Abstract
NHE-RF, a regulatory cofactor for NHE (Na+-H+ exchanger) type 3, interacts with ion transporters and receptors through its PDZ domains and with the MERM proteins (merlin, ezrin, radixin and moesin) via its carboxyl terminus. Thus, NHE-RF may act as a multifunctional adaptor protein and play a role in the assembly of signal transduction complexes, linking ion channels and receptors to the actin cytoskeleton. NHE-RF expression is up-regulated in response to estrogen in estrogen receptor-positive breast carcinoma cell lines, suggesting that it may be involved in estrogen signaling. To further understand NHE-RF function and its possible role in estrogen signaling, we analyzed NHE-RF expression in normal human tissues, including cycling endometrium, and in breast carcinomas, tissues in which estrogen plays an important role in regulating cell growth and proliferation. NHE-RF is expressed in many epithelia, especially in cells specialized in ion transport or absorption, and is often localized to apical (luminal) membranes. NHE-RF expression varies markedly in proliferative versus secretory endometrium, with high expression in proliferative (estrogen-stimulated) endometrium. Furthermore, estrogen receptor status and NHE-RF expression correlate closely in breast carcinoma specimens. These findings support a role for NHE-RF in estrogen signaling.
NHE-RF (Na+-H+ exchanger regulatory factor), a cytoplasmic phosphoprotein, was originally identified in the rabbit renal brush border as a cofactor required for cAMP/protein kinase A-mediated inhibition of NHE (Na+-H+ exchanger) type 3. 1 The human homologue was identified from human placenta and has also been referred to as EBP50 (ezrin binding protein). 2 NHE-RF is a 358-amino-acid protein with two homologous PDZ domains, which mediate protein-protein interactions. A related protein, NHE-RF2 (also known as E3KARP), was isolated from small intestine and renal brush border and shares 51% identity with NHE-RF, primarily in the PDZ domains. 3 PDZ-containing proteins are often localized at the plasma membrane and appear to promote the assembly of membrane-bound macromolecular complexes (transduction complexes), into which target molecules are recruited. Many PDZ domain-containing-proteins are involved in cell signaling. 4-6
NHE-RF interacts, via its PDZ domains, with a variety of ion transport proteins such as the cystic fibrosis transmembrane regulator, the sodium bicarbonate cotransporter, as well as with membrane receptors such as the β2 adrenergic and the purinergic P2Y1. Recently, we and others have shown that NHE-RF directly interacts with merlin, the NF2 (neurofibromatosis 2)-encoded tumor suppressor protein, and with ezrin, radixin, and moesin (MERM proteins), which are involved in cytoskeletal reorganization and signal transduction. 7 The MERM binding region is a non-PDZ site in the C-terminus. 8 Thus, NHE-RF may also be involved in the assembly of transduction complexes that link membrane receptors and transporters with intracellular signaling components.
Interestingly, NHE-RF expression is up-regulated in response to estrogen in estrogen receptor (ER)-positive breast carcinoma cell lines (see Ref. 9 and unpublished data). NHE-RF expression in these cells is mediated by the estrogen receptor and suppressed by antiestrogens. 9 These findings suggest a role for NHE-RF in the estrogen signal transduction cascade in estrogen-responsive tissues. To gain more insight into the physiological relevance of NHE-RF, we performed a comprehensive expression study of the protein in normal adult human tissues. To evaluate the relationship between estrogen and NHE-RF expression in normal and malignant estrogen-responsive tissues, we also examined NHE-RF expression in cycling endometrium, as well as in ER-positive and ER-negative breast carcinomas.
