Abstract
Nitric oxide (NO) has been implicated in the local regulation of bone metabolism. However, the contribution made by specific NO synthase (NOS) enzymes is unclear. Here we show that endothelial NOS gene knockout mice (eNOS−/−) have marked abnormalities in bone formation. Histomorphometric analysis of eNOS−/− femurs showed bone volume and bone formation rate was reduced by up to 45% (P < 0.01) and 52% (P < 0.01), respectively. These abnormalities were prevalent in young (6 to 9 weeks old) adults but by 12 to 18 weeks bone phenotype was restored toward wild-type. Dual energy X-ray absorptiometry analysis confirmed the age-related bone abnormalities revealing significant reductions in femoral (P < 0.05) and spinal bone mineral densities (P < 0.01) at 8 weeks that were normalized at 12 weeks. Reduction in bone formation and volume was not related to increased osteoclast numbers or activity but rather to dysfunctional osteoblasts. Osteoblast numbers and mineralizing activity were reduced in eNOS−/− mice. In vitro, osteoblasts from calvarial explants showed retarded proliferation and differentiation (alkaline phosphatase activity and mineral deposition) that could be restored by exogenous administration of a NO donor. These cells were also unresponsive to 17β-estradiol and had an attenuated chemotactic response to transforming growth factor-β. In conclusion, eNOS is involved in the postnatal regulation of bone mass and lack of eNOS gene results in reduced bone formation and volume and this is related to impaired osteoblast function.
Bone is a vital dynamic connective tissue that has evolved to maintain a balance between its two major functions: provision of mechanical integrity for locomotion and modulation and control of mineral homeostasis. 1 Mineralized bone is continuously resorbed by osteoclasts and new bone is formed by osteoblasts. This process, known as bone remodeling, is highly regulated with maintenance of normal integrity and structure. 2 Systemic hormones including calcitonin, parathyroid hormone, and sex steroids, particularly estrogen, are known to be important regulators of bone cell function. Their effects on bone turnover are in general exerted by activation of local mediators and second messengers present within bone cells. 3 Recent investigations have focused on the role of nitric oxide (NO) as one of these possible local regulators of bone metabolism and bone cell activity. NO is a short-lived radical gas generated from l-arginine by nitric oxide synthase (NOS) isoenzymes. 4 Three distinct isoforms of NOS have been identified: a neuronal form (type I; nNOS) originally isolated from brain, 5 an endothelial form (type III; eNOS) originally isolated from bovine aortic endothelial cells, 6 and an inducible form (type II; iNOS) originally isolated from murine macrophages. 7 Both eNOS and nNOS are expressed constitutively and are characterized by highly regulated rapid but low-output NO production. 4 In contrast the iNOS pathway is generally only activated after stimulation by certain pro-inflammatory cytokines such as interferon-γ, interleukin-1β, and tumor necrosis factor-α. The inducible NOS isoform is characterized by production of persistent and high concentrations of NO. 8
There is now ample evidence to indicate that expression and activity of NOS isoforms are significant in osteoblast and osteoclast biology. NO seems to have an overall suppressive effect on bone resorption inhibiting both osteoclast activity and precursor recruitment and this may be associated with iNOS activity. 9-16 However, constitutive eNOS activity may also be of consequence, stimulating osteoclast function. 9,16 Basal, constitutive NO synthesis within osteoblasts supports proliferation and activity of these cells. 17 Moreover, osteoblast NO synthesis/activity is augmented by osteogenic hormones such as estrogen 18 and involves up-regulation of eNOS. 19 Stimulation of eNOS and synthesis of NO by osteoblasts and osteocytes is also involved in mediating the osteogenic effects induced in response to mechanical strain or shear flow. 20-24 Conversely, iNOS activity (stimulated in response to interleukin-1β, tumor necrosis factor-α, and interferon-γ) causes marked suppression of osteoblast function. 25-27 Numerous studies have also shown that administration of NOS inhibitors to rats in vivo leads to a reversible osteopenia. 12,28,29 Rat models of ovariectomy-induced osteopenia have also revealed that osteoporotic bone loss can be alleviated by administration of NO donors and conversely, inhibition of endogenous NO suppresses the bone-conserving action of estradiol. 18
These observations have demonstrated that the synthesis and activity of both eNOS and iNOS is significant in bone biology although there is consensus that under physiological conditions eNOS probably represents the major NOS activity regulating bone formation. 17-24 Despite this, many of the previous studies have been based on the use of NOS inhibitors and are therefore subject to potential inconsistencies arising from the lack of isoform-specific selectivity of these compounds as well as effects independent of NOS inhibition. 4,29 Consequently, it has not been previously possible to define clearly the contribution made by a specific NOS isoform to the control of bone turnover.
