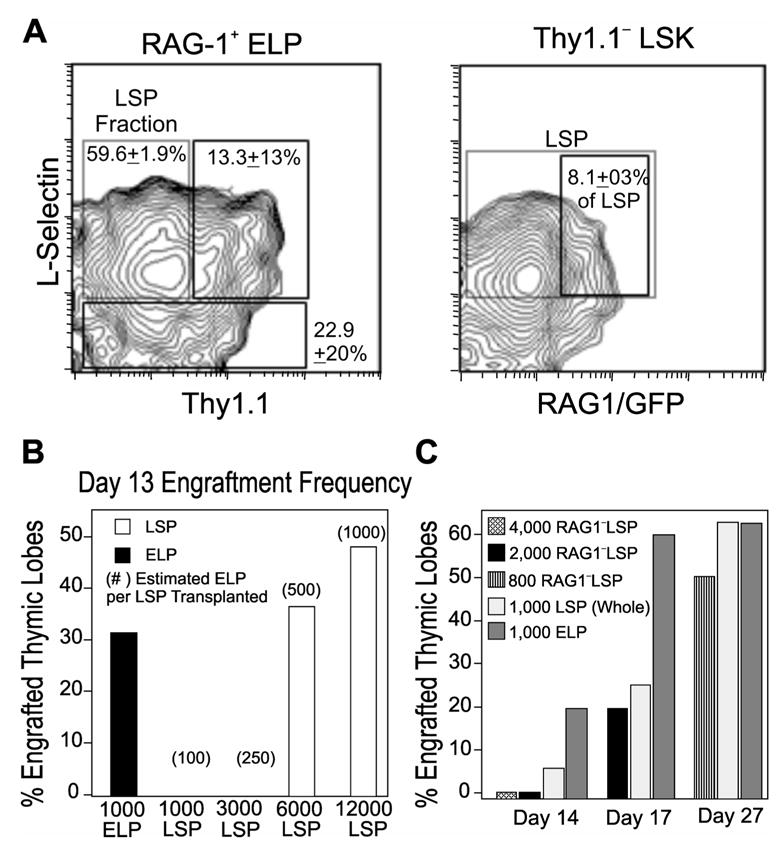
Six color flow cytometric analysis was used to determine the degree of coincidence between ELP and LSP marrow fractions. (A) The left panel shows that 60% of RAG-1+ ELP express L-selectin and lack Thy 1.1, while an additional 13% of these progenitors display low levels of Thy1.1 (right box). The right panel demonstrates that 8% of LSP (large box) express RAG-1/GFP (small box). The percentages represent means ±SEM from 6 independent experiments. (B) Increasing numbers of progenitors were transplanted to assess competency for rapid regeneration. Thymic lobes containing at least 105 donor thymocytes 13 days post i.v. transplant of ELP (filled bars) and LSP (open bars) are shown. Numbers in parentheses are estimates of the ELP included at each dose of LSP administered, as predicted from the overlap between these populations described in Figure 4 below. A minimum of 10 thymic lobes were analyzed at each transplant dose. (C) T progenitor activity associated with RAG-1− LSP. Percentages are given for thymic lobes with at least 105 donor thymocytes after transplant with LSP depleted of RAG-1+ cells (RAG-1− LSP). Transplantation results with unseparated LSP and ELP (open and gray bars, respectively) are given for reference. Data in C represents two independent experiments, the smaller number of thymic lobes studied could explain the different in frequency of ELP thymic engraftment seen from B and C.
LSP failed to generate a peak of thymocytes on day 13 post transplant, but many RAG-1+ ELP are included in the LSP subset. An explanation could be the low frequency of RAG-1+ ELP in this fraction (Fig. 4A, right panel). Therefore, we transplanted increasing numbers of LSP and evaluated the frequencies of engrafted thymic lobes on day 13 (Fig. 4B). Engraftment was detectable with LSP when transplant doses exceeded 6 x 103 cells (which would include ~500 RAG-1+). Thus, rare RAG-1-expressing cells in the LSP category could account for a very small early wave of T lymphopoiesis.