Materials and Methods
Tissues
Formalin-fixed, paraffin-embedded sections of normal adult human organs from surgical specimens and autopsies were obtained from the Department of Pathology at Massachusetts General Hospital. We examined two to five samples from each paraffin-embedded organ. Organs examined included: brain and spinal cord, peripheral nerve, pituitary gland, placenta, kidney, skin, muscle, endometrium, breast, esophagus, stomach, small and large intestines, liver, spleen, pancreas, salivary glands, thyroid, tonsils, heart, and lungs. To evaluate the possible association between estrogen and NHE-RF expression in normal tissues, we examined multiple samples of proliferative and secretory endometrium. To assess the possible association between estrogen receptor status and NHE-RF expression in breast carcinomas, we studied eighteen invasive breast carcinomas for which estrogen receptor status has been previously determined by immunohistochemistry.
Cell Lines
Breast cancer cell lines MCF-7, ZR-75-B, T-47D, MCF-7-ADR, and MDA-MB-231 were obtained from the Massachusetts General Hospital Cancer Center, and normal breast epithelial cell lines HBL-100 and MCF-12-F were obtained from ATCC. Cell lines were maintained in DMEM with 10% fetal calf serum.
Antibodies
The polyclonal IC270 antibody is directed at the GST-NHE-RF fusion protein and has been characterized elsewhere. 10,11 A commercial antibody (Estrogen Receptor, Clone 1D5, DAKO, Carpinteria, CA) was used to evaluate estrogen receptor status; this antibody recognizes both the α and β forms of the receptor.
Western Blot Analysis
Protein lysates were prepared from cells in phosphate buffered saline (PBS) containing 2% sodium dodecyl sulfate (SDS) and a cocktail of protease inhibitors (Boehringer Mannheim, Indianapolis, IN). Protein concentrations were measured using the DC protein assay system (Bio-Rad, Melville, NY). Three hundred μg of total cellular protein were subjected to SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad). Blots were then probed with the NHE-RF antibody IC270 (affinity eluted 1:50. Proteins were visualized with anti-rabbit horseradish peroxidase-conjugated secondary antibody and the ECL chemiluminescence system (Amersham Inc, Arlington Heights, IL). Signal intensity was quantified by densitometric scanning of autoradiographs using transmittance analysis (Fluor-S, Multiimager, Bio-Rad).
Immunohistochemistry
Immunohistochemistry for NHE-RF was performed using IC270. Formalin-fixed, paraffin-embedded, 8-μm-thick sections were deparaffinized, rehydrated, immersed in 0.5% H2O2/methanol for 20 minutes, and rehydrated in graded ethanols. For antigen retrieval, sections were microwaved in 0.01 mol/L sodium citrate buffer (pH 6.0) for 15 minutes. Sections were then blocked in 10% normal goat serum and 5% milk in 1% bovine serum albumin (BSA) in PBS, followed by incubation with primary antibody overnight at 4°C. Incubation with biotinylated goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA) for 30 minutes at room temperature was followed by the standard avidin-biotin-complex (ABC) process (Vectastain Elite ABC kit, Vector). Diaminobenzidine (DAB) was used as a chromogen, followed by counterstaining with hematoxylin. Positive controls included paraffin-embedded human placenta sections as well as formalin fixed, paraffin-embedded cell pellets from MCF-7 and MCF-7-ADR cell lines, which express high levels or low levels of NHE-RF, respectively, as shown in Western blot analysis (see below). For negative controls, the primary antibody was omitted and prior immunostaining with preabsorbed serum did not reveal any specific reactivity. 12 To control for possible effects of fixation and antigen retrieval, frozen sections of human placenta were also immunostained.
Immunohistochemistry for estrogen receptors was performed according to standard procedures. Briefly, antigen retrieval was achieved by microwaving the sections in Tris buffer (pH 10) for 10 minutes. Sections were blocked with 10% normal horse serum and incubated with ER antibodies (1:100 dilution) overnight at 4°C. Sections were incubated with secondary antibodies at room temperature for 45 minutes followed by the ABC reaction, visualization with DAB and counterstaining with hematoxylin.
