Abstract
Notch family receptors control critical events in the production and replenishment of specialized cells in the immune system. However, it is unclear if Notch signaling regulates abrupt binary lineage choices in homogeneous progenitors or has more gradual influence over multiple aspects of the process. A recently developed co-culture system with Delta 1 transduced stromal cells is being extensively used to address such fundamental questions. Different from fetal progenitors, multiple types of adult marrow cells expanded indefinitely in OP9-DL1 co-cultures, progressed to a DN2/DN3 thymocyte stage, and slowly produced TCR+ and NK cells. Long-term cultured cells of this kind retained some potential for T lymphopoiesis in vivo. Adult marrow progressed through DP and SP stages only when IL-7 concentrations were low and passages were infrequent. Lin− c-Kitlo GFP+ IL-7Rα+/− pro-lymphocytes were the most efficient of adult bone marrow cells in short term cultures, but the assay does not necessarily reflect cells normally responsible for replenishing the adult thymus. While marrow derived progenitors with immunoglobulin DH-JH rearrangements acquired T lineage characteristics in this model, that was not the case for more B committed cells with VH-DHJH rearrangement products.
The following disclaimer is a requirement of The Journal of Immunology: "This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
Keywords: Notch, stem cells, thymocyte, lymphocyte
Introduction
The Notch family of receptor/transcription factors has attracted great interest because of its potential importance in establishment of immune system, stem cell self-renewal and lineage fate decisions (1). Knockout and over-expression experiments have generated an impressive body of data indicating that Notch determines whether lymphoid progenitors generate B or T lineage lymphocytes (2, 3). Moreover, two laboratories demonstrated that the behavior of normal progenitors could be modified by contact with ligand modified stromal cells (4, 5). Indeed, experimental models were described that support all stages of T lymphopoiesis and even when embryonic stem (ES) cell lines were used to initiate the cultures (6).
However, the range of target cells that can respond to Notch receptor ligation with a lineage choice decision, and the extent to which partially committed progenitors can be reprogrammed, remain unclear. Indeed, we need to rigorously test if there are single branch points where progenitors at a discrete stage are irreversibly diverted into a particular pathway. Moreover, it is not known if B lineage inhibition and T lineage induction represent completely separate or related events (7, 8). Controversy remains about the role of Notch in determining the relative production of TCRαβ versus TCRγδ lymphocytes and biasing the formation of CD4 and CD8 subsets (9–11) . Additionally, most of the studies to date have utilized fetal lymphoid progenitors and direct comparisons with their counterparts in adult bone marrow have not been reported.
One of the most unique and important features of hematopoietic stem cells is their ability to extensively self-renew, while retaining the ability to give rise to multiple specialized types of blood cells. Understanding how this process is regulated may someday facilitate expansion of stem cells in culture, opening possibilities for genetic engineering and other therapeutic applications. In that context, it is interesting that human stem cells were expanded 10 fold in culture by ligation of Notch receptors (12), and stem cell lines were established by introduction of constitutively active Notch 1 (13). Therefore, it is possible that Notch receptor ligation might trap cells in a proliferative mode and block their differentiation. If so, lympho-hematopoietic cells might be expanded and maintained for prolonged periods on Delta 1 transduced stromal cells.
We have now utilized RAG-1/GFP knock-in mice, cell sorting and co-culture with Notch ligand transduced stromal cells to address these questions. Unlike their fetal counterparts, multiple subsets of adult bone marrow cells were able to generate long-term, Notch ligand and IL-7 dependent cell lines paused at the DN2/3 thymocyte stage. Progression of adult progenitors in the T lineage was blocked in the presence of 5 ng/ml IL-7. These findings provide a basis for further investigation of adult lymphoid progenitors in short and long-term cultures.
Materials and Methods
Mice and Embryos
RAG1/GFP knock-in mice have been described (14). Female B6.PL-Thy1a/CyJ (Thy -1/CD90.1) congenic and C57BL/B6 mice were bought from the Jackson Laboratory (Bar Harbor, ME). Mating homozygous male RAG1/GFP knock-in mice with wild-type C57BL/6 strain female mice generated heterozygous RAG1/GFP embryos, as well as mice that were studied as adults. Mating homozygous male RAG1/GFP knock-in mice with female B6.PL-Thy1a/CyJ mice yielded heterozygous RAG1/GFP animals bearing the Thy-1/CD90.1 antigen. Timed pregnancies were determined by designating the day of vaginal plug observation as day 0.5 post-coitus (E0.5). For experiments with adult mice, 5–10 weeks old male mice were used. All of the studies were reviewed and approved by an institutional review committee.
Antibodies
FITC-conjugated CD45R/B220 (RA3-6B2), CD3ε(145-2C11), CD8α(53-6.7), CD43, CD25, and Mac-1 antibodies, as well as PE conjugated CD3ε(145-2C11), TCRγδ, NK1.1(PK136), CD45R/B220 (RA3/6B2), CD19 (ID3), and IL-7Rα(SB/199) antibodies were all purchased from BD Pharmingen (San Diego, CA). The same source was used for biotinylated-CD106/VCAM-1, biotinylated-CD19 (clone 1D3), CD90.1(OX-7) and allophycocyanin (APC) conjugated-CD11b/Mac-1 (M1/70), DX-5, c-Kit (2B8), CD44 (1M7), TCRβ, CD4 and CD45R/B220. APC-Cy-7-CD62L(MEL-14) and PE-Cy5-Sca-1(D7) were obtained from eBioscience (San Diego, CA). A phycoerythrin-Texas red tandem-conjugated streptavidin (PE-TR-streptavidin) was purchased from Caltag (Burlingame, CA). Purified rabbit anti-TdT polyclonal antibody and a FITC-conjugated F(ab′)2 fraction of goat antirabbit IgG antibody were purchased from Supertechs (Rockville, MD). FITC-conjugated goat antimouse IgM antibody was purchased from Zymed (San Francisco, CA).
OP9 Cocultures
Transduced OP9-MigR1(OP9-Vector) and OP9-DL1 stromal cell lines have been previously described (5). They were maintained in modified minimum essential medium (α-MEM; Invitrogen, Carlsbad, CA) supplemented with 20% FCS (Hyclone, Logan, UT). Flt3-L, SCF and IL-7 were all purchased from R&D Systems Inc. (Minneapolis, MN ) and used at concentrations specified in the text. Co-culture of hematopoietic progenitors was modified to allow calculation and comparisons of proliferation and differentiation from stem cells/progenitors (5, 15). Briefly, one day before plating of progenitors, 2 × 104 stromal cells were plated into each well of 24-well plates. Progenitors were then added to the stromal cells at different densities to determine the numbers of input cells that gave optimal lymphocyte yields. On a weekly basis, stromal cells and hematopoietic cells were harvested using 0.53 mM EDTA/PBS (pH 7.4). Then, 3000~10000 hematopoietic cells together with stromal cells were subcultured onto freshly prepared stromal cells. For weekly monitoring of differentiation, stromal cells were excluded by staining with an antibody to VCAM-1 and dead cells were excluded by staining with 7-Amino-actinomycin D (7-AAD). Culture conditions and cell densities were considered acceptable when more than 95% of the recovered hematopoietic cells were viable.
Cell Sorting and Flow Cytometry
E13.5-15.5 fetal livers were harvested and subjected to cell sorting as described previously (16). Bone marrow cells were harvested and enriched for lineage-negative cells by incubation with a cocktail of antibodies to lineage markers, CD45RA (supernatant from clone 14.8), Gr-1 (Ly-6G; RB6-8C5), CD11b/Mac-1 (M1/70), CD19 (1D3) and Ter-119, followed by negative selection with the MACS cell separation system (Miltenyi Biotec, Auburn, CA). These partially lineage-depleted cells were further stained with rat anti-mouse lineage markers (biotin-Gr-1, Mac-1, CD2, CD3ε, CD8, Ter-119, CD45R, DX-5, CD19) as well as APC- labeled c-Kit and PE-Sca-1). Lineage-positive cells were electronically gated out and lineage negative fractions were sorted on the basis of RAG1/GFP, c-Kit and Sca-1 expression with a MoFlo (DakoCytomation, Ft. Collins, CO). Similar separations were done to isolate the hematopoietic stem cell (HSC; Lin−Sca-1+c-KithiCD90.1lo), L-selectin positive progenitors LSP; Lin−CD90.1−Sca-1+c-KithiCD62L+), early lymphoid progenitors (ELP; Lin−Sca-1+c-KithiRAG1+) and c ommon lymphoid progenitors, (CLP); Lin−Sca-1loc-KitloIL-7Rα+) described in Figure 6. These previously described fractions (17–20) were sorted on a FacsAria (BD Biosciences, San Jose, CA) after thoroughly lineage depleting bone marrow cells and then staining with Biotin-CD90.1 revealed by PE-TR, APC-Cy7-CD62L, PE-IL-7Rα, PE-Cy-5-Sca-1 and APC-c-Kit. Post-sort evaluation of sorted preparations revealed purities of greater than 97%. All analyses were performed on a FACSCalibur (Becton Dickinson, San Diego, CA).
