Abstract
The C57BL/6, 129, and B6,129 mouse strains or stocks have been commonly used to generate targeted mutant mice. The pathology of these mice is not well characterized. In studies of these aging mice, we found high incidences of hyalinosis (eosinophilic cytoplasmic change) in the glandular stomach, respiratory tract, bile duct, and gall bladder of B6,129 CYP1A2-null and wild-type mice as well as in both sexes of the background 129S4/SvJae strain. The gastric lesions of the glandular stomach were found in 95.7% of female CYP1A2-null mice as well as in 45.7% of female 129S4/SvJae animals. The eosinophilic protein isolated from characteristic hyaline gastric lesions was identified as Ym2, a member of the chitinase family. Immunohistochemistry, using rabbit polyclonal antibodies to oligopeptides derived from the Ym1 sequence, detected focal to diffuse reactivity within both normal and abnormal nasal olfactory and respiratory epithelium, pulmonary alveolar macrophages, bone marrow myeloid cells, and the squamous epithelium of the forestomach and epithelium of the glandular stomach. Alveolar macrophages in acidophilic pneumonia, a major cause of death of aging 129 mice, and in mice with the me mutation also were highly immunoreactive. The possible cause of this protein excess in gastric and other lesions and its possible functions are discussed.
CYP1A2 carries out oxidation and N-hydroxylation of arylamine carcinogens and heterocyclic amine food mutagens which, when followed by O-esterification by acetate or sulfate transferases, results in unstable electrophilic derivatives that can cause cell toxicity, cell death, or cell transformation. 1-3 CYP1A2 catalyzes caffeine 3-demethylation and metabolically activates aflatoxin B1 to its ultimate carcinogenic intermediate 4 and paradoxically catalyzing the hydroxylation of aflatoxin B1 to aflatoxin M1 in a detoxification pathway. 5 In addition, CYP1A2 was found to be involved in both hamster and human catechol estrogen metabolism. 6 Although CYP1A2 is constitutively expressed in the liver of mice, rats, and humans and is inducible by ligands of the aryl hydrocarbon receptor (Ahr) in all mammalian species analyzed, no expression has been demonstrated in the stomach, even after treating animals with known inducers of this enzyme. 7 However, the mechanisms involved in CYP1A2 gene regulation are less well established than CYP1A1 because it is difficult to induce expression in vitro and the sensitivities of current methodologies may simply be too low under normal in vivo conditions. The mammalian conservation of the CYP1A subfamily argues for an essential function in metabolism not limited to activation and detoxification of exogenous chemicals and toxins. As with most of the cytochromes P-450, determination of function has primarily been a consequence of exposures to man-made compounds, but it must be remembered that their evolution predates industrialization. The genes of the two recognized members of the CYP1A subfamily, CYP1A1 and CYP1A2, are located in tandem on chromosome 9 in the mouse and such proximity, as well as the homology that exists between them, may indicate a cooperation or overlap in activity. 8,9
A great many, if not the majority, of genetically engineered mice are constructed using embryonic stem cells derived from a 129/Sv strain injected into C57BL/6 mouse blastocysts. The resulting chimeric animals are then bred back to either the B6 or 129 to fix the new genotype on one or the other background strains with the new designation of B6,129. The natural history of the more standardized C57BL/6 strain has been fairly well chronicled. However, the 129 mouse, although used extensively for genetic studies, has diverged into many sometimes uncharacterized lines and sublines 10 with subsequent limited and conflicting information as to endogenous pathology. 11 Not surprisingly, the pathology of the relatively new B6,129 mouse, as a co-isogenic strain to 129 and/or C57BL/6, is also not well known. 11
In a study of the effect of targeted deletion of the CYP1A2 gene, we discovered high incidences of unusual hyaline gastric, biliary, and respiratory lesions both in one of the progenitor strains,129S4/SvJae, and in the co-isogenic B6,129 mice. 11 We identified the hyaline protein and showed distribution of it in normal tissues and in hyalinosis.
Materials and Methods
Experimental Design and Histopathology
Control 129S4/SvJae mice were originally derived from animals bred and maintained in Dr. Heiner Westphal’s laboratory at the National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD. The wild-type and CYP1A2-null B6,129 populations (C57BL6/NCr × 129S4/SvJae, brother × sister matings) were derived from those used in previous CYP1A2 targeted deletion mouse experiments 11-14 transferred to National Cancer Institute at Frederick, Frederick, MD, for line maintenance and studies of aging. Groups of 25 to 50 mice of each sex were housed at no more than five per cage; all received NIH 31 diet (PMI, St. Louis, MO) ad libitum and acidified water and were allowed to live out their life spans in compliance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Research Council. Mice were sacrificed when conditions suggested progressive deterioration because of aging changes; few mice died spontaneously.
A complete necropsy was performed on all animals with tissues fixed in 10% neutral-buffered formalin, trimmed, and embedded in paraffin. The stomach was inflated with formalin at the time of necropsy, tied off at the pyloric end, and fixed for 5 minutes. It was then opened by cutting along the greater curvature, flattened by sandwiching between filter paper, and fixed further. When adequately flattened and preserved, sequential longitudinal sections were trimmed and embedded according to a specific protocol. 15
Tissues from 2- to 4-month-old wild-type B6,129 (ie, CYP1A2 +/+) mice and from our archival motheaten (me/me) mice 16 were also collected for histopathological comparisons. Sections were stained with hematoxylin and eosin (H&E). Selected tissues were also stained by the periodic acid-Schiff method, acid fast, Masson’s trichrome, and Dominici and Steiner’s silver.
Immunohistochemistry and Electron Microscopy
Immunohistochemistry was performed on formalin-fixed tissues embedded in paraffin and sectioned at 4 to 6 μm. They were incubated with a rabbit serum raised against two peptide sequences identified on analysis of the gastric lesion protein (see following section) at dilutions of 1:1,000 through 1:64,000 using the Vectastain ABC Rabbit Elite Kit (Vector Laboratories, Inc., Burlingame, CA). Diaminobenzidine was the chromagen with hematoxylin as the counterstain.
Two stomach lesions were chosen for electron microscopy; they were fixed in 2.5% glutaraldehyde, postfixed in osmium tetroxide, and embedded in epoxy resin. Thin sections were cut and stained with uranyl acetate and lead citrate before electron microscopic evaluation.
Gastric Protein Isolation and Identification
Gastric lesions and normal glandular stomach tissues adjacent to the lesions were obtained from 15- to 17-month-old female CYP1A2-null (B6,129) mice. Control stomach tissue was obtained from a 12-month-old wild-type B6,129 female mouse. Tissues were minced with scissors in 5 volumes of ice-cold phosphate-buffered saline (pH 7.5) containing 10 mmol/L ethylenediaminetetraacetic acid, 15 mmol/L MgCl2, 10 mmol/L dithiothreitol, 1 mmol/L phenylmethylsulfonyl fluoride, 10 mg/ml leupeptin, and 10 mg/ml aprotinin. Two volumes of sodium dodecyl sulfate (SDS)-loading buffer 17 were added and the suspension boiled for 10 minutes. After brief sonication for 30 seconds, the solution was spun at 10,000 × g for 10 minutes and the supernatant loaded onto 10% SDS-polyacrylamide gels. Protein concentration was determined by use of the BCA protein assay reagent (Pierce Chemical Company, Rockford, IL) using bovine serum albumin as standard.