Results
Western Blotting and Immunohistochemistry for NHE-RF in Cell Lines
Quantitative Western blot analysis, performed three times, confirmed that three estrogen receptor-positive breast cancer cell lines, MCF-7, ZR-75-B, and T-47D, had 9.50 ± 2.20-fold higher levels of NHE-RF when compared to the normal mammary lines HBL-100 and MCF-12-F (Figure 1) ▶ . Expression of NHE-RF was expressed at only low levels in the estrogen receptor-negative breast cancer lines MCF-7-ADR and MDA-MB-231 and was not detectable in the breast cancer cell line DU4475. Immunocytotochemical staining of cell pellets from MCF-7 and MCF-7-ADR with the IC270 antibody showed strong expression only in MCF-7, the ER-positive cell line (Figure 2A) ▶ .
Figure 1.
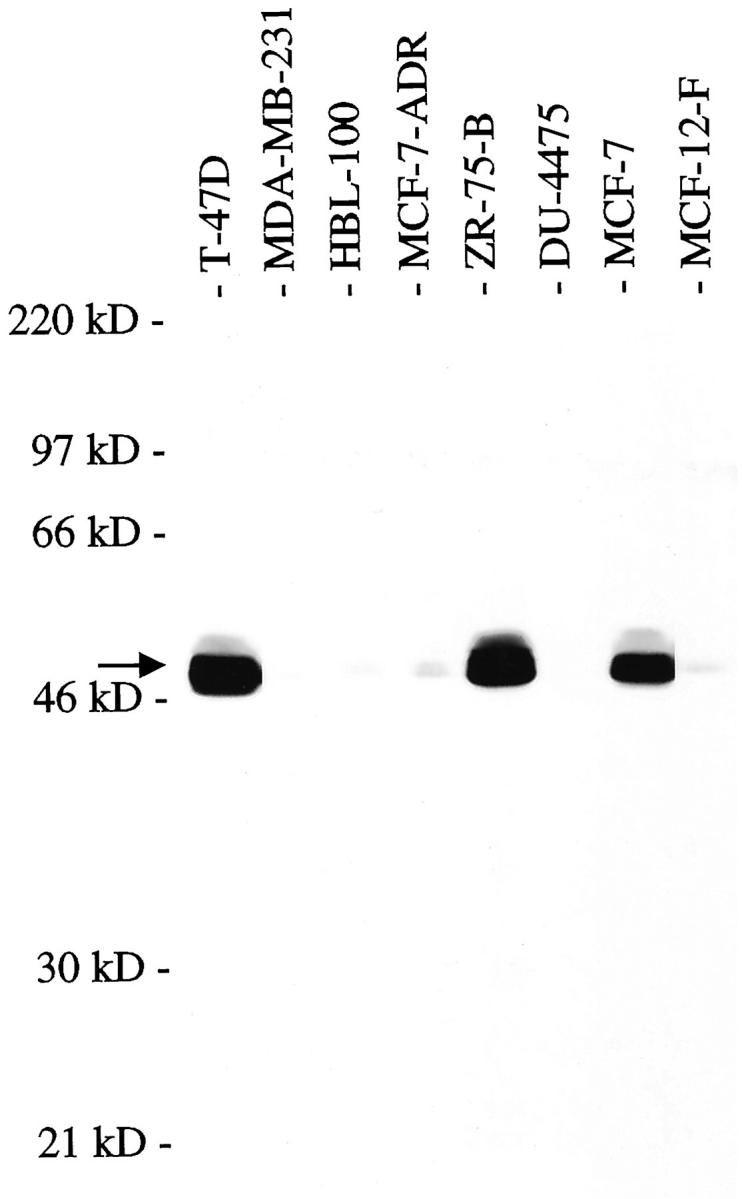
Western blot analysis, using affinity eluted IC270 antibody at 1:50 dilution, of mammary epithelial and breast cancer cell lines showing high NHE-RF expression in the ER-positive cell lines T-47D, ZR-75-B, and MCF-7. The arrow indicates NHE-RF at 50 kd.
