Abstract
Type I and type II macrophage scavenger receptors (SR-A I/II) recognize a variety of polyanions including bacterial cell-wall products such as lipopolysaccharide, suggesting a role for SR-A I/II in immunity against bacterial infection. SR-A I/II-deficient (MSR-A−/−) mice were more susceptible to infection with listeriolysin-O (LLO)-producing Listeria monocytogenes. After infection, Kupffer cells in wild-type (MSR-A+/+) mice phagocytized larger numbers of Listeria than those in MSR-A−/− mice. The number and the diameter of hepatic granulomas were larger in MSR-A−/− mice than MSR-A+/+ mice. L. monocytogenes replicated at higher levels in the liver of MSR-A−/− mice compared with MSR-A+/+ mice, and macrophages from MSR-A−/− mice showed impaired ability to kill Listeria in vitro. However, macrophages from MSR-A+/+ and MSR-A−/− mice showed similar levels of listericidal activity against isogenic mutant L. monocytogenes with an inactivated LLO gene. The listerial phagocytic activities of MSR-A+/+ macrophages treated with an anti-SR-A I/II antibody (2F8) and MSR-A−/− macrophages were significantly impaired compared with untreated MSR-A+/+ macrophages, indicating that SR-A I/II function as a receptor for L. monocytogenes. Electron microscopy revealed that most L. monocytogenes had been eliminated from the lysosomes of MSR-A+/+ macrophages in vivo and in vitro. In contrast, L. monocytogenes rapidly lysed the phagosomal membrane and escaped to the cytosol in MSR-A−/− macrophages and in MSR-A+/+ macrophages treated with 2F8 before phagosome-lysosome fusion. These findings imply that SR-A I/II plays a crucial role in host defense against listerial infection not only by functioning as a receptor but also by mediating listericidal mechanisms through the regulation of LLO-dependent listerial escape from the macrophages.
Macrophage scavenger receptors are implicated in the deposition of cholesterol in arterial walls during atherogenesis through receptor-mediated endocytosis of chemically modified low density lipoproteins (LDL). 1-6 Because of the broad ligand-binding capacity of these receptors, they have a wide spectrum of biological roles in not only atherogenesis but also host defense against pathogens as well as the removal and clearance of various arrays of negatively charged macromolecules. The scavenger receptors are classified into class A [type I and type II macrophage scavenger receptors (SR-AI/II), 1-6 and macrophage receptor with collagenous structure (MARCO) 7,8 )]; class B (CD36 9 and SR-BI 10,11 ); class C (dSR-CI 12,13 ); class D (CD68/macrosialin 14,15 ); class E [(lectin-like oxidized low-density lipoprotein receptor 1 (LOX-1) 16 ]; class F [scavenger receptor expressed by endothelial cells (SREC) 17 ]; and Fc receptors (Fc γ RII-B2). 18 SR-AI/II is a trimeric glycoprotein expressed in macrophages in various tissues and binds to a precursor of gram-negative bacterial lipid A 19,20 and lipoteichoic acid of gram-positive bacteria. 21 Recently it was shown that SR-AI/II-knockout (MSR-A−/−) mice were more susceptible than wild-type mice to Listeria monocytogenes, herpes simplex virus, and malaria infections, 22-24 indicating important roles for SR-AI/II in host defense. Recent reports have demonstrated that MSR-A-deficient macrophages are defective in the uptake ability of dead and live bacteria. 25,26 However, the precise role of SR-AI/II in bactericidal mechanism has been little investigated.
L. monocytogenes is a facultative, intracellular gram-positive bacterium responsible for severe infections in newborn, aged, and immunocompromised individuals, 27,28 that has been used as a model to study cell-mediated immunity. 29-31 In murine infections, the bacteria accumulate predominantly in the liver and replicate until the host develops an acquired cellular immune response. 27,28,32 A key aspect of the pathogenicity of L. monocytogenes is its ability to invade and multiply in macrophages. 27,28 Although most Listeria are killed during the first 6 hours after primary infection, some of them lyse the host vacuole membrane, and escape from phagosomes into the host cytoplasm where the bacteria multiply and spread from cell to cell. 32,33 A critical factor implicated in the escape of Listeria from phagosomes is listeriolysin O (LLO), a sulfhydryl-activated pore-forming hemolysin secreted by Listeria. 34-36 It has been reported that mutants lacking LLO remained in vacuoles without proliferating. 35,36 However, the mechanism controlling this process has not been fully elucidated. In this study, we compared susceptibility of MSR-A−/− and MSR-A+/+ mice to L. monocytogenes. Phagocytic and listericidal activity of macrophages obtained from MSR-A+/+ and MSR-A−/− mice was also investigated using LLO-producing and -nonproducing strains.
Materials and Methods
Animals
MSR-A−/− mice were generated by disrupting exon 4 of the MSR-A gene 22 and maintained under standard conditions at the Laboratory Animal Center, Niigata University School of Medicine. Eight-week-old male mice were used in the experiments and were killed by ether anesthesia. The liver was removed at various times after intravenous injection with L. monocytogenes for the preparation of tissue sections and enumeration of bacteria.
Microorganisms and Infection of Mice
Virulent L. monocytogenes, strain EGD, was used in all experiments. Bacterial virulence was maintained by serial passage in BALB/c mice. Fresh isolates were obtained from infected spleens, grown in tryptic soy broth (Difco Laboratories, Detroit, MI), washed repeatedly, resuspended in sterile phosphate-buffered saline (PBS), and then stored at −80°C in small aliquots. Mice were inoculated intravenously with L. monocytogenes at various doses. For the observation of granuloma formation, we injected intravenously 1 × 10 4 colony-forming units (CFUs) of Listeria, a dose not lethal to either MSR-A−/− or MSR-A+/+ mice. An isogenic LLO-defective mutant, L. monocytogenes EGD hlyA::pLSV2, was obtained from Eva Ng (University of Wurzburg, Wurzburg, Germany). This mutant has been constructed by insertional inactivation of gene encoding LLO (hlyA) by using a plasmid integration into L. monocytogenes EGD. 37 This avirulent strain was also used in the in vitro experiments.
Evaluation of Listerial Growth by Determination of CFU Counts
The number of viable bacteria in the inoculum, homogenates of the liver and spleen, and infected cells was determined by plating 10-fold serial dilutions on brain-heart infusion agar (Difco Laboratories) plates. The numbers of CFUs were counted after incubation for 24 hours at 37°C.
Antibodies
The rat monoclonal antibodies F4/80 and Mac-1 (BMA Biomedicals, Augst, Switzerland) were used at a dilution of 1:100. F4/80 recognizes mature tissue macrophages and monocytes. 38 Mac-1 recognizes exudate macrophages and neutrophils. 39,40 The rat monoclonal antibody for SR-AI/II, 2F8, 41 was kindly provided by Prof. S. Gordon (Oxford University).
Histology and Histochemistry
Tissues were fixed in 10% phosphate-buffered formalin and processed routinely for paraffin sections. Five-μm-thick sections were prepared, deparaffinized, and hydrated before incubation with the staining solution. Sections were stained with hematoxylin and eosin. For detecting bacilli, Gram staining was performed. Neutrophils were stained by the 3-hydroxy-2-naphtholic acid o-toluidide-chloroacetate esterase method. 42
Immunohistochemistry
The livers were fixed for 4 hours at 4°C in periodate-lysine-paraformaldehyde, washed for 4 hours with PBS containing 10%, 15%, and 20% sucrose, and embedded in OCT compound (Miles, Elkhart, IN). These tissue specimens were frozen in dry-ice-acetone and cut by a cryostat (Bright, Huntington, UK) into 6-μm-thick sections. After inhibition of endogenous peroxidase activity by the method of Isobe and colleagues, 43 we performed immunohistochemistry using the monoclonal antibodies described above. As a secondary antibody, we used anti-rat Ig-horseradish peroxidase-linked F(ab)2 fragment (Amersham, Poole, UK). After visualization with 3,3′-diaminobenzidine (Dojin Chemical Co., Kumamoto, Japan), and nuclear staining with methylene green, the sections were mounted with resin.