These observations raised the question of what T-lineage potential in the LSP fraction corresponds to RAG-1− progenitors. Therefore, we sorted and transplanted several doses of RAG-1/GFP-negative LSP (RAG-1− LSP) and evaluated thymocyte production (Fig. 4C). By day 17, 2,000 RAG-1− LSP produced thymic engraftment comparable to 1,000 whole LSP and by day 27, 800 RAG-1− LSP were comparable in thymocyte potential to un-separated LSP and ELP. These results suggest that the early thymic potential of LSP may be a function of the RAG-1+ cells. Even so, the LSP subset includes engrafting thymic progenitors apart from the RAG-1+ fraction. We also found that RAG-1− LSP are more T lineage restricted than the LSP population as a whole (data not shown).
Cells with ELP and LSP characteristics in blood and thymus
Marrow cells that colonize the thymus must traverse the blood stream. To determine if LSP or ELP were present in the circulation, we analyzed peripheral blood leukocytes for expression of surface antigens. Analysis for L-selectin and RAG-1/GFP in Lin− Thy1.1− peripheral blood showed a fraction of ELP and LSP in the circulation (Fig. 5A). The ratio of LSP to ELP was reduced relative to the marrow (~3:1 in blood versus ~12:1 in marrow). In contrast to a previous report (13), we found CLP in the circulation (Fig. 5A, far right panel) and approximately 85% of them were RAG-1/GFP+ (data not shown).
Fig. 5. Characteristics of progenitors in blood and thymus.

(A) A flow cytometry analysis is shown for Lin– Thy1.1– cells present in peripheral blood. The left panel resolves LSK (R1) and c-kitLo Sca-1+ (R2) subsets. The R1 population is further separated according to L-selectin and RAG-1/GFP in the middle panel to show RAG-1+ ELP and L-selectin+ LSP. In addition, c-KitLo IL-7Rα + CLP are gated from the R2 subset and illustrated in the far right panel. (B) DN1 thymocytes prepared as described in Materials & Methods were analyzed with respect to c-kit density and intracellular TdT. (C) In two separate experiments, purified bone marrow progenitors were stained for TdT. Average frequencies TdT+ cells relative to negative controls are shown. (D) The thymus DN subsets are resolved in the dot plot and used to set gates for analysis of RAG-1/GFP expression (histograms). (E) RAG-1/GFP is not expressed by DN1 thymocytes with high levels of c-Kit (left panel). The two gated DN1 subsets were placed in fetal thymic organ cultures and only the c-KitHi subsets (R1) generated double positive T lineage lymphocytes (middle and right panels).
ELP were originally defined on the basis of lymphoid gene expression, including terminal deoxynucleotidyl transferase (TdT) (18). Analysis of the primitive cKitHi DN1 fraction of thymocytes revealed uniform expression of TdT (Fig. 5B). The cKitHi DN1 thymus subset also expresses L-selectin (16), so we suspected that TdT might be indicative of thymic progenitors within the LSP subset. Stained in separate experiments, half of ELP and a fourth of LSP expressed TdT. For comparison, only 4% of the common myeloid progenitor subset expressed TdT (Fig. 5C). These results indicate that some markers associated with the most primitive of thymocytes precursors are expressed in marrow cells.
The DN1 fraction of the thymus includes cells with LSP characteristics (16). We next asked if RAG-1+ ELP overlapped with these progenitors. Figure 5D shows RAG-1/GFP expression in the DN subsets. All cKitHi DN1 thymocytes were RAG-1/GFP–, and only this fraction generated thymocytes in fetal thymic organ culture (Fig. 5E). Therefore, cells phenotypically similar to ELP and LSP were found in the circulation, but L-selectin+ TdT+ primitive thymocytes did not include RAG-1+ cells.