Figure 2.
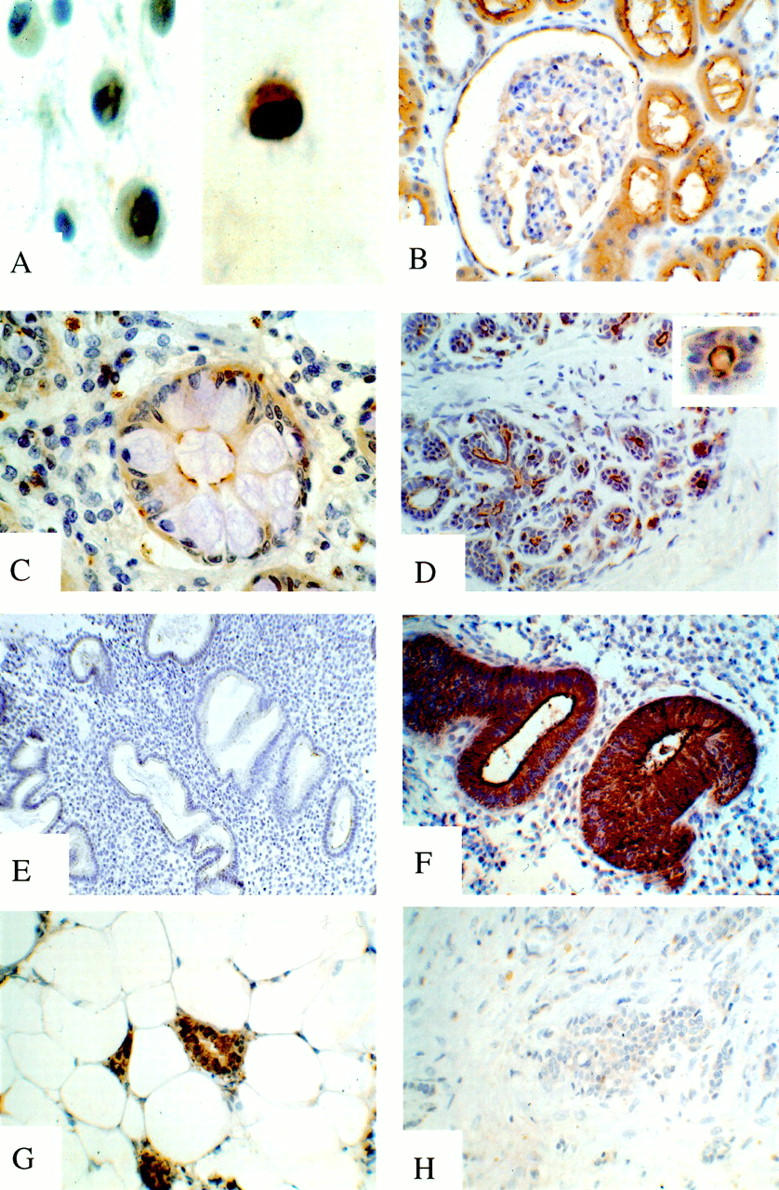
NHE-RF expression in normal and neoplastic tissues immunostained with IC270 antibody. A: NHE-RF expression in breast carcinoma cell lines: low expression in estrogen receptor (ER) negative MCF-7-ADR (left); strong expression in ER positive MCF-7 cells (right). B-F: NHE-RF expression in normal tissues. B, strong membranous expression of NHE-RF in proximal tubular epithelium and parietal epithelial cells of Bowman’s capsule; C, NHE-RF expression in apical surface of absorptive cells in colonic glands; goblet cells are immunonegative; D, strong expression of NHE-RF in luminal membrane of ducts and acini in the breast; E, low NHE-RF expression in secretory endometrium; F, high expression of NHE-RF in proliferative endometrium. G-H: Expression of NHE-RF in breast carcinomas. G, high expression of NHE-RF in ER-positive breast carcinoma; H, ER-negative breast carcinoma immunonegative for NHE-RF.