In this respect eNOS gene-deficient (eNOS−/−) mice 30 and their wild-type controls (eNOS+/+) could be a useful model to study the contribution made by NO to the control of bone metabolism and bone regulation by circumventing the lack of isoform specificity of the current generation of NOS inhibitors. Moreover, this can provide useful information to test previous studies on this field based on pharmacological inhibition of this NO synthase. The aim of this study therefore, was to test the hypothesis that the eNOS isoform plays a major role in regulation of bone growth and remodeling and that disruption of the eNOS gene alters this process. Histomorphometry and dual energy X-ray absorptiometry (DEXA) was used to analyze the bones of young adult eNOS−/− and eNOS+/+ mice. We also performed in vitro studies on osteoblasts isolated from calvarial explants to further investigate the mechanism of action of eNOS.
Materials and Methods
Animals
eNOS−/− mice and their respective wild-type controls were generated on a mixed SV129/C57BL/6 background by intercrossing heterozygous parents as described previously. 30 In all experiments we used wild-type or heterozygous littermates as controls. Mice were viable, fertile, and indistinguishable from wild type and heterozygous littermates in gross physical appearance and routine behavior.
Reagents
Except where indicated all chemicals and reagents were obtained from Sigma-Aldrich, Poole, UK.
Genotyping
Mice from each litter were genotyped by polymerase chain reaction (PCR) to confirm presence or absence of the eNOS gene. Tail snips (3 mm) were taken from mice between 8 and 15 days of age under local anesthesia. Samples were placed in 0.4 ml of lysis buffer (10 mmol/L Tris, pH 8.0, 50 mmol/L ethylenediaminetetraacetic acid, 100 mmol/L NaCl, 0.5% sodium dodecyl sulfate, and 500 μl/ml proteinase K) at 55°C with agitation overnight. Lysate was then extracted once with phenol-chloroform (1:1) and chloroform, (1:1), respectively, by mixing gently six times and centrifugation for 10 minutes at 9,000 rpm. The DNA was precipitated by absolute ethanol (2:1), washed with 1 ml of 70% ethanol, and resuspended in 30 μl of distilled water. Amplification of DNA products was accomplished by subjecting 2 to 4 μl of resuspended DNA to 35 cycles of PCR (94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1 minute) and then to one cycle of PCR (72°C for 10 minutes) in the presence of dNTPs (50 μmol/L) and 2.5 U of Taq polymerase (Promega, Madison, WI) in a standard buffer containing 1.8 mmol/L MgCl2. The sense oligonucleotide 5′-GGGCTCCCTCCTTCCGGCTGCCACC-3′ and the antisense oligonucleotide 5′-GGATCCCTGGAAAAGGCGGTGAGG-3′, which amplify a 900-bp PCR product of eNOS cDNA identified eNOS+/+ mice. In contrast, the sense oligonucleotide 5′-ATGAACTGCAGGACGAGGCAGCG-3′ and the antisense 5′-GGCGATAGAAGGCGATGCGCTG-3′, which amplify a 603-bp PCR product, corresponding to the neo resistance gene and therefore, identified eNOS−/− mice. For each experiment, positive and negative control samples were always run. The amplified products were electrophoresed in 1% agarose gel with 50 μl/ml ethidium bromide.
Histology
Mice were killed by cervical dislocation and lungs, heart, liver, kidneys, and spleen were removed and fixed in 10% buffered formalin. The long bones were also removed and processed as described below. Paraffin-embedded sections (4 μm) were cut and stained with hematoxylin and eosin (H&E).
