Summary
The earliest progenitors of lymphocytes are extremely rare and typically present among very complex populations of hematopoietic cells. Additionally, it is difficult to know how cells with any given set of characteristics are developmentally related to stem cells and maturing lymphoid precursors. However, it is now possible to divide bone marrow into progressively smaller fractions and exploit well defined culture systems to determine which ones contain cells that can turn into lymphocytes. Analysis of steroid hormone sensitive cells and use of two-step cultures is providing additional information about the most likely differentiation pathways for B and NK lineage lymphocytes. A newly identified category early lymphoid progenitors (ELP) can now be sorted to high purity from RAG1/GFP knock in mice. Furthermore, the same experimental model makes it possible to image lymphoid progenitors in fetal and adult hematopoietic tissues.
Introduction
Magnetic bead depletion, genetic cell marking and high speed cell sorting now make it possible to extensively dissect bone marrow, and to determine the differentiation potential of very small subpopulations of cells in culture or transplantation experiments. When considered together with results from gene targeting studies, the resulting information should lead to more precise models of lymphopoiesis. Indeed, progress is being made in understanding a sequence of critical differentiation events required for multipotential hematopoietic stem cells to give rise to B, T and NK cells. A longer-term goal will be to construct a coherent image of physical relationships between cells and molecules within the bone marrow, so that we can appreciate how lymphoid progenitors receive cues for survival, differentiation, and migration.
Divergence of lymphoid from myelo-erythroid differentiation pathways
The existence of multipotential hematopoietic stem cells has long been recognized and it was thought that there might also be some type of “lymphoid stem cells” (1). Among the indirect evidence was the fact that humans and mice can have selective deficiencies of B and T cells. Also, cells dedicated to myeloid and erythroid lineages could be readily detected in short term spleen (CFU-s) and in vitro colony (CFU-GEMM) assays that did not yield lymphocytes (2). On the other hand, lymphoid stem cells were not demonstrated by retroviral insertion experiments and, if the term “stem cells” is reserved for primitive cells with extensive self-renewal capability, are unlikely to exist (3).
Kondo and Weissman provided the first clear demonstration that single progenitors in mouse bone marrow have the potential for short-term generation of B and T cells (4). These lineage marker (CD3, CD4, CD8, CD45R/B220, CD11b/Mac-1, Ly-6G/GR-1, TER119) negative cells were isolated on the basis of IL-7Rα expression and other properties that would distinguish them from stem cells. Long-term repopulating stem cells in a Lin− Thy-1.1Lo Sca-1Hi c-KitHi fraction were eliminated from the sorted population. At least one out of 5 proliferated in culture and one third of those clones generated T lineage cells following thymic injection. Additionally, some cells in this marrow fraction gave rise to NK cells on transplantation. Importantly, no potential for myeloid differentiation was found in either transplantation or colony assays. The cells were designated “common lymphoid progenitors” (CLP), a name that was being used in textbooks (5). It was unclear at the time if cells with these particular characteristics represent major intermediates in lymphopoiesis and it remains uncertain how many lymphocytes derive from “common” progenitors with homogeneous characteristics. However, subsequent findings support the conclusion that lymphoid differentiation pathways diverge from those responsible for myeloerythroid differentiation and in fact do so at an even earlier stage (6, 7).
Hormones as experimental tools and regulators of lymphopoiesis
Many of our recent studies were driven by attempts to understand how steroid hormones influence steady-state rates of lymphocyte production in bone marrow. Their importance is suggested by the marked suppression of lymphopoiesis in pregnant or estrogen treated mice, contrasted with abnormally elevated pre-B cell numbers in castrated male, ovariectomized female and hypogonadal animals (8–10). However, patterns of bone marrow changes that we observed in hormone treated mice did not easily fit the most popular differentiation models (11). It eventually became obvious that the most estrogen sensitive populations were rare, very primitive and not previously well described. Hormone treatment protocols proved to be quite helpful in establishing precursor-product relationships between lymphoid progenitors in bone marrow and establishing the most likely pathways for their differentiation.
Confusion surrounding heterogeneous CD45R/B220+ CD19− cells
Acquisition of CD19 represents an important milestone in murine B lineage differentiation and corresponds to the ability of late pro-B cells to proliferate in IL-7 without other stromal cell derived factors (12–14). Although very small numbers of CD19+ cells may be capable of non-B lymphoid differentiation (15), expression of this marker generally signals lineage commitment in bone marrow. Primitive CD19+ precursors are appropriately designated “fraction B” or “Pre-B1” in the Hardy and Rolink/Melchers schemes, respectively (16, 17).
Although suggested by the name “B220”, none of the various epitopes on CD45R are B lymphocyte lineage restricted (18). The CD45R/B220+ CD19− fraction of mouse bone marrow can be subdivided into a surprisingly large number of subsets and we attempted to learn which ones are capable of generating CD19+ lymphocytes in short term cultures (13). It appeared from these experiments that all of the functional lymphocyte progenitors were Flk-2/Flt3+, CD24+ and Ly-6C−. Hardy and colleagues had previously shown that a particular CD24 specific antibody, 30F1 could be used to resolve a clear subset of CD45R/B220+ CD19− CD24− marrow cells, and especially when the reagent was used under sub-saturating concentrations (12). Furthermore, they divided this “fraction A” into subsets (A1 and A2) according to common expression of AA4.1 and differential display of CD4 (19). Their studies demonstrated low-level transcription of some B lymphocyte lineage genes, expression of a human μ heavy chain transgene, and at least some immunoglobulin D-J rearrangements in fraction A (16, 19). However, we found that all functional B lymphocyte precursors in the CD45R/B220+ CD19− subset were CD24+, regardless of which of two CD24 specific antibodies were used (13). Furthermore, it was frustrating to find that fraction A cells accumulate in estrogen treated mice, while progenitors that appeared to be before or after that stage were hormone sensitive (11). Low-level expression of CD11b/Mac-1 and absence of IL-7Rα, raised further suspicion surrounding the fate of fraction A cells. Recent findings support earlier indications that a large fraction of NK lineage progenitors express CD45R/B220+ (17, 20). Furthermore, it is now obvious that neither transcription of genes required for B lymphopoiesis or the initial D-J step in Ig gene rearrangement restrict progenitors to the B lineage differentiation pathway (7). Thus, the CD45R/B220+ CD19− fraction of bone marrow contains a mixture of cells whose destiny is only revealed by functional assays (see below). It is likely that very few of them are in the main pathway that generates B lymphocytes (13).
A Lin− c-KitLo pro-lymphocyte fraction contains most lymphoid progenitors
Expression of CD45R/B220 does provide an extremely useful distinction between lineage marker positive (Lin+) and Lin− lymphocyte progenitors in bone marrow, and it is worth stressing the importance of selecting appropriate reagents for the preparative enrichment of rare Lin− cells. For example, Kondo and Weissman used CD4 for lineage depletion although this marker can be expressed at low levels on several categories of potential lymphoid progenitors (4, 7, 17, 19). In our experience, positive selection for CD4+ cells that are otherwise Lin− can give some enrichment for functional B lineage progenitors (Kouro, unpublished observations). Therefore, use of anti-CD4 for lineage depletion could discard some cells of potential interest. In contrast, CD11b/Mac-1 has been detected on some populations of short term repopulating cells and putative lymphocyte progenitors, but did not correlate with potential for differentiation in our cultures (13, 19, 21). It is obvious that cell separation protocols are not standardized between research groups, and populations being studied will not necessarily be comparable. Complicating matters further, expression of the Thy-1.2, Sca-1 and CD34 cell surface markers varies in a mouse sub-strain and age related manner (22, 23) and unpublished observations). We systematically divided bone marrow cells according to one marker at a time, comparing positive and negative fractions for functional lymphocyte differentiation potential (6, 13, 24, 25). While the resulting data may not necessarily be generalized to all experimental circumstances, it hopefully provides a picture of the most preferred pathways through which lymphocytes are produced.