Electron Microscopy, Ultrastructural Acid Phosphatase Cytochemistry, and Immunoelectron Microscopy
The liver tissues and cultured macrophages were fixed with 1.5% glutaraldehyde, postfixed with 1% osmium tetroxide (OsO4), dehydrated in a graded ethanol series, and embedded in Epok. Ultrathin sections were observed under an electron microscope (H-800; Hitachi, Tokyo, Japan) after staining with lead citrate.
For visualizing lysosomes in macrophages, ultrastructural acid phosphatase staining was performed. Infected cells were washed in Hanks’ balanced salt solution (Sigma Chemical, Co., St. Louis, MO), fixed for 30 minutes at 4°C with 1.0% glutaraldehyde in cold sodium-cacodylate buffer (SCB) (0.1 mol/L sodium cacodylate, 0.25 mol/L sucrose, pH 7.4), and then washed again with SCB. Washing was followed by two 30-minute incubations in acid-phosphatase reaction buffer (0.1 mol/L sodium acetate, 1 mmol/L glyceroacetate, 2 mmol/L CeCl3), pH 5.2, at 37°C with gentle shaking. Thereafter, the cells were rinsed three times with the acid-phosphatase reaction buffer, and refixed in 3% glutaraldehyde in SCB for 1 hour at 4°C. After three more washes in SCB, monolayers were postfixed with 1% OsO4 in cold SCB. The cells then were washed, dehydrated, and processed for electron microscopy.
For identifying the location of SR-AI/II, macrophages were fixed with periodate-lysine-paraformaldehyde, washed in 0.05 mol/L cacodylate buffer, and incubated with 2F8 as reported. 5
Listericidal Assay
Resident peritoneal cells were washed from the peritoneal cavity of mice by lavage with 10 ml of cold sterile PBS. Peritoneal exudate cells were elicited by intraperitoneal injection of 2 ml of Brewer’s Thioglycollate Medium (Difco Laboratories, Sparks, MD). Cells were harvested by peritoneal lavage 48 hours after stimulation and centrifuged (1,000 rpm, 10 minutes). After a wash, peritoneal macrophages were resuspended in RPMI 1640 (Flow Laboratories, Inc., MacLean, VA) containing heat-inactivated 10% (v/v) fetal calf serum and the cells were counted. Cells (1 × 106/ml) were plated on 35-mm tissue-culture Petri dishes (Corning Costar Corp., Cambridge, MA) and then incubated for 2 hours at 37°C in 5% CO2 in air. For characterization of the peritoneal exudate cells, ultrastructural peroxidase cytochemistry 44,45 and immunoelectron microscopy were performed on harvested cells as described above before infection. L. monocytogenes were added to a dish and incubated for 30 minutes at 37°C in 5% CO2 in air. Listeria macrophage ratios were set at 10:1 46 for evaluation of listericidal activity, and at 100:1 and 200:1 for listerial uptake ability. For determination of listerial counts, dishes were washed five times with 4°C PBS and deposited into sterile distilled water. After mixing vigorously for 15 seconds to lyse the infected cells, 10-fold serial dilutions were plated on brain-heart infusion agar to enumerate the number of the bacteria from 0 to 4 hours after infection. At 30 minutes after infection, gentamicin sulfate was added to kill extracellular Listeria without affecting the viability of intracellular bacteria. 47,48 To test the involvement of SR-AI/II in the listerial uptake, peritoneal exudate cells of MSR-A+/+ mice were pretreated by 2F8 in some experiments. Electron microscopy and listerial multiplication as described above were performed at given time points from 30 minutes to 4 hours after infection. The monolayers of cells were also processed for electron microscopy as described above.
Statistics
For the analysis of survival rate, the Wilcoxon method was used. The significance of the other data were evaluated by Student’s t-test.
Results
Decreased Survival of MSR-A−/− Mice after L. monocytogenes Infection
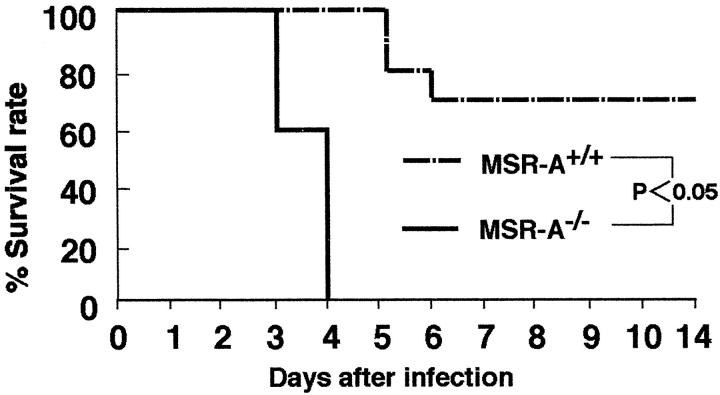
The survival rates for MSR-A−/− mice after injection of 1 × 10 5 CFU of Listeria were significantly lower than those for wild-type mice (Figure 1) ▶ .
Figure 1.
The survival rate of MSR-A+/+ and MSR-A−/− mice injected intravenously with 1 × 10 5 CFU of L. monocytogenes per mouse. Each genotype consisted of 10 mice. Their survival was assessed daily for 14 days.