Bone Histomorphometry
Static and dynamic bone histomorphometry was performed on the femurs from 22 eNOS−/− and 19 eNOS+/+ male mice. All mice were maintained in a temperature-controlled room (24°C) with free access to sterilized water and food. Mice were divided into the following groups: 1) 6 weeks old (six eNOS+/+ and eight eNOS−/− mice); 2) 9 weeks old (seven eNOS+/+ and six eNOS−/− mice); 3) 18 weeks old (six eNOS+/+ and eight eNOS−/− mice).
For the purposes of dynamic histomorphometry all mice were injected intraperitoneally with oxytetracycline (30 mg/kg body weight) and with calcein (30 mg/kg body weight) 9 and 3 days before killing, respectively.
Femurs were removed from each mouse immediately after killing. Soft tissues were gently excised and length of the bones recorded. Bones were cut with an electrical saw at the exact midshaft to facilitate bone marrow embedding. Fixation was performed immediately after collection by immersion in 70% ethanol for 48 hours at 4°C. The bones were then dehydrated in increasing concentrations of ethanol and embedded in methyl-methacrylate without decalcification. 31-33 After polymerization of the resin, blocks were sectioned using a Reichert-Jung 1140 autocut (Leica UK Ltd., Milton Keynes, UK) equipped with a tungsten carbide-tipped Jung D profile knife (Leica UK Ltd.). Five 3-μm serial sections were cut, deplasticized with 2-methoxyethylacetate for 20 minutes, and stained with Von Kossa with toluidine blue at acid pH as counterstain 31-33 for the static histomorphometry analysis. For dynamic histomorphometry two 12-μm sections from distal femur, neither deplasticized nor stained, were used to investigate fluorochrome labeling.
Histomorphometry of trabecular bone was made in longitudinal sections of distal femur within an area between 500 to 2,700 μm from the epiphyseal growth plate, to exclude the primary spongiosa. 31-33 Thirty-two to 40 fields were analyzed from each distal femur metaphysis. Evaluation of bone turnover during remodeling included static evaluation using serial sections for various parameters and dynamic evaluation using fluorochrome labeling of bone in situ. Stereology using a Merz grid was performed. 31-33 Table 1 ▶ shows the histomorphometric parameters used in this study. They were reported according to the recommended American Society of Bone and Mineral Research nomenclature. 34
Table 1.
Measurements Used in the Histomorphometry Analysis of Cancellous Bone Tissue with Their Abbreviations, Units, and Definitions
| 1) | Bone volume (BV/TV) (%): the percentage of the total bone marrow volume occupied by trabecular bone |
| 2) | Osteoid volume (OV/TV) (%): the percentage of the total bone marrow volume occupied by osteoid |
| 3) | Osteoclast surface (Oc.S/BS) (%): the percentage of the total trabecular bone surface (BS/TV) that is occupied by osteoclasts |
| 4) | Eroded surface (ES/BS) (%): the percentage of the BS/TV that is occupied by howship’s lacunae, whether or not there are osteoclasts present |
| 5) | Osteoblast surface (Ob.S/BS) (%): the percentage of the BS/TV that is occupied by osteoblasts |
| 6) | Osteoid surface (OS/BS) (%): the percentage of the BS/TV that is occupied by osteoid whether or not there are osteoblasts present |
| 7) | Mineralising surface (MS/BS) (%): the percentage of the BS/TV that is double labelled (2LS) plus one half single labelled (1LS) |
| 8) | Mineral appositional rate (MAR) (μm per day): the rate of addition of new layers of mineral at trabecular surfaces. It is obtained by dividing the distance between labels by the time interval between labels. The measurements are made at three different points from the middle of one label to the middle of the other. The mean value is taken and corrected for the randomness of the angle between the section plane and the plane of the trabecular surface (π/4) |
| 9) | Bone formation rate at tissue level (BFR/BV) (μm3 per μm2 per day): 2LS plus one half 1LS divided by BS/TV and multiplied by MAR. Therefore, BFR/BV expresses the amount of new mineralised bone per micrometer of BS/TV per day |
| 10) | Mean trabecular thickness (MTT) (μm): a derived value obtained by dividing the BV/TV by the BS/TV |
DEXA Analysis
DEXA analysis was performed on female mice aged 8 weeks (seven eNOS−/− and seven eNOS+/+) and 12 weeks (seven eNOS−/− and seven eNOS+/+). The mice were maintained under conditions identical to those described above. Animals were killed by intraperitoneal overdose of pentabarbitone sodium (Euthatal; RMB Animal Health Ltd., UK). Total and regional (femur/pelvis and spine) bone mineral density was measured using a Lunar DPX bone densitometer (Lunar Corp., Madison, WI) and analysis performed using ultra-high resolution software designed for small animals.