Discussion
A major objective of these experiments was to perform a side by side comparision of three categories of lymphoid progenitors. Early and sustained thymic reconstitution was obtained with LSP and RAG-1+ ELP subsets of the LSK fraction of bone marrow. Flow cytometry analysis revealed a substantial degree of overlap between these two populations. While purified according to their original descriptions, both of these subsets were heterogeneous with respect to other parameters and only some of them had characteristics similar to early thymic progenitors (ETP). Consistent with previous studies (18,24), Lin− c-KitLo IL-7Rα + CLP were much less effective T lineage precursors when assessed by intravenous transplantation.
While a wealth of information has been obtained by many laboratories, the nature of marrow cells that replenish the adult thymus under normal circumstances remains unclear (3). This could be an infrequent event and it almost certainly involves very few cells. The issue is further complicated by the fact that many marrow fractions have T lineage potential under experimental conditions. Rigorous comparisions of previously defined progenitors could be helpful, especially when information is available about their relative potency, abundance in the bone marrow and presence in the circulation.
Spangrude and colleagues described candidate T lineage progenitors among the Thy1.1− subset of the LSK fraction of bone marrow that expressed the CD62L/L-selectin adhesion molecule (16,35). These LSP comprise approximately 17% of the LSK fraction and we have confirmed that they are better T progenitors than B progenitors in transplanted mice. We estimate that LSP are 12 fold more numerous than RAG-1+ ELP and 4 times more abundant than CLP. As in a previous study (16), we found that LSP contributed to T lymphopoiesis within 13 days, nine days faster than reported for stem cells (12). Thus, the abundance of LSP relative to ELP and the increased frequency of thymic seeding progenitors indicate that LSP represent a rich source of thymus replenishing cells.
Early lymphoid progenitors were originally identified as a hormone sensitive Flt3/Flk-2+ CD27+ subset of LSK and some of them can be sorted in viable form from RAG-1/GFP mice (17,18). We have now determined that 60% of RAG-1+ ELP express L-selectin and lack Thy1.1. That is, they would be sorted as LSP. However, RAG-1+ ELP comprise only 8% of the total LSP subset. Approximately 23% of LSP were TdT+, another characteristic used to distinguish ELP (17). Overlaps between LSP/ELP subsets are depicted in Figure 6.
Fig. 6. Relationship between progenitors within the LSK fraction of bone marrow.

Gating and resolution of progenitor populations was performed as illustrated in Figure 4A. The relative incidences among total marrow nucleated cells are depicted with circles of proportional size. The degree that subsets have overlapping properties is also indicated.
Serial examination of mice transplanted with these populations revealed that RAG-1+ ELP accounted for the first wave of T cell production (Day 13; Fig. 3B and Fig. 4B,C). This very small peak was followed one week later by robust thymic regeneration, when LSP and RAG-1+ ELP made equivalent contributions. ELP and LSP both generated large numbers of thymocytes, but neither are stem cells because T lymphopoiesis declined 21 days after transplantation. Limiting dilution analyses performed 17 days after transplantation indicated that active T cell progenitors were approximately two fold more enriched in the RAG-1+ ELP subset than in the LSP fraction. Considering the two fold higher percentages of successfully engrafted lobes on day 17 (Fig. 3C), RAG-1+ ELP may have been superior with respect to thymic homing.
Elegant single cell studies performed by Kondo and colleagues revealed that CLP have high T potential when injected into the thymus (23). This was confirmed in another study where CD93/AA4.1 was used as a gating parameter for CLP (24). CD93+ CLP produced lymphocytes as early as Flt3/Flk-2+ LSK following intrathymic injection, but lost activity more rapidly (24). Therefore, we were surprised that T lymphopoiesis was slow and gradual following intravenous transplantation of CLP (Fig. 2D and Fig. 3A). It may be that these cells are inefficient with respect to thymic homing and, as suggested by others (3,23,24,44) would normally differentiate to B and NK lineages if left in the marrow.