Increased Hepatic Granuloma Formation in MSR-A−/− Mice
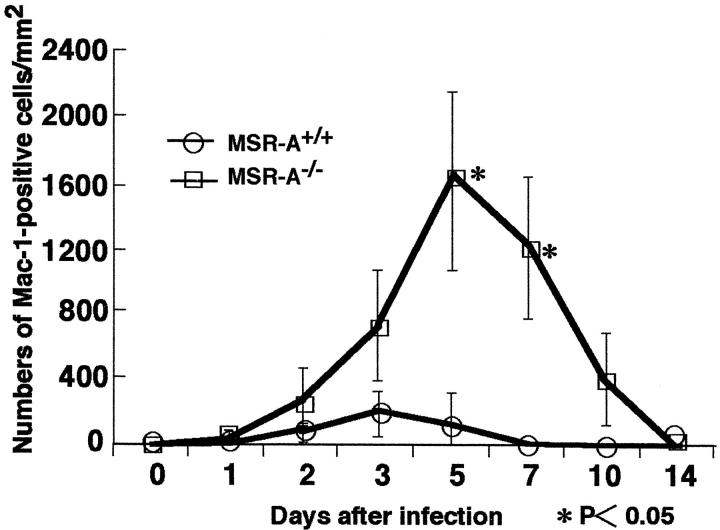
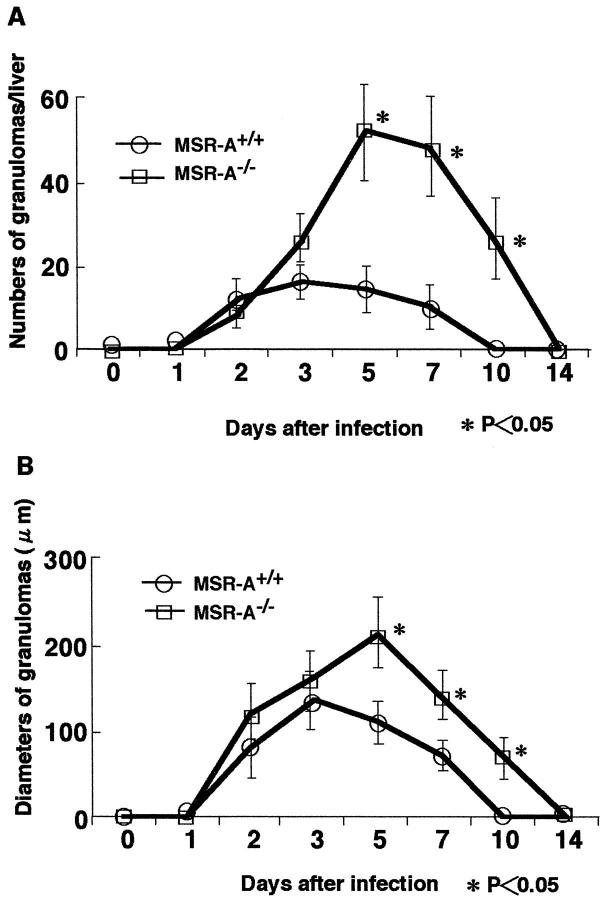
In both MSR-A−/− and MSR-A+/+ mice injected intravenously with 1 × 10 4 CFU of L. monocytogenes, abscesses started to form in the liver from 2 days, and these changed into granulomas at 3 days. In MSR-A+/+ mice, SR-AI/II was expressed in Kupffer cells, sinusoidal endothelial cells, and macrophages in the granulomas (Figure 2A) ▶ . In contrast, SR-AI/II was not expressed in any cell types in MSR-A−/− mice (Figure 2B) ▶ . Significantly larger numbers of Mac-1-positive cells were accumulated in the granulomas of MSR-A−/− than MSR-A+/+ mice (Figure 2, C and D ▶ , and Figure 3 ▶ ). The numbers of granulomas in MSR-A+/+ mice peaked at 3 days and decreased thereafter. In MSR-A−/− mice, however, the numbers of granulomas increased remarkably up to day 5. At 7 and 10 days, significantly more granulomas remained in the liver of MSR-A−/− than MSR-A+/+ mice (Figure 4A) ▶ . Granulomas in MSR-A+/+ mice had disappeared by day 10. In MSR-A−/− mice, granulomas were still found at day 10 (Figure 4A) ▶ . The mean diameters of granulomas of MSR-A−/− mice were significantly larger than those of MSR-A+/+ mice (Figure 4B) ▶ .
Figure 2.
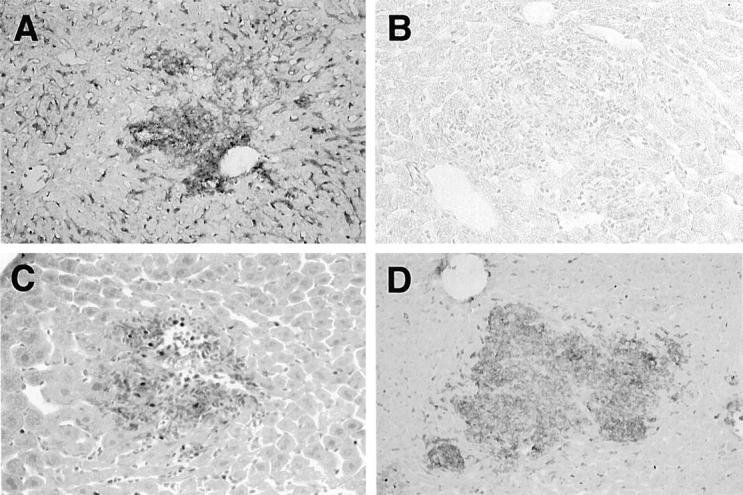
Immunohistochemistry of Listeria-infected livers of MSR-A+/+ and MSR-A−/− mice after intravenous injection of 1 × 10 4 CFU of L. monocytogenes. A: SR-AI/II is expressed in Kupffer cells, sinusoidal endothelial cells, and macrophages in the granulomas of MSR-A+/+ mice at 3 days after infection. Immunohistochemical staining using 2F8; original magnification, ×200. B: SR-AI/II is not expressed in any cell types in MSR-A−/− mice at 3 days after infection. Immunohistochemical staining using 2F8; original magnification, ×200. C: Mac-1-positive cells appear in granulomas of MSR-A+/+ mice at 3 days after infection. Immunohistochemical staining using Mac-1; original magnification, ×400. D: Abundant Mac-1-positive cells accumulate in large granulomas of MSR-A−/− mice at 3 days after infection. Immunohistochemical staining using Mac-1; original magnification, ×400.
Figure 3.
Changes in the numbers of Mac-1-positive cells in the granulomas of MSR-A+/+ and MSR-A−/− mice after intravenous injection of 1 × 10 4 CFU of L. monocytogenes. Data are expressed as the mean ± SD of five mice. *, P < 0.05.
Figure 4.
Changes in the numbers (A) and mean diameters (B) of granulomas in livers of MSR-A+/+ and MSR-A−/− mice after intravenous injection of 1 × 10 4 CFU of L. monocytogenes. Data are expressed as the mean ± SD for five mice. *, P < 0.05.
Decreased Listerial Phagocytosis by Kupffer Cells in MSR-A−/− Mice
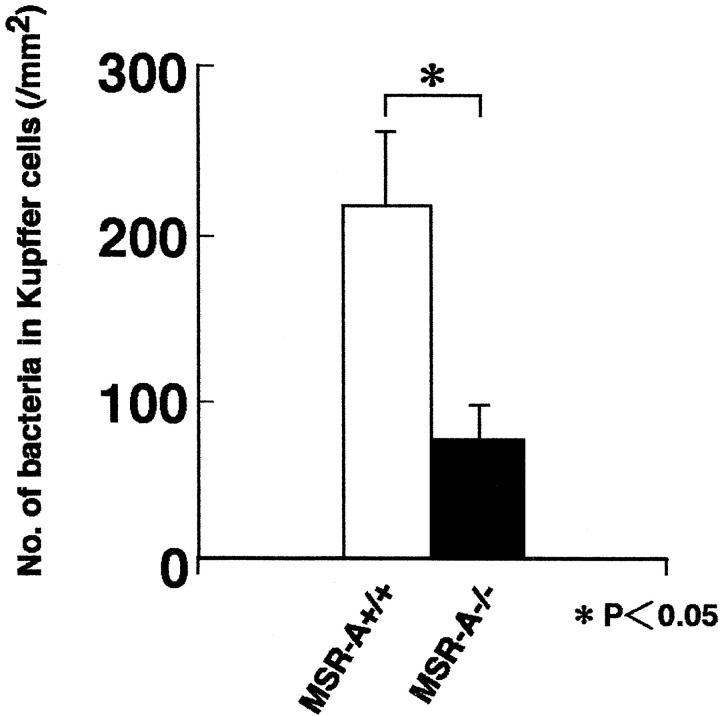
At 30 minutes after infection, several Gram-positive rods were frequently present in Kupffer cells of MSR-A+/+ mice (Figure 5A and 6) ▶ ▶ . In MSR-A−/− mice, however, only a few bacilli were detected in Kupffer cells (Figures 5B and 6) ▶ ▶ . At 3 and 5 days, a few bacteria were detected in granulomas of MSR-A+/+ mice (Figure 5E) ▶ . In contrast, a large number of bacilli were observed in the granulomas of MSR-A−/− mice (Figure 5F) ▶ . Electron microscopy revealed that there was a remarkable difference in the fate of Listeria in Kupffer cells between MSR-A+/+ and MSR-A−/− mice. In MSR-A+/+ mice, the majority of bacteria had been incorporated into lysosomes of Kupffer cells and macrophages in the granulomas (Figure 5, C and G) ▶ . In contrast, most of the bacteria were observed in single forms in MSR-A−/− mice, and endosomal membranes were often unclear (Figure 5, D and H) ▶ .