Immunohistochemistry for NHE-RF Expression in Normal Adult Tissues
NHE-RF is expressed in many tissues but has a highly selective cellular and subcellular distribution. Intense NHE-RF expression was seen in epithelial cells of many organ systems, especially in cells that perform an absorptive function, possess microvilli, or are involved in ion transport. Immunohistochemistry of paraffin-embedded sections of placenta demonstrated a characteristic pattern of NHE-RF expression, with prominent expression in the syncytiotrophoblast, the outer cell layer of placental villi, and its brush border, whereas the villous mesenchyme was immunonegative. Frozen sections of placenta showed the same pattern of immunostaining. All negative controls showed no staining.
As previously demonstrated using immunofluorescence, 12 strong expression of NHE-RF was seen in the renal proximal tubules with prominent staining of the luminal cell membrane and the microvillous brush border (Figure 2B) ▶ . The parietal epithelium lining Bowman’s capsule of the renal glomeruli also showed prominent positivity, while the mesangium and glomerular endothelium were immunonegative and the distal tubules and collecting tubules had only weak cytoplasmic staining.
A highly selective pattern of expression of NHE-RF was also seen in the gastro-intestinal system. In the stomach, fundic glands showed strong expression in parietal cells, whereas chief cells, mucin-secreting cells, and surface epithelium were immunonegative. In the small bowel, the protein is abundant at the apical surface of absorptive cells and in microvilli extending from the cell surface, but is absent in intercalated goblet cells. Similarly, in the large bowel, absorptive cells of the surface epithelium and colonic crypts show strong membranous staining of the luminal border, but adjacent intercalated goblet cells were immunonegative (Figure 2C) ▶ .
Stratified squamous epithelium in the esophagus, skin, and tonsils showed only weak to moderate cytoplasmic staining, more pronounced in the deep layers. Eccrine glands in the skin, however, showed cytoplasmic immunostaining with strong apical membranous expression, highlighting intercalated canaliculi. In contrast, the underlying myoepithelial cells were immunonegative. Other skin adenexa, such as the pilar unit, showed weak diffuse cytoplasmic staining. In the salivary glands, NHE-RF was weakly expressed in the cytoplasm of serous acinar cells, but there was prominent linear luminal membranous immunostaining. The mucinous acinar cells of the salivary glands were immunonegative. Similarly, acinar cells of the pancreas showed weak cytoplasmic staining and luminal linear immunopositivity, while cells of the islands of Langerhans were immunonegative. In the anterior pituitary, selected cells were moderately immunopositive. The posterior pituitary was immunonegative, as was the thyroid gland. In the breast, ductal and acinar epithelia were immunopositive, with apical membranous staining of the acinar epithelial cells (Figure 2D) ▶ .
Skeletal muscle and myocardium were immunonegative for NHE-RF, while smooth muscle, particularly vascular smooth muscle, showed moderate immunopositivity in most tissues. In the lung, bronchial epithelium was only weakly positive and alveolar pneumocytes were immunonegative. In the liver, hepatocytes and Kupffer cells showed no expression of NHE-RF and only weak to moderate expression was seen in ductal epithelium. Focal, moderate positivity was seen in the white pulp of the spleen and in scattered lymphocytes in germinal centers of lymph nodes.
In the brain and spinal cord, neurons and resting glia did not express NHE-RF. Strong expression of NHE-RF, however, was observed in glial processes in areas of chronic reactive gliosis, such as subpial region, or in proliferating Bergmann glia in the cerebellum. Marked NHE-RF expression was also observed at the apical membranes of ependymal cells. Interestingly, this pattern of expression was altered in buried ependymal cells found just below the ventricular surface, in which NHE-RF was seen as an area of strong cytoplasmic immunopositivity without polarized apical or membranous staining. Arachnoid cells were immunonegative, as were Schwann cells in peripheral nerves.