In Vitro Studies
Culture of Primary Calvarial Osteoblasts
Primary mouse osteoblast-enriched cultures were isolated by sequential enzymatic digestion of neonatal calvariae, as previously described. 35 Briefly, the calvariae were dissected from 25 neonatal eNOS+/+ and 25 eNOS−/− mice and digested with collagenase for sequential periods of 2 × 10 minutes and 3 × 20 minutes. Cells isolated from the final three digests were then plated into 75-cm 2 flasks in α-modified Eagle’s medium with nucleotides supplemented with 15% v/v fetal calf serum, 10 mmol/L sodium β-glycerophosphate, 50 μg/ml ascorbic acid, 50 U/ml penicillin G, 50 μg/ml streptomycin, and 0.3 μg/ml amphotericin B. After 24 hours cells were released by 0.25% trypsin-ethylenediaminetetraacetic acid treatment, pooled, and then plated into 24-well culture plates at a density of 5 × 10 3 cells/well forsubsequent assessment of proliferation, alkaline phosphatase activity, and formation of mineralized bone nodules.
Osteoblast Proliferation
Cells were plated into 24-well culture plates at a density of 5 × 10 3 cells/well and allowed to settle overnight. Cells were then temporarily growth arrested by incubation for 24 hours in α-modified Eagle’s medium supplemented with 0.1% serum. Thereafter cells were maintained for 5 days in full culture media (with one change after 3 days), released by 0.25% v/v trypsin-ethylenediaminetetraacetic acid treatment, and resuspended in 10% v/v fetal calf serum-containing media. After centrifugation (1,000 × g × 5 minutes) cells were resuspended in 10% v/v fetal calf serum-containing media mixed with 0.4% w/v trypan blue in 1:1 ratio and a viable cell count performed using a hemocytometer (nonviable cells were stained with trypan blue).
Osteoblast Differentiation
i) Alkaline Phosphatase Activity: Cells were plated into 24-well culture plates at a density of 5 × 10 3 cells/well and maintained in full culture media for 12 days (with fresh changes every 3 days). Total alkaline phosphatase enzyme activity was assessed as described previously. 27 Briefly, total cellular protein was extracted by digestion in buffer containing 0.1% lauryl sulfate (SDS), 0.1% Nonidet P-40, 10 μl/ml phenylmethylsulfonyl fluoride, 30 μl/ml aprotinin, and 10 μl/ml sodium orthovanadate. Cell lysates were collected and either assayed immediately or stored at −20°C. To 50 μl of lysate, 50 μl of alkaline buffer solution and 50 μl of substrates were added. The mixture was incubated at 37°C for 30 minutes, and the reaction was then stopped by adding 100 μl of 0.5 mol/L NaOH and absorbance was read at 405 nm using a Multiscan RC plate reader (Labsystems, Life Sciences International Ltd., Hampshire, UK). A standard curve was constructed using p-nitrophenol as a standard concentration range (6.25 to 400 μmol/L) and alkaline phosphatase activity was calculated. One unit of activity is defined as the amount of enzyme activity that liberates 1 nmol of p-nitrophenol per minute under the assay conditions. The results were normalized for total protein concentration by the Bradford dye-binding method. The absorbance was read at 750 nm using a Multiscan RC plate reader against a protein standard curve generated using bovine serum albumin (0.2 to 10 mg/ml). Measurements were made from a total of nine replicates representing three separate experiments.