After separation on SDS-polyacrylamide gels, stomach proteins were transferred to polyvinylidene difluoride membranes (Schleicher & Schuell, Keene, NH) for peptide sequencing and to nitrocellulose membranes (Schleicher & Schuell) for Western blotting. Whole proteins were visualized by gel staining with Coomassie Brilliant Blue R250. Proteins transferred to polyvinylidene difluoride membranes for peptide sequencing were stained with Ponceau S before cutting and subsequent analyses. The antibody was prepared in rabbits using two peptide sequences (Macromolecular Resources, Fort Collins, CO), QYNFDGLNLDWQYPGSRGSPPK and DLHDPKDGYTGENSPLYKSPYD, a part of which was derived from those sequences that were determined by peptide sequencing of the band from the SDS gel and the rest from the published Ym1 sequence (see below). The peptides corresponded to the amino acid residues 130 to 151 and 203 to 234, respectively, based on the first amino acid residue in the precursor Ym1 protein as position 1. Western blots were developed using rabbit anti-peptide antibody and secondary antibody coupled with horseradish peroxidase for enhanced chemiluminescence detection (ECL; Amersham, Arlington Heights, IL).
RNA Analyses
Total RNAs were isolated from various tissues as indicated using TRIzol reagent (Life Technologies, Inc., Gaithersburg, MD) according to the manufacturer’s protocol. RNAs were electrophoresed on a 0.22 mol/L formaldehyde-1% agarose gels, blotted onto a GeneScreen Plus membrane (NEN Life Science Products, Boston, MA), and hybridized with a Ym2 cDNA probe that encodes the N-terminal one-fourth of the protein. Because the 5′ region of the Ym2 sequence is not known, a Ym2 cDNA probe of ∼340 bp was prepared using reverse transcriptase-polymerase chain reaction (RT-PCR) with stomach RNA and primers designed based on the published Ym1 sequence, 18 assuming that high similarity is present between entire Ym1 and Ym2 sequences. RT-PCR was also performed to confirm the identity of mRNAs expressed in various parts of the stomach. In this case, primer sequences were chosen from the region where sequences of Ym1 and Ym2 are identical. RT-PCR products were analyzed with an Applied Biosystems DNA sequencer (model 373A; Applied Biosystems, Foster City, CA), and MacVector software (Oxford Molecular Group, Beaverton, OR).
Results
The 129S4/SvJae mice showed good survival with more than half of both sexes surviving to 2 years of age. Survival of B6,129 wild-type and targeted mutants was somewhat lower; <50% of wild-type male and female B6,129 mice reached 2 years of age, and the number of male and female CYP1A2-null mice still alive at 18 months was ∼50%. The complete aging pathology of these mice is reported elsewhere. 11 In Table 1 ▶ , the incidence data are based on the pathology of the mice that were sacrificed because of clinical signs or that died of natural causes.
Table 1.
Incidence of Hyalinosis in Aging 129S4/SvJae and Wild-Type and CYP1A2-Null B6, 129 Mice*
| Tissue | Strain/sex | |||||
|---|---|---|---|---|---|---|
| 129S4/SvJae female (%) | 129S4/SvJae male (%) | B6, 129 CYP1A2 +/+ female (%) | B6, 129 CYP1A2 +/+ male (%) | B6,129 CYP1A2 −/− female (%) | B6, 129 CYP1A2 −/− male (%) | |
| Glandular stomach* | 21/46 (45.7) | 8/39 (20.5) | 3/48 (6.3) | 4/47 (8.5) | 22/23 (95.7) | 8/24 (33.3) |
| Nasal cavity, olfactory | 9/47 (19.1) | 2/39 (3.1) | 38/49 (77.6) | 14/48 (29.2) | 20/23 (87) | 18/26 (69.2) |
| Nasal cavity, respiratory | 36/47 (76.6) | 12/39 (30.8) | 46/49 (93.9) | 25/48 (52.1) | 23/23 (100) | 24/26 (92.3) |
| Lung† | 42/48 (87.5) | 27/40 (67.5) | 7/49 (14.3) | 3/48 (6.3) | 6/23 (26.1) | 4/26 (15.4) |
| Bile duct | 6/47 (12.8) | 2/40 (5) | 0/49 (0) | 0/48 (0) | 1/23 (4.3) | 0/26 (0) |
| Gall bladder | 5/45 (11.1) | 1/38 (2.6) | 7/46 (15.2) | 3/41 (7.3) | 11/22 (50) | 5/21 (23.8) |
| Trachea | 12/47 (25.5) | 7/37 (18.9) | 1/49 (2) | 0/48 (0) | 4/23 (17.4) | 7/26 (26.9) |
* Number of animals with lesions/number reviewed histologically. Mean survival was approximately 102 to 104 weeks for male or female 129S4/SvJae mice, 80 weeks for male wild-type B6, 129 mice, 104 weeks for female wild-type B6, 129 mice, 80 weeks for male CYP1A2-null mice, and 80 weeks for female CYP1A2 null mice.
† Number of animals with acidophilic macrophage pneumonia/number histologically reviewed.
Pathology
Histologically, an eosinophilic cytoplasmic degenerative change, ie, hyalinosis, was found in multiple epithelial tissues, but differed in incidence by mouse strain and sex (Table 1) ▶ . The incidence was particularly high in the nasal mucosa, trachea, lung, and stomach of 129S4/SvJae and CYP1A2-null mice.
Epithelial cells in the nasal mucosa, trachea, lung, glandular stomach, gall bladder, bile ducts, and pancreatic ducts showed large cytoplasmic eosinophilic inclusions, diffuse hyaline cytoplasm, or other cytoplasmic changes. The respiratory epithelium tended to be most affected, particularly in the regions of the nasal glands, with olfactory epithelial involvement occurring primarily near the olfactory/respiratory transition areas characterized by focal hyalinosis in the form of eosinophilic inclusions (Figure 1A) ▶ . Affected respiratory epithelial cells were frequently swollen with peripheral displacement of the nucleus. In affected olfactory epithelium, the inclusions tended to be more common and to originate at the basal aspects of lining cells. Inflammation was usually not associated with epithelial changes. The occurrence and severity of the lesions varied from mouse to mouse. Nasal inclusions were also found in 4-month-old wild-type B6,129 mice and crystal formation seen in 9 to 12% of aged mice of the same genotype. Crystals were variably needle-like, rectangular, or square-shaped (Figure 1A) ▶ .
Figure 1.