Primitive hematopoietic cells express low levels of transcripts corresponding to multiple lineages (45,46), and there is no evidence that lymphoid genes are activated in synchronous fashion. While some transcription factors are essential for initiating the process, patterns of cell surface marker expression are variable and a precise sequence need not be followed to produce lymphocytes of a given type (7,47,48). Early lymphoid cells sorted according to selected markers are heterogeneous with respect to others, and we have documented many combinations of CD62L, TdT, RAG-1 and a human μ transgene in individual cells (17,18). It remains unclear if acquisition or loss of any marker corresponds to lymphoid lineage specification. Mice transplanted with RAG-1+ ELP or large numbers of LSP produced early (day 13) and later (day 20) peaks of thymocytes. Large numbers (up to 64 lobes) of recipient thymuses were evaluated and the phenomenon was consistent. This could reflect heterogeneity in the populations and may also indicate that some of the cells colonize other hematopoietic organs in sub-lethally irradiated recipients before seeding the thymus (49).
The degree of lymphoid lineage restriction of progenitors has been controversial and may be assay dependent. For example, CLP produce no myeloid cells in clonal assays driven by recombinant cytokines, but do so when placed in stromal cell co-cultures (23,43). This has also been our experience, and we observed small numbers of GR-1+ cells in marrow of CLP recipients soon after transplantation (<day 13, data not shown). It is possible that cells sorted in our laboratory were contaminated with myeloid progenitors, but we used all four of the sort criteria originally described by Kondo and similar results were obtained when CLP were sorted twice. The time after engraftment (before or after 4 weeks) and tissues examined (blood versus marrow) represent additional variables between studies. In any case, myeloid potential is lost in progressive rather than abrupt fashion as multipotent progenitors acquire LSP or ELP and then CLP characteristics (50,51).
The marrow cells we studied were sources of B and NK cells and our experiments do not reveal when T progenitors completely lose those options (Fig. 2). However, there was notable bias associated with the subsets. LSP were particularly effective for producing T cells, while recipients of RAG-1+ ELP made all lymphoid cell types. Our findings may indicate that segregation of cell fates starts at an extremely early stage in bone marrow. There is some evidence for this in lymphoid progenitors present in the fetus (52,53). As a possible indication of maturity, B lineage lymphocytes emerged in the marrow of CLP recipients faster (<12 days) than in mice transplanted with LSP or ELP (14 days). CLP2 were not included in our study and it would be useful to assess their potency as T cell progenitors relative to the other subsets (27,28).
As noted in the Introduction, Lin− Sca-1+ c-KitHi CD62L+ CD44Hi cells resident in the thymus are potent T progenitors and produce lymphocytes within 9 days of transplantation (14,33,54,55). This pattern of surface marker expression corresponds to the characteristics of marrow LSP and ELP. Flt3/Flk-2 is expressed on 15% of ETP while almost all ELP have this marker (15,17,18) and our unpublished observations). TdT uniformly marked ETP in the thymus, while 23% of LSP and 43% of ELP had this intracellular marker (Fig. 5C). Given their effectiveness for producing T cells following transplantation, it was surprising to find no RAG-1+ ELP in the thymus (Fig. 5D,E). None of the RAG-1+ DN1 cells were c-KitHi and RAG-1+ DN1 cells did not produce T cells in fetal thymic organ culture. This suggests that RAG-1+ ELP may only colonize the thymus under conditions of stress, as would result from the sub-lethal irradiation treatment we used. However, RAG-1+ ELP were detected in the circulation of unconditioned mice, which suggests they have access to the thymus. If RAG-1+ ELP colonize the organ under normal conditions, they must rapidly down-regulate RAG-1 or become cKitHi RAG-1+ CD25+ DN2 thymocytes.