Figure 5.
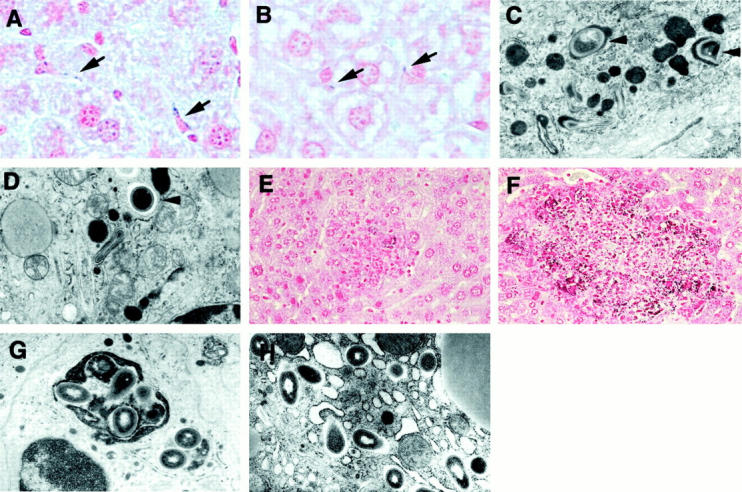
Gram staining and electron micrographs of Listeria-infected livers of MSR-A+/+ and MSR-A−/− mice after intravenous injection of 1 × 10 4 CFU of L. monocytogenes. A: Several Listeria are phagocytosed by Kupffer cells in MSR-A+/+ mice at 30 minutes after infection. Gram staining; original magnification, ×1,000. B: In MSR-A−/− mice, only a few bacilli are detected in Kupffer cells at 30 minutes after infection. Gram staining; original magnification, ×1,000. C: Most Listeria are incorporated into lysosomes (arrowheads) at 30 minutes after infection in Kupffer cells of MSR-A+/+ mice. Electron microscopy; original magnification, ×10,000. D: A few bacteria are present in Kupffer cells of MSR-A−/− mice at 30 minutes after infection. Some bacteria are free in the cytoplasm (arrowhead). Electron microscopy; original magnification, ×10,000. E: The bacteria are observed sparsely in the granulomas of MSR-A+/+ mice at 5 days after infection. Gram staining; original magnification, ×400. F: Numerous bacteria are observed in the granulomas of MSR-A−/− mice at 5 days after infection. Gram staining; original magnification, ×400. G: Most Listeria are confined within large phagolysosomes in macrophages of MSR-A+/+ mice at 5 days after infection. Electron microscopy; original magnification, ×10,000. H: In the macrophages of MSR-A−/− mice, many bacilli are located free in the cytoplasm at 5 days after infection. Electron microscopy; original magnification, ×10,000.
Figure 6.
The numbers of L. monocytogenes in Kupffer cells of MSR+/+ and MSR−/− mice at 20 minutes after intravenous injection of 1 × 10 4 CFU of L. monocytogenes. *, P < 0.05.
Enhanced Multiplication of L. monocytogenes in the Liver of MSR-A−/− Mice
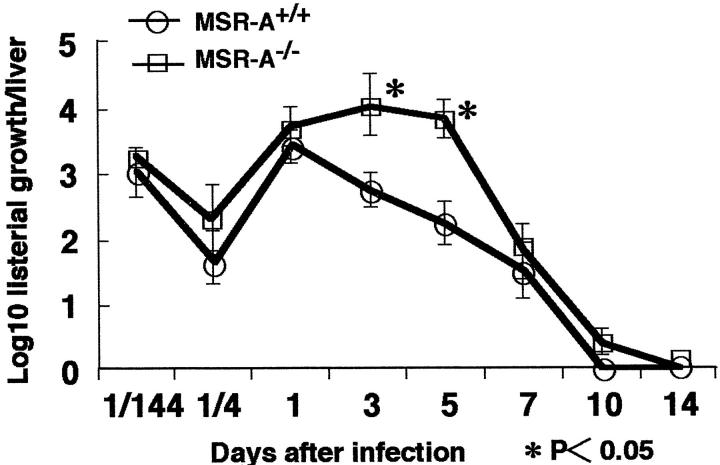
The multiplication of L. monocytogenes in liver is shown in Figure 7 ▶ . The Listeria proliferated more in the liver of MSR-A−/− than MSR-A+/+ mice and the numbers of CFU in the liver of MSR-A−/− mice significantly increased from 3 to 5 days after infection. These results were consistent with the results of Gram staining, and indicated that listericidal ability was impaired in MSR-A−/− mice compared with that in MSR-A+/+ mice. The numbers of Listeria in the spleen did not differ significantly between the genotypes of mice (data not shown).
Figure 7.
Proliferation of L. monocytogenes in livers of MSR-A+/+ and MSR-A−/− mice after intravenous injection of 1 × 10 4 CFU of L. monocytogenes. Data are expressed as the mean ± SD of five mice. *, P < 0.05.
Decreased Phagocytosis of L. monocytogenes by MSR-A−/− Macrophages in Vitro
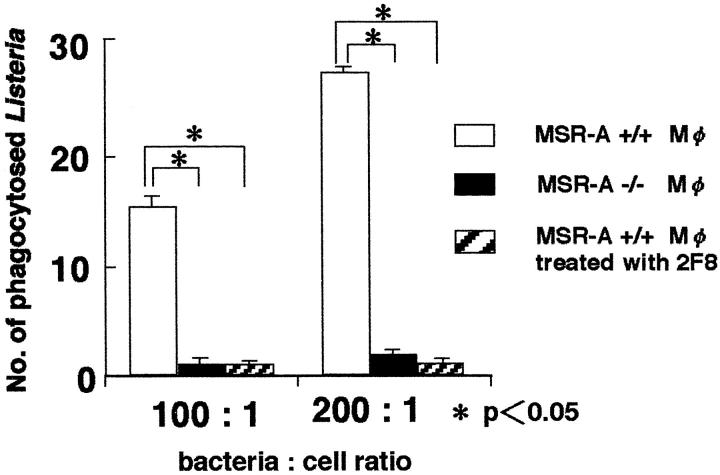
The numbers of phagocytosed Listeria of MSR-A−/− exudate peritoneal macrophages at 20 minutes after infection were significantly smaller than those of MSR-A+/+ macrophages (Figure 8) ▶ . In addition, 2F8, 41 a monoclonal antibody for murine SR-AI/II, markedly inhibited the listerial uptake of MSR-A+/+ macrophages (Figure 8) ▶ . These findings indicate that SR-AI/II functions as a crucial receptor for the uptake of L. monocytogenes.
Figure 8.
The numbers of phagocytosed L. monocytogenes per cell of MSR-A+/+, MSR-A−/−, and 2F8-treated MSR-A+/+ peritoneal macrophages at 20 minutes after infection in vitro. The Listeria cell ratios were set at 100:1 (1 × 10 7 Listeria for 1 × 10 5 cells), and 200:1 (2 × 10 7 Listeria for 1 × 10 5 cells). Data are the mean for 100 cells in each group. *, P < 0.05.
Resident macrophages of both types of mice phagocytosed smaller numbers of Listeria than exudate macrophages, indicating that the listerial uptake of exudate macrophages is more active than resident macrophages (data not shown).