Osmond and colleagues identified a population of CD45R/B220− terminal deoxynucleotidyl transferase positive (TdT+) cells in marrow and designated them “early pro-B cells” (26). We found that Lin− TdT+ cells are selectively sensitive to steroid hormones and present in populations that have the most potential to generate B lineage lymphocytes (6, 7, 25). Most of them have a very distinctive low density of c-kit, a transmembrane tyrosine kinase type receptor for stem cell factor (24). Approximately 60% of Lin− c-KitLo TdT+ cells express IL-7Rα, and would thus be included in Kondo & Weissman’s CLP fraction (25). We now refer to cells in the Lin− c-KitLo fraction of bone marrow as “pro-lymphocytes” because it includes a mixture of progenitors/precursors that are poised to become B, T and NK cells. The relative abundance of pro-lymphocyte containing fractions in bone marrow and the differentiation activity on a per cell basis in short term cultures are illustrated in Fig 1.
Fig. 1. Localization of B and NK lineage progenitor activity in bone marrow.
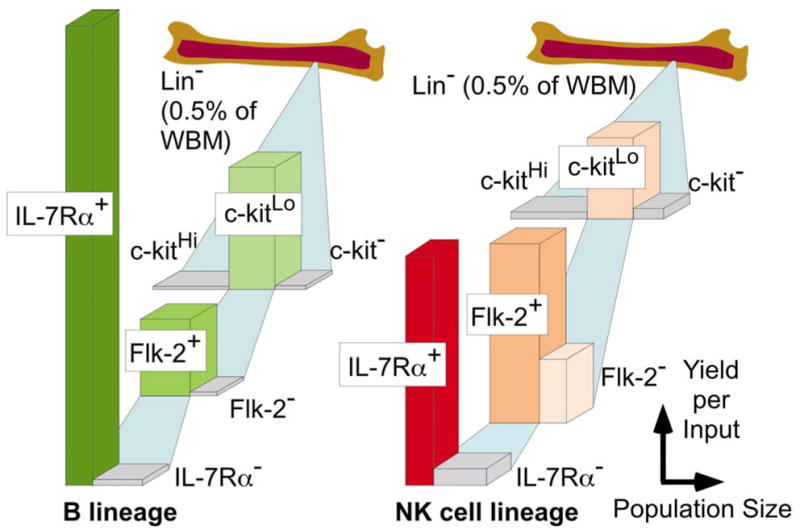
The small subset that lacks markers associated with differentiated cells (Lin−) can be subdivided into many fractions. Here we depict the relative sizes of the populations (width) and yields (height) of B or NK lineage cells that were obtained when cells of each type were placed in defined short term cultures.
Early lymphoid progenitors identified in RAG1/GFP mice
An additional category of Lin− c-KitHi cells contains a more primitive population of lymphocyte progenitors. The relatively long time required for cells in this fraction to generate lymphocytes in culture and their tendency to down-regulate c-Kit suggested a possible differentiation sequence (24). However, these initial findings might also be explained in terms of pro-lymphocyte heterogeneity and dilution in the Lin− c-KitHi fraction by non-lymphoid progenitors. Fortunately, the notion of a distinct population of early progenitors was supported by the identification of estrogen sensitive Lin− c-KitHi Sca-1Hi CD27+ Flk-2+ TdT+ cells (6). At least some cells with the same surface characteristics expressed a human μ transgene protein, although it is probably worth noting that this transgene is also expressed in the brain (27). Importantly, myeloid progenitors in the Lin− c-KitHi fraction were spared in estrogen treated mice while cells able to generate T and B lineage lymphocytes were suppressed. Although long term repopulating stem cells are present in the Lin− c-KitHi Sca-1Hi fraction, they can be distinguished from progenitors without extensive self renewal potential on the basis of their lack of either CD27 or Flk-2 (28, 29). Thus, the hormone sensitive progenitors we identified in that fraction were unlikely to be stem cells.
The extremely small Lin− c-KitHi Sca-1+ CD27+ Flk-2+ subset of mouse bone marrow is heterogeneous. Furthermore, fixation and cell permeabilization were required to detect the most informative markers, TdT and human μ (6). It was therefore a delightful surprise to find that RAG1/GFP mice can be used to isolate viable early lymphoid progenitors (ELP) (7). Expression of GFP in these animals correlates perfectly with the onset of transcription from the RAG1 and RAG2 genes. Also, functional progenitors of B, T and NK cells can be highly enriched from heterozygous mice with one knock-in allele on the basis of GFP expression. GFP+ progenitors are unlike the otherwise identical cells in the Lin− c-KitHi Sca-1+ CD27+ Flk-2+ fraction in one important respect; they have less than 10% the potential to generate blood cells in the myelo-erythroid lineages. A sorted fraction with just above background levels of GFP included many myeloid progenitors, suggesting that lineage choice restriction accompanies RAG1 transcription at this early stage (7). Changes that subsequently occur in the progeny of ELP will be discussed below.
The EBF transcription factor is essential to B lymphopoiesis and it was not surprising to find detectable mRNA in pooled populations of sorted ELP (7). Similarly, progression in lymphoid lineages requires a net balance of E47/E12 members of the helix-loop helix family of transcription factors relative to the Id family of transcriptional repressors (30, 31). ELP had detectable transcripts for E47 and reduced amounts of Id-1 as compared to the GFP− cohort population. It was also the earliest population with detectable D-J rearrangment products. The Pax-5 transcription factor is essential for supporting and maintaining progression in the B lineage, and may also repress myeloid differentiation options (32, 33). However, we think it is unlikely to play a role in the initial B lineage choice because expression was not detectable before the pro-lymphocyte stage (7). This is consistent with the fact that hematopoietic cell clones containing D-J rearrangements have been isolated from Pax-5 knock out mice (33). It is extremely interesting that pro-B cells continuously require Pax-5 and can regain options to become T and myeloid lineage cells if it is subsequently lost (34). The GATA-3 transcription factor required for T lymphopoiesis is expressed by at least some cells in the ELP population, but Aiolos transcripts were only detectable in Lin− c-KitLo cells. We conclude from these and other studies that EBF and E47, along with PU1 and Ikaros family transcription factors, as well as signals from c-kit and Flk-2 cytokine receptors represent minimal requirements for the establishment and maintenance of ELP.