Figure 6. T lineage differentiation from marrow progenitors is influenced by IL-7 concentration and subculture.
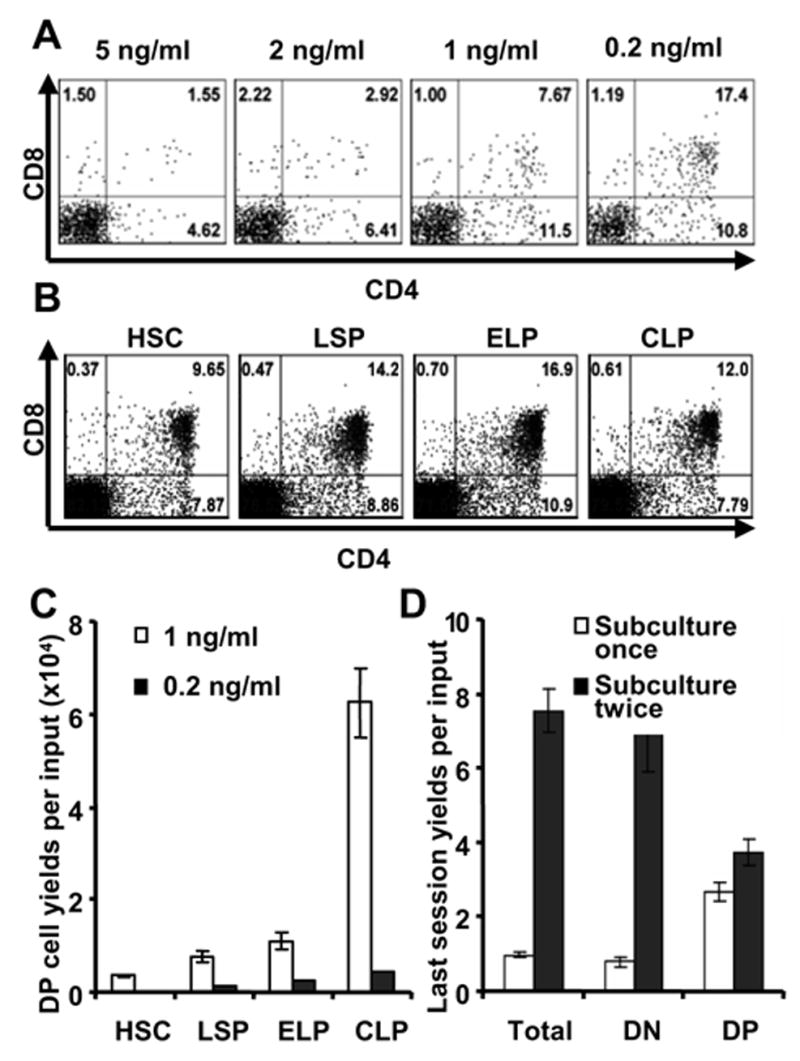
A. Lin−c-KitloIL-7Rα+ pro-lymphocytes were cultured first on OP9-DL1 with 5 ng/ml IL-7 and 5 ng/ml Flt3-Lfor two weeks. They were then subcultured to fresh stromal cells with the indicated concentrations of IL-7 plus Flt3-L and subcultured again one week later. After a total of four weeks of culture, cells were harvested and stained for flow cytometry analysis.
B. HSC, LSP, ELP and CLP fractions were cultured on OP9-DL1 with 1 ng/ml IL-7 and 5 ng/ml Flt3-L for eight days. They were then subcultured under the same conditions for another 16 days before harvest and staining for CD4 and CD8.
C. Total yields of CD4+ CD8+ (DP) cells were calculated per input HSC, LSP, ELP or CLP in cultures that were maintained for the whole time on OP9-DL1 with Flt3-L and either 1 ng/ml IL-7 or 0.2 ng/ml IL-7. After the first eight days, all groups were subcultured with the original conditions for another 16 days before harvest and analysis. The absolute numbers of DP cells per sorted progenitor were calculated by multiplying yields of the first culture interval with that of the second interval and the frequency of DP cells.
D. HSC were cultured on OP9-DL1 with Flt3-L and 1 ng/ml IL-7 for 8 days, and then subcultured in two groups . One group (shown by open bars) was harvested and stained on day 27, The parallel group (closed bars) was maintained in the same way, but passaged one additional time on day 24. Numbers of total cells, CD4− CD8− ( DN) and DP cells are shown.
Transplantation
Congenic Ly 5.1 mice were lethally irradiated with a single dose of 960 rads 4 hours before transplantation. Then, 105 recipient whole bone marrow cells were mixed with 105 sorted Lin−c-Kit+Sca-1+ cultured cells and injected intravenously into recipients. Cells were harvested from bone marrows, thymuses and spleens 21–28 days after injection and stained to check lineage differentiation. Additional transplanted animals were observed for two months and autopsied to see if tumors developed.
Reverse Transcriptase (RT)-PCR Analysis of Gene Expression
The mRNAs were isolated from sorted cells using MicroPoly(A) Pure (Ambion, Austin, TX) and converted to cDNA with Moloney murine leukemia virus RT (GIBCO/BRL). cDNA amounts were normalized by comparing the PCR products using probes specific for β-actin. The PCR was conducted by using combination with ampli-Taq DNA polymerase (Takara, Shiga, Japan) and TaqStart antibody (CLONTECH) at three different cycles to confirm the reaction is in the exponential phase. To compare the relative expression level of relevant genes, serial 5-fold dilutions of cDNA were amplified at appropriate cycles. In the case of no amplification, secondary PCR was performed by using 2 μl for 20-μl reaction volume from first reaction as a template to confirm the lack of transcripts. Anti-Taq antibody was inactivated by heating at 95°C for 7 min before amplification carried out as follows: 15 s at 94°C, 15 s at 57°C, and 30 s at 72°C. Gene specific primer sequences are listed in Table 1.
Table 1.