It has been previously demonstrated that stem cells and progenitors with LSK characteristics are in the circulation (56,57). This includes cells that express a RAG-2 BAC transgene. We now report that LSP, RAG-1+ ELP and CLP are detectable in the blood. Resolution of the CLP subset was done with the original Kondo gating parameters and may explain a small discrepancy with a previous study (57).
Our analysis was not designed to establish developmental relationships between HSC and lymphoid progenitors present in marrow, blood and thymus. However, the kinetics of thymocyte production and patterns of marker expression would be consistent with LSP and ELP being immediately downstream of HSC. While it is difficult to define rare cells that infrequently replenish the thymus, we have characterized defined categories of marrow progenitors and compared them for T lineage potential. It seems probable that some specification for T lymphopoiesis begins in the marrow, but loss of other differentiation options is clearly a gradual process.
Since completion of these experiments, Hardy and colleagues compared marrow progenitors separated according to different parameters (58). While not as lymphoid restricted as RAG-1+ ELP, a CD24Lo CD93+ subset of Lin− c-KitHi bone marrow cells were superior to CLP and later fractions with respect to T lineage reconstitution.
Acknowledgments
We are grateful for the technical help offered by Viji Dandapani, Jacob Bass, Diana Hamilton, Tara Khamphanthala, Erin Beeston, and Amanda Johnson. We appreciate the secretarial assistance of Shelli Wasson.
Footnotes
This work was supported by grants from the National Institutes of Health (X-H.S.: AI 33597 and AI 056129; P.W.K.: AI 20069 and AI 58162; and S.S.P.: T32-AI 07633). X-H.S. holds the Eli Lilly Distinguished Chair in Biomedical Research and P.W.K. holds the William H. and Rita Bell Chair in Biomedical Research.
Publisher's Disclaimer: The following disclaimer is a requirement of The Journal of Immunology: “This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
References
- 1.Scollay R, Smith J, Stauffer V. Dynamics of early T cells: prothymocyte migration and proliferation in the adult mouse thymus. Immunol Rev. 1986;91:129–157. doi: 10.1111/j.1600-065x.1986.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 2.Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med. 2001;193:365–374. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhandoola A, Sambandam A. From stem cell to T cell: one route or many? Nat Rev Immunol. 2006;6:117–126. doi: 10.1038/nri1778. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Garrett KP, Pelayo R, Zúñiga-Pflücker JC, Petrie HT, Kincade PW. Propensity of adult lymphoid progenitors to progress to DN2/3 stage thymocytes with Notch receptor ligation. J Immunol. 2005;175:4858–4865. doi: 10.4049/jimmunol.175.8.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balciunaite G, Ceredig R, Fehling HJ, Zúñiga-Pflücker JC, Rolink AG. The role of Notch and IL-7 signaling in early thymocyte proliferation and differentiation. Eur J Immunol. 2005;35:1292–1300. doi: 10.1002/eji.200425822. [DOI] [PubMed] [Google Scholar]
- 6.Balciunaite G, Ceredig R, Rolink AG. The earliest subpopulation of mouse thymocytes contains potent T, significant macrophage, and natural killer cell but no B-lymphocyte potential. Blood. 2005;105:1930–1936. doi: 10.1182/blood-2004-08-3087. [DOI] [PubMed] [Google Scholar]