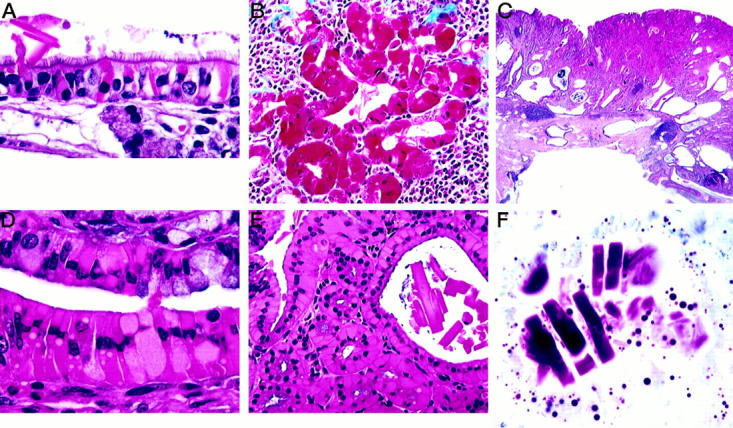
Histological appearance of hyalinosis in various tissues. A: Respiratory portion of nasal cavity showing hyaline intracellular inclusions and an extracellular crystal. H&E. B: Epithelium of hyaline bile duct epithelium stains deep red with Masson’s trinchrome. C: Portion of a large gastric lesion showing hyperplastic epithelium and cystic epithelium extending into the muscle wall with foci of lymphocytic inflammation. Note the eosinophilic epithelial areas within the hyperplastic lesion. H&E. D: Higher magnification of a gastric lesion showing eosinophilic epithelial cells. H&E. E: Gastric lesion showing large extracellular crystals within an area of hyalinosis. H&E. F: Dominici’s stain showing metachromatic extracellular crystals in a gastric lesion.
Pulmonary macrophages, a component of acidophilic macrophage pneumonia in the 129S4/SvJae mice, 11 frequently contained both intracellular and extracellular crystals. They were sometimes also present in alveolar and bronchiolar lumens unassociated with macrophages. This macrophage pneumonia was frequently severe enough to produce clinical illness and was a major cause of death in the 129S4/SvJae control population. 11
Gall bladders were often grossly enlarged with thickened opaque walls in 129S4/SvJae mice and had eosinophilic cytoplasmic hyaline change. Extracellular crystals, when present, tended to be large and rectangular to square in shape. Affected bile ducts had associated mucoid metaplasia and fibrosis. Masson’s trichrome revealed intense red staining of eosinophilic gall bladder and bile duct epithelium (Figure 1B) ▶ . In a few cases, smaller pancreatic ducts had lesions similar to those in the bile ducts.
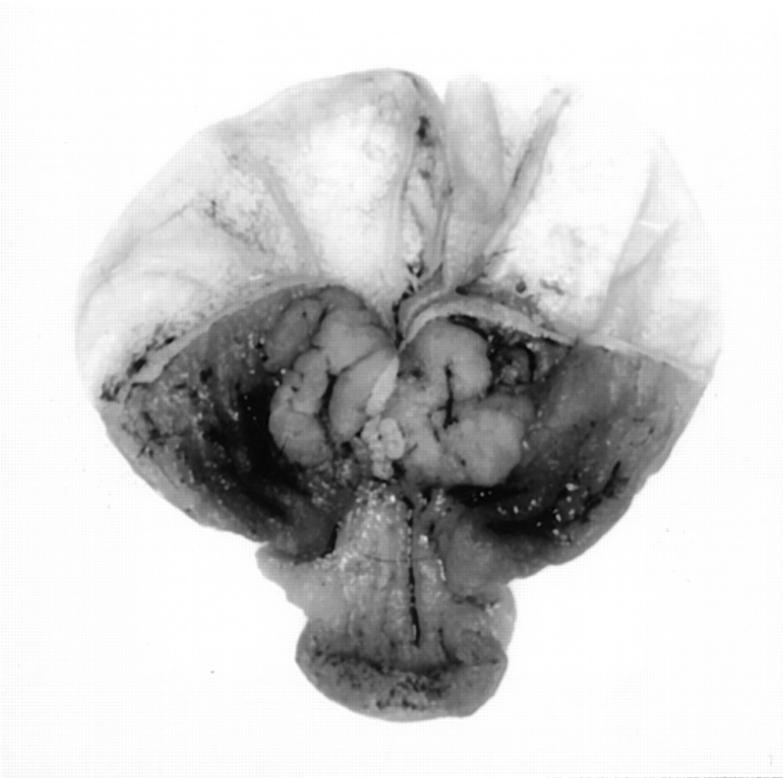
Gastric lesions were most common in the cardiac glandular stomach at or near the limiting ridge (Figure 2) ▶ . These lesions were most often plaque-like and were frequently multiple, but localized within the same periesophageal area of the cardia; some were hemorrhagic. The size of the lesion was apparently a function of age as the largest lesions were found in the oldest mice. The glandular epithelium was disorganized focally in the plaque and had loss of normal differentiation patterns (Figure 1C) ▶ . It was usually also hyperplastic and contained focal areas of hyalinized epithelial cells (Figure 1, D and E) ▶ that seemed to arise from chief cells in the mid-region of the glands. Some epithelial cells contained only a few intracytoplasmic droplets, whereas other cells were swollen by the brightly eosinophilic material with a peripherally displaced nucleus. Extracellular eosinophilic crystals were often focally abundant in the lumen of the glands, being present in 83% of the CYP1A2-null females and in only 12.5% of the null males (Figure 1, E and F) ▶ . These crystals were most often rectangular, as opposed to needle- or square-shaped; they were metachromatic with Dominici stain (Figure 1F) ▶ as were the hyaline granules within epithelial cells.
Figure 2.
Gross appearance of two plaques in the glandular stomach at the limiting ridge of a 19-month-old female CYP1A2-null mutant (B6,129).
In 8 to 9% of the CYP1A2-null mice, glandular diverticula were seen to extend into the muscle wall and occasionally into the serosa (Figure 1C) ▶ . Chronic to chronic-active inflammatory foci in the wall of the glandular stomach, often with eosinophils or a mixed inflammatory cell response, were often associated with the plaques of the CYP1A2-null mice (41 to 96%) but were less frequently found in 129S4/SvJae gastric lesions (30%). Steiner’s staining usually revealed no helical organisms in the lumen or glands. Forestomach squamous hyperplasia was observed in some mice and eosinophilic inclusions were sometimes present in the stratum spinosum.
Ultrastructure of two gastric lesions revealed abundant pale granular material within dilated rough endoplasmic reticulum in what appeared to be chief cells; in addition, these cells often contained some secretory granules (Figure 3) ▶ . Some cells had one large dilated cisterna whereas in others multiple cisternae were affected. We found no evidence of obvious parietal cell origin.
Figure 3.

Ultrastructure of hyalin in the chief cells of a CYP1A2-null mouse. Note the dilated rough endoplasmic reticulum filled with fine granular material and few secretory granules. Uranyl acetate, lead citrate; original magnification, ×6,000.
Protein Identification
Proteins obtained from the lesion, normal part of the glandular stomach adjacent to the lesion, and normal glandular stomach were subjected to SDS-polyacrylamide gel electrophoresis, followed by protein staining. A protein band of ∼45 kd was detected in only the lesion sample, but not the others (Figure 4) ▶ . After proteins were transferred to a membrane and were visualized, the corresponding band was excised from the gel and subjected to peptide sequencing. Two peptide sequences were obtained that matched those of secretory protein Ym1 18 when subjected to a GenBank search. This protein belongs to a chitinase family, and is also known as a chitinase-related protein (GenBank U56900) or T lymphocyte-derived eosinophil chemotactic factor (ECF-L). 19 Antibody was prepared using a part of these peptide sequences with the extension of several amino acid residues based on the published Ym1 sequence, 18,19 which exhibited good antigenicity by computer analyses.