Primers used for RT-PCR analyses
| Notch 1 | 5′-CGGTGTGAGGGTGATGTCAATG-3′ | 5′-GAATGTCCGGGCCAGCGCCACC-3′ |
| Notch 2 | 5′-CTACAACTGTATCTGCCGCAGC-3′ | 5′-CCTTGTCGGCGCAGTACTGACTC-3′ |
| IL-7Rα | 5′-CCCCATAACGATTACTTCAAAGGC-3′ | 5-AGAGTTTGGCAGCAAGTCTTGATA-3′ |
| Bcl-2 | 5′-TCGCTACCGTCGTGACTTC-3′ | 5′-AAACAGAGGTCGCATGCTG-3′ |
| EBF | 5′-GAGATTTTTCCACAAGAAAAGGTTG-3′ | 5′-GGAAGAACCTGTCAATTATCACTGG-3′ |
| λ 5 | 5′-CTTGAGGGTCAATGAAGCTCAGAAGA-3′ | 5′-CTTGGGCTGACCTAGGATTG-3′ |
| mb1 | 5′-GCCAGGGGGTCTAGAAGC-3′ | 5′-TCACTTGGCACCCAGTACAA-3′ |
| PU.1 | 5′-CGGATGACTTGGTTACTTACG-3′ | 5′-GTAGGAAACCTGGTGACTGAG-3′ |
| GATA1 | 5′-TCATACCACTAAGGTGGCTGAATC-3′ | 5′-ACACAGTTGAGGCAGGGTAGAG-3′ |
| GATA2 | 5′-CGGAATTCGACACACCACCCGATACC-3′ | 5′-CGGAATTCGCCTACGCCATGGCAGT-3′ |
| GATA3 | 5-GGCCAGGCAAGATGAGAAAGA-3′ | 5′-TCTGACAGTTCGCGCAGGATG-3′ |
| Rag 1 | 5′-TGCAGACATTCTAGCACTCTGG-3′ | 5′-ACATCTGCCTTCACGTCGAT-3′ |
| pTα | 5-CATGCTTCTCCACGAGTG-3′ | 5′-CTATGTCCAAATTCTGTGGGTG-3′ |
| Id1 | 5′-TCCAACTTCTTGTTCTCTCCC-3′ | 5′-CACAAGATGCGATCGTCG-3′ |
| E47 | 5′-CGCACTGACCACGAGCTTCAC-3′ | 5′-TCCAGGGACAGCACCTCATCTG-3′ |
| Hes-1 | 5′-GCCAGTGTCAACACGACACCGG-3′ | 5′-TCACCTCGTTCATGCACTCG-3′ |
| Irf 5 | 5′-TTCCAGAAGGGCCAGACTAAT-3′ | 5′-TGACATCAGGCCATTCTTCTC-3′ |
| BhllhB2 | 5′-AGCCGTGGACTTGAAAGAGA-3′ | 5′-TGGATGACTGGCACACAGTT-3′ |
| cFos | 5′-ATCCTTGGAGCCAGTCAAGA-3′ | 5′-TCCCAGTCTGCTGCATAGAA-3′ |
| IL2Rα | 5′-AACGGCACCATCCTAAACTG-3′ | 5′-CTGTGTTGGCTTCTGCATGT-3′ |
Results
Fetal and adult lymphoid progenitors can respond quite differently to Notch receptor ligation
Primitive cells in the AGM and fetal liver can efficiently give rise to T lineage lymphocytes (defined as TCRαβ+CD4+CD8+ or TCRγδ+ ) when cultured on OP9-DL1 in alpha MEM supplemented with 5 ng/ml IL-7 and 5 ng/ml Flt3-L (Yokota et al. submitted) and we assumed the same would be true for adult bone marrow. However, this was not the case, and only very small yields of γδ+ T lymphocytes were obtained (Figure 1). In the example shown, RAG-1/GFPlo progenitors from day 14 fetal liver were directly compared to Lin− c-Kitlo RAG-1/GFP+ IL-7Rα+ cells isolated from adult bone marrow and found to generate at least 1000 times more T cells (Figure 1B).
Figure 1. Fetal/adult disparity in T lymphopoietic potential in OP9-DL1 co-cultures.
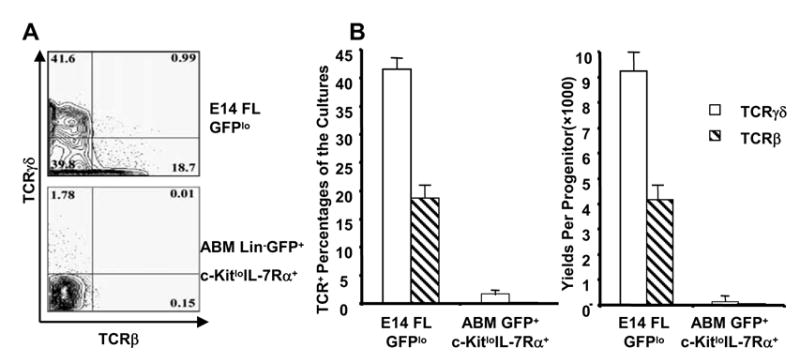
A. E14 Fetal liver progenitors and adult bone marrow Lin−RAG1/GFP+c-KitloIL-7Rα+ cells were cultured with 5 ng/ml IL-7 and 5 ng/Flt3-L on OP9-DL1 for fourteen days. Representative plots are shown for cultured cells stained for TCRβ and TCRγδ. Similar results were obtained in experiments with 8 other fractions of adult bone marrow.
B. Percentages of TCRβ+ and TCRγδ+ cells present at the end of cultures are shown along with yields per input progenitor. The values are means of duplicate cultures ± S.D. in this typical experiment.
It seemed possible that this might not have represented an appropriate Notch ligand responding subset of marrow progenitors, so subsequent experiments utilized a series of Lin− fractions. Many different subsets of marrow were capable of B lymphopoiesis when placed on OP9-Vector stromal cells, but this differentiation option was completely blocked in OP9-DL1 co-cultures (Figure 2 and data not shown). The production of CD11b/Mac-1+ myeloid cells was also inhibited by Notch receptor ligation. Percentages and total numbers of recovered TCR+ cells were always extremely low in OP9-DL1 co-cultures initiated with marrow fractions. In addition, the cultures usually contained approximately the same numbers of NK1.1+ and an unidentified population of DX5+NK1.1− cells (data not shown). Similar observations were obtained in more than ten experiments where different parameters were exploited for separation of a total of nine marrow cell populations. This was also the case when fetal and adult progenitors were isolated at the same time using the same sorting strategy and cultured side by side. Therefore, it is highly unlikely that any subset in adult bone marrow has comparable activity to fetal progenitors, suggesting that adult progenitors have requirements not recapitulated by these particular culture conditions. This issue is addressed in more detail below.
Figure 2. Delta1 mediated Notch signals inhibit B and myeloid lineage differentiation from adult progenitors in short term (9 days) cultures.
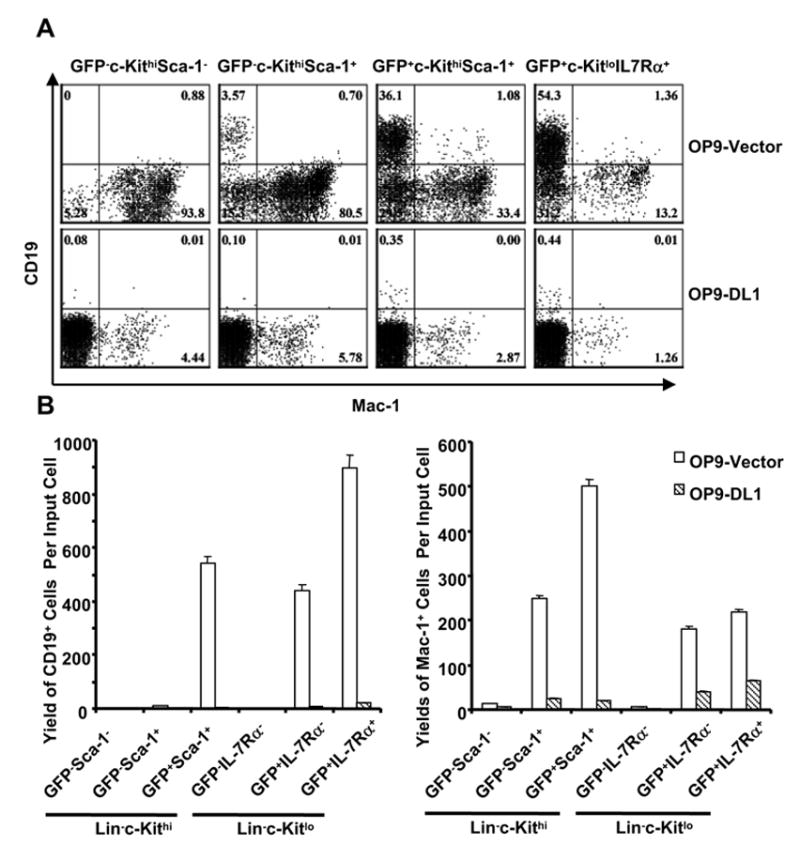
A. Six subsets of adult progenitors were sorted according to expression of RAG1/GFP, c-Kit and Sca-1 after lineage depletion before culture on OP9-Vector (top row) and OP9-DL1 (bottom row) for 9 days (5 ng/ml IL-7 and 5 ng/ml Flt-L). Recovered cells were then stained for CD11c/Mac-1 and CD19 and results from four of the major populations are illustrated here.
B. Yields of B lineage cells (left panel) and myeloid cells (right panel) per input progenitor were calculated for all six groups of cultures. Open bars represent cells recovered from OP9-Vector cultures, while shaded bars correspond to OP9-DL1 cultures. The values are means of duplicate cultures ± S.D. in this representative of three similar experiments.