- 7.Pelayo R, Welner R, Perry SS, Huang J, Baba Y, Yokota T, Kincade PW. Lymphoid progenitors and primary routes to becoming cells of the immune system. Curr Opin Immunol. 2005;17:100–107. doi: 10.1016/j.coi.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Pear WS, Tu L, Stein PL. Lineage choices in the developing thymus: choosing the T and NKT pathways. Curr Opin Immunol. 2004;16:167–173. doi: 10.1016/j.coi.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Bhandoola A, Sambandam A, Allman D, Meraz A, Schwarz B. Early T lineage progenitors: new insights, but old questions remain. J Immunol. 2003;171:5653–5658. doi: 10.4049/jimmunol.171.11.5653. [DOI] [PubMed] [Google Scholar]
- 10.Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci U S A. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nat Immunol. 2004;5:953–960. doi: 10.1038/ni1101. [DOI] [PubMed] [Google Scholar]
- 12.Perry SS, Pierce LJ, Slayton WB, Spangrude GJ. Characterization of thymic progenitors in adult mouse bone marrow. J Immunol. 2003;170:1877–1886. doi: 10.4049/jimmunol.170.4.1877. [DOI] [PubMed] [Google Scholar]
- 13.Spangrude GJ, Scollay R. Differentiation of hematopoietic stem cells in irradiated mouse thymic lobes: Kinetics and phenotype of progeny. J Immunol. 1990;145:3661–3668. [PubMed] [Google Scholar]
- 14.Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zúñiga-Pflücker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–745. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Sambandam A, Maillard I, Zediak VP, Xu L, Gerstein RM, Aster JC, Pear WS, Bhandoola A. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat Immunol. 2005;6:663–670. doi: 10.1038/ni1216. [DOI] [PubMed] [Google Scholar]
- 16.Perry SS, Wang H, Pierce LJ, Yang AM, Tsai S, Spangrude GJ. L-selectin defines a bone marrow analog to the thymic early T-lineage progenitor. Blood. 2004;103:2990–2996. doi: 10.1182/blood-2003-09-3030. [DOI] [PubMed] [Google Scholar]
- 17.Medina KL, Garrett KP, Thompson LF, Rossi MID, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nature Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- 18.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 19.Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Lai AY, Lin SM, Kondo M. Heterogeneity of Flt3-expressing multipotent progenitors in mouse bone marrow. J Immunol. 2005;175:5016–5023. doi: 10.4049/jimmunol.175.8.5016. [DOI] [PubMed] [Google Scholar]
- 21.Searles AE, Pohlmann SJ, Pierce LJ, Perry SS, Slayton WB, Mojica MP, Spangrude GJ. Rapid, B lymphoid-restricted engraftment mediated by a primitive bone marrow subpopulation. J Immunol. 2000;165:67–74. doi: 10.4049/jimmunol.165.1.67. [DOI] [PubMed] [Google Scholar]
- 22.Mojica MP, Perry SS, Searles AE, Elenitoba-Johnson KS, Pierce LJ, Wiesmann A, Slayton WB, Spangrude GJ. Phenotypic distinction and functional characterization of pro-B cells in adult mouse bone marrow. J Immunol. 2001;166:3042–3051. doi: 10.4049/jimmunol.166.5.3042. [DOI] [PubMed] [Google Scholar]
- 23.Kondo M, I, Weissman L, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 24.Allman D, Sambandam A, Kim S, Miller JP, Pagan A, Well D, Meraz A, Bhandoola A. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4:168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 25.Benz C, Bleul CC. A multipotent precursor in the thymus maps to the branching point of the T versus B lineage decision. J Exp Med. 2005;202:21–31. doi: 10.1084/jem.20050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benz C, Heinzel K, Bleul CC. Homing of immature thymocytes to the subcapsular microenvironment within the thymus is not an absolute requirement for T cell development. Eur J Immunol. 2004;34:3652–3663. doi: 10.1002/eji.200425248. [DOI] [PubMed] [Google Scholar]