Figure 4.
Protein analyses of the stomach. Tissue homogenates (20 μg/lane) from normal wild-type glandular stomach (GS+/+), from a gastric lesion in a CYP1A2-null mouse (L−/−), and from a normal part of the same glandular stomach adjacent to the lesion (GS−/−) were run on 10% SDS-polyacrylamide gel and stained for protein.
Western blotting analyses of various parts of the stomach revealed the presence of another protein band of ∼55 kd, in addition to the 45-kd band (Figure 5A) ▶ . This 55-kd protein was detected in the normal part of the glandular stomach adjacent to the lesion as well as in the normal glandular stomach, whereas the 45-kd protein was seen only in the lesion and in normal forestomach. The gastric limiting ridge exhibited both 45- and 55-kd bands. Expression level of the 45-kd protein in the lesion varied depending on samples, from as low as 20-fold to as high as 200-fold greater than the level of the 55-kd protein present in the adjacent normal part of the glandular stomach or normal glandular stomach. This is based on the fact that the amount of total lesion protein loaded on a gel was reduced such that the intensity of the 55-kd band and the lesion 45-kd band were equivalent. The antibody also reacted to a reduced extent, with a protein of ∼50 kd expressed in the lung and spleen, and two bands of ∼42 kd in the liver (Figure 5B) ▶ .
Figure 5.
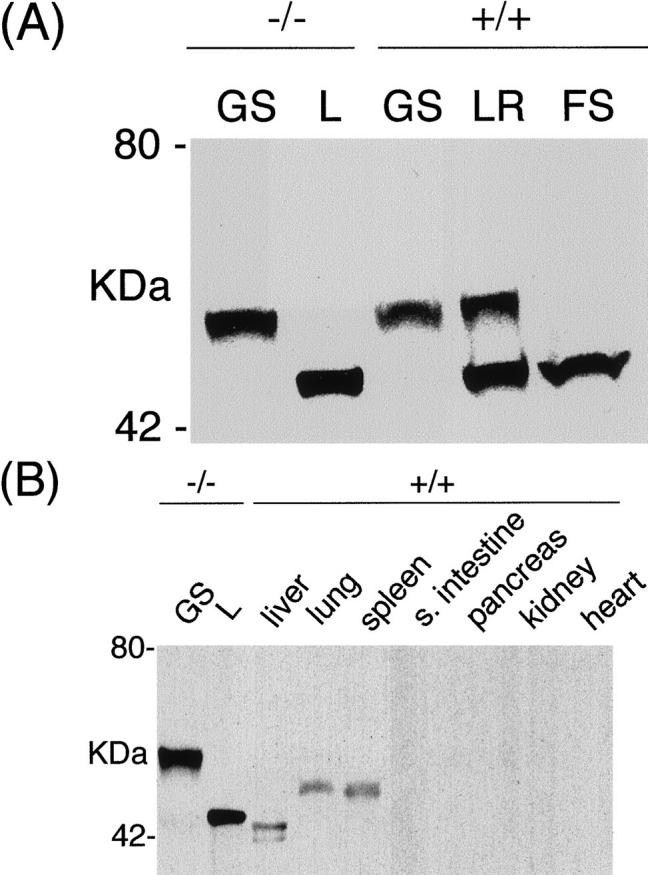
Western blotting analyses of Ym2/1 proteins. Representative results are shown. A: Tissue homogenates from a normal part of the CYP1A2-null glandular stomach adjacent to a lesion (GS−/−), from a gastric lesion in a CYP1A2-null mouse (L−/−), from a normal wild-type glandular stomach (GS +/+), from a normal limiting ridge (LR+/+), and from a normal forestomach (FS +/+). Twenty μg of total protein was used per each lane, except for the lesion homogenate, L(−/−), in which 0.1 μg was used. B: Tissue homogenates from a normal part of a glandular stomach in a CYP1A2-null mouse adjacent to a gastric lesion (GS−/−), from a lesion in a CYP1A2-null mouse (L−/−), and from various normal organs of wild-type (+/+) mice as indicated were subjected to Western blotting. Twenty μg was used per each lane, except for the plaque-like lesion homogenate, L, in which 0.1 μg was used.
To determine the nature of the 55- and 45-kd bands, RT-PCR was performed and the products sequenced using RNAs isolated from the stomach lesion, normal part of the glandular stomach adjacent to the lesion, normal glandular stomach, and the whole normal stomach. All RT-PCR products had nucleotide sequences identical to those of Ym2. 18
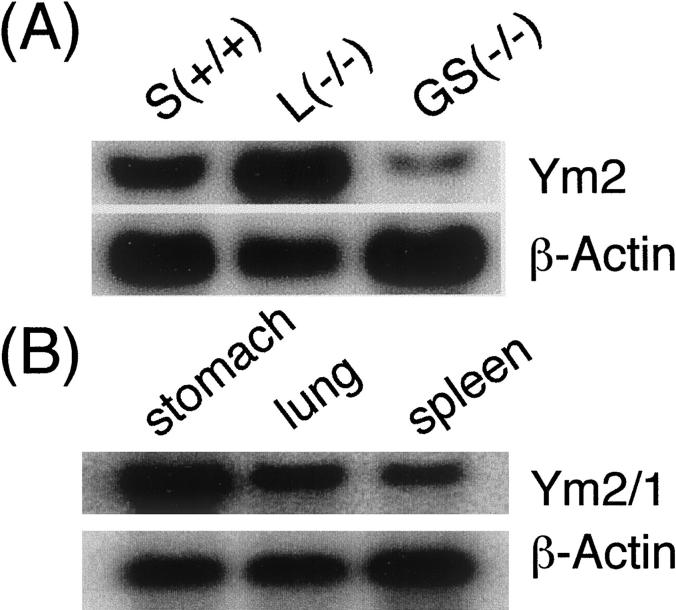
Northern blotting analyses were also performed to examine the level of Ym2 mRNA expression (Figure 6, A and B) ▶ . Ym2 mRNA was ∼1.6 kb, and the expression level in the stomach lesion was at least 20 times higher than the normal part of the glandular stomach adjacent to the lesion. Northern blotting analyses also revealed that a Ym2 cDNA probe cross-hybridized with mRNA expressed in the lung and spleen, which is slightly larger than the stomach-specific Ym2 mRNA (Figure 6B) ▶ .
Figure 6.
Northern blotting analyses of Ym2/1 mRNAs. Representative results are shown. A: Total RNAs from whole normal stomach (S +/+; glandular stomach + limiting ridge + forestomach), from a gastric lesion in a CYP1A2-null mouse (L−/−), and from a normal part of the glandular stomach adjacent to the lesion (GS−/−). Ten μg of total RNA/lane was used for the normal stomach samples, and 3 μg/lane for the lesion, L. B: Total RNAs from various organs as indicated were subjected to Northern blotting. Ten and 5 μg total RNA/lane were used for lung, spleen, and stomach samples, respectively.