Homogenous populations of early T lineage lymphocytes expand in OP9-DL1 co-cultures initiated with adult bone marrow
It seemed possible that adult bone marrow progenitors of T cells might benefit from extended culture intervals. However, this was not the case, and surface TCR+ cells represented a minor fraction of recovered populations even after five weeks of co-culture (data not shown). Note that stromal cell overgrowth was detrimental to total cell yields and we sub-cultured lymphoid cells at weekly intervals to freshly prepared stromal cell layers. Lymphocyte growth could not be sustained when long-term OP9-DL1 cultured cells were transferred to OP9-Vector stromal cells or when IL-7 was withdrawn, indicated that survival and/or proliferation required sustained Notch and IL-7 signaling (data not shown). In contrast, OP9-DL1 co-cultures with added cytokines continued to thrive for at least three months (Figure 3A and data not shown). While cultures could be established from six discrete fractions of bone marrow, the efficiency of growth during the first week was not uniform. The most efficient Lin− c-Kitlo RAG-1/GFP+ IL-7Rα+/− pro-lymphocytes/CLP produced 5,000 cells per input progenitor during this interval, while the yield from other populations was one log less. From the second week, each cell that was subcultured produced an average of 65 cells, regardless of the starting population. After becoming established, the cultures could be propagated indefinitely and we estimate that one progenitor produced at least 1016 lymphocytes within two months (Figure 3A).
Figure 3. A range of adult bone marrow progenitors representing various stages of differentiation expand under the influence of Delta 1.
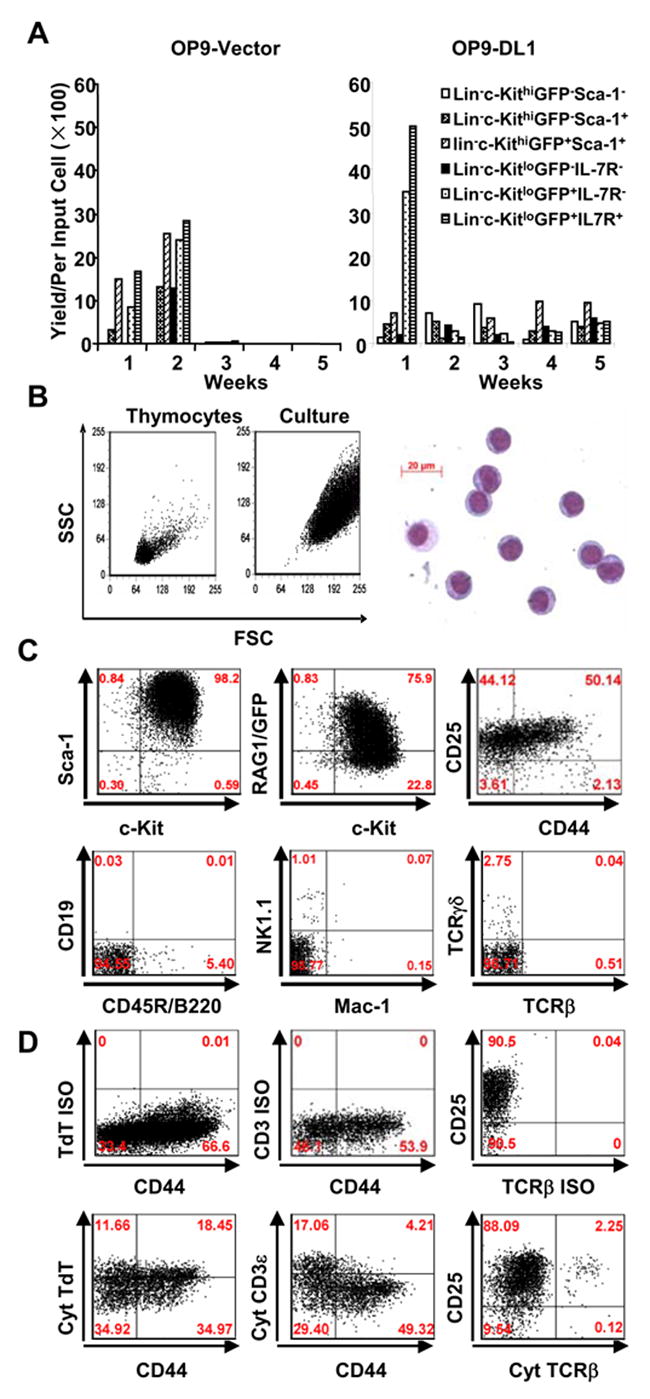
A. Six fractions of bone marrow progenitors were sorted on the basis of RAG1/GFP expression and cultured for 5 weeks on stromal cells plus 5 ng/ml IL-7 and 5 ng/ml of FL. The yields per input were calculated for each weekly expansion.
B. Light scatter properties of cultured cells are compared to freshly isolated lymphocytes (left panel). May-Grunwald-Giemsa staining of a cytocentrifuge preparation is also shown (right panel).
C. Representative flow cytometry results are shown for Lin− c-Kitlo IL-7Rα+ marrow cells expanded on OP9-DL1 for four weeks and then stained for progenitor and lineage specific markers. Identical results were obtained when eight other fractions of bone marrow were cultured in this way.
D. Expanded cells were first stained for surface lineage markers (VCAM-1 for stromal cells, DX-5 for NK cells, CD3ε for T cells, Mac-1 for myeloid cells), along with CD25 and CD44. They were then permeabilized and stained for cytoplasmic T lineage stage-specific proteins. Quadrants were set based on isotype matched controls (ISO) and positive controls using bone marrow and thymocytes (data not shown). Cultured cells were gated for VCAM-1− DX-5− CD3ε− Mac-1− cells, Isotype control stainings for cytoplasmic (cyt) TdT (Rabbit IgG and FITCGoat anti-rabbit IgG), cyt CD3ε (FITC IgG1), cyt TCRβ (PE IgG2) were also prepared with cultured cells and are shown in the upper panel. Cytoplasmic TdT, CD3ε and TCRβ are shown in the lower panel
Weekly examination of cells by flow cytometry revealed that the long-term propagated cells were nearly homogeneous. Indeed, recovered cells had very similar characteristics even when very different subsets of marrow were used to initiate the cultures. That is, most cells propagated for extended periods on OP9-DL1 were medium to large in size and lacked markers of erythroid (TER119), myeloid (CD11b/Mac-1 and Gr-1), NK (DX-5 and NK1.1), dendritic (CD11c) and B lymphocyte (CD45R/B220, BP-1 and CD19) lineages (Figure 3B, C and data not shown). Markers associated with mature lymphoid cells (CD40, CD23, CD21, CD28 and B7) were also undetectable (data not shown). Other hematopoietic markers that were detectable include CD43, Ly6C, Fas and CD26 while the AA4.1 antigen associated with fetal stem cells and developing B lineage lymphocytes was absent.
Long-term cultured cells were CD45+ Sca-1+ c-Kit+ CD43+ CD44+/− CD25+ CD5+ and thus resembled thymocytes at the DN2/DN3 stage (Figure 3C). Flow cytometry revealed low to high levels of RAG-1/GFP and RAG-1 transcripts were detected by RT-PCR (see below). TdT is a valuable marker of lymphopoiesis that is expressed from the early lymphoid progenitor (ELP) stage in bone marrow and by thymocytes. Long-term cultured cells were TdTlo/+(Figure 3D). Components of the TCR complex were also evaluated. Small numbers of cells with cytoplasmic CD3ε or TCRβ were detectable by flow cytometry (Figure 3D). RT-PCR revealed mRNA for pTα (data not shown). We conclude that while only pro-lymphocytes expand rapidly, many other discrete fractions of adult bone marrow progenitors can be extensively propagated in response to Notch receptor mediated signals and acquire indistinguishable properties. Our subsequent experiments focused on extensive characterization of these long-term cultured cells.