TdT+ cells only partially overlap with RAG-1 expressing cells in the Lin− c-KitHi Sca-1+ CD27+ Flk-2+ subset (7). This suggests that the initial remodeling of chromatin and transcription of genes required for lymphopoiesis are unlikely to be synchronized in ELP. Furthermore, low level expression of genes for proteins like Ig-β, that are required for B lymphopoiesis need not preclude adoption of T or NK lineage differentiation fates (35). Similarly, there is no known functional significance to rearrangement of Ig genes in T or NK cells. However, this is the case in many T lineage cells (36) and we estimate that more than half of NK lineage progenitors express RAG1 at some point during their formation. Our findings are compatible with those of a single cell study of factor dependent cell lines (37). They concluded that multiple loci become transcriptionally active before the differentiation fate of early myeloid progenitors is established. Still other groups showed that the normal pattern of lineage option restriction in lymphocyte progenitors could be abrogated by delivery of signals from cytokine receptors and proto-oncogenes (38–40). Those findings indicate that down-regulation of receptors for myeloid growth factors may be important for directing cells to lymphoid fates. Our observations suggest that the reciprocal is not the case, i.e. expression of receptors for the lymphoid cytokine IL-7 occurs after cells have lost myelo-erythroid potential (7). It is convenient to think of ELP as a category of cells with high potential for lymphopoiesis, but a degree of uncertainty about the final outcome. Presumably, signals from the microenvironment support and reinforce the decisions that culminate in the production of B, T and NK cells.
The major route from stem cells to B cells & NK cells
Over 90% of the Lin− c-KitLo IL-7Rα+ population in RAG1/GFP mice, i.e. cells that would be designated CLP by Kondo, et. al. (4) expressed the fluorescent protein (7). However, it is important to stress that 40% of the GFP+ cells in the Lin− c-KitLo pro-lymphocyte fraction lacked detectable levels of IL-7Rα and many Lin− c-KitLo TdT+ cells would also not be included in the CLP fraction (7, 25). An unresolved issue is whether all or most lymphocyte progenitors must pass through such a “stage” in sufficiently synchronous fashion to be defined with surface markers. Alternatively, it may be useful to consider that even extremely small “fractions” arbitrarily capture heterogeneous populations of cells that are undergoing continuous change.
The total number of functional lymphocyte precursors in any given subset can be appreciated by multiplying the absolute numbers of cells in the population by the activity achieved in a differentiation assay. A pictorial representation of such information is given in Fig. 1, where sorted cells were placed in serum-free, stromal cell free cultures for one week with recombinant SF, FL, IL-7, and IL-15. Such short-term assays only detect those progenitors that are substantially differentiated, and ELP require much longer in the same cultures to generate CD19+ lymphocytes (7, 24). Furthermore, additional strategies are needed to establish a probable sequence of subsequent events.
All lymphocytes derive from hormone sensitive progenitors, including those enriched in the Lin− c-kitLo pro-lymphocyte fraction. However, we found that NK progenitors were often more estrogen resistant than B cell precursors when pro-lymphocytes were first exposed to the hormone in culture. Single cell studies indicated that this fraction is a mixture of B restricted, NK restricted and bipotential progenitors. A two-step culture strategy was then used to follow progression of cells in these two pathways. After just 3 days in defined conditions, some pro-lymphocytes acquired CD45R/B220 and/or the CD122 receptor for IL-15, a cytokine that supports NK lineage differentiation. Returning them to culture for an additional 4 days allowed the ultimate fate and hormone sensitivity of these partially differentiated populations to be established (20). Interestingly, over half of the precursors destined to become NK cells at least transiently expressed CD45R/B220 (Fig. 2). Acquisition of CD122 occurred at, or just before commitment to the NK lineage and loss of sensitivity to estrogen (20).
Fig. 2. The main routes to B and NK cell lineages.
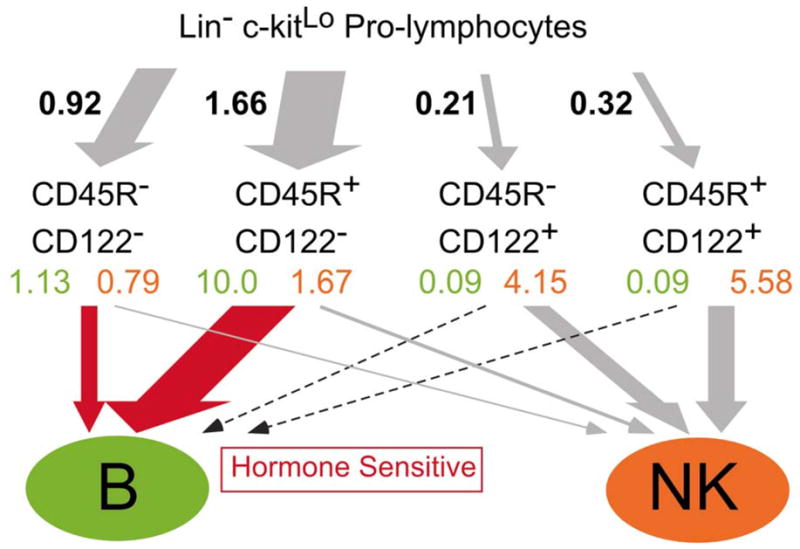
Lin− c-kitLo pro-lymphocytes were cultured 3 days with SCF, FL and IL-7 under defined conditions and harvested. The CD19− Mac-1− fraction was then sorted according to the expression of CD122 and CD45R as shown. Each of the resulting four subsets was then cultured for an additional 4 days with SCF, FL, IL-7 and IL-15. The figures given in black represent numbers of cells obtained in each subset divided by the starting cell numbers (yield). Yields of B and NK cells after the 2nd step cultures (shown in green and orange, respectively) were also calculated in the same way but multiplied by the yields in the representative 1st step culture to show how many B or NK cells were generated from one pro-lymphocyte through each pathway. When β-estradiol was included in the 2nd step cultures, differentiation of B cells (red arrows) was more significantly reduced than formation of NK cells.
Assuming that the results obtained with defined culture conditions reflect normal events in bone marrow, a sequence of early NK lineage differentiation events can be established (Fig. 3). The precise point when single cells lose the potential for differentiation in all lymphoid lineages is not known. Therefore, it is confusing to use the adjective “common” in referring to lymphoid progenitors. However, it is likely that most conventional NK cells are created in bone marrow from Lin− c-kitLo Flk-2+ IL-7Rα+ pro-lymphocytes that subsequently express CD45R before acquiring CD122, NK-1.1, and DX5 (20). Approximately one third of the NK lineage cells acquire CD94/NKG2, but progress no further in these stromal cell-free cultures.
Fig. 3. Early stages of NK lineage differentiation.
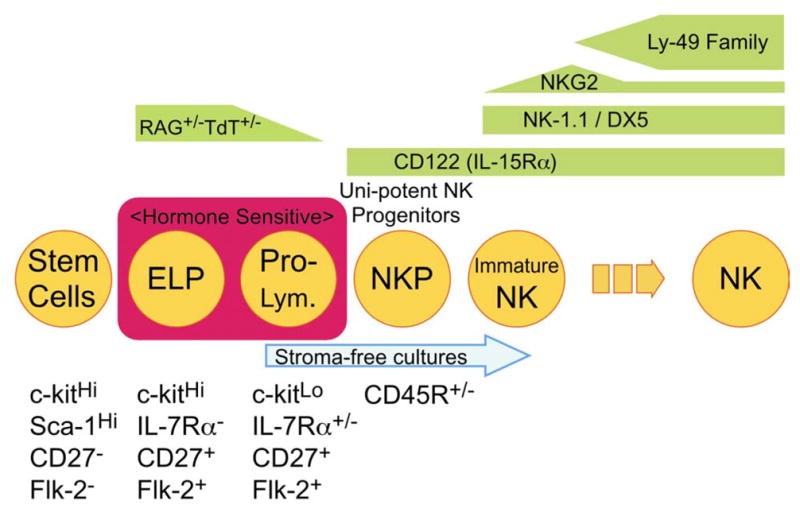
The acquisition and loss of markers by differentiating NK cell progenitors is shown in this summary of our culture studies.