Immunohistochemistry
Using the rabbit polyclonal antibody raised against the two oligopeptides whose sequences are derived from Ym1, immunohistochemical evaluation of tissues from normal young mice of both the 129S4/SvJae and B6,129 lines revealed immunoreactivity in olfactory and respiratory nasal epithelium (Figure 7A) ▶ , most pulmonary alveolar macrophages (Figure 7B) ▶ , and some alveolar type II cells, bone marrow myeloid cells (Figure 7C) ▶ , and the squamous epithelium of the oral cavity, esophagus, and limiting ridge of the stomach (Figure 7D) ▶ . In wild-type B6,129 mice, some chief and parietal cells in the lower portion of the gastric glands in the fundus had either diffuse cytoplasmic reactivity or peripheral cytoplasmic granular reactivity (Figure 7E) ▶ . The staining pattern varied from granular cytoplasmic in alveolar type II cells, diffuse cytoplasmic in squamous epithelium and bone marrow, and vacuolated-diffuse cytoplasmic in normal nasal epithelium.
Figure 7.
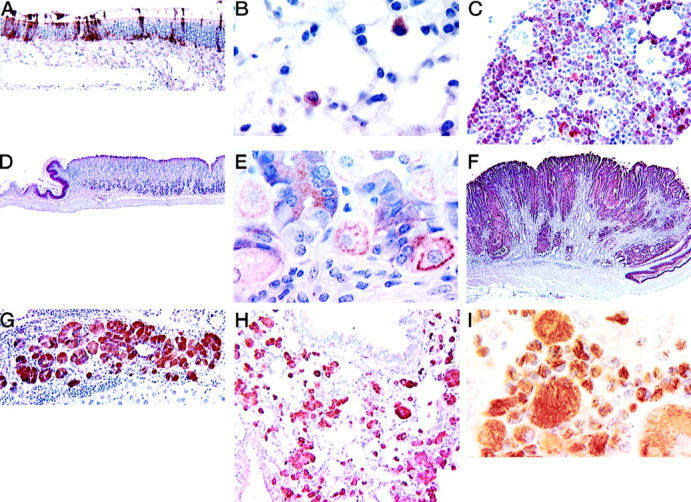
Expression of Ym1/2 in tissues of mice as determined by ABC immunohistochemistry using a rabbit polyclonal antibody prepared against oligopeptides derived from the Ym1 protein sequence. All figures from formalin-fixed tissues unless otherwise noted and with hematoxylin counterstain. A: Olfactory region of a nasal cavity from 2-month-old wild-type B6,129 mouse. Note the many immunoreactive epithelial cells. B: Immunoreactive alveolar macrophages in a normal lung of a 4-month-old wild-type B6,129 mouse. C: Bone marrow from wild-type B6,129 mouse showing reactive myeloid cells. D: Normal stomach of a wild-type (CYP1A2+/+) mouse showing immunoreactivity in the squamous epithelium of the limiting ridge but no reactive staining in the glandular stomach. E: Higher magnification of the normal glandular stomach shown in D. Immunoreactivity is seen within chief and parietal cells. F: A gastric lesion from a CYP1A2-null animal showing abundant multifocal immunoreactivity. G: Hyalinosis in bile duct hyperplasia and cholangitis; the epithelium is strongly immunoreactive in a CYP1A2 null mouse. H: Macrophage pneumonia in a 129S4/SvJae mouse. The pulmonary macrophages are strongly immunoreactive. I: Macrophages in the lungs of a motheaten mouse. The intracytoplasmic needle-like crystals are reactive whereas the large extracellular crystals are not immunoreactive. Bouin’s fixation.
In hyaline lesions of the nasal cavity, glandular stomach (Figure 7F) ▶ , normal, hyperplastic, or inflammatory gall bladder and bile ducts (Figure 7G) ▶ , the immunostaining pattern was often diffuse and cytoplasmic but focal or granular patterns were also seen. The pulmonary macrophages of 129S4/SvJae (Figure 7H) ▶ and motheaten mice (Figure 7I) ▶ with macrophage pneumonias displayed diffuse cytoplasmic staining. Gastric plaques in both B6,129 and 129S4/SvJae animals demonstrated a diffuse cytoplasmic immunostaining of inclusions and hyperplastic cells; extracellular crystals were also immunoreactive. CYP1A2-null mice sometimes showed focal to diffuse reactivity throughout epithelium distant from the gastric plaques themselves. Within the gastric lesions, immunostaining was usually focal and not all cells with inclusions were positive.
Discussion
We have found a hyalinosis syndrome in 129S4/SvJae and in B6,129 mice that seemed to be exacerbated in the glandular stomachs of CYP1A2-null animals. Stomach lesions similar to those we describe have been reported at a low incidence in Han:NMRI mice 20 and, although disruption of normal cellular differentiation in the glandular stomach has also been reported in transgenic mice, 21 associated gastric hyalinosis has not been described previously in a mutant model. In both cases, there is some loss of normal cellular differentiation and migration in the glandular epithelium. In the transgenic mice, however, this loss is found throughout the glandular stomach epithelium whereas it is primarily in the region of the limiting ridge in our mice.
In contrast, hyaline lesions of the nasal cavity and respiratory tract identical to those described in our study, have been found in other conventional mice. 11,22-26 Hyalinosis in gall bladders and in bile and pancreatic ducts has also been reported previously in these same and several other mouse strains or stocks. 11,13,14,27-29 The characteristic pulmonary lesions containing macrophages with eosinophilic crystal formation seen in our 129S4/SvJae population, furthermore, resemble those reported in the motheaten mouse 16,22,30,31 and are sometimes associated with lung tumors 32,33 as well as with macrophage pneumonias in a number of strains. 22,27 In motheaten mice, the pulmonary macrophage lesion is associated with a deficiency of SHP-1, a protein tyrosine phosphatase. 34
We have identified that extracellular and intracellular eosinophilic crystals in the gastric lesion are composed of secretory protein Ym2. Ym2 is highly related to Ym1 (GenBank M94584), 18 the latter of which is also called eosinophil chemotactic factor (ECF-L, GenBank D87757), 19 a chitinase-related protein. Ym1 and Ym2 share ∼95% identity but show tissue-specific expression; whereas Ym1 expression is high in lung and spleen and barely detectable in stomach, Ym2 expression is high in stomach and nondetectable in lung and spleen. 18 Ym2 is also detectable in the thymus to a lesser extent than the stomach. Together with these results, our findings suggest that Ym2 may be a stomach-specific eosinophil chemotactic cytokine.
The mature form of Ym1/ECF-L is a processed product of the precursor that has a signal peptide at the N-terminus. 19 On the analogy of Ym1, it is likely that the 55-kd protein is a precursor and the 45-kd protein is a mature form of Ym2. Our Western blotting results demonstrate that the 55-kd precursor protein is predominantly produced in the glandular stomach whereas the 45-kd protein is abundant in the forestomach. This may suggest that the precursor protein is mainly produced in the glandular stomach, which moves to the forestomach as the protein is processed. Because the junction (limiting ridge) between the glandular stomach and the forestomach contains both 55- and 45-kd proteins, the protein processing may take place at or near the limiting ridge. The mechanism for this processing relative to the histological structure of the stomach remains unclear.