Continued, low-level T and NK lineage differentiation from long-term propagated lymphocytes arrested at the DN 2/3 stage
It has been reported that Notch can promote self-renewal of hematopoietic stem cells (21) and the above results show that bone marrow cells could be expanded for months on the Delta 1 ligand. Thus, it seemed possible that long-term propagated cells generated from the various marrow fractions were reprogrammed and assumed stem cell properties. This was addressed with a more extensive RT-PCR and functional analysis of the cultured cells. Semiquantitative RT-PCR analyses revealed that the GATA-3 and PU.1 and E47 transcription factors were s expressed, and the Id-1 repressor of the bHLH family of transcription factors was just detectable. Consistent with the growth selection conditions, the cells expressed Bcl-2, Notch 1, Hes-1 and IL-7Rα transcripts. Levels of the IL-7 receptor were beneath that detectable by flow cytometry even though their survival required the continuous presence of this cytokine. In contrast, the γc (CD132) cytokine receptor component was easily seen by staining. While the cells expressed the IL-2Rα chain (CD25), the IL-2Rβ (CD122) component was absent (data not shown).
A recent study identified genes whose expression changes sharply as cells progress through early differentiation stages in the thymus (22). Therefore, we evaluated four of these by RT-PCR analysis, comparing long-term cultured lymphocytes to freshly sorted CD4− CD8− CD3− CD19− CD11c− DX-5− thymocytes (Figure 4). This revealed that cells propagated in Notch dependent cultures resembled CD44+ CD25+ (DN2) and CD44− CD25+ (DN3) cells within a normal thymus. Additional PCR analyses were performed to assess the status of receptor gene rearrangements. As expected, evidence was obtained for rearrangement of all of four TCR gene segments (Vβ3, Vβ6, Vβ8, Vβ17) that were evaluated (data not shown). Although long-term cultures always contained very small percentages (~3%) of surface TCR+ or NK1.1+ lymphocytes, it seemed possible that these were self-renewing. Therefore, we rigorously depleted these differentiated cells by cell sorting and returned the remaining cells to culture. While the majority population remained immature Sca-1+ c-Kit+ cells one week later, T and NK lineage cells were again detectable (Figure 5A).
Figure 4. Expanded cells have properties similar to DN2/DN3 stage thymocytes.
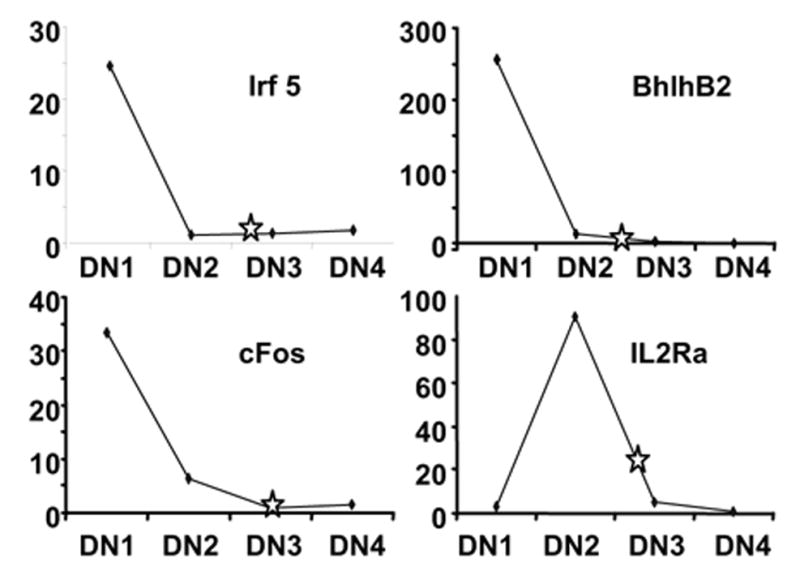
Lin−Sca-1+c-Kit+ cells were prepared by sorting cells recovered from 28 day cultures of Lin− c-Kitlo IL-7Rα+ bone marrow progenitors. This eliminated the small numbers of stromal, T and NK cells present at that time Highly enriched lymphocytes from the long term cultures were then compared to freshly sorted CD3ε− CD4− CD8− NK1.1− Mac-1− CD19− adult thymocytes that were further resolved into DN subsets according to expression patterns of CD44 and CD25. RT-PCR analyses were performed to detect transcripts that vary in a stage dependent fashion (22). The plots depict values obtained with freshly isolated thymocytes and the stars show results obtained with cultured cells.
Figure 5. Expanded cells retain the potential to become T cells and natural killer cells.
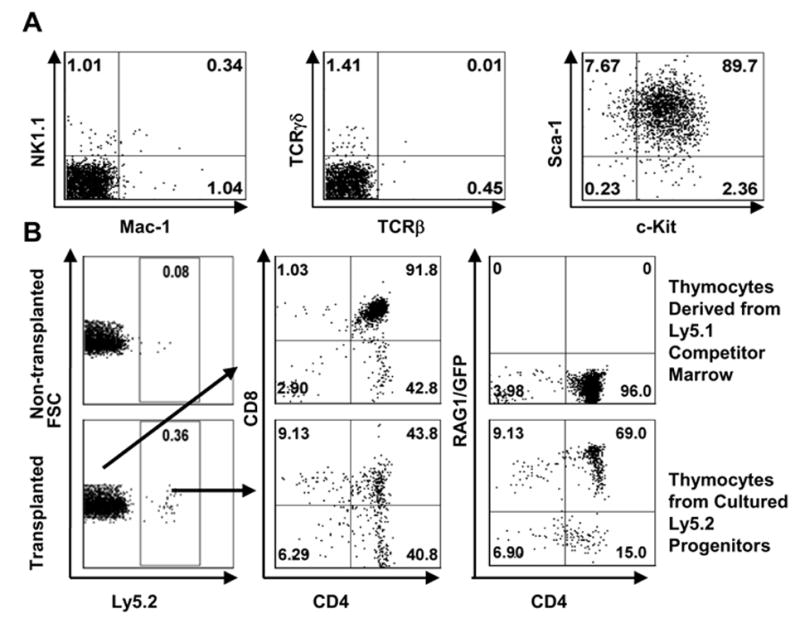
A. Long-term (28 days) cultured lymphocytes derived from the Lin− c-Kitlo IL-7Rα+ subset of marrow were purified by sorting for Lin−c-Kit+Sca-1+ cells. They were then re-cultured on OP9-DL1 for another week before staining for NK1.1, Mac-1, TCRβ, TCRγδ, c-Kit and Sca-1 antigens.
B. A total of 105 sorted c-Kit+Sca-1+ cells were mixed with the same amount of host type, whole bone marrow cells and intravenously injected into lethally irradiated Ly5.1 mice. Twenty-eight days after transplantation, thymocytes from non-transplanted host and transplanted animals were collected and assayed for thymus reconstitution. The left panels show gating for donor Ly5.2+ lymphocytes, while the middle and right panels illustrate their properties.
Ly-5.2 marked long-term cultured lymphocytes (1 x 105 cells) were then transferred with an equal number of Ly-5.1 competitor bone marrow cells to irradiated (9.6 Gys) Ly-5.1 recipient mice to assess their differentiation potential (Figure 5B). In two experiments of this kind, a total of 3.9(±0.4)x105 donor-type cells were recovered from the thymus 21–28 days later, where they expressed RAG-1/GFP and had progressed through CD4+ CD8+ to SP stages. However, cultured cells competed poorly with Ly5.1 bone marrow cells and no donor-type cells were detected in either the spleen or bone marrow. The fact that there was no evidence of malignant transformation in transplanted animals suggests that long-term cultured lymphocytes retained normal properties.
Adult lymphoid progenitors have unique requirements for T Lineage differentiation in culture
Recent studies have demonstrated remarkable differences in cytokine requirements for fetal and adult lymphopoiesis (23–25). In particular, IL-7 is only essential for formation of T and B cells beyond the early neonatal period (26). Therefore, we considered that T cell progenitors within adult bone marrow might require special conditions. Therefore, cultures were initiated with pro-lymphocytes and maintained for the first two weeks in the presence of 5ng/ml IL7. They were then subcultured weekly with a range of IL-7 concentrations for an additional two weeks (Figure 6A). This revealed that low doses of IL-7 dramatically increased percentages of CD4 CD8 DP T lineage cells. However, total yields of TCRγδ+ cells were much less and slightly fewer TCRβ+ cells were produced when IL-7 was low during the last two weeks (data not shown).