ii) Bone Nodule Assay: To assess the ability of osteoblasts to express their mature phenotype the bone nodule assay was performed, which is a well-established quantitative assay of in vitro bone formation. 35 Cells were grown in 4-cm wells for 21 days with an initial seeding density of 5 × 10 4 cells/well, in which the media was changed every 3 days. Cultures were fixed in 10% v/v formol saline for 5 minutes. Mineralized bone nodules were detected by Alizarin Red (pH 7.4) staining. Bone nodules per well were counted macroscopically.
Osteoblast Response to 17β-Estradiol
For 48 hours before experimentation, cultures of eNOS+/+ and eNOS−/− osteoblasts were maintained in 0.1% v/v serum and phenol red-free α-modified Eagle’s medium supplemented with antibiotics/antimycotics. After 48 hours the cells were placed in phenol red-free α-modified Eagle’s medium supplemented with 10% v/v dextran-charcoal stripped fetal bovine serum and antibiotics/antimycotics. Osteoblasts were dose-dependently treated with 17β-E2 (10−10 to 10−6 mol/L; data not shown). A final concentration of 10−7 mol/L was used in all experiments. Cell proliferation in response to 17β-estradiol was assessed as described above. Cells were also treated with the inactive stereoisomer 17α-estradiol (17α-E2, 10−7 mol/L; data not shown).
Response to S-Nitrosoacetylpenicillamine (SNAP)
To assess the effects of exogenous application of NO on osteoblast proliferation and differentiation, osteoblasts from eNOS−/− and eNOS+/+ were dose-dependently treated with SNAP (0.01 to 50 μmol/L; data not shown). A final concentration of 1 μm SNAP was used in all experiments. SNAP was replenished each time the cells were fed and its effects on proliferation, alkaline phosphatase activity, and bone nodule formation was assessed as described above.
Chemotaxis Assay
Chemotactic responses were measured by a modified Boyden Chamber assay 36 using 6-well Transwell chambers (Costar U.K. Ltd., High Wycombe, UK) that is divided by polycarbonate filters with 8-μm pores. Media was added to both the lower and upper chambers and left for 30 minutes to equilibrate. Then 3 × 10 3 cells/well were placed in the upper chamber and left for 2 hours to attach. Transforming growth factor-β1 (10 ng/ml, 1 ng/ml, 100 ρg/ml) was added to the lower chamber and left for 4 hours. The filter was immersed in alcohol (4°C) to fix the cells. The cells on the upper aspect of the filter were carefully removed by scraping with the edge of a glass coverslip, and then the filter was rinsed in 0.05 mol/L Tris buffer (pH 9). The filter was then stained with a DNA-binding fluorescent reagent 4,6-diamidino-2-phenylindole, enabling visualization and direct counting of cell nuclei, of cells that had migrated across the filter, by fluorescent microscopy (Olympus BX60 photomicroscope). Data presented as percentage increase relative to control, unstimulated cells.
Statistical Methods
Results of the static and dynamic histomorphometry and cell culture experiments were expressed as the mean and SEM. Cell culture experiments were performed in triplicate and repeated four times. Differences between eNOS−/− and eNOS+/+ mice were determined by statistical analysis using Student’s t-test or by analysis of variance. A value of P < 0.05 was taken as a significant difference.
Results
Genotyping
An example of genotyping eNOS−/− and eNOS+/+ mice by PCR is shown in Figure 1 ▶ . The set of primers (A) that amplified a 900-bp PCR product identified eNOS+/+ mice. The set of primers (B) that amplified a 603-bp PCR product identified eNOS−/− mice.
Figure 1.
Genotyping of eNOS+/+ and eNOS−/− mice. The set of primers (A) that amplified a 900-bp PCR product identified wild-type. The set of primers (B) that amplified a 603-bp PCR product identified the eNOS gene-deficient phenotype.
Histology
Histological examination of eNOS−/− mice organs revealed no pathological findings or abnormalities except for a mild glomerular hypercellularity observed in ∼10% of 9-week-old eNOS−/− mice.