Our Western blotting results demonstrated that the level of Ym2 protein massively increased in the gastric lesion. Especially of interest is that the majority of increased Ym2 protein is a mature form. This explains why the 45-kd protein was initially detected only in the lesion, but not in the other stomach samples on SDS-polyacrylamide gels. In contrast, normal stomach has both the precursor and the mature form at comparable levels. Increased expression of Ym2 mRNA was also observed in the lesion, indicating that the increased level of Ym2 protein expression is mainly because of increased level of Ym2 mRNA. The mechanism for this increase in mRNA is not known. Increased levels of Ym2 precursor may facilitate the processing machinery in massively accumulating mature form of Ym2, which results in crystallization and formation of the lesion.
Because the antibody used in the current study was raised against peptides, whose sequences are derived from the Ym1 sequence and a high degree of sequence similarity is present between Ym1 and Ym2, and possibly other members of chitinase family, 18,35 we are most likely showing a certain amount of cross-reactivity for murine chitinase-like protein products. In fact, Western blots of lung and spleen exhibited a protein band of ∼50 kd and additional protein bands of ∼42 kd were identified in liver. Because Ym1 is purported to be expressed in lung and spleen rather than in stomach 18 it is reasonable to assume that the 50-kd protein may be Ym1. This is further supported by a recent study 22 in which Ym1 was isolated from endogenously formed eosinophilic crystals in the lungs of motheaten (mev/mev) mice. Another member of the chitinase gene family, Ym3, has been described that shares ∼95% identity with Ym1 and Ym2. 18 Although the total length and sequence is not known for Ym3, the degree of homology would not preclude detection of this isoform as well. The ∼42-kd proteins present in liver could therefore be Ym3 although Ym3 expression was not detected in the liver. 18 The ∼42-kd proteins consist of two bands, the second band being smaller in size and fainter than the main band, suggesting that the second band may be a degradation product. Alternatively, the upper band is a precursor and the second band is a mature form of the protein, given that the protein is Ym3, and this protein also goes through the same processing as Ym1 and Ym2. The identity of the immunoreactive proteins found in the olfactory and nasal cavity, bone marrow, oral cavity, esophagus, bile duct, and gall bladder could be Ym1, 2, 3, or as yet unidentified members of the chitinase-like family.
The data presented in this experiment suggest that sex and strain genetics can highly influence the prevalence of gastric lesions and that CYP1A2 seems to exert a sex-specific moderating effect on the expression/production of particular protein secretions by some means. Exactly which hormone or hormones may be involved is not clear since ancillary investigations of other gastric products and modifiers have not yet been performed. However, the markedly higher female incidence of these hyaline lesions in both the control 129S4/SvJae and the wild-type and 1A2-null B6,129 animals implicates a sex-specific signal and some form of estrogen is, at least on the surface, a likely candidate. The highest frequencies of hyalinosis in the background 129S4/SvJae strain occurred in the lung and in the respiratory epithelium of the nasal cavity; the primary cause of death in this population was, in fact, macrophage pneumonia. 11 Although the incidence in an equivalently aged population of naïve C57BL/6 was not examined in this study, the B6,129 descendents of these strains demonstrate a shift of hyaline lesions toward involvement of the respiratory and olfactory epithelia of the nasal mucosa and away from lung. C57BL/6 mice seem to be refractory to the formation of spontaneous lung tumors, 32,36 as well as to the acidophilic macrophage pneumonia seen in this and other aging studies. 33,37 Despite genetic make-up, however, female mice are always more frequently affected. Only in the CYP1A2-null groups do the male incidences approach those of the females, again suggesting some hormonal influence kept in check by CYP1A2.
The positive immunoreactivity of the Ym1/2 antibodies with macrophages in archival motheaten mouse lung implies that at least part of the etiology of this condition is based in normal physiology gone awry. There are some similarities of the hyalinosis syndrome in our mice to autoimmune disorders. The motheaten syndrome is an immunodeficient and autoimmune condition comprising abnormal neutrophil accumulations in tissues and abnormal macrophage physiology that includes the formation of eosinophilic crystals. 16,30,38 Being a spontaneous mutant in the B6 strain implicates a possible susceptibility of the respiratory tract in this strain to macrophage-mediated insult. Noteworthy is the chronic ulcerative dermatitis frequently encountered in C57BL/6 and C57BL/10 populations that is characterized by a mixed cell infiltrate including macrophages and neutrophils. 39 Many, if not most, autoimmune diseases are more prevalent and/or more severe in females 40,41 and control of autoimmune reactions might be a P450 homeostatic function. Chitinases can act on the acetylglucosamine moieties found not only in exogenous chitins of fungi, bacteria, plants, parasites, and insects, but possibly on similar glycosaminoglycans formed by endogenous polysaccharides in the extracellular matrices as well; patients suffering from rheumatoid arthritis and Gaucher’s disease have been shown to have elevated levels of chitinase-like proteins. 22
The surprising increase in the incidence of female gastric lesions on deletion of CYP1A2 may be the clearest evidence of involvement of this cytochrome subfamily in normal physiological events. Even though the function of CYP1A2 has been fairly well determined within the context of its exogenous substrates, eg, aromatic and heterocyclic amines, 5 its endogenous substrates have not been fully identified, possibly because high induction may not be necessary in homeostatic reactions. This would be especially true if CYP1A2 served as a watchdog of metabolic activities directly mediated by other substances, such as hormones and cytokines. Although CYP1A2 expression has been detected only in liver, 42 the absence of expression in other organs does not necessarily mean lack of activity, either directly within tissues and/or via feedback from extrahepatic sites.
In higher vertebrates, chitinases are believed to act as sentinels against parasites as well as having some digestive capabilities for chitin moieties in plant and insect ingesta. 43 ECF-L has been shown to cause extravasation of eosinophils and recruitment of T cells in response to parasitic infections, especially invasive helminth exposures. 19 Targeted mutation of CYP1A2 apparently allows for the overproduction of Ym2 protein, which may be the gastric-specific isoform of ECF-L that accumulates in the lumens of the glands of the gastric cardia and eventually leads to the characteristic hyperplastic lesions. The incidence in CYP1A2-null females is nearly threefold greater than in null males, in contrast to the low but slightly elevated male incidences in WT animals. This argues that CYP1A2 exerts some regulatory effect, but that it is possibly indirect and exerting the effect through a hormone with sexual dimorphism, if not sex-specificity. Thus, a candidate would logically be estrogen. The CYP1A1 gene has been found to be regulated by glucocorticoids via the XRE-Ahr-Arnt binding complex. 5
Another candidate, maybe more pertinent in the case of the gastric lesions induced in CYP1A2-null mice, is bombesin or, specifically gastrin-releasing peptide, which initiates the release of gastrin and other substances in the glandular stomach, duodenum, and pancreatic islets. 44,45 Both bombesin and gastrin-releasing peptide have been shown to play roles in lung morphogenesis 46,47 and in maintenance of mucosal immunity in both the gastrointestinal and respiratory tracts. 48,49 Two other possibilities are growth hormone (somatotropin) and insulin, possibly mediated by gastrin-releasing peptide. All operate within liver feedback systems and can be tissue-specific; in fact, glucose levels have been shown to regulate the effect of both bombesin and gastrin-releasing peptide. 45 In addition, another cytochrome, CYP2E1 has been shown to be regulated by growth hormone. 50
The data from the lungs and upper respiratory tract seems to parallel those in the stomach, and probably for the same reasons. Ingestion and inhalation are the two most frequent methods of exposure to any parasite or toxin and, therefore, it is possible that similar systems be operating along the same routes. It is also conceivable that for exogenous substrates similar to those found endogenously, a moderating surveillance mechanism be in place to control an overexuberant response that might prove harmful to the body. This study suggests a role for CYP1A2 moderating a complex hormonal control of YM1/2 as surveillance proteins in the mouse respiratory and gastrointestinal tracts.