Most B lineage cells also derive from Lin− c-kitLo Flk-2+ IL-7Rα+ pro-lymphocytes, but never express CD122 and could remain estrogen sensitive slightly longer than NK-lineage progenitors. As discussed above and in contrast with some other models, our findings suggest that they never lose CD24 or IL-7Rα and do not acquire either Ly-6C or CD11b/Mac-1 in bone marrow before expressing CD19 (13). Subsequent steps in the Ig gene rearrangement process, expansion of precursor numbers, display of surface markers and selection of newly formed B cells have been reviewed elsewhere (32, 41, 42).
Where do thymocytes come from?
Some type of lymphoid progenitors periodically replenishes the thymus in time-gated fashion (43). Stem cells, ELP and pro-lymphocytes from bone marrow are all capable of colonizing the thymus under experimental circumstances, but it is difficult to know which normally does so (Fig. 4). The most primitive cells in the thymus are included in a CD3− CD4Lo/− CD8− c-kitHi CD44+ CD25− triple negative (TN1) fraction (44, 45). This compartment includes cells with potential for dendritic, B, T and NK lineage differentiation, but could represent a mixture of progenitors. For example, one study showed that cells with B lineage potential in that compartment could be resolved on the basis of their lack of fluorescence in Lck promoter/GFP transgenic mice (46). Also, single cell PCR analysis revealed that only 17% of TN1 cells express RAG1 (47). We have found that a similarly small and variable subset of TN1 cells in RAG1/GFP mice express RAG1 or TdT and are depleted in estrogen or glucocorticoid treated mice (Igarashi and Kouro, unpublished observations). Thus, they are similar in many, but not necessarily all respects to the ELP in bone marrow. Acquisition of CD25 by thymocytes corresponds to reduced B lineage potential and progress is being made in understanding extracellular cues that could be involved (44). A series of compelling experiments have been done to show that signals delivered from Notch receptors normally suppress the option for progression in the B lymphoid lineage that would otherwise be possible in the thymus (48–50). This role is made especially clear when confounding influences of transformation are blocked (51). It will be interesting to learn how late in B lineage differentiation Notch is effective and whether the signal is typically delivered in the marrow before thymus seeding. The marrow is known to be replete in Notch ligands, that could be selectively modulated in discrete microenvironments by molecules of the Fringe and Numb families (52, 53).
Fig. 4. What type of cell normally seeds the thymus?
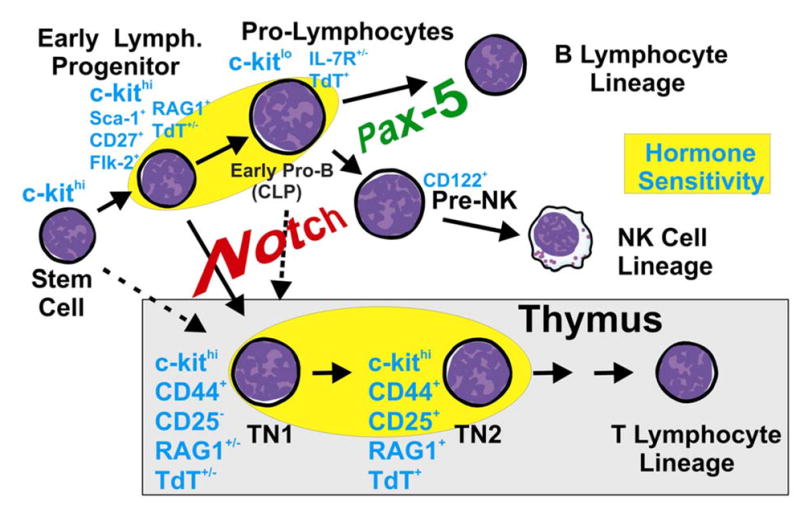
Three types of bone marrow cells are known to be able to colonize the thymus under experimental circumstances. Early lymphoid progenitors in marrow most closely resemble the least differentiated, triple negative (TN1) thymocytes and both are steroid hormone sensitive. The Pax-5 transcription factor supports cells committed to the B lineage, while signals from Notch receptors are required for T lymphopoiesis and suppress the B lineage option.
Another important issue is whether the B versus T lineage choice decision occurs before expression of Pax-5 at the ELP stage because this transcription factor is potentially able to preclude the T option (34). In this context, it is interesting that Spangrude and colleagues subdivided the pro-lymphocyte fraction, finding that upregulation of Pax-5 and loss of T cell potential both correlated with acquisition of AA4.1 (54). On the other hand, another group reported astonishingly high T potential in a Lin− IL-7Rα+ AA4.1+ fraction (55). Single cell PCR experiments might reveal what percentage of cells in the Lin− c-kitLo pro-lymphocyte fraction are Pax-5+, because at least some cells in that population can colonize the thymus and generate T cells (4, 7, 25, 55).
A technical note
The RAG1/GFP mouse model enables the isolation of extremely rare subsets of lymphoid progenitors and may be extensively used in many experimental designs. However, we would like to share our experience with one potential artifact that can be easily avoided. Cells with relatively high levels of green fluorescence are specific to the knock-in mice and are restricted lymphoid progenitors (7). However, wild type mice have hematopoietic cells that autofluoresce to the same degree as GFPLo cells in RAG1/GFP animals. We have found that this background problem is usually not significant with extensively lineage depleted adult bone marrow. However, it can be eliminated completely by use of two parameter, uncompensated flow cytometry gating. Autofluorescent cells are easily visualized as a diagonal when plotted as FL1 versus FL2, and can be detected equally well in both channels. In contrast, signals are preferentially received in the FL1 (FITC) channel from authentic GFP+ cells. Use of this method allows specific detection and sorting of cells that have just threshold levels of RAG1 expression.
Fetal/adult differences may be substantial
There has been impressive recent progress in understanding the embryonic derivation of stem cells in association with vasculogenesis (56, 57). Their first appearance in intra-embryonic sites is marked by dependence on the Runx1(AML1) transcription factor as well as by expression of endothelial cell genes. Waves of lymphoid progenitors with various differentiation options then emerge most conspicuously in the fetal liver, thymus and spleen. We recently noted that lymphopoiesis during fetal life differs in important respects from the adult steady state process (58). Therefore, only a few questions about the emergence and migration of early lymphoid progenitors will be considered here.
