Abstract
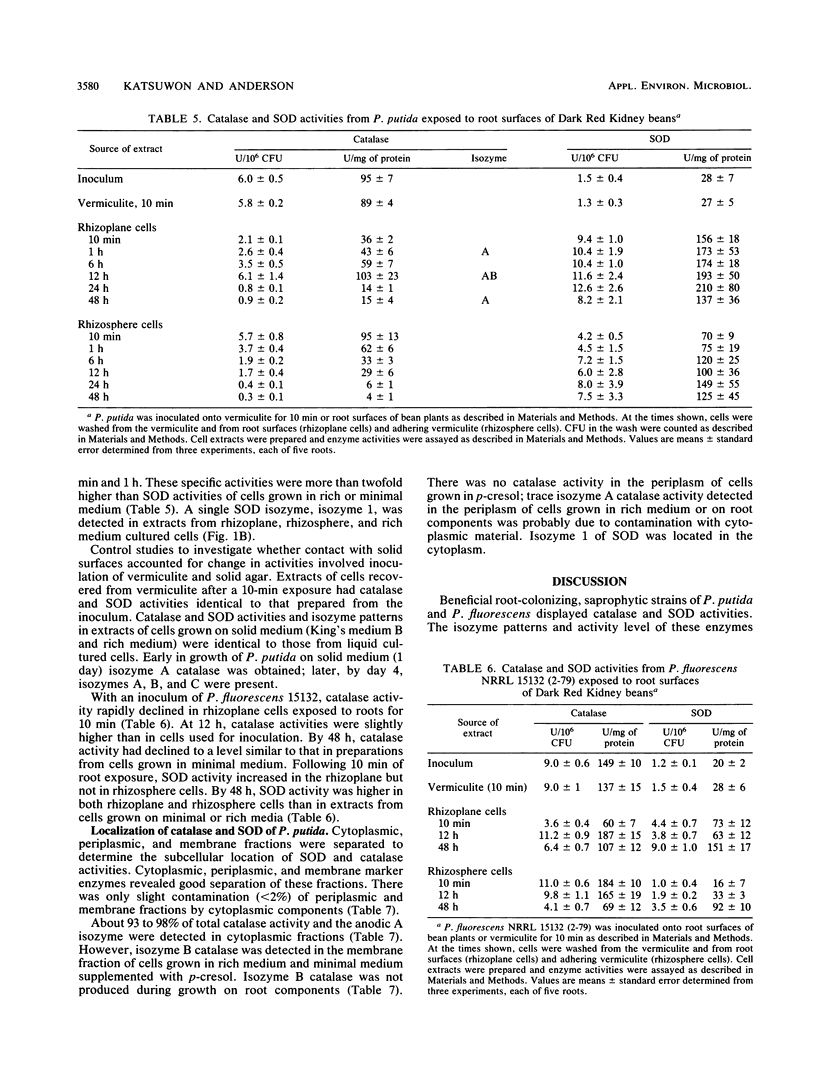
Root-colonizing, saprophytic fluorescent pseudomonads of the Pseudomonas putida-P. fluorescens group express similar levels of catalase and superoxide dismutase activities during growth on a sucrose- and amino acid-rich medium. Increased specific activities of catalase but not superoxide dismutase were observed during growth of these bacteria on components washed from root surfaces. The specific activities of both enzymes were also regulated during contact of these bacteria with intact bean roots. Increased superoxide dismutase and decreased catalase activities were observed rapidly, by 10 min upon inoculation of cells onto intact bean roots. Catalase specific activity increased with time to peak at 12 h before declining. By 48 h, the cells displayed this low catalase but maintained high superoxide dismutase specific activities. Catalase with a low specific activity and a high superoxide dismutase activity also were present in extracts of cells obtained from 7-day-old roots colonized from inoculum applied to seed. This specific activity of superoxide dismutase of root-contacted cells was about fourfold-higher in comparison to cells grown on rich medium, whereas the specific activity for catalase was reduced about fivefold. A single catalase isozyme, isozyme A, and one isozyme of superoxide dismutase, isozyme 1, were detected during growth of the bacteria on root surface components and during exposure of cells to intact bean roots for 1 h. An additional catalase, isozyme B, was detected from bacteria after exposure to the intact bean roots for 12 h. Catalase isozyme A and superoxide dismutase isozyme 1 were located in the cytoplasm and catalase band B was located in the membrane of P. putida.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Habibzadegah-Tari P., Tepper C. S. Molecular Studies on the Role of a Root Surface Agglutinin in Adherence and Colonization by Pseudomonas putida. Appl Environ Microbiol. 1988 Feb;54(2):375–380. doi: 10.1128/aem.54.2.375-380.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram I., Shaklai N. The association of human erythrocyte catalase with the cell membrane. Arch Biochem Biophys. 1981 Dec;212(2):329–337. doi: 10.1016/0003-9861(81)90373-8. [DOI] [PubMed] [Google Scholar]
- Beaman B. L., Black C. M., Doughty F., Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985 Jan;47(1):135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Scates S. M., Moring S. E., Deem R., Misra H. P. Purification and properties of a unique superoxide dismutase from Nocardia asteroides. J Biol Chem. 1983 Jan 10;258(1):91–96. [PubMed] [Google Scholar]
- Franzon V. L., Arondel J., Sansonetti P. J. Contribution of superoxide dismutase and catalase activities to Shigella flexneri pathogenesis. Infect Immun. 1990 Feb;58(2):529–535. doi: 10.1128/iai.58.2.529-535.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T., Cardini G. E., Maseles F. C., Kallio R. E. Incorporation of oxygen-18 into benzene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1631–1635. doi: 10.1021/bi00809a024. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Hayaishi O. Studies on indoleamine 2,3-dioxygenase. I. Superoxide anion as substrate. J Biol Chem. 1975 Aug 10;250(15):5960–5966. [PubMed] [Google Scholar]