Immunohistochemistry for NHE-RF Expression in Endometrium
Proliferative endometrium showed strong expression of NHE-RF in the cytoplasm and luminal membrane of glandular epithelium as well as in scattered stromal cells (Figure 2F) ▶ . NHE-RF was expressed in essentially all epithelial cells in the proliferative endometrium samples. In contrast, there was only weak expression of NHE-RF in the glandular epithelium of secretory endometrium and adjacent stroma (Figure 2E) ▶ .
Immunohistochemistry for NHE-RF Expression in Primary Breast Tumors
Examination of sections from 18 infiltrating breast adenocarcinomas showed strong correlation between positive immunostaining for ER and high expression of the NHE-RF protein: 10 of 11 (>90%) of the ER-positive tumors strongly expressed NHE-RF. On the contrary, 5 of 7 ER-negative tumors did not express NHE-RF (Figure 2, G–H ▶ , and Table 1 ▶ ). In those tumors that were NHE-RF immunopositive, staining was observed in nearly all tumor cells, contrasting with the adjacent immunonegative stroma. NHE-RF positivity was present as membranous staining, especially at the luminal aspects of cells, and as diffuse cytoplasmic staining.
Table 1.
NHE-RF and ER Expression in 18 Primary Breast Carcinomas
| Tumor sample | NHE-RF IHC | ER status |
|---|---|---|
| BC1 | +++ | Positive |
| BC3 | +++ | Positive |
| BC5 | +++ | Positive |
| BC9 | +++ | Positive |
| BC10 | +++ | Positive |
| BC11 | +++ | Positive |
| BC4 | ++ | Positive |
| BC7 | ++ | Positive |
| BC12 | ++ | Positive |
| BC14 | ++ | Positive |
| BC8 | - | Positive |
| BC6 | ++ | Negative |
| BC15 | ++ | Negative |
| BC2 | - | Negative |
| BC13 | - | Negative |
| BC16 | - | Negative |
| BC17 | - | Negative |
| BC18 | - | Negative |
NHE-RF immunostaining intensity: +++, strong staining; ++, moderate staining; -, no or minimal staining. When positive, nearly all cells were positive.
Discussion
NHE-RF is highly expressed in epithelia of many tissues, particularly in cells with numerous microvilli, and is often concentrated at the luminal membrane. Prominent NHE-RF expression is seen in cells specialized in ion transport: renal proximal tubules, eccrine glands, colonic absorptive cells, parietal cells in gastric glands and ependymal cells. These findings support the proposed role of NHE-RF as a regulator of membrane protein transporters, and confirm Northern blot data showing high NHE-RF mRNA levels in human tissues containing polarized epithelia such as the mammary gland, kidney, small intestines and salivary gland. 9
The association between estrogen stimulation and NHE-RF expression in normal endometrium and in breast carcinomas suggests that NHE-RF must be a multifunctional protein, with additional roles to those of ion transport. Specific and early induction of NHE-RF mRNA by estrogen has been observed in breast carcinoma cells. 9 The present observations extend the correlation between ER status and NHE-RF expression in breast cancer to primary human tumors, and raise the possibility that NHE-RF may play a role in estrogen-mediated cell growth. The proliferative effects of the estrogen are mediated through ER, an intracellular nuclear receptor that, when bound to estrogen, is transformed into an active transcription factor and regulates the expression of a variety of genes. Antiestrogens may therefore be therapeutically effective in ER-positive breast cancers. ER-negative tumors, however, are more aggressive tumors, associated with early recurrence and poor patient survival, and do not generally respond to antiestrogen treatment. However, about 40% of patients with ER-positive cancer do not respond to endocrine manipulations, and about 10% of ER-negative tumors respond. 13,14 The factors that lead to the conversion of an ER-positive, responsive breast cancer into a hormone-refractory tumor are poorly understood, and encourage further study of mechanisms of ER-mediated cell growth.