RAG1/GFP mice represented a powerful tool for identifying and isolating the earliest known lymphoid progenitors in adult mice (7) and we hope they will be similarly useful for fetal studies. Lymphoid progenitors have been repeatedly described in the fetal liver (2, 59–62), so it was not surprising to find substantial numbers of GFP+ cells there and in the developing marrow RAG1/GFP knock-in embryos (Fig. 5A, B). However, many of these cells were not present in discrete aggregates as might be expected if liver and marrow contained large foci of proliferating progenitors. It is possible that migration rates are sufficiently high to separate daughter cells soon after mitosis and we need to know if generation times are as fast as they can be in adult thymus and bone marrow. Careful examination of all stages of embryogenesis is also needed to exclude the possibility that cells transcribing RAG1 are actually produced elsewhere and simply colonize the liver. Identification of additional sites of expression could also be informative about the origin of stem cells and suggest experiments to learn which ones colonize developing bones. Adhesion molecules and chemokines required for retention and movement of lymphoid progenitors should be studied in parallel (63, 64).
Fig. 5. Remarkable distribution of lymphoid progenitors in tissues.
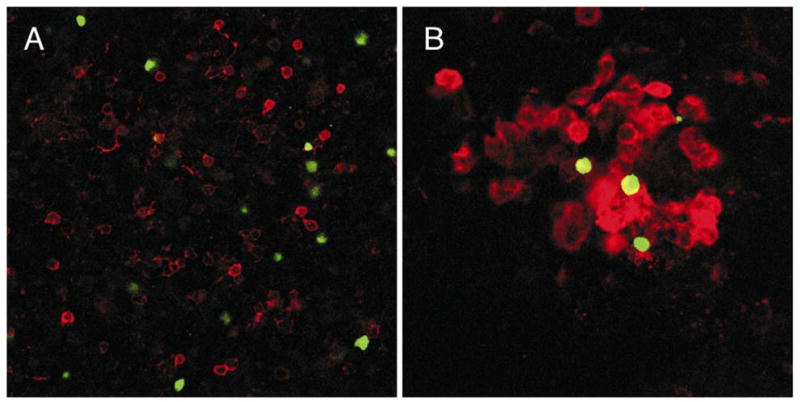
GFP+ cells (green) are shown along with CD11b/Mac-1+ cells (red) in 15 day fetal liver (A) or 16 day bone marrow (B) from heterozygous RAG-1/GFP knock in mice. These images of stained viable preparations were recorded as single optical sections with a Zeiss confocal microscope.
One important issue is whether adult marrow ELP and pro-lymphocytes have fetal counterparts, with equivalent differentiation potential. In contrast to bone marrow, clonal studies have so far revealed no restricted progenitors for all lymphoid lineages that do not also generate myeloid or erythroid cells (65, 66). Spangrude has recently reviewed the technical limitations to clonal assays, stressing that direct comparisons between laboratories is difficult and that some fetal/adult differences may be more apparent than real (67). This is certainly possible, and our own characterization of fetal cells is incomplete. However, we do know that lymphoid progenitors in fetal liver differ from ones in adult marrow with respect to hormone receptor expression and estrogen sensitivity (68). Furthermore, fetal cells with low levels of RAG1 expression have different patterns of surface marker expression from adult cells (T. Yokota and J. Hirose, unpublished observations).
Katsura and colleagues described the early emergence of a wave of restricted T lineage progenitors that predominate in the thymus of 12 day embryos (61, 69). The thymus of fetal RAG1/GFP mice contains large numbers of GFP+ cells (Fig. 6) and we hope to determine if RAG1 transcription begins before colonization of that organ at around 11 days gestation.
Fig. 6. Expression of RAG-1 by lymphoid progenitors in the fetal thymus.
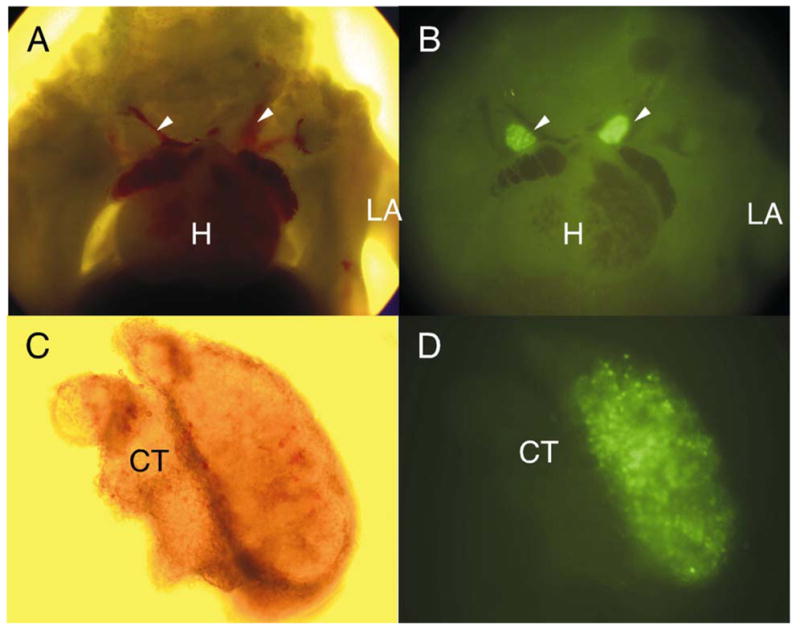
Bright field (A, C) and fluorescent (B, D) images were made of a 13 day heterozygous RAG-1/GFP knock in embryo. White arrowheads indicate the fetal thymus, while the heart (H) and left arm (LA) are also marked (panels A and B). The thymus and surrounding connective tissue (CT) was then dissected to illustrate the rich population of GFP+ cells (C, D). Corresponding FACS analysis of similar lobes revealed that approximately 80% of cells expressed GFP at that stage (data not shown).
Questions about the microanatomy of bone marrow
Our knowledge of cells in the various lymphoid differentiation pathways far exceeds information about their localization in central tissues. Classical descriptions long ago provided details about the blood supply, organization of extravascular hematopoietic zones and adipose tissue in bone marrow (70). However, we need to identify specialized sites for lymphopoiesis and track the progression of differentiating progenitors within them. Comparable questions concerning the thymus have recently been effectively addressed (71 and Petrie, this volume). However, the marrow is more complex and there are technical obstacles associated with bone and lipid rich tissue. Nonetheless, we have begun to collect images of RAG1 transcribing cells within marrow and the initial findings have been somewhat surprising (Fig. 7). Stem cells, and very primitive lymphocytes are thought to be concentrated near the sub-endosteal surface of marrow and are even so depicted in one textbook (72–74). While there are some GFP+ cells just beneath the bone surface, we have found much larger numbers scattered throughout the marrow (Fig. 7A). This was even the case for marrow from homozygous RAG1/GFP mice, where progression beyond the pro-lymphocyte stage is blocked (Fig. 7B). As with fetal liver, conspicuous foci of GFP+ cells were rare. Again, this could mean that sessile lymphoid progenitors are uncommon in adult marrow and/or that they are less mitotically active than generally believed. The spatial orientation of lymphoid progenitors in marrow must be vitally important, controlling their access to essential survival, growth and differentiation stimuli. While information is accumulating about the importance of chemokines for controlling adhesiveness and migration of cells within marrow (75, 76), three dimensional images are needed to fully appreciate this process.
Fig. 7. Lymphoid progenitors are dispersed in adult bone marrow.