- Hopper D. J., Jones M. R., Causer M. J. Periplasmic location of p-cresol methylhydroxylase in Pseudomonas putida. FEBS Lett. 1985 Mar 25;182(2):485–488. doi: 10.1016/0014-5793(85)80359-8. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Katsuwon J., Anderson A. J. Response of plant-colonizing pseudomonads to hydrogen peroxide. Appl Environ Microbiol. 1989 Nov;55(11):2985–2989. doi: 10.1128/aem.55.11.2985-2989.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982 May 25;257(10):5751–5754. [PubMed] [Google Scholar]
- Kranz R. G., Barassi C. A., Gennis R. B. Immunological analysis of the heme proteins present in aerobically grown Escherichia coli. J Bacteriol. 1984 Jun;158(3):1191–1194. doi: 10.1128/jb.158.3.1191-1194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Effects of superoxide on the erythrocyte membrane. J Biol Chem. 1978 Mar 25;253(6):1838–1845. [PubMed] [Google Scholar]
- Mandell G. L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J Clin Invest. 1975 Mar;55(3):561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire W., Singer T. P. Resolution of p-cresol methylhydroxylase into catalytically active subunits and reconstitution of the flavocytochrome. FEBS Lett. 1982 Jul 5;143(2):316–318. doi: 10.1016/0014-5793(82)80124-5. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Mottley C., Mason R. P. An electron spin resonance study of free radical intermediates in the oxidation of indole acetic acid by horseradish peroxidase. J Biol Chem. 1986 Dec 25;261(36):16860–16864. [PubMed] [Google Scholar]
- Richter H. E., Loewen P. C. Induction of catalase in Escherichia coli by ascorbic acid involves hydrogen peroxide. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1039–1046. doi: 10.1016/0006-291x(81)91928-8. [DOI] [PubMed] [Google Scholar]
- Römheld V., Marschner H. Mechanism of iron uptake by peanut plants : I. Fe reduction, chelate splitting, and release of phenolics. Plant Physiol. 1983 Apr;71(4):949–954. doi: 10.1104/pp.71.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O. Organization, expression, and evolution of genes for mercury resistance. Annu Rev Microbiol. 1986;40:607–634. doi: 10.1146/annurev.mi.40.100186.003135. [DOI] [PubMed] [Google Scholar]
- Tari P. H., Anderson A. J. Fusarium Wilt Suppression and Agglutinability of Pseudomonas putida. Appl Environ Microbiol. 1988 Aug;54(8):2037–2041. doi: 10.1128/aem.54.8.2037-2041.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist L., Rannug U., Rannug A., Ramel C. Protection from toxic and mutagenic effects of H2O2 by catalase induction in Salmonella typhimurium. Mutat Res. 1984 Nov-Dec;141(3-4):145–147. doi: 10.1016/0165-7992(84)90087-3. [DOI] [PubMed] [Google Scholar]