- 27.Gounari F, Aifantis I, Martin C, Fehling HJ, Hoeflinger S, Leder P, Von Boehmer H, Reizis B. Tracing lymphopoiesis with the aid of a pTalpha-controlled reporter gene. Nat Immunol. 2002;3:489–496. doi: 10.1038/ni778. [DOI] [PubMed] [Google Scholar]
- 28.Martin CH, Aifantis I, Scimone ML, Von Andrian UH, Reizis B, Von Boehmer H, Gounari F. Efficient thymic immigration of B220+ lymphoid-restricted bone marrow cells with T precursor potential. Nat Immunol. 2003;4:866–873. doi: 10.1038/ni965. [DOI] [PubMed] [Google Scholar]
- 29.Ceredig R, Sekaly RP, MacDonald HR. Differentiation in vitro of Lyt 2+ thymocytes from embryonic Lyt 2- precursors. Nature. 1983;303:248–250. doi: 10.1038/303248a0. [DOI] [PubMed] [Google Scholar]
- 30.Pearse M, Wu L, Egerton M, Wilson A, Shortman K, Scollay R. A murine early thymocyte developmental sequence is marked by transient expression of the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1989;86:1614–1618. doi: 10.1073/pnas.86.5.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fowlkes BJ, Edison L, Mathieson BJ, Chused TM. Early T lymphocytes. Differentiation in vivo of adult intrathymic precursor cells. J Exp Med. 1985;162:802–822. doi: 10.1084/jem.162.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple- negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 33.Ceredig R, Rolink T. A positive look at double-negative thymocytes. Nat Rev Immunol. 2002;2:888–897. doi: 10.1038/nri937. [DOI] [PubMed] [Google Scholar]
- 34.Laurent J, Bosco N, Marche PN, Ceredig R. New insights into the proliferation and differentiation of early mouse thymocytes. Int Immunol. 2004;16:1069–1080. doi: 10.1093/intimm/dxh108. [DOI] [PubMed] [Google Scholar]
- 35.Wu L, D’Amico A. Functional analysis of Mel-14+ and Mel-14- early precursor cells in the adult mouse thymus. Immunol Lett. 1994;40:89–92. doi: 10.1016/0165-2478(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 36.Massa S, Balciunaite G, Ceredig R, Rolink AG. Critical role for c-kit (CD117) in T cell lineage commitment and early thymocyte development in vitro. Eur J Immunol. 2006;36:526–532. doi: 10.1002/eji.200535760. [DOI] [PubMed] [Google Scholar]
- 37.Prockop SE, Petrie HT. Regulation of thymus size by competition for stromal niches among early T cell progenitors. J Immunol. 2004;173:1604–1611. doi: 10.4049/jimmunol.173.3.1604. [DOI] [PubMed] [Google Scholar]
- 38.Dunon D, Allioli N, Vainio O, Ody C, Imhof BA. Quantification of T-cell progenitors during ontogeny: thymus colonization depends on blood delivery of progenitors. Blood. 1999;93:2234–2243. [PubMed] [Google Scholar]
- 39.Kuwata N, Igarashi H, Ohmura T, Aizawa S, Sakaguchi N. Cutting edge: Absence of expression of RAG1 in peritoneal B-1 cells detected by knocking into RAG1 locus with green fluorescent protein gene. J Immunol. 1999;163:6355–6359. [PubMed] [Google Scholar]
- 40.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. III. Validity tests for the single-hit Poisson model. J Immunol Meth. 1984;72:29–40. doi: 10.1016/0022-1759(84)90430-7. [DOI] [PubMed] [Google Scholar]
- 41.Spangrude GJ, Brooks DM, Tumas DB. Long-term repopulation of irradiated mice with limiting numbers of purified hematopoietic stem cells: In vivo expansion of stem cell phenotype but not function. Blood. 1995;85:1006–1018. [PubMed] [Google Scholar]