Decreased Killing of LLO-Producing L. monocytogenes by Macrophages from MSR-A−/− Mice
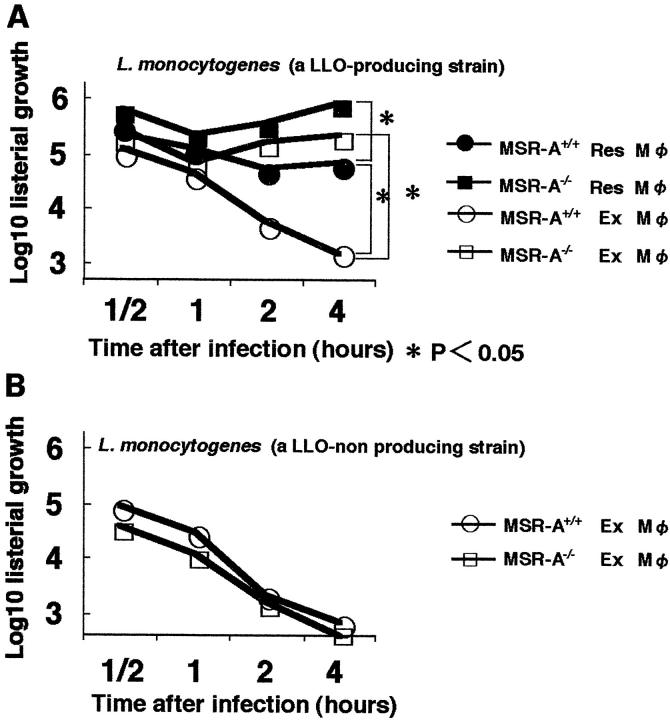
The multiplication of the EGD strain of L. monocytogenes in peritoneal macrophages was evaluated by determining CFUs (Figure 9A) ▶ . The decreased number of bacterial colonies with time indicates bactericidal activity of macrophages. The listericidal capacity of MSR-A−/− macrophages was more impaired than that of MSR-A+/+ macrophages in both resident and exudate peritoneal macrophages. The listericidal activity of resident macrophages was impaired more in MSR-A−/− mice. MSR-A+/+ exudate macrophages efficiently suppressed the growth and continued to exhibit listericidal activity during the 4-hour experiment.
Figure 9.
Growth of the bacteria in vitro. Listeria cell ratio was set at 10:1 (1 × 10 6 Listeria for 1 × 10 5 cells). A: Growth of a LLO-producing strain of L. monocytogenes in resident and exudate peritoneal macrophages obtained from MSR-A+/+ and MSR-A−/− mice. Data are the mean of three experiments. *, P < 0.05. B: Growth of a LLO-nonproducing mutant of L. monocytogenes in MSR-A+/+ and MSR-A−/− exudate peritoneal macrophages. Data are the mean for three experiments. Res, resident; Ex, exudate.
The replication of LLO-nonproducing isogenic mutant in exudate peritoneal macrophages was also examined. There was no significant difference in listericidal activity between MSR-A−/− and MSR-A+/+ exudate peritoneal macrophages (Figure 9B) ▶ , indicating that LLO is one of the molecules responsible for the difference in listericidal capacity between MSR-A−/− and MSR-A+/+ mice.
Rapid Escape of Listeria from the Phagosomes of MSR-A−/− Macrophages
Electron microscopically, Listeria were rapidly phagocytosed by MSR-A+/+ and MSR-A−/− exudate macrophages in vitro (Figure 10, A and B) ▶ . At 30 minutes after infection, most of the Listeria had been incorporated into large endosomes of MSR-A+/+ exudate macrophages (Figure 10, C and E) ▶ . In contrast, most of the bacteria were observed in single forms at 30 minutes after infection in MSR-A−/− exudate macrophages, and a few bacteria were surrounded by an endosomal membrane (Figure 10, D and F) ▶ . Some of the Listeria in MSR-A−/− macrophages were in the process of dividing (Figure 10F) ▶ . These features of listerial behavior in macrophages in vitro were quite similar to those in vivo.
Figure 10.
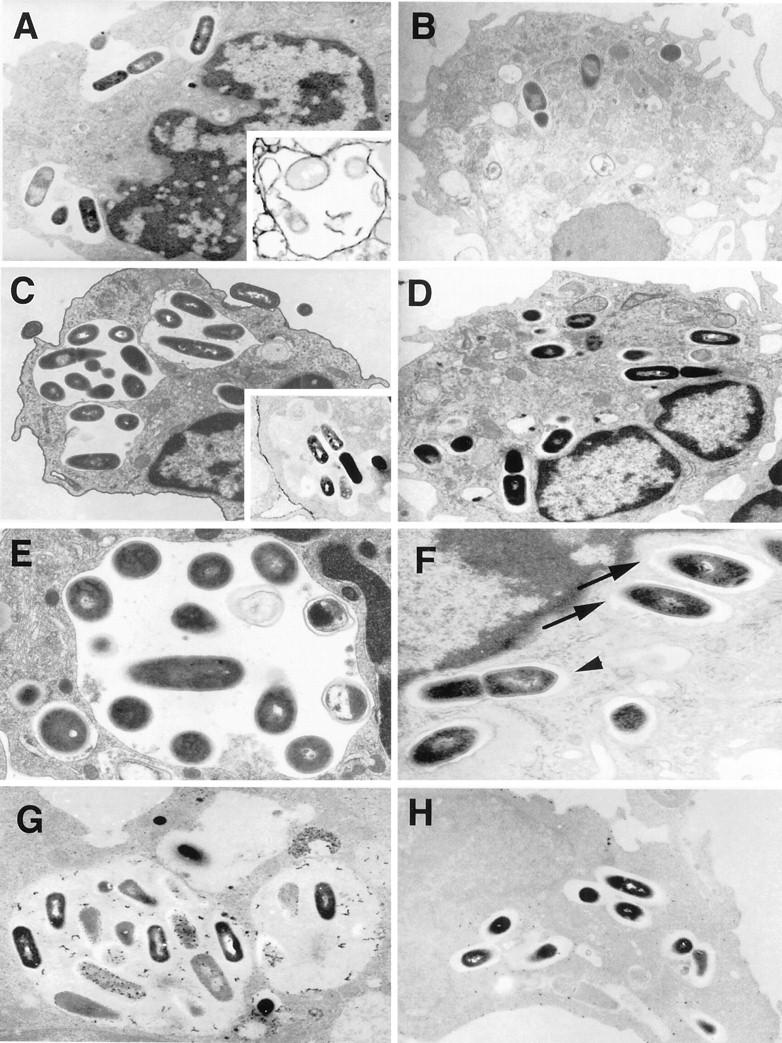
Electron micrographs of Listeria-infected macrophages. Listeria cell ratio was set at 10:1 (1 × 10 6 Listeria for 1 × 10 5 cells). A: Listeria are phagocytosed and incorporated in phagosomes of MSR-A+/+ macrophages at 20 minutes after infection. Original magnification, ×5,000. Inset: 2F8 is expressed on the phagosomal membrane. Immunoelectron microscopy using 2F8; original magnification, ×4,000. B: In MSR-A−/− macrophages, the majority of Listeria are present in single form at 20 minutes after infection. Original magnification, ×4,000. C: Most Listeria are confined within large vacuoles (phagolysosomes) at 30 minutes after infection in MSR-A+/+ macrophages. Original magnification, ×5,000. Inset: 2F8 is not expressed on the phagosomal membrane. Immunoelectron microscopy using 2F8; original magnification, ×4,000. D: Most Listeria are remaining in single form, and no large phagosomes are found at 30 minutes after infection in MSR-A−/− macrophages; original magnification, ×4,000. E: Vacuolar membranes encircle Listeria in MSR-A+/+ macrophages, and several Listeria are degraded at 30 minutes after infection. Original magnification, ×10,000. F: In MSR-A−/− macrophages, the vacuolar membranes are partially disrupted (arrows). A division of Listeria in the cytoplasm (arrowhead). Original magnification, ×20,000. G: In MSR-A+/+ macrophages, phagosomes are fused with lysosomes, and most Listeria are undergoing degradation at 1 hour after infection. Acid phosphatase staining; original magnification, ×4,000. H: Few phagosomes of MSR-A−/− macrophages have fused with lysosomes at 1 hour after infection. Acid phosphatase staining; original magnification, ×4,000.