Four subsets of adult marrow were then isolated and placed on OP9-DL1 stromal cells with 1ng/ml of IL-7. They were passaged to fresh OP9-DL1 cells just once after 8 days. The Lin− Sca-1lo c-Kitlo IL-7Rα+ (CLP) fraction generated lymphocytes on day 18 and they emerged in other cultures somewhat later. Impressive percentages of CD4 CD8 DP cells, and even CD4+ cells were present in all of the cultures when they were evaluated after a total of 24 days (Figure 7B). Similar results were obtained in parallel cultures that were held with 0.2ng/ml of IL-7. Total yields of DP T lineage cells were highest in cultures initiated with CLP and when the higher concentration of IL-7 was used (Figure 6C).
Figure 7. Long-term OP9-DL1 co-cultures selectively expand bone marrow cells that lack immunoglobulin VH-DHJH rearrangements.
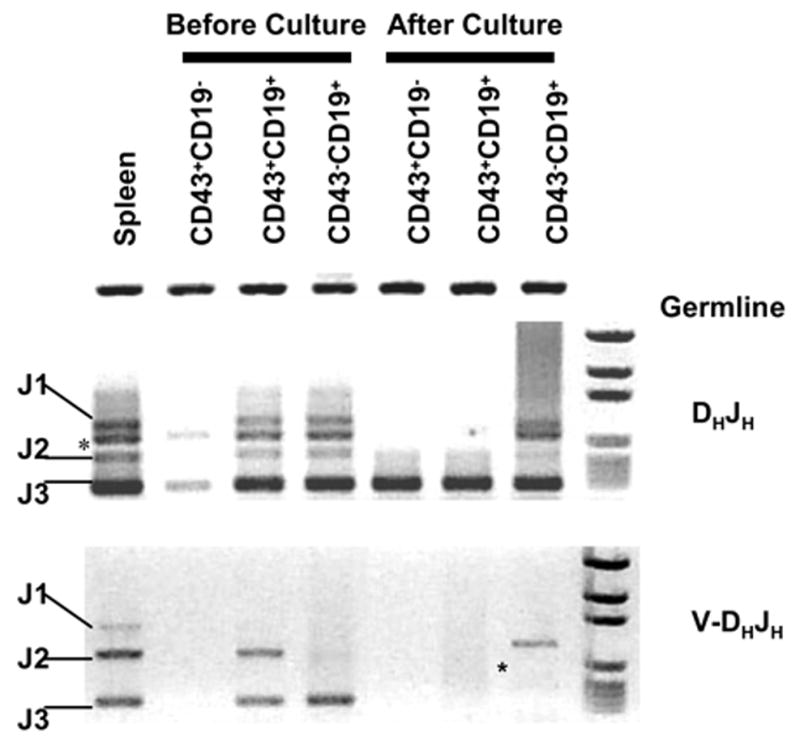
B220+IgM− B progenitors from adult bone marrow were sorted into CD43+CD19−, CD43+CD19+and CD43−CD19+ fractions before culture for 4 weeks. CD19−c-Kit+Sca-1+ cells were then sorted to exclude differentiated and stromal cells. Genomic DNA was extracted and DH-JH (upper panel) and VH-DHJH immunoglobulin gene rearrangement were tested and compared with those prepared from initiating progenitors. Germline and rearrangement products are labeled, while two artifact bands are designated with asterisks.
The frequency of subculture is another variable that might influence T lineage differentiation and we sought conditions that might favor T cell production from stem cells. Therefore, Lin− Sca-1+ c-Kithi Thy-1/CD90.1Lo HSC were isolated from adult bone marrow and placed in OP9-DL1 co-cultures for 8 days with 1 ng/ml IL-7. A subset of those cultures was passaged and held for an additional 19 days (Figure 6D). An additional group was sub-cultured a second time after 16 days, and all were evaluated at the same time. While absolute yields of DP cells were not dramatically changed by the additional passage, numbers of DN cells substantially increased.
Unlike fetal progenitors, which produced large numbers of DP cells in two weeks, adult progenitors required more time and some subculture was required to maintain optimal yields. While adult progenitors progressed to the DN2/3 stage and proliferated indefinitely in high IL-7 concentrations, further differentiation was favored by much lower concentrations of this cytokine and a minimal number of passages. In addition, long-term Notch dependent cell cultures could be easily established from adult, but not fetal cells.
Long-term OP9-DL1 Co-cultures expand marrow progenitors prior to VHDHJH rearrangement
There are at least two possible explanations why long-term cultures initiated with a wide variety of bone marrow cells are indistinguishable. Rare cells with uniform properties might have been selected for expansion or, alternatively, progenitors dedicated to alternate fates could have been re-directed towards the T lineage. Therefore, we prepared cultures with three types of relatively differentiated CD45R/B220+ marrow cells. We know from other studies that several types of stromal cells are suppressive for committed B lineage progenitors (Yokota, et al. manuscript submitted) and that was the case in these experiments. That is, in constrast to pro-lymphocytes, the three CD45R/B220+ fractions generated very small numbers of cells in one week OP9-DL1 co-cultures (data not shown). It is also important to note that even when cultures were initiated with CD19+ cells, numbers of CD19+ cells present in OP9-DL1 cultures one week later were only a fraction of those present in OP9 stromal cell co-cultures. However, these could be expanded with three subsequent passages and sufficient cells were harvested at four weeks for analysis. At that time, no CD19+ cells were present, and the lymphocytes resembled DN2/DN3 stage thymocytes as described above. That is, the cultures contained 3–8% TCRγδ+ , 2–15% NK+ and 75–90% CD44+/− CD25+ cells. We reasoned that CD45R/B220+ CD19+ CD43+IgM− and CD45R/B220+ CD19+ CD43− IgM− fractions would be highly enriched for B lineage cells and genomic PCR analyses confirmed that at least some in the starting populations had undergone VH to DHJH recombination (Figure 7). However, DHJH, but not VH-DHJH rearrangement products were found in long-term cultured lymphocytes. We conclude that long-term OP9-DL1 co-cultures can suppress expansion of cells that would normally become B cells and provide a very powerful selective pressure to cells not fully committed to the B lymphocyte lineage.
Discussion
This study demonstrates that numerous types of progenitors derived from adult marrow can be used to establish long-term T lineage cell cultures on DL1 transduced stromal cells However, while fetal cells progressed efficiently to CD4+ CD8+ and even single positive T lineage cells, progenitors from adult bone marrow expanded continuously but failed to progress past a stage equivalent to DN2/DN3 thymocytes in the presence of 5 ng/ml IL-7. Reduced cytokine concentrations and a minimum of subcultures supported the production of TCRβ+ lymphocytes from adult progenitors. This information extends previous indications that fetal and adult progenitors differ in differentiation requirements. A pro-lymphocyte fraction, including cells designated CLP, grew most efficiently during the first week on OP9-DL1, but established cultures initiated with a wide range of marrow subsets were indistinguishable. This resulted at least in part from the selective expansion of rare progenitors that were not fully committed to the B lymphocyte lineage.
Adult marrow progenitors were dramatically different from their fetal counterparts in several respects. Two Lin− c-Kitlo RAG-1/GFP+ IL-7Rα+/− pro-lymphocyte fractions of bone marrow expanded as efficiently as fetal liver in one-week OP9-DL1 co-cultures (Figure 3A and data not shown). However, the yield of TCR+ lymphocytes one week later was at least one thousand fold less than that obtained from cultures initiated at the same time from fetal liver progenitors (Figure 1). While co-cultures initiated with fetal cells usually lasted no longer than 4 weeks, we found that adult marrow progenitors could be serially transferred to fresh stromal cells an indefinite number of times. These adult derived cells retained dependence on IL-7 and Notch receptor ligation, while acquiring homogenous characteristics. Indeed, cultures initiated with six distinctly different subsets of bone marrow were remarkably similar by the second passage and subsequently expanded at approximately the same rate. Other fetal/adult differences in T lymphopoiesis have been previously reported (27, 28). In addition, a very recent study indicates that IL-7 blocks adult, but not fetal thymocyte differentiation. (29, 30)
The density and complexity of Notch ligands, antigen-presenting cells and cytokines found in the normal thymus is unlikely to be fully replicated in this co-culture model. Despite that, progression of T lymphopoiesis and production of TCR+ cells from fetal progenitors is surprisingly robust. All three of our labs routinely obtain lower cell yields and slower expansion from adult marrow progenitors, but only one routinely observes efficient T cell production from that source (6). We show here that low cytokine concentrations and a minimal number of passages favor lineage progression from adult cells. Subsequent analysis may reveal important technical variables such as serum batches, cytokine source, passage number of stromal cells and mouse strains may represent other important technical variables.