Acknowledgments
We thank Chris Perella, Kunio Nagashima, Barbara Kasprzak, and Drs. Hideki Morishima and Mayumi Kawabe for their assistance and Deborah Devor-Henneman for valuable editorial assistance.
Footnotes
Address reprint requests to Dr. Jerrold M. Ward, National Cancer Institute, NCI-FCRDC, Fairview 201, PO Box B, Frederick, MD 21702-1201. E-mail: ward@mail.ncifcrf.gov.
Supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract NO1-CO-5600 to SAIC Frederick.
G. K.’s current address: Daiichi Pharmaceutical Co., Tokyo 134-0081 Japan.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Guengerich FP, Humphreys WG, Yun CH, Hammons GJ, Kadlubar FF, Seto Y, Okazaki O, Martin MV: Mechanisms of cytochrome P450 1A2-mediated formation of N-hydroxy arylamines and heterocyclic amines and their reaction with guanyl residues. Princess Takamatsu Symp 1995, 23:78-84 [PubMed] [Google Scholar]
- 2.Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, Waterman MR, Gotoh O, Coon MJ, Estabrook RW, Gunsalus IC, Nebert DW: P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 1996, 6:1-42 [DOI] [PubMed] [Google Scholar]
- 3.Windmill KF, McKinnon RA, Zhu X, Gaedigk A, Grant DM, McManus ME: The role of xenobiotic metabolizing enzymes in arylamine toxicity and carcinogenesis: functional and localization studies. Mutat Res 1997, 376:153-160 [DOI] [PubMed] [Google Scholar]
- 4.Eaton DL, Gallagher EP, Bammler TK, Kunze KL: Role of cytochrome P4501A2 in chemical carcinogenesis: implications for human variability in expression and enzyme activity. Pharmacogenetics 1995, 5:259-274 [DOI] [PubMed] [Google Scholar]
- 5.Kawajiri K, Hayashi S-I: The CYP1 family. Ioannides C eds. Cytochromes P450: Metabolic and Toxicological Aspects. 1996, :pp 77-97 CRC Press, London [Google Scholar]
- 6.Liehr JG: Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev 2000, 21:40-54 [DOI] [PubMed] [Google Scholar]
- 7.Dey A, Jones JE, Nebert DW: Tissue- and cell-specific expression of cytochrome P450 1A1 and cytochrome P450 1A2 mRNA in the mouse localized in situ hybridization. Biochem Pharmacol 1999, 58:525-537 [DOI] [PubMed] [Google Scholar]
- 8.Tukey RH, Lalley PA, Nebert DW: Localization of cytochrome P1-450 and P3-450 genes to mouse chromosome 9. Proc Natl Acad Sci USA 1984, 81:3163-3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hildebrand CE, Gonzalez FJ, Kozak CA, Nebert DW: Regional linkage analysis of the dioxin-inducible P450 gene family on mouse chromosome 9. Biochem Biophys Res Commun 1985, 130:396-406 [DOI] [PubMed] [Google Scholar]
- 10.Threadgill DW, Yee D, Matin A, Nadeau JH, Magnuson T: Genealogy of the 129 inbred strains: 129/SvJ is a contaminated inbred strain. Mamm Genome 1997, 8:390-393 [DOI] [PubMed] [Google Scholar]
- 11.Ward JM, Anver MR, Mahler JF, Devor-Henneman DE: Pathology of mice commonly used in genetic engineering (C57BL/6, 129, B6,129, and FVB/N). Ward JM Mahler JF Maronpot RR Sundberg JP eds. Pathology of Genetically Engineered Mice. 2000, :pp 161-179 Iowa State University Press, Ames [Google Scholar]
- 12.Pineau T, Fernandez-Salguero P, Lee SS, McPhail T, Ward JM, Gonzalez FJ: Neonatal lethality associated with respiratory distress in mice lacking cytochrome P450 1A2. Proc Natl Acad Sci USA 1995, 92:5134-5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters JM, Morishima H, Ward JM, Coakley CJ, Kimura S, Gonzalez FJ: Role of CYP1A2 in the toxicity of long-term phenacetin feeding in mice. Toxicol Sci 1999, 50:82-89 [DOI] [PubMed] [Google Scholar]
- 14.Kimura S, Kawabe M, Ward JM, Morishima H, Kadlubar FF, Hammons GJ, Fernandez-Salguero P, Gonzalez FJ: CYP1A2 is not the primary enzyme responsible for 4-aminobiphenyl-induced hepatocarcinogenesis in mice. Carcinogenesis 1999, 20:1825-1830 [DOI] [PubMed] [Google Scholar]
- 15.Tamano S, Jakubczak J, Takagi H, Merlino G, Ward JM: Increased susceptibility to N-nitrosomethylurea gastric carcinogenesis in transforming growth factor alpha transgenic mice with gastric hyperplasia. Jpn J Cancer Res 1995, 86:435-443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward JM: Pulmonary pathology of the motheaten mouse. Vet Pathol 1978, 15:170-178 [DOI] [PubMed] [Google Scholar]
- 17.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 18.Jin HM, Copeland NG, Gilbert DJ, Jenkins NA, Kirkpatrick RB, Rosenberg M: Genetic characterization of the murine Ym1 gene and identification of a cluster of highly homologous genes. Genomics 1998, 54:316-322 [DOI] [PubMed] [Google Scholar]
- 19.Owhashi M, Arita H, Hayai N: Identification of a novel eosinophil chemotactic cytokine (ECF-L) as a chitinase family protein. J Biol Chem 2000, 275:1279-1286 [DOI] [PubMed] [Google Scholar]
- 20.Rehm S, Sommer R, Deerberg F: Spontaneous nonneoplastic gastric lesions in female Han: NMRI mice, and influence of food restriction throughout life. Vet Pathol 1987, 24:216-225 [DOI] [PubMed] [Google Scholar]
- 21.Sharp R, Babyyatsky MW, Takagi H, Tagerud S, Wang TC, Bockman DE, Brand SJ, Merlino G: Transforming growth factor α disrupts the normal program of cellular differentiation in the gastric mucosa of transgenic mice. Development 1995, 121:149-161 [DOI] [PubMed] [Google Scholar]
- 22.Guo L, Johnson RS, Schuh JCL: Biochemical characterization of endogenously formed eosinophilic crystals in the lungs of mice. J Biol Chem 2000, 275:8032-8037 [DOI] [PubMed] [Google Scholar]
- 23.Herbert RA, Leninger JR: Nose, larynx, and trachea. Maronpot RR Boorman GA Gaul BW eds. Pathology of the Mouse: Reference and Atlas. 1999, :pp 259-292 Cache River Press, Vienna [Google Scholar]