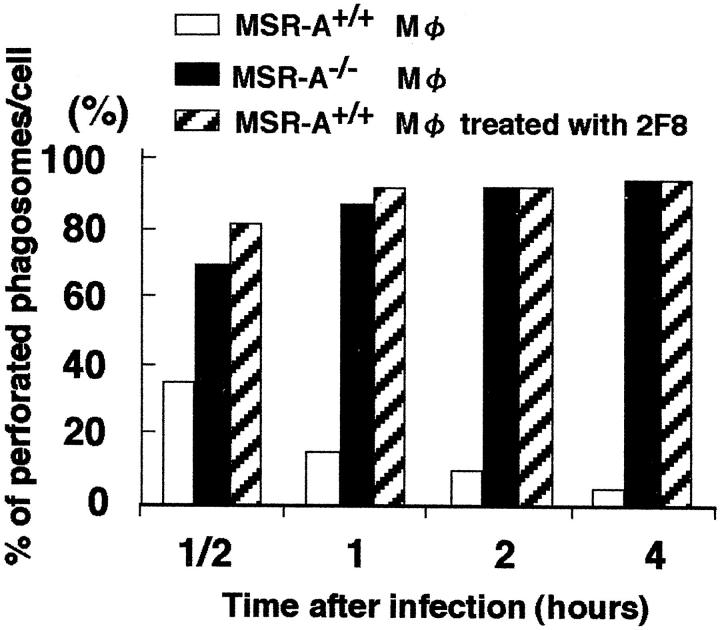
A striking difference was that approximately 90% of the phagosomes were perforated in MSR-A−/− exudate macrophages at 1 hour after infection, compared to <10% in MSR-A+/+ exudate macrophages (Figure 11) ▶ . Anti-SR-AI/II antibody also enhanced the numbers of perforated phagosomes in MSR-A+/+ exudate macrophages (Figure 11) ▶ .
Figure 11.
Changes in the proportion of perforated phagosomes in MSR-A+/+, MSR-A−/−, and 2F8-treated MSR-A+/+ peritoneal macrophages in vitro. The Listeria cell ratio was set at 10:1 (1 × 10 6 Listeria for 1 × 10 5 cells). Data are the mean for 100 cells in each group.
Ultrastructural acid phosphatase staining for lysosomes revealed that most of the Listeria-bearing endosomes fused with lysosomes in MSR-A+/+ macrophages and many bacteria were undergoing degradation at 1 hour (Figure 10G) ▶ . In contrast, few lysosomes fused with phagosomes in MSR-A−/− exudate macrophages (Figure 10H) ▶ . In MSR-A+/+ resident macrophages, approximately half of the phagocytosed L. monocytogenes were eliminated in the same manner as in MSR-A+/+ exudate macrophages, but the residual Listeria were viable in nonperforated endosomes, supporting the fact that the listericidal activity of MSR-A+/+ resident macrophages was lower than that of MSR-A+/+ exudate macrophages (Figure 9A) ▶ . These results imply that, in MSR-A−/− macrophages, especially MSR-A−/− exudate macrophages, Listeria rapidly escape from their phagosomes into cytoplasm before phagosome-lysosome fusion. The efficient replication of Listeria in macrophages reflects an impaired listericidal ability in MSR-A−/− mice.
Immunoelectron microscopy revealed that SR-AI/II was expressed continuously on the cell membranes of MSR-A+/+ macrophages as reported previously. 5 Immediately after bacterial phagocytosis, SR-AI/II was expressed on the endosomal membrane (Figure 10A ▶ , inset). However, no SR-AI/II expression was observed on the phagolysosomal membrane at 30 minutes after infection (Figure 10C ▶ , inset), suggesting the dissociation of SR-AI/II and bacteria before phagosome-lysosome fusion as reported previously. 49 Expression of SR-AI/II was not detected in any organelles of MSR-A−/− macrophages.
Discussion
The impaired capacity of macrophages from MSR-A−/− mice to phagocytose Listeria, the increased susceptibility of MSR-A−/− versus MSR-A+/+ mice to lethal challenge with Listeria, prolonged and remarkable granulomatous inflammation in the liver of MSR-A−/− mice, and the impaired bactericidal activity in MSR-A−/− macrophages provide the direct evidence that SR-AI/II plays an essential role in host defense against Listeria by promoting phagocytosis and killing of the microorganisms. The impaired listericidal activity of macrophages from MSR-A−/− mice was attributed to the rapid escape of Listeria from the phagosomes into the host macrophage cytoplasm. LLO is in part responsible for the evasion.
Granulomas are part of host defense against pathogens including L. monocytogenes. The numbers of Mac-1-positive exudate macrophages 39,40 increased remarkably during the granuloma formation in MSR-A−/− mice. Therefore, we used thioglycollate-elicited exudate peritoneal macrophages for in vitro analysis and demonstrated the strong activity of elimination of Listeria by exudate macrophages, compared with that of resident macrophages. Hence, the impaired listericidal activity of MSR-A−/− exudate macrophages may chiefly account for the prominent granuloma formation in MSR-A−/− mice.
It has been reported that Kupffer cells are mainly responsible for the initial elimination of Listeria at the early stage of infection. 32 Several molecules, such as complement receptor type 3 (CR3), 50,51 and the receptor of internalin A (InlA) 52 mediate the uptake of Listeria. Previous reports have demonstrated a crucial role of SR-AI/II for the uptake of not only dead bacteria, 25 but live bacteria. 26 Thomas and colleagues 26 demonstrated that both opsonin-dependent and SR-AI/II mediated opsonin-independent phagocytosis of Gram-positive bacteria play an important role in host defense against bacterial infections. The SR-AI has been reported to recognize lipoteichoic acid and bind to Gram-positive bacteria including L. monocytogenes in vitro. 21 In the present in vitro study, listerial uptake activities of MSR-A−/− peritoneal macrophages and 2F8-treated MSR-A+/+ macrophages were more impaired than those of MSR-A+/+ macrophages. Furthermore, in the present in vivo study, initial uptake of Listeria by Kupffer cells were defective in MSR-A−/− mice. These findings clearly imply that SR-AI/II mediate the phagocytosis of L. monocytogenes.
The mechanism underlying the role of SR-AI/II in host defense against infections with intracellular pathogens is unclear. It is speculated that the impaired uptake of L. monocytogenes in MSR-A−/− macrophages results in decreased clearance of bacteria from the site of infection and extensive bacterial dissemination in various tissues. This is supported by the present study that demonstrated defective listericidal activity of MSR-A−/− macrophages. Decreased listericidal activity and increased listerial proliferation in MSR-A−/− macrophages may have resulted in recruitment of larger numbers of phagocytes and other immune cells, more remarkable formation of granulomas, and higher susceptibility to listerial infection in MSR-A−/− mice. This is the first evidence that SR-AI/II plays a critical role in the listericidal processes.