There is growing evidence that many discrete cell types in bone marrow have the potential to become T lymphocytes, and it is difficult to discern which ones normally replenish the thymus (32, 33). The most primitive Lin− Sca-1+ c-Kithi fraction of bone marrow contains stem cells, L-selectin positive progenitors and early lymphoid progenitors with the potential for sustained T cell production (20, 34). Moreover, cells with these general properties are detectable within the bloodstream and resemble canonical pro-thymocytes in some respects (7, 35). On the other hand, cells with at least short term T lymphopoietic potential can be found within Lin− c-Kitlo and even CD45R/B220+ fractions and it is possible they contribute under some circumstances (18, 20, 36). While direct comparisons under physiological conditions could be informative, it seems likely that the T versus B fate decision is not as abrupt as generally believed. There are likely to be two factors that contribute to this misconception. One is that multiple types of progenitors can be diverted into the T cell lineage under the conditions found in OP9-DL1 cocultures (present data and 7), although this is not recapitulated under normal physiologic conditions (7). The other is that Notch signaling can, to some extent, redirect the lineage fate of cells that would normally not become T cells.
In any event, the Lin− c-Kitlo RAG-1-GFP+ IL-7Rα+/− category of bone marrow gave the most impressive short-term expansion in OP9-DL1 co-cultures. Although this includes cells originally described as common lymphoid progenitors, there are reasons to believe they are not the most efficient for replenishing the adult thymus (20,33). It is also important to note that at least two stromal cell lines can actually suppress the activity of fully committed B lineage progenitors (16). We do not understand the basis for this inhibition, but could speculate that there is constitutive low level expression of Notch ligands. Any functional assessment of differentiation potential is subject to bias and caution is advised when interpreting culture results in terms of in vivo relevance.
Long-term propagated adult lymphocytes closely resembled primitive DN2/ DN3 stage thymocytes with respect to expression of four genes (22). Although largely arrested at that stage, the cultures continued to contain small numbers of cytoplasmic TCRβ+ cells and slowly generated NK and T cells. In addition, they successfully competed with normal bone marrow and progressed to CD4+ and CD8+ lymphocytes in the thymuses of irradiated recipients. Furthermore, we found evidence of polyclonal rearrangement of four TCRβ genes (data not shown). It is curious that fetal progenitors, like those freshly isolated from the thymus (5, 7) efficiently complete maturation in the OP9-DL1 culture system while adult bone marrow progenitors transiently arrest at the DN2/DN3 stage when 5 ng/ml IL-7 is added. When the purified Lin−c-Kit+Sca-1+ cells from long-term cultures were transplanted with equal number of total recipient bone marrow cells, only 0.3% of thymic cells were derived from the cultured cells. This indicates that thymic homing and/or T cell differentiation of BM progenitors cultured on OP9-DL1 may decrease over time. It has been previously shown that T lineage progression from fetal progenitors in a fetal thymic organ culture system is inhibited by high cytokine concentrations (37). However, in the OP9-DL1 co-culture system this is only the case for adult progenitors (this report and 30). These findings again highlight fetal/adult differences and indicate that the cultures do not optimally provide all environmental cues required by adult marrow derived progenitors. Further study might implicate other Notch ligands (38) or additional components of the normal adult thymus.
The bone marrow, rather than the thymus, is likely to be the source of NK cells during adult life (39). Indeed, the Lin− c-Kitlo Flk-2+ pro-lymphocyte fraction of marrow is the best source of NK progenitors and, prior to acquisition of the CD122 receptor for IL-15, many of them still retain B lineage potential (40, 41). We did not intentionally promote development of NK cells by addition of IL-15 in the present study. However, small numbers of DX-5+ NK1.1+ NK lineage cells were produced in all of our OP9-DL1 cultures, regardless of whether they were initiated with fetal liver or adult bone marrow. These emerged during the first week on OP9 vector control stromal cells and from the second week in OP9-DL1 co-cultures (data not shown). Other than this apparent difference in kinetics, we saw no strong evidence that the NK lineage choice was either positively or negatively influenced by Notch receptor ligation. A recent analysis of fetal thymocytes demonstrated that residual NK lineage potential is normally down-regulated at the DN2 stage and suppressed by Notch receptor ligation (8).
Notch is thought to influence a variety of events in hematopoiesis, including the self-renewal of stem cells (12, 13, 21, 42). However, recombinant forms of Delta 1 have been shown to suppress myelopoiesis (43, 44). We consistently observed a reduction in production of myeloid cells in OP9-DL1 co-cultures, regardless of whether fetal or adult hematopoietic cells were used. It is unclear if strong Notch signals killed myeloid progenitors, held them in an undifferentiated, cytokine unresponsive state or directed them to alternative fates.
It is critical to know if long-term cultured marrow cells were similar because of reprogramming or alternatively, if rare cells contaminating all of the sorted fractions were selected over time. This is a difficult determination when the starting populations to be compared are all very primitive. Therefore, we started some cultures with highly enriched (98% by post-sort analysis) and relatively late CD45R/B220+ CD19+ CD43− sIgM− (Hardy Fraction E) cells. Although there is almost no proliferative activity in the starting population (31) and we saw no expansion during the first week of co-culture, long-term lines were established within three weeks of serial passage. Remarkably, there was no evidence for Ig VH to DHJH rearrangements and no cytoplasmic μ heavy chain staining in the expanded lymphocytes, even though small pre-B cells made up almost all of the starting population. We interpret these results as showing that the OP9-DL1 co-culture system has tremendous potential for selection and expansion of rare, undifferentiated lymphocytes that might represent contaminants in sorted populations. While cells can progress substantially in the B lineage and remain Notch ligation sensitive, late stage progenitors with productively rearranged immunoglobulin genes are not reprogrammed to adopt a T cell fate.We demonstrate that an impressive range of different progenitors in adult marrow could be propagated indefinitely in OP9-DL1 co-cultures. Regardless of starting population, the cells assumed properties of DN2/DN3 stage thymocytes and retained some potential for terminal T lineage differentiation. While a degree of lineage reprogramming may occur in this culture model, it can preferentially expand rare, relatively undifferentiated cells. The findings are compatible with a model where Notch regulates multiple events and differentiation options become gradually restricted (45).
Acknowledgments
We thank Rob Welner and Scott Perry for help in sorting some of the adult marrow cells. We also thank Shelli Wasson for assistance with preparing the manuscript.
Abbreviations used in this paper:
- OP9-DL1
murine Delta –like 1 transduced OP9 cells
- DN
CD4−CD8− thymocyte
- DP
CD4+CD8+ Thymocyte
- SP
CD4+CD8− or CD4−CD8+ thymocyte
Footnotes
This work was supported by grants AI20069, AI58162, AI33940, AI53739 from the National Institutes of Health and P20 RR15577 from the COBRE Program of the National Center for Research Resources. PWK holds the William H. and Rita Bell Endowed Chair in biomedical research. JCZP is supported by an Investigator Award from the Canadian Institutes of Health Research.