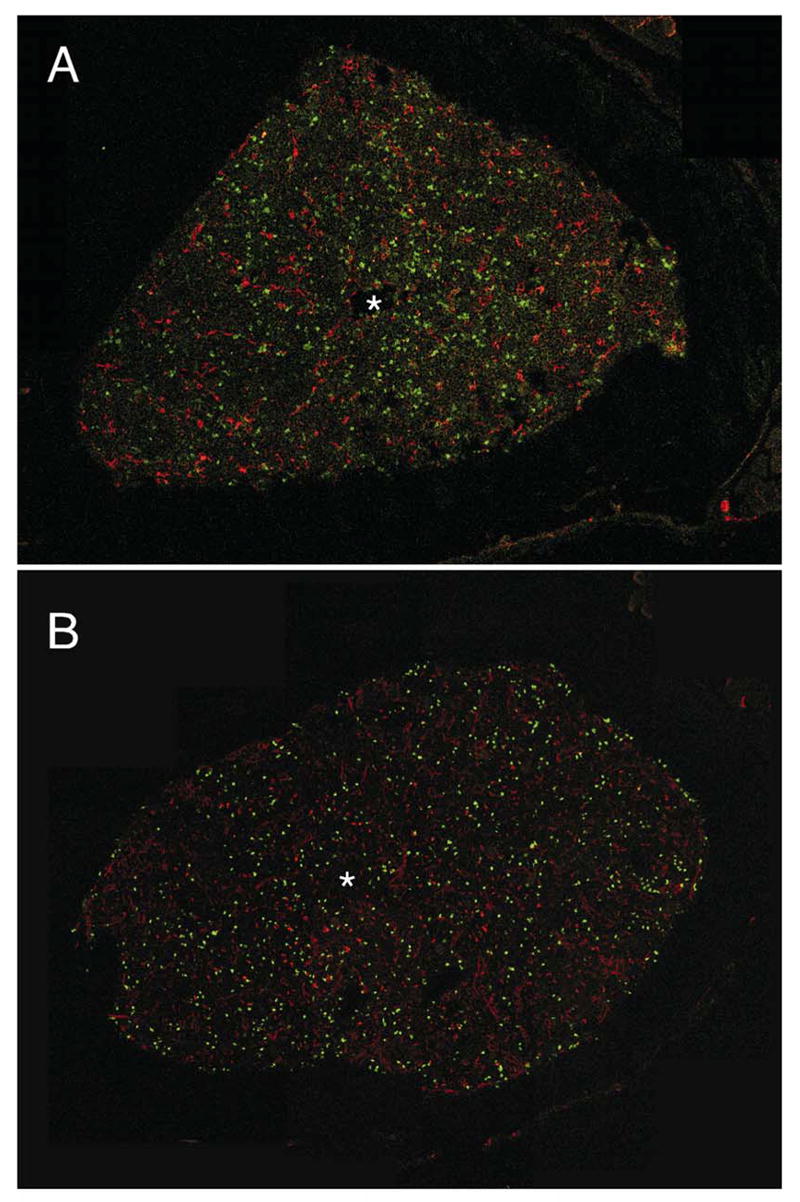
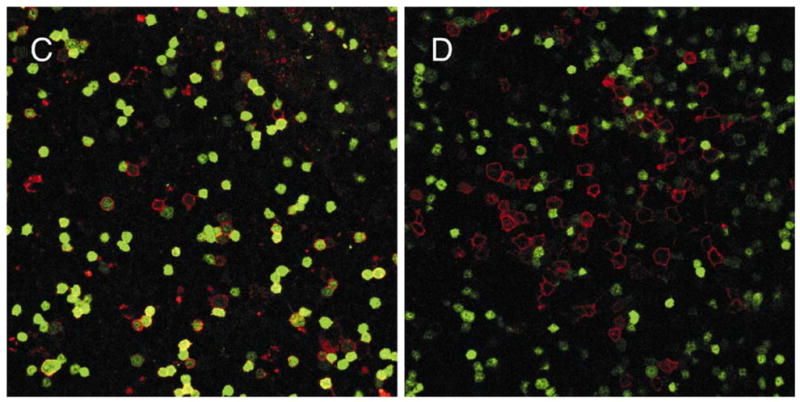
Frozen sections of femoral bones were prepared from heterozygous (A) or homozygous (B) RAG-1/GFP knock in mice. Before sacrifice, TRITC-labeled BS-1 lectin was injected to provide red staining of the vasculature, and asterisks indicate the presence of the central sinus in each section. The photographs represent 12–22 adjacent confocal optical sections, which were assembled to form the final montage. Adult marrow plugs were then prepared, stained and examined as viable preparations. Dispersed RAG-1 expressing cells (green) are contrasted with those stained red for CD19 (C) or CD11b/Mac-1 (D). These images were also recorded with a Zeiss confocal microscope.
Concluding remarks
Model diagrams depicting the process of lymphocyte differentiation have been extremely useful, if misleading and incomplete is some respects. For example, they allow rapid interpretation of results from gene targeting experiments and prediction of order in a complex hierarchy of events. An attempt was made here to show that details of the plans are still being learned, and especially with respect to very rare, primitive lymphoid progenitors. While it is convenient to talk about “stages” as though they were discrete and homogeneous, more often the data refers to “subsets”, “fractions” and “categories” of progenitors that are undergoing rapid change. Given the powerful experimental tools now available, it is hoped that differentiation models will become even more instructive, acquiring a third, more physiologically relevant dimension.
Acknowledgments
This work was supported by grants AI 20069, AI 33085, AI 45864 from the National Institutes of Health and P20 RR15577 from the COBRE Program of the National Center for Research Resources. P.W. K. holds the William H. and Rita Bell Chair in biomedical research.