Bone Histomorphometry
Results of measurements made in distal femur metaphyses of eNOS−/− and eNOS+/+ mice are shown in Table 2 ▶ . Gross measurements made on the femur revealed that eNOS−/− mice had a significant reduction in femur length at 6 (P < 0.01), 9 (P < 0.05), and 18 (P < 0.05) weeks of age (Table 2) ▶ .
Table 2.
Histomorphometry Analysis of Distal Femur in 6, 9, and 18-Week-Old eNOS Gene-Deficient (eNOS−/−) and Wild-Type Control (eNOS+/+) Mice
| N* | 6 | 9 | 18 | |||
|---|---|---|---|---|---|---|
| eNOS+/+ | eNOS−/− | eNOS+/+ | eNOS−/− | eNOS+/+ | eNOS−/− | |
| 6 | 8 | 7 | 6 | 6 | 8 | |
| Length† | 14.90 ± 0.10 | 13.94 ± 0.06§ | 14.96 ± 0.20 | 14.43 ± 0.14‡ | 15.70 ± 0.19 | 14.96 ± 0.15‡ |
| BV/TV | 18.99 ± 1.59 | 13.64 ± 0.78‡ | 22.01 ± 1.19 | 12.04 ± 1.27§ | 20.10 ± 2.24 | 14.24 ± 1.73 |
| MTT | 52.92 ± 2.40 | 36.32 ± 1.07§ | 44.88 ± 1.58 | 37.96 ± 3.30 | 43.30 ± 2.06 | 37.57 ± 1.36‡ |
| OV/TV | 0.93 ± 0.18 | 0.57 ± 0.07 | 0.55 ± 0.34 | 0.32 ± 0.17 | 0.30 ± 0.10 | 0.25 ± 0.04 |
| OS/BS | 12.61 ± 1.39 | 8.17 ± 0.79‡ | 6.04 ± 0.69 | 5.25 ± 1.43 | 4.11 ± 1.66 | 3.42 ± 0.36 |
| Ob.S/BS | 7.79 ± 1.35 | 4.38 ± 0.50‡ | 1.75 ± 0.51 | 1.48 ± 0.59 | 0.68 ± 0.22 | 0.30 ± 0.14 |
| ES/BS | 2.90 ± 0.42 | 1.44 ± 0.14‡ | 1.44 ± 0.18 | 1.21 ± 0.39 | 0.90 ± 0.35 | 0.79 ± 0.29 |
| Oc.S/BS | 0.32 ± 0.14 | 0.20 ± 0.25 | 0.25 ± 0.21 | 0.10 ± 0.21 | 0.12 ± 0.09 | 0.03 ± 0.06 |
| MS/OS | 22.32 ± 1.90 | 12.69 ± 1.88‡ | 13.32 ± 1.75 | 9.89 ± 1.14 | 10.20 ± 0.63 | 9.90 ± 1.01 |
| MAR | 1.78 ± 0.07 | 1.50 ± 0.09‡ | 1.37 ± 0.07 | 1.24 ± 0.10 | 0.81 ± 0.02 | 0.72 ± 0.04 |
| BFR/BV | 0.41 ± 0.03 | 0.20 ± 0.02§ | 0.22 ± 0.01 | 0.13 ± 0.02‡ | 0.08 ± 0.01 | 0.07 ± 0.01 |
*Number of mice used per group.
†Length of femur expressed in mm.
‡P < 0.05.
§P < 0.01.
For definitions, abbreviations, and units of measurements, see Table 1 ▶ . Results are means ± SEM.