References
- 1.Maillard I, Adler SH, Pear WS. Notch and the immune system. Immunity. 2003;19:781–791. doi: 10.1016/s1074-7613(03)00325-x. [DOI] [PubMed] [Google Scholar]
- 2.Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 3.Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, Lee JY, Kadesch T, Hardy RR, Aster JC, Pear WS. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 4.Jaleco AC, Neves H, Hooijberg E, Gameiro P, Clode N, Haury M, Henrique D, Parreira L. Differential effects of Notch ligands Delta-1 and Jagged-1 in human lymphoid differentiation. J Exp Med. 2001;194:991–1002. doi: 10.1084/jem.194.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zúñiga-Pflücker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 7.HE Porritt, Rumfelt LL, Tabrizifard S, Schmitt TM, Zúñiga-Pflücker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–745. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 8.TM Schmitt, Ciofani M, Petrie HT, Zúñiga-Pflücker JC. Maintenance of T cell specification and differentiation requires recurrent notch receptor-ligand interactions. J Exp Med. 2004;200:469–479. doi: 10.1084/jem.20040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Peydro M, V, de Yebenes G, Toribio ML. Sustained Notch1 signaling instructs the earliest human intrathymic precursors to adopt a γδ T-cell fate in fetal thymus organ culture. Blood. 2003;102:2444–2451. doi: 10.1182/blood-2002-10-3261. [DOI] [PubMed] [Google Scholar]
- 10.Maekawa Y, Tsukumo S, Chiba S, Hirai H, Hayashi Y, Okada H, Kishihara K, Yasutomo K. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19:549–559. doi: 10.1016/s1074-7613(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 11.Robey E, Chang D, Itano A, Cado D, Alexander H, Lans D, Weinmaster G, Salmon P. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell. 1996;87:483–492. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]
- 12.Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34+CD38− cord blood cells. J Clin Invest. 2002;110:1165–1174. doi: 10.1172/JCI16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, Pear WS, Bernstein ID. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 14.Kuwata N, Igarashi H, Ohmura T, Aizawa S, Sakaguchi N. Cutting edge: Absence of expression of RAG1 in peritoneal B-1 cells detected by knocking into RAG1 locus with green fluorescent protein gene. J Immunol. 1999;163:6355–6359. [PubMed] [Google Scholar]
- 15.de Pooter RF, Cho SK, Carlyle JR, Zuniga-Pflucker JC. In vitro generation of T lymphocytes from embryonic stem cell-derived prehematopoietic progenitors. Blood. 2003;102:1649–1653. doi: 10.1182/blood-2003-01-0224. [DOI] [PubMed] [Google Scholar]
- 16.Yokota T, Kouro T, Hirose J, Igarashi H, Garrett KP, Gregory SC, Sakaguchi N, Owen JJ, Kincade PW. Unique properties of fetal lymphoid progenitors identified according to RAG1 gene expression. Immunity. 2003;19:365–375. doi: 10.1016/s1074-7613(03)00231-0. [DOI] [PubMed] [Google Scholar]
- 17.Perry SS, Pierce LJ, Slayton WB, Spangrude GJ. Characterization of thymic progenitors in adult mouse bone marrow. J Immunol. 2003;170:1877–1886. doi: 10.4049/jimmunol.170.4.1877. [DOI] [PubMed] [Google Scholar]
- 18.Kondo M, I, Weissman L, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 19.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 21.Varnum-Finney B, Brashem-Stein C, Bernstein ID. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003;101:1784–1789. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- 22.Tabrizifard S, Olaru A, Plotkin J, Fallahi-Sichani M, Livak F, Petrie HT. Analysis of transcription factor expression during discrete stages of postnatal thymocyte differentiation. J Immunol. 2004;173:1094–1102. doi: 10.4049/jimmunol.173.2.1094. [DOI] [PubMed] [Google Scholar]
- 23.Douagi I, Vieira P, Cumano A. Lymphocyte commitment during embryonic development, in the mouse. Semin Immunol. 2002;14:361–369. doi: 10.1016/s1044532302000702. [DOI] [PubMed] [Google Scholar]
- 24.Crompton T, Outram SV, Buckland J, Owen MJ. Distinct roles of the interleukin-7 receptor α chain in fetal and adult thymocyte development revealed by analysis of interleukin-7 receptor α-deficient mice. Eur J Immunol. 1998;28:1859–1866. doi: 10.1002/(SICI)1521-4141(199806)28:06<1859::AID-IMMU1859>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 25.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(−/)- mice. J Exp Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vosshenrich CA, Cumano A, Muller W, Di Santo JP, Vieira P. Thymic stromal-derived lymphopoietin distinguishes fetal from adult B cell development. Nat Immunol. 2003;4:773–779. doi: 10.1038/ni956. [DOI] [PubMed] [Google Scholar]
- 27.Kawamoto H, Ohmura K, Hattori N, Katsura Y. Hemopoietic progenitors in the murine fetal liver capable of rapidly generating T cells. J Immunol. 1997;158:3118–3124. [PubMed] [Google Scholar]
- 28.Watanabe Y, Aiba Y, Katsura Y. T cell progenitors in the murine fetal liver: differences from those in the adult bone marrow. Cell Immunol. 1997;177:18–25. doi: 10.1006/cimm.1997.1094. [DOI] [PubMed] [Google Scholar]
- 29.DeLuca D, Clark DR. Interleukin-7 negatively regulates the development of mature T cells in fetal thymus organ cultures. Dev Comp Immunol. 2002;26:365–384. doi: 10.1016/s0145-305x(01)00085-4. [DOI] [PubMed] [Google Scholar]
- 30.Balciunaite G, Ceredig R, Fehling HJ, Zúñiga-Pflücker JC, Rolink AG. The role of Notch and IL-7 signaling in early thymocyte proliferation and differentiation. Eur J Immunol. 2005;35:1292–1300. doi: 10.1002/eji.200425822. [DOI] [PubMed] [Google Scholar]
- 31.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhandoola A, Sambandam A, Allman D, Meraz A, Schwarz B. Early T lineage progenitors: new insights, but old questions remain. J Immunol. 2003;171:5653–5658. doi: 10.4049/jimmunol.171.11.5653. [DOI] [PubMed] [Google Scholar]
- 33.Allman D, Sambandam A, Kim S, Miller JP, Pagan A, Well D, Meraz A, Bhandoola A. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4:168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 34.Perry SS, Wang H, Pierce LJ, Yang AM, Tsai S, Spangrude GJ. L-selectin defines a bone marrow analog to the thymic early T-lineage progenitor. Blood. 2004;103:2990–2996. doi: 10.1182/blood-2003-09-3030. [DOI] [PubMed] [Google Scholar]
- 35.Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nat Immunol. 2004;5:953–960. doi: 10.1038/ni1101. [DOI] [PubMed] [Google Scholar]
- 36.Martin CH, Aifantis I, Scimone ML, Von Andrian UH, Reizis B, Von Boehmer H, Gounari F. Efficient thymic immigration of B220+ lymphoid-restricted bone marrow cells with T precursor potential. Nat Immunol. 2003;4:866–873. doi: 10.1038/ni965. [DOI] [PubMed] [Google Scholar]
- 37.Plum J, De Smedt M, Leclercq G. Exogenous IL-7 promotes the growth of CD3−CD4−CD8− CD44+CD25+/− precursor cells and blocks the differentiation pathway of TCR-αβ cells in fetal thymus organ culture. J Immunol. 1993;150:2706–2716. [PubMed] [Google Scholar]
- 38.Lehar SM, Dooley J, Farr AG, Bevan MJ. Notch ligands Delta 1 and Jagged1 transmit distinct signals to T-cell precursors. Blood. 2005;105:1440–1447. doi: 10.1182/blood-2004-08-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 40.Hirose J, Kouro T, Igarashi H, Yokota T, Sakaguchi N, Kincade PW. A developing picture of lymphopoiesis in bone marrow. Immunol Rev. 2002;189:28–40. doi: 10.1034/j.1600-065x.2002.18904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kouro T, Kumar V, Kincade PW. Relationships between early B- and NK-lineage lymphocyte precursors in bone marrow. Blood. 2002;100:3672–3680. doi: 10.1182/blood-2002-02-0653. [DOI] [PubMed] [Google Scholar]
- 42.Stier S, Cheng T, Dombkowski D, Carlesso N, Scadden DT. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood. 2002;99:2369–2378. doi: 10.1182/blood.v99.7.2369. [DOI] [PubMed] [Google Scholar]
- 43.Han W, Ye Q, Moore MA. A soluble form of human Delta-like-1 inhibits differentiation of hematopoietic progenitor cells. Blood. 2000;95:1616–1625. [PubMed] [Google Scholar]
- 44.Varnum-Finney B, Wu L, Yu M, Brashem-Stein C, Staats S, Flowers D, Griffin JD, Bernstein ID. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J Cell Sci. 2000;113(Pt 23):4313–4318. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- 45.Baba Y, Pelayo R, Kincade PW. Relationships between hematopoietic stem cells and lymphocyte progenitors. Trends Immunol. 2004;25:645–649. doi: 10.1016/j.it.2004.09.010. [DOI] [PubMed] [Google Scholar]