References
- 1.Kincade PW, Phillips RA. B lymphocyte development. Fed Proc. 1985;44:2874–2881. [PubMed] [Google Scholar]
- 2.Paige CJ, Kincade PW, Moore MAS, Lee G. The fate of fetal and adult B-cell progenitors grafted into immunodeficient CBA/N mice. J Exp Med. 1979;150:548–563. doi: 10.1084/jem.150.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemischka IR, Raulet DH, Mulligan RC. Developmental potential and dynamic behavior of hemopoietic stem cells. Cell. 1986;45:917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- 4.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 5.Roitt IM, Brostoff J, Male D. Immunology. St. Louis; C.V. Mosby: 1985. [Google Scholar]
- 6.Medina KL, Garrett KP, Thompson LF, Rossi MID, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- 7.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002 doi: 10.1016/s1074-7613(02)00366-7. in press. [DOI] [PubMed] [Google Scholar]
- 8.Medina KL, Smithson GM, Kincade PW. Suppression of B lymphopoiesis during normal pregnancy. J Exp Med. 1993;178:1507–1515. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smithson G, Beamer WG, Shultz KL, Christianson SW, Shultz LD, Kincade PW. Increased B lymphopoiesis in genetically sex steroid-deficient hypogonadal (hpg) mice. J Exp Med. 1994;180:717–720. doi: 10.1084/jem.180.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J Immunol. 1998;161:27–34. [PubMed] [Google Scholar]
- 11.Medina KL, Strasser A, Kincade PW. Estrogen influences the differentiation, proliferation and survival of early B lineage precursors. Blood. 2000;95:2059–2067. [PubMed] [Google Scholar]
- 12.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tudor K-SRS, Payne KJ, Yamashita Y, Kincade PW. Functional assessment of precursors from murine bone marrow suggests a sequence of early B-lineage differentiation events. Immunity. 2000;12:335–345. doi: 10.1016/s1074-7613(00)80186-7. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi S-I, Kunisada T, Ogawa M, Sudo T, Kodama H, Suda T, et al. Stepwise progression of B lineage differentiation supported by Interleukin 7 and other stromal cell molecules. J Exp Med. 1990;171:1683–1695. doi: 10.1084/jem.171.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montecino-Rodriguez E, Leathers H, Dorshkind K. Bipotential B-macrophage progenitors are present in adult bone marrow. Nat Immunol. 2001;2:83–88. doi: 10.1038/83210. [DOI] [PubMed] [Google Scholar]
- 16.Li Y-S, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolink A, Ten Boekel E, Melchers F, Fearon DT, Krop I, Andersson J. A subpopulation of B220+ cells in murine bone marrow does not express CD19 and contains natural killer cell progenitors. J Exp Med. 1996;183:187–194. doi: 10.1084/jem.183.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheid MP, Landreth KS, Tung JS, Kincade PW. Preferential but nonexclusive expression of macromolecular antigens on B-lineage cells. Immunol Rev. 1982;69:141–159. doi: 10.1111/j.1600-065x.1983.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 19.Li YS, Wasserman R, Hayakawa K, Hardy RR. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5:527–535. doi: 10.1016/s1074-7613(00)80268-x. [DOI] [PubMed] [Google Scholar]
- 20.Kouro T, Kumar V, Kincade PW. Relationships between early B and NK lineage lymphocyte precursors in bone marrow. Blood. 2002 doi: 10.1182/blood-2002-02-0653. in press. [DOI] [PubMed] [Google Scholar]
- 21.Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- 22.Spangrude GJ, Brooks DM. Mouse strain variability in the expression of the hematopoietic stem cell antigen Ly-6A/E by bone marrow cells. Blood. 1993;82:3327–3332. [PubMed] [Google Scholar]
- 23.Ogawa M. Changing phenotypes of hematopoietic stem cells. Exp Hematol. 2002;30:3–6. doi: 10.1016/s0301-472x(01)00770-6. [DOI] [PubMed] [Google Scholar]
- 24.Payne KJ, Medina KL, Kincade PW. Loss of c-kit accompanies B lineage commitment and acquisition of CD45R in most murine B lymphocyte precursors. Blood. 1999;94:713–723. [PubMed] [Google Scholar]
- 25.Kouro T, Medina KL, Oritani K, Kincade PW. Characteristics of early murine B lymphocyte precursors and their direct sensitivity to negative regulators. Blood. 2001;97:2708–2715. doi: 10.1182/blood.v97.9.2708. [DOI] [PubMed] [Google Scholar]
- 26.Park Y-H, Osmond DG. Dynamics of early B lymphocyte precursor cells in mouse bone marrow: Proliferation of cells containing terminal deoxynucleotidyl transferase. Eur J Immunol. 1989;19:2139–2144. doi: 10.1002/eji.1830191125. [DOI] [PubMed] [Google Scholar]
- 27.Nussenzweig MC, Shaw AC, Sinn E, Danner DB, Holmes KL, Morse HC, et al. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin mu. Science. 1987;236:816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- 28.Wiesmann A, Phillips RL, Mojica M, Pierce LJ, Searles AE, Spangrude GJ, et al. Expression of CD27 on murine hematopoietic stem and progenitor cells. Immunity. 2000;12:193–199. doi: 10.1016/s1074-7613(00)80172-7. [DOI] [PubMed] [Google Scholar]
- 29.Adolfsson J, Borge OJ, Bryder D, Theilgaard-Mönch K, Åstrand-Grundström I, Sitnicka E, et al. Upregulation of Flt3 expression within the bone marrow Lin−Sca1+ c- kit+ stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 30.Romanow WJ, Langerak AW, Goebel P, Wolvers-Tettero IL, van Dongen JJ, Feeney AJ, et al. E2A and EBF act in synergy with the V(D)J recombinase to generate a diverse immunoglobulin repertoire in nonlymphoid cells. Mol Cell. 2000;5:343–353. doi: 10.1016/s1097-2765(00)80429-3. [DOI] [PubMed] [Google Scholar]
- 31.Sun X-H. Constitutive expression of the Id1 gene impairs mouse B cell development. Cell. 1994;79:893–900. doi: 10.1016/0092-8674(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 32.Rolink AG, Schaniel C, Busslinger M, Nutt SL, Melchers F. Fidelity and infidelity in commitment to B-lymphocyte lineage development. Immunol Rev. 2000;175:104–111. [PubMed] [Google Scholar]
- 33.Morrison AM, Nutt SL, Thevenin C, Rolink A, Busslinger M. Loss- and gain-of-function mutations reveal an important role of BSAP (Pax-5) at the start and end of B cell differentiation. Semin Immunol. 1998;10:133–142. doi: 10.1006/smim.1998.0115. [DOI] [PubMed] [Google Scholar]
- 34.Mikkola I, Heavey B, Horcher M, Busslinger M. Reversion of B Cell Commitment upon Loss of Pax5 Expression. Science. 2002;297:110–113. doi: 10.1126/science.1067518. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Diamond RA, Rothenberg EV. Cross-lineage expression of Ig-beta (B29) in thymocytes: positive and negative gene regulation to establish T cell identity. Proc Natl Acad Sci USA. 1998;95:6831–6836. doi: 10.1073/pnas.95.12.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Born W, White J, Kappler J, Marrack P. Rearrangement of IgH genes in normal thymocyte development. J Immunol. 1988;140:3228–3232. [PubMed] [Google Scholar]
- 37.Hu M, Krause D, Greaves M, Sharkis S, Dexter M, Heyworth C, et al. Multilineage gene expression precedes commitment in the hemopoietic system. Genes Dev. 1997;11:774–785. doi: 10.1101/gad.11.6.774. [DOI] [PubMed] [Google Scholar]