Estrogens induce cytoskeletal changes in ER-positive breast cancer cells that include an increase in the number and size of microvilli, 15,16 as well as increased expression of cytokeratins associated with the nuclear matrix-intermediate filament system. 17 Interestingly, alterations of the nuclear matrix-intermediate filament system, an extensive network that connects the plasma membrane and cytoskeleton with the nuclear membrane and nuclear matrix, can mediate changes in gene expression. 18,19 NHE-RF, an early response gene to estrogen stimulation, may therefore play a role in the assembly of an estrogen transduction complex by linking the actin cytoskeleton to an anchored membrane protein. NHE-RF could bind a transduction complex via interaction with MERM proteins at a C-terminal, non-PDZ site, and to a membrane protein via one of the PDZ domains. NHE-RF may also be involved in cross-talk among signal transduction pathways. Several signaling cascades, including epidermal growth factor and tyrosine kinase/MAP-kinase pathways, are involved in estrogen signaling; 20-23 such overlapping pathways may partly explain how breast cancer cells adapt to and bypass estrogen receptors blocked by antiestrogen therapy. It is possible that an alternative pathway for estrogen signaling in ER-negative breast carcinomas might involve NHE-RF expression, via ER-independent up-regulation, which could explain the 30% of ER-negative tumors expressing NHE-RF in our series.
These studies suggest that NHE-RF acts as a multifunctional protein and has potential roles in the apical surfaces of ion transporting epithelium as well as in estrogen-mediated growth control. The data encourage further study of NHE-RF in breast carcinomas to increase understanding of the growth control mechanisms in these hormonally-regulated tumors, and to develop novel means to interfere therapeutically in such growth pathways.
Footnotes
Address reprint requests to David N. Louis, MD, Department of Pathology, Massachusetts General Hospital, WRN 3, 55 Fruit St., Boston, MA 02114. E-mail: louis@helix.mgh.harvard.edu.
Supported by National Institutes of Health grant NS24279 and a U.S. Army grant. T. W. was supported by a Gottlieb Daimler and Karl Benz predoctoral fellowship.
References
- 1.Weinman EJ, Steplock D, Wang Y, Shenolikar S: Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane Na(+)-H+ exchanger. J Clin Invest 1995, 95:2143-2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reczek D, Berryman M, Bretscher A: Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol 1997, 139:169-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yun CH, Oh S, Zizak M, Steplock D, Tsao S, Tse CM, Weinman EJ, Donowitz M: cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA 1997, 94:3010–3015 [published erratum appears in Proc Natl Acad Sci USA 1997, 94:10006] [DOI] [PMC free article] [PubMed]
- 4.Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R: Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell 1996, 85:1067-1076 [DOI] [PubMed] [Google Scholar]
- 5.Morais Cabral JH, Petosa C, Sutcliffe MJ, Raza S, Byron O, Poy F, Marfatia SM, Chishti AH, Liddington RC: Crystal structure of a PDZ domain. Nature 1996, 382:649-652 [DOI] [PubMed] [Google Scholar]
- 6.Fanning AS, Anderson JM: Protein-protein interactions: PDZ domain networks. Curr Biol 1996, 6:1385-1388 [DOI] [PubMed] [Google Scholar]
- 7.Murthy A, Gonzalez-Agosti C, Cordero E, Pinney D, Candia C, Solomon F, Gusella J, Ramesh V: NHE-RF, a regulatory cofactor for Na(+)-H+ exchange, is a common interactor for merlin and ERM (MERM) proteins. J Biol Chem 1998, 273:1273-1276 [DOI] [PubMed] [Google Scholar]