- 24.Leninger JR, Herbert RA, Morgan KT: Aging changes in the upper respiratory tract. Mohr U Dungworth DL Capen CC Carlton WW Sundberg JP Ward JM eds. Pathobiology of The Aging Mouse. 1996, :pp 247-260 DC, ILSI Press, Washington [Google Scholar]
- 25.Chan PC, Eustis SL, Huff JE, Haseman JK, Ragan H: Two-year inhalation carcinogenesis studies of methyl methacrylate in rats and mice: inflammation and degeneration of nasal epithelium. Toxicology 1988, 52:237-252 [DOI] [PubMed] [Google Scholar]
- 26.Rosli Y, Breckenridge LJ, Smith RA: An ultrastructural study of age-related changes in mouse olfactory epithelium. J Electron Microscopy 1999, 48:77-84 [DOI] [PubMed] [Google Scholar]
- 27.Yang YH, Campbell JS: Crystalline excrements in bronchitis and cholecystitis of mice. Am J Pathol 1964, 45:337-345 [PMC free article] [PubMed] [Google Scholar]
- 28.Nakao Y, Ueki R, Tada T, Furukawa M, Uratani M, Takahashi T, Nomura M, Nakao Y: A tumor-like lesion with crystalline excrements in bile duct system and certain other organs in mice: a light and electron microscopic study. J Clin Electron Microscopy 1985, 18:141-161 [Google Scholar]
- 29.Rabstein LS, Peters RL, Spahn GJ: Spontaneous tumors and pathologic lesions in SWR/J mice. J Natl Cancer Inst 1973, 50:751-758 [DOI] [PubMed] [Google Scholar]
- 30.Green MC, Shultz LD: Motheaten, an immunodeficient mutant of the mouse. I. Genetics and pathology. J Hered 1975, 66:250-258 [DOI] [PubMed] [Google Scholar]
- 31.Thrall RS, Vogel SN, Evans R, Shultz LD: Role of tumor necrosis factor-alpha in the spontaneous development of pulmonary fibrosis in viable motheaten mutant mice. Am J Pathol 1997, 151:1303-1310 [PMC free article] [PubMed] [Google Scholar]
- 32.Rittinghausen S, Dungworth DL, Ernst H, Mohr U: Primary pulmonary tumors. Pathobiology of the Aging Mouse, vol.1. Edited by U Mohr, DL Dungworth, CC Capen, WW Carlton, JP Sunfberg, JM Ward. Washington, DC, ILSI Press, 1996, pp 301–314
- 33.Ernst H, Dungworth DL, Kamino K, Rittinghausen S, Mohr U: Nonneoplastic lesions in the lungs. Mohr U Dungworth DL Capen CC Carlton WW Sundberg JP Ward JM eds. Pathobiology of The Aging Mouse. 1996, :pp 281-300 DC, ILSI Press, Washington [Google Scholar]
- 34.Khaled AR, Butfiloski EJ, Sobel ES, Schiffenbauer J: Functional consequences of the SHP-1 defect in motheaten viable mice: role of NF-kappa B. Cell Immunol 1998, 185:49-58 [DOI] [PubMed] [Google Scholar]
- 35.Rehli M, Krause SW, Andreesen R: Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics 1997, 43:221-225 [DOI] [PubMed] [Google Scholar]
- 36.Theiss JC, Shimkin MB: Neoplasms of the respiratory system. The Mouse in Biomedical Research, vol. IV. Experimental Biology and Oncology. Edited by HL Foster, JD Small, JG Fox. New York, Academic Press, 1982, pp 477–484
- 37.Murray AB, Luz A: Acidophilic macrophage pneumonia in laboratory mice. Vet Path 1990, 27:274-281 [DOI] [PubMed] [Google Scholar]
- 38.Doolittle DP, Davisson MT, Guidi JN, Green MC: Catalogue of mutant genes and polymorphic loci. Genetic Variants and Strains of the Laboratory Mouse, vol.1, ed 3. Edited by MF Lyon, S Rastan, SDM Brown. New York, Oxford University Press, 1996, pp 17–854
- 39.Sundberg JP, Sundberg BA, King LE, Jr: Cutaneous changes in commonly used inbred mouse strains and mutant stocks. Mohr U Dungworth DL Capen CC Carlton WW Sundberg JP Ward JM eds. Pathobiology of the Aging Mouse, 1996, vol. 2.:pp 325-337 DC, ILSI Press, Washington [Google Scholar]
- 40.Cotran RS, Kumar V, Collins T: Diseases of immunity. ed 6 Cotran RS Kumar V Collins T eds. Pathologic Basis of Disease, 1999, :pp 188-259 W. B. Saunders Company, Philadelphia [Google Scholar]
- 41.Whitacre CC, Reingold SC, O’Looney PA: Task Force on Gender, Multiple Sclerosis and Autoimmunity: a gender gap in autoimmunity. Science 1999, 283:1277-1278 [DOI] [PubMed] [Google Scholar]
- 42.Kimura S, Gonzalez FJ, Nebert DW: Tissue-specific expression of the mouse dioxin-inducible P1450 and P3450 genes: differential transcriptional activation and mRNA stability in liver and extrahepatic tissue. Mol Cell Biol 1986, 6:1471-1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon MS: Nutrition. Animal Physiology: Principals and Adaptations. 1972, :pp 14-43 The Macmillan Company, New York [Google Scholar]
- 44.Linder MC: Human nutrition in context. Linder MC eds. Nutritional Biochemistry and Metabolism With Clinical Applications. 1991, :pp 1-15 Elsevier, New York [Google Scholar]
- 45.Kloss H, Wahl MA, Holger N, Verspohl EJ: Modulation of gastrin-releasing peptide (GRP) receptors in insulin secreting cells. Cell Biochem Function 1999, 17:229-236 [DOI] [PubMed] [Google Scholar]
- 46.Al Moustafa A-E, Tsao M-S, Battey JF, Viallet J: Expression of the gastrin-releasing peptide receptor confers a growth response to bombesin in immortalized human bronchial epithelial cells. Cancer Res 1995, 55:1853-1855 [PubMed] [Google Scholar]
- 47.Wang D, Yeger H, Cutz E: Expression of gastrin-releasing peptide receptor gene in developing lung. Am J Respir Cell Mol Biol 1996, 14:409-416 [DOI] [PubMed] [Google Scholar]
- 48.DeWitt RC, Wu Y, Renegar KB, King BK, Li J, Kudsk KA: Bombesin recovers gut-associated lymphoid tissue and preserves immunity to bacterial pneumonia in mice receiving total parenteral nutrition. Ann Surg 2000, 231:1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Kudsk KA, Hamidian M, Gocinski BL: Bombesin affects mucosal immunity in gut-associated lymphoid tissue in intravenously fed mice. Arch Surg 1995, 130:1164-1170 [DOI] [PubMed] [Google Scholar]
- 50.Ronis MJJ, Lindros KO, Ingelmann-Sundberg M: The CYPE subfamily. Ioannides C eds. Cytochromes P450: Metabolic and Toxicological Aspects. 1996, :pp 211-239 CRC Press, London [Google Scholar]