- 38.Borzillo GV, Ashmun RA, Sherr CJ. Macrophage lineage switching of murine early pre-B lymphoid cells expressing transduced fms genes. Mol Cell Biol. 1990;10:2703–2714. doi: 10.1128/mcb.10.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kondo M, Scherer DC, Miyamoto T, King AG, Akashi K, Sugamura K, et al. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407:383–386. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- 40.King AG, Kondo M, Scherer DC, Weissman IL. Lineage infidelity in myeloid cells with TCR gene rearrangement: a latent developmental potential of proT cells revealed by ectopic cytokine receptor signaling. Proc Natl Acad Sci USA. 2002;99:4508–4513. doi: 10.1073/pnas.072087899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 42.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 43.Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med. 2001;193:365–374. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore TA, Zlotnik A. T-cell lineage commitment and cytokine responses of thymic progenitors. Blood. 1995;86:1850–1860. [PubMed] [Google Scholar]
- 45.Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu C, Kawamoto H, Yamashita M, Kimura M, Kondou E, Kaneko Y, et al. Progression of T cell lineage restriction in the earliest subpopulation of murine adult thymus visualized by the expression of lck proximal promoter activity. Int Immunol. 2001;13:105–117. doi: 10.1093/intimm/13.1.105. [DOI] [PubMed] [Google Scholar]
- 47.Lambolez F, Azogui O, Joret AM, Garcia C, Von Boehmer H, Di Santo J, et al. Characterization of T cell differentiation in the murine gut. J Exp Med. 2002;195:437–449. doi: 10.1084/jem.20010798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pui JC, Allman D, DeRocco S, Karnell FG, Bakkour S, Lee JY, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 49.Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 50.Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14:637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- 51.Allman D, Karnell FG, Punt JA, Bakkour S, Xu L, Myung P, et al. Separation of Notch1 promoted lineage commitment and expansion/transformation in developing T cells. J Exp Med. 2001;194:99–106. doi: 10.1084/jem.194.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koch U, Lacombe TA, Holland D, Bowman JL, Cohen BL, Egan SE, et al. Subversion of the T/B lineage decision in the thymus by lunatic fringe- mediated inhibition of Notch-1. Immunity. 2001;15:225–236. doi: 10.1016/s1074-7613(01)00189-3. [DOI] [PubMed] [Google Scholar]
- 53.French MB, Koch U, Shaye RE, McGill MA, Dho SE, Guidos CJ, et al. Transgenic expression of numb inhibits notch signaling in immature thymocytes but does not alter T cell fate specification. J Immunol. 2002;168:3173–3180. doi: 10.4049/jimmunol.168.7.3173. [DOI] [PubMed] [Google Scholar]
- 54.Mojica MP, Perry SS, Searles AE, Elenitoba-Johnson KS, Pierce LJ, Wiesmann A, et al. Phenotypic distinction and functional characterization of pro-B cells in adult mouse bone marrow. J Immunol. 2001;166:3042–3051. doi: 10.4049/jimmunol.166.5.3042. [DOI] [PubMed] [Google Scholar]
- 55.Izon D, Rudd K, DeMuth W, Pear WS, Clendenin C, Lindsley RC, et al. A common pathway for dendritic cell and early B cell development. J Immunol. 2001;167:1387–1392. doi: 10.4049/jimmunol.167.3.1387. [DOI] [PubMed] [Google Scholar]
- 56.Nishikawa SI. A complex linkage in the developmental pathway of endothelial and hematopoietic cells. Curr Opin Cell Biol. 2001;13:673–678. doi: 10.1016/s0955-0674(00)00270-2. [DOI] [PubMed] [Google Scholar]
- 57.Mikkola HK, Orkin SH. The search for the hemangioblast. J Hematother Stem Cell Res. 2002;11:9–17. doi: 10.1089/152581602753448504. [DOI] [PubMed] [Google Scholar]
- 58.Kincade PW, Owen JJT, Igarashi H, Kouro T, Yokota T, Rossi MID. Nature or Nurture? Steady state lymphocyte formation in adults does not recapitulate ontogeny. Immunol Rev. 2002 doi: 10.1034/j.1600-065x.2002.18710.x. in press. [DOI] [PubMed] [Google Scholar]
- 59.Landreth KS, Kincade PW, Lee G, Medlock ES. Phenotypic and functional characterization of murine B lymphocyte precursors isolated from fetal and adult tissues. J Immunol. 1983;131:572–580. [PubMed] [Google Scholar]
- 60.Kee BL, Cumano A, Iscove NN, Paige CJ. Stromal cell independent growth of bipotent B cell-macrophage precursors from murine fetal liver. Int Immunol. 1993;6:401–407. doi: 10.1093/intimm/6.3.401. [DOI] [PubMed] [Google Scholar]
- 61.Kawamoto H, Ikawa T, Ohmura K, Fujimoto S, Katsura Y. T cell progenitors emerge earlier than B cell progenitors in the murine fetal liver. Immunity. 2000;12:441–450. doi: 10.1016/s1074-7613(00)80196-x. [DOI] [PubMed] [Google Scholar]
- 62.Douagi I, Colucci F, Di Santo JP, Cumano A. Identification of the earliest prethymic bipotent T/NK progenitor in murine fetal liver. Blood. 2002;99:463–471. doi: 10.1182/blood.v99.2.463. [DOI] [PubMed] [Google Scholar]
- 63.Potocnik AJ, Brakebusch C, Fassler R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 2000;12:653–663. doi: 10.1016/s1074-7613(00)80216-2. [DOI] [PubMed] [Google Scholar]
- 64.Egawa T, Kawabata K, Kawamoto H, Amada K, Okamoto R, Fujii N, et al. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15:323–334. doi: 10.1016/s1074-7613(01)00185-6. [DOI] [PubMed] [Google Scholar]
- 65.Katsura Y, Kawamoto H. Stepwise lineage restriction of progenitors in lympho-myelopoiesis. Int Rev Immunol. 2001;20:1–20. doi: 10.3109/08830180109056720. [DOI] [PubMed] [Google Scholar]
- 66.Mebius RE, Miyamoto T, Christensen J, Domen J, Cupedo T, Weissman IL, et al. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+CD4+CD3− cells, as well as macrophages. J Immunol. 2001;166:6593–6601. doi: 10.4049/jimmunol.166.11.6593. [DOI] [PubMed] [Google Scholar]
- 67.Spangrude GJ. Divergent models of lymphoid lineage specification: Do clonal assays provide all the answers? Immunol Rev. 2002 doi: 10.1034/j.1600-065x.2002.18704.x. in press. [DOI] [PubMed] [Google Scholar]
- 68.Igarashi H, Kouro T, Yokota T, Comp PC, Kincade PW. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proc Natl Acad Sci USA. 2001;98:15131–15136. doi: 10.1073/pnas.011513098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawamoto H, Ohmura K, Katsura Y. Presence of progenitors restricted to T, B, or myeloid lineage, but absence of multipotent stem cells, in the murine fetal thymus. J Immunol. 1998;161:3799–3802. [PubMed] [Google Scholar]
- 70.Weiss L. Bone Marrow. In: Osler AG, Weiss L, editors. The Cells and Tissues of the Immune System: Structure, Functions, Interactions. Englewood Cliffs, N.J.: Prentice-Hall, Inc.; 1972. pp. 12–29. [Google Scholar]
- 71.Lind EF, Prockop SE, Porritt HE, Petrie HT. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J Exp Med. 2001;194:127–134. doi: 10.1084/jem.194.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lord BI, Testa NG, Hendry JH. The relative spatial distributions of CFU-s and CFU-c in the normal mouse femur. Blood. 1975;46:65. [PubMed] [Google Scholar]
- 73.Jacobsen K, Osmond DG. Microenvironmental organization and stromal cell associations of B lymphocyte precursor cells in mouse bone marrow. Eur J Immunol. 1990;20:2395–2404. doi: 10.1002/eji.1830201106. [DOI] [PubMed] [Google Scholar]
- 74.Janeway CA, Jr, Travers P, Walport M, Shlomchik MJ. Immunobiology. 5. New York: Garland Publishing; 2001. [Google Scholar]
- 75.Bowman EP, Campbell JJ, Soler E, Dong A, Manlongat N, Picarella D, et al. Developmental switches in chemokine response profiles during B cell differentiation and maturation. J Exp Med. 2000;191:1303–1318. doi: 10.1084/jem.191.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]