Which mechanism is responsible for the decreased listericidal capacity in MSR-A−/− macrophages? The present electron microscopy in vivo and in vitro demonstrated a significantly higher proportion of perforated Listeria-bearing phagosomes in MSR-A−/− macrophages than in MSR-A+/+ macrophages. Ultrastructural acid-phosphatase staining revealed that few perforated phagosomes of MSR-A−/− macrophages fused with lysosomes, whereas phagosome-lysosome fusion occurred frequently in MSR-A+/+ macrophages after infection. The importance of LLO for listerial escape was confirmed in the experiment using a LLO-nonproducing mutant strain. These findings suggest that Listeria use LLO efficiently to escape from the phagosomes before phagosome-lysosome fusion in MSR-A−/− macrophages. It has been shown that a key step to regulate the LLO activation is phagosomal acidification in macrophages. Vacuolar membrane perforation by LLO occurs frequently at acidic vacuolar pH, with a mean near 6.0. 53 On the other hand, phagosome-lysosome fusions require a more acidic pH, near 5.0 or less. 54,55 Our preliminary study demonstrated that bafilomycin A1, a proton pump inhibitor, 56,57 suppressed both the listerial multiplication and the perforation of the Listeria-containing phagosomes of MSR-A−/− macrophages (data not shown), suggesting that SR-AI/II may be involved in the mechanism of phagosomal acidification. Further studies are necessary to clarify the SR-AI/II-mediated listericidal mechanism in macrophages.
In conclusion, SR-AI/II functions positively in host defense against listerial infection not only by functioning as a receptor but also by mediating listericidal mechanisms through the regulation of LLO-dependent listerial escape.
Acknowledgments
We thank Mr. K. Sato, S. Momozaki, H. Sano, K. Ohyachi, Ms. M. Saito, and Ms. K. Moriki for their excellent technical assistance; and Dr. I. Fraser, Dr. D. A. Hughes, and Prof. S. Gordon, Oxford University, for the 2F8 monoclonal antibody.
Footnotes
Address reprint requests to Makoto Naito, M.D., Second Department of Pathology, Niigata University School of Medicine, Asahimachi-dori 1, Niigata 951-8510, Japan. E-mail: 2byori@med.niigata-u.ac.jp.
Supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan, and Japan Foundation Grant for Aging and Health.
References
- 1.Kodama T, Freeman M, Rohrer L, Zabrewky J, Matsudaira P, Krieger M: Type I macrophage scavenger receptor contains α-helical and coiled-like coils. Nature 1990, 343:531-535 [DOI] [PubMed] [Google Scholar]
- 2.Rohrer L, Freeman M, Kodama T, Penman M, Krieger M: Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature 1990, 343:570-572 [DOI] [PubMed] [Google Scholar]
- 3.Freeman M, Ashkenas J, Rees KJG, Kingsley DM, Copeland NG, Jenkins NA, Krieger M: An ancient, highly conserved family of cystein-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc Natl Acad Sci USA 1990, 87:8810-8814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto A, Naito M, Itakura H, Ikemoto S, Asaoka H, Hayakawa I, Kanamori H, Aburatani H, Takaku F, Suzuki H, Kobari Y, Miyai T, Takahashi K, Cohen EH, Wydro R, Housman DE, Kodama T: Human macrophage scavenger receptors: primary structure, expression, and localization in atherosclerotic lesions. Proc Natl Acad Sci USA 1990, 87:9133-9137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naito M, Kodama T, Matsumoto A, Doi T, Takahashi K: Tissue distribution, intracellular localization, and in vitro expression of bovine scavenger receptors. Am J Pathol 1991, 139:1411-1423 [PMC free article] [PubMed] [Google Scholar]
- 6.Mato M, Ookawara S, Sakamoto A, Aikawa E, Ogawa T, Mitsuhashi U, Masuzawa T, Suzuki H, Honda M, Yazaki Y, Watanabe E, Luoma J, Yla-Herttuala S, Fraser I, Gordon S, Kodama T: Involvement of specific macrophage-lineage cells surrounding arterioles in barrier and scavenger function in brain cortex. Proc Natl Acad Sci USA 1996, 93:3269-3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elomaa O, Kangas M, Sahlberg C, Tuukkanen J, Sormunen R, Liakka A, Thesleff I, Kraal G, Tryggvason K: Cloning of a novel bacteria binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell 1995, 80:603-609 [DOI] [PubMed] [Google Scholar]
- 8.van der Laan LJW, Kangas M, Dopp ED, Bloug-Holub E, Elomaa O, Tryggvason K, Kraal G: Macrophage scavenger receptor MARCO: in vitro and in vivo regulation and involvement in anti-bacterial host defense. Immunol Lett 1997, 57:203-208 [DOI] [PubMed] [Google Scholar]
- 9.Endermann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA: CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem 1993, 268:11811-11816 [PubMed] [Google Scholar]
- 10.Acton SL, Scherer PE, Lodish HF, Krieger M: Expression cloning of SR-BI, a CD36 related class B scavenger receptor. J Biol Chem 1994, 269:21003-21009 [PubMed] [Google Scholar]
- 11.Acton S, Rigotti A, Landschuluz KT, Xu S, Hobbs HH, Krieger M: Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996, 271:518-520 [DOI] [PubMed] [Google Scholar]
- 12.Pearson A, Lux A, Krieger M: Expression cloning of dSR-CI, a class C macrophage-specific scavenger receptor from Drosophila melanogaster. Proc Natl Acad Sci USA 1995, 92:4056-4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landschulz KT, Pathak RK, Rigotti A, Krieger M, Hobbs HH: Regulation of scavenger receptor, class B, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J Clin Invest 1995, 98:984-995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramprasad MP, Fisher JL, Witztum JL, Sambrano GR, Quehenberger O, Steinberg D: The 94- to 97-kDa mouse macrophage membrane protein that recognizes oxidized low density lipoprotein and phosphatidyl serine-rich liposomes is identical to macrosialin, the mouse homologue of human CD68. Proc Natl Acad Sci USA 1995, 92:9580-9584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holness CL, da Silva RP, Fawsett J, Gordon S, Simmonds DL: Macrosialin, a mouse macrophage-restricted glycoprotein, is a member of the lamp/lgp family. J Biol Chem 1993, 268:9661-9666 [PubMed] [Google Scholar]
- 16.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T: An endothelial receptor for oxidized low-density lipoprotein. Nature 1997, 386:73-77 [DOI] [PubMed] [Google Scholar]
- 17.Adachi H, Tsujimoto M, Arai H, Inoue K: Expression cloning of a novel scavenger receptor from human endothelial cells. J Biol Chem 1997, 272:31217-31220 [DOI] [PubMed] [Google Scholar]
- 18.Stanton LW, White RT, Bryant CM, Protter AA, Endemann G: A macrophage Fc receptor for IgG is also a receptor for oxidized low density lipoprotein. J Biol Chem 1992, 267:22446-22451 [PubMed] [Google Scholar]
- 19.Hampton RY, Golenbock DT, Penman M, Krieger M, Raetz CR: Recognition and plasma clearance of endotoxin by scavenger receptors. Nature 1991, 352:342-344 [DOI] [PubMed] [Google Scholar]
- 20.Ashkenas J, Penman M, Vasile E, Freeman M, Krieger M: Structures and high and low affinity ligand binding properties of murine type I and type II macrophage scavenger receptors. J Lipid Res 1993, 34:983-1000 [PubMed] [Google Scholar]
- 21.Dunn DW, Resnick K, Greenberg J, Krieger M, Joiner KA: The type I macrophage scavenger receptor binds to gram-positive bacteria and recognizes lipoteichoic acid. Proc Natl Acad Sci USA 1994, 91:1863-1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Horiuchi S, Takahashi K, Kruijt JK, van Berkel TJC, Steinbrecher UP, Ishibashi S, Maeda N, Gordon S, Kodama T: A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 1997, 386:292-296 [DOI] [PubMed] [Google Scholar]