- 8.Reczek D, Bretscher A: The carboxyl-terminal region of EBP50 binds to a site in the amino-terminal domain of ezrin that is masked in the dormant molecule. J Biol Chem 1998, 273:18452-18458 [DOI] [PubMed] [Google Scholar]
- 9.Ediger TR, Kraus WL, Weinman EJ, Katzenellenbogen BS: Estrogen receptor regulation of the Na+/H+ exchange regulatory factor. Endocrinology 1999, 140:2976-2982 [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Agosti C, Wiederhold T, Herndon ME, Gusella J, Ramesh V: Interdomain interaction of merlin isoforms and its influence on intermolecular binding to NHE-RF. J Biol Chem 1999, 274:34438-34442 [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Agosti C, Xu L, Pinney D, Beauchamp R, Hobbs W, Gusella J, Ramesh V: The merlin tumor suppressor localizes preferentially in membrane ruffles. Oncogene 1996, 13:1239-1247 [PubMed] [Google Scholar]
- 12.Breton S, Wiederhold T, Marshansky V, Nsumu NN, Ramesh V, Brown D: The B1 subunit of the H+ATPase is a PDZ-domain binding protein: Colocalization with NHE-RF in renal B-intercalated cells. J Biol Chem 2000, 275:18219-18224 [DOI] [PubMed] [Google Scholar]
- 13.Nagai R, Kataoka M, Kobayashi S, Ishihara K, Tobioka N, Nakashima K, Naruse M, Saito K, Sakuma S: Estrogen and progesterone receptors in human breast cancer with concomitant assay of plasma 17beta-estradiol, progesterone, and prolactin levels. Cancer Res 1979, 39:1834-1840 [PubMed] [Google Scholar]
- 14.Henderson BE, Bernstein L, Ross R: Etiology of cancer: hormonal factors. ed 5 Cancer: Principles and Practice of Oncology, 1997, Lippincott-Raven, Philadelphia
- 15.Vic P, Vignon F, Derocq D, Rochefort H: Effect of estradiol on the ultrastructure of the MCF7 human breast cancer cells in culture. Cancer Res 1982, 42:667-673 [PubMed] [Google Scholar]
- 16.Antakly T, Pelletier G, Zeytinoglu F, Labrie F: Changes of cell morphology and prolactin secretion induced by 2-Br-alpha-ergocryptine, estradiol, and thyrotropin-releasing hormone in rat anterior pituitary cells in culture. J Cell Biol 1980, 86:377-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coutts AS, Davie JR, Dotzlaw H, Murphy LC: Estrogen regulation of nuclear matrix-intermediate filament proteins in human breast cancer cells. J Cell Biochem 1996, 63:174-184 [DOI] [PubMed] [Google Scholar]
- 18.Blum JL, Wicha MS: Role of the cytoskeleton in laminin induced mammary gene expression. J Cell Physiol 1988, 135:13-22 [DOI] [PubMed] [Google Scholar]
- 19.Seely KA, Aggeler J: Modulation of milk protein synthesis through alteration of the cytoskeleton in mouse mammary epithelial cells cultured on a reconstituted basement membrane. J Cell Physiol 1991, 146:117-130 [DOI] [PubMed] [Google Scholar]
- 20.Katzenellenbogen BS: Estrogen receptors: bioactivities and interactions with cell signaling pathways. Biol Reprod 1996, 54:287-293 [DOI] [PubMed] [Google Scholar]
- 21.Smith CL: Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biol Reprod 1998, 58:627-632 [DOI] [PubMed] [Google Scholar]
- 22.Aronica SM, Katzenellenbogen BS: Stimulation of estrogen receptor-mediated transcription and alteration in the phosphorylation state of the rat uterine estrogen receptor by estrogen, cyclic adenosine monophosphate, and insulin-like growth factor-I. Mol Endocrinol 1993, 7:743-752 [DOI] [PubMed] [Google Scholar]
- 23.Migliaccio A, Pagano M, Auricchio F: Immediate and transient stimulation of protein tyrosine phosphorylation by estradiol in MCF-7 cells. Oncogene 1993, 8:2183-2191 [PubMed] [Google Scholar]