- 42.Kawamoto H, Ohmura K, Hattori N, Katsura Y. Hemopoietic progenitors in the murine fetal liver capable of rapidly generating T cells. J Immunol. 1997;158:3118–3124. [PubMed] [Google Scholar]
- 43.Balciunaite G, Ceredig R, Massa S, Rolink AG. A B220+ CD117+ CD19+/− hematopoietic progenitor with potent lymphoid and myeloid developmental potential. Eur J Immunol. 2005;35:2019–2030. doi: 10.1002/eji.200526318. [DOI] [PubMed] [Google Scholar]
- 44.Plotkin J, Prockop SE, Lepique A, Petrie HT. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J Immunol. 2003;171:4521–4527. doi: 10.4049/jimmunol.171.9.4521. [DOI] [PubMed] [Google Scholar]
- 45.Traver D, Akashi K. Lineage commitment and developmental plasticity in early lymphoid progenitor subsets. Adv Immunol. 2004;83:1–54. doi: 10.1016/S0065-2776(04)83001-3. [DOI] [PubMed] [Google Scholar]
- 46.Miyamoto T, Iwasaki H, Reizis B, Ye M, Graf T, Weissman IL, Akashi K. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev Cell. 2002;3:137–147. doi: 10.1016/s1534-5807(02)00201-0. [DOI] [PubMed] [Google Scholar]
- 47.Warren LA, Rothenberg EV. Regulatory coding of lymphoid lineage choice by hematopoietic transcription factors. Curr Opin Immunol. 2003;15:166–175. doi: 10.1016/s0952-7915(03)00011-6. [DOI] [PubMed] [Google Scholar]
- 48.Baba Y, Pelayo R, Kincade PW. Relationships between hematopoietic stem cells and lymphocyte progenitors. Trends Immunol. 2004;25:645–649. doi: 10.1016/j.it.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Maillard I, Schwarz BA, Sambandam A, Fang T, Shestova O, Xu L, Bhandoola A, Pear WS. Notch-dependent T-lineage commitment occurs at extrathymic sites following bone marrow transplantation. Blood. 2006;107:3511–3519. doi: 10.1182/blood-2005-08-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu M, Tayu R, Ikawa T, Masuda K, Matsumoto I, Mugishima H, Kawamoto H, Katsura Y. The earliest thymic progenitors in adults are restricted to T, NK, and dendritic cell lineage and have a potential to form more diverse TCRbeta chains than fetal progenitors. J Immunol. 2005;175:5848–5856. doi: 10.4049/jimmunol.175.9.5848. [DOI] [PubMed] [Google Scholar]
- 51.Kawamoto H. A close developmental relationship between the lymphoid and myeloid lineages. Trends Immunol epub ahead of print. 2006 doi: 10.1016/j.it.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Ikawa T, Masuda K, Lu M, Minato N, Katsura Y, Kawamoto H. Identification of the earliest prethymic T-cell progenitors in murine fetal blood. Blood. 2004;103:530–537. doi: 10.1182/blood-2003-06-1797. [DOI] [PubMed] [Google Scholar]
- 53.Harman BC, Jenkinson WE, Parnell SM, Rossi SW, Jenkinson EJ, Anderson G. T/B lineage choice occurs prior to intrathymic Notch signaling. Blood. 2005;106:886–892. doi: 10.1182/blood-2004-12-4881. [DOI] [PubMed] [Google Scholar]
- 54.Godfrey DI, Zlotnik A, Suda T. Phenotypic and functional characterization of c-kit expression during intrathymic T cell development. J Immunol. 1992;149:2281–2285. [PubMed] [Google Scholar]
- 55.Moore TA, Zlotnik A. T-cell lineage commitment and cytokine responses of thymic progenitors. Blood. 1995;86:1850–1860. [PubMed] [Google Scholar]
- 56.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 57.Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nat Immunol. 2004;5:953–960. doi: 10.1038/ni1101. [DOI] [PubMed] [Google Scholar]
- 58.Rumfelt LL, Zhou Y, Rowley BM, Shinton SA, Hardy RR. Lineage specification and plasticity in CD19- early B cell precursors. J Exp Med. 2006;203:675–687. doi: 10.1084/jem.20052444. [DOI] [PMC free article] [PubMed] [Google Scholar]