- 23.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Sakaguchi H, Kruijt JK, Higashi T, Suzuki T, Berkel TJC, Horiuchi S, Takahashi K, Yazaki Y, Kodama T: The multiple roles of macrophage scavenger receptors (MSR) in vivo: resistance to atherosclerosis and susceptibility to infection in MSR knockout mice. J Atheroscler Thromb 1997, 4:1-11 [DOI] [PubMed] [Google Scholar]
- 24.Nogami S, Watanabe J, Nakazaki K: Involvement of macrophage scavenger receptors in protection against murine malaria. Am J Trop Med Hyg 1998, 59:843-845 [DOI] [PubMed] [Google Scholar]
- 25.Hagiwara S, Takeya M, Suzuki H, Kodama T, van der Laan LJW, Kraal G, Kitamura N, Takahashi K: Role of macrophage scavenger receptors in hepatic granuloma formation in mice. Am J Pathol 1999, 154:705-720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas CA, Li Y, Kodama T, Suzuki H, Silverstein SC, El Khoury J: Protection from lethal gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J Exp Med 2000, 191:147-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gellin BG, Broome CV: Listeriosis. JAMA 1989, 261:1313-1320 [PubMed] [Google Scholar]
- 28.Gray ML, Killinger AH: Listeria monocytogenes and listeric infections. Bacteriol Rev 1966, 30:309-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.North RJ: The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med 1970, 132:521-534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.North RJ: Suppression of cell-mediated immunity to infection by an antimitotic drug. J Exp Med 1970, 132:535-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn H, Kaufman SHF: The role of cell mediated immunity in bacterial infections. Rev Infect Dis 1981, 3:1221-1250 [DOI] [PubMed] [Google Scholar]
- 32.Mitsuyama M, Takeya K, Nomoto K, Shimotori S: Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol 1978, 106:165-171 [DOI] [PubMed] [Google Scholar]
- 33.Tilney LG, Portnoy DA: Actin filaments and the growth, movement, and the spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol 1989, 109:1597-1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cossart P, Vincente MF, Mengaud J, Baquelo F, Perez-Diaz JC, Berche P: Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun 1989, 57:3629-3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portnoy DA, Jacks PS, Hinrichs DJ: Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med 1988, 167:1459-1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaillard JL, Berche P, Mounier J, Richard S, Sansonetti P: In vivo model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell lone Caco-2. Infect Immun 1987, 55:2822-2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wuenscher MD, Kohler S, Goebel W, Chakrabortz T: Gene disruption by plasmid integration in Listeria monocytogenes: insertional inactivation of the listeriolysin determinant lisA. Mol Gen Genet 1991, 228:177-182 [DOI] [PubMed] [Google Scholar]
- 38.Austyn JM, Gorden S: F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 1981, 11:805-815 [DOI] [PubMed] [Google Scholar]
- 39.Leenen PJM, Melis M, Slieker WAT, van Ewijk W: Murine macrophage precursor characterization. II. Monoclonal antibodies against macrophage precursor antigens. Eur J Immunol 1990, 20:27-34 [DOI] [PubMed] [Google Scholar]
- 40.Leenen PJM, de Bruijin MFTR, Voerman JSA, Campbell PA, van Ewijk W: Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods 1994, 176:5-19 [DOI] [PubMed] [Google Scholar]
- 41.Hughes DA, Fraser IP, Gordon S: Murine macrophage scavenger receptor in vivo expression and function as receptor for macrophage adhesion in lymphoid and non-lymphoid organs. Eur J Immunol 1995, 25:466-473 [DOI] [PubMed] [Google Scholar]
- 42.Maloney WC, McPherson K, Fliegelman L: Esterase activity in leukocytes demonstrated by the use of naphthol AS-D chloroacetate substrate. J Histochem Cytochem 1960, 8:200-207 [DOI] [PubMed] [Google Scholar]
- 43.Isobe Y, Chen ST, Nakane PK, Brown WR: Studies on translocation of immunoglobulins across intestinal epithelium. I. Improvements in the peroxidase-labeled antibody method for application to study of human intestinal mucosa. Acta Histochem Cytochem 1977, 10:161-171 [Google Scholar]
- 44.Wisse E: Observation on the fine structure and peroxidase cytochemistry of normal rat liver Kupffer cells. J Ultrastruct Res 1974, 46:393-426 [DOI] [PubMed] [Google Scholar]
- 45.Graham RC, Karnovsky MJ: The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem 1966, 14:291-302 [DOI] [PubMed] [Google Scholar]
- 46.Portnoy DA, Schreiber RD, Connelly P, Tillney LG: Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J Exp Med 1989, 170:2141-2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohya S, Xiong H, Tanabe Y, Arakawa M, Mitsuyama M: Killing mechanism of Listeria monocytogenes in activated macrophages as determined by an improved assay system. J Med Microbiol 1998, 47:211-215 [DOI] [PubMed] [Google Scholar]
- 48.Ohya S, Tanabe Y, Makino M, Nomura T, Xiong H, Arakawa M, Mitsuyama M: The contribution of reactive oxygen intermediates and reactive nitrogen intermediates to listericidal mechanisms differ in macrophages activated pre- and postinfection. Infect Immun 1998, 66:4043-4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mori T, Takahashi K, Naito M, Kodama T, Hakamata H, Sakai M, Miyazaki A, Horiuchi S, Ando M: Endocytic pathway of scavenger receptors via trans-Golgi system in bovine alveolar macrophages. Lab Invest 1994, 71:409-416 [PubMed] [Google Scholar]
- 50.Drevets DA, Canono BP, Campbell PA: Listericidal and nonlistericidal mouse macrophages differ in complement receptor type 3-mediated phagocytosis of L. monocytogenes and in preventing escape of the bacteria into the cytoplasm. J Leukoc Biol 1992, 52:70-79 [DOI] [PubMed] [Google Scholar]
- 51.Drevets DA, Leenen PJM, Campbell PA: Complement receptor type 3 (CD11b/CD18) involvement is essential for killing of Listeria monocytogenes by mouse macrophages. J Immunol 1993, 151:5431-5439 [PubMed] [Google Scholar]
- 52.Sawyer RT, Drevets DA, Campbell PA, Potter TA: Internalin A can mediate phagocytosis of Listeria monocytogenes by mouse macrophage cell lines. J Leukoc Biol 1996, 60:603-610 [DOI] [PubMed] [Google Scholar]
- 53.Beauregard KE, Lee KD, Collier RJ, Swanson JA: pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J Exp Med 1997, 186:1159-1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fok AK, Ueno MS, Azada EA, Allen RD: Phagosomal acidification in Paramecium: effects on lysosomal fusion. Eur J Cell Biol 1987, 43:412-420 [PubMed] [Google Scholar]
- 55.Beaman BL, Beaman L: Nocardia species: host-parasite relationships. Clin Microbiol Rev 1994, 7:213-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bowman EJ, Siebers A, Altendorf K: Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA 1988, 85:7972-7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tapper H, Sundler R: Bafilomycin A1 inhibits lysosomal, phagosomal, and plasma membrane H(+)-ATPase and induces lysosomal enzyme secretion in macrophages. J Cell Physiol 1995, 163:137-144 [DOI] [PubMed] [Google Scholar]