Static and dynamic histomorphometric analysis of distal femur showed that eNOS−/− mice had a reduction in bone volume (BV/TV) at 6 and 9 weeks of age decreasing from 18.99 ± 1.59% to 13.64 ± 0.78% (P < 0.05) and from 22.01 ± 1.19% to 12.04 ± 1.27% (P < 0.01) (Table 2 ▶ and Figures 2 and 3 ▶ ▶ ). There was also a significant reduction in mean trabecular thickness at 6 weeks of age (Table 2) ▶ . Osteoid surface (OS/BS) decreased from 12.61 ± 1.4% in eNOS+/+ mice to 8.17 ± 0.8% in eNOS−/− mice (P < 0.05) at 6 weeks of age (Table 2) ▶ . Osteoblast surface (Ob.S/BS) was also decreased from 7.8 ± 1.4% in the femurs of 6-week-old eNOS+/+ mice to 4.4 ± 0.5% (P < 0.05) in age-matched eNOS−/− mice (Table 2) ▶ . Analysis of the eroded surface (ES/BS) showed that there was a reduction from 2.90 ± 0.42% observed in the femurs of 6-week-old eNOS+/+ mice to 1.44 ± 0.14% (P < 0.05) in eNOS−/− mice (Table 2) ▶ ; however, there were no significant differences in the osteoclast surface (Table 2) ▶ .
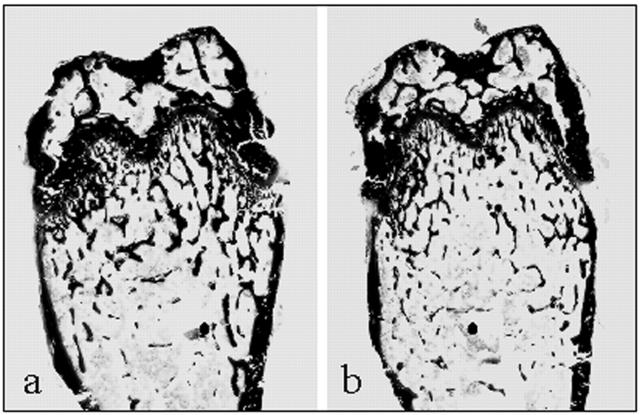
Figure 2.
Sections (3 μm) of distal femur from 6-week-old eNOS+/+ (a) and eNOS−/− (b) mice stained with von Kossa and toluidine blue. Fewer, thinner trabeculae are seen in the femurs of eNOS−/− mice compared to age-matched eNOS+/+ mice. Original magnification, ×3.
Figure 3.

Sections (3 μm) of distal femur from 9-week-old eNOS+/+ (a and c) and eNOS−/− (b and d) mice stained with von Kossa and toluidine blue. Fewer, thinner trabeculae are seen in the femurs of eNOS−/− mice compared to age-matched eNOS+/+ mice. Original magnifications: ×3 (a and b); ×10 (c and d).
The mineralizing surface (MS/OS) was almost twice as great in 6-week-old eNOS+/+ mice (22.32 ± 1.9%) as compared with age-matched eNOS−/− mice (12.69 ± 1.88%; P < 0.01) (Table 2) ▶ . Mineral apposition rate (MAR) showed a modest decrease from 1.75 ± 0.09 to 1.52 ± 0.060 μm/day (P < 0.05) in the femurs of 6-week-old eNOS +/+ and eNOS−/− mice, respectively (Table 2) ▶ . Bone formation rate fell in the femurs of both 6-week-old (0.4 ± 0.03 to 0.2 ± 0.02 μm3/μm2/day; P < 0.01) and 9-week-old (0.22 ± 0.01 to 0.13 ± 0.02 μm3/μm2/day; P < 0.05) eNOS−/− mice compared to wild-type mice.
Histomorphometric analysis of femurs from 18-week-old animals revealed a significant reduction in mean trabecular thickness decreasing from 43.30 ± 2.06 μm to 37.57 ± 1.36 μm (P < 0.05) in eNOS−/− mice. All other parameters were found not to be significantly different from wild-type mice (Table 2 ▶ , Figure 4 ▶ ).
Figure 4.
Sections (3 μm) from 18-week-old eNOS +/+ (a) and eNOS−/− (b) mice stained with von Kossa and toluidine blue. There are no obvious differences between wild-type and knockout in bone architecture. Original magnification, ×3.
Dual-Energy X-Ray Absorptiometry
Analysis of 8-week-old eNOS+/+ and eNOS−/− female mice revealed a significant reduction in both femoral/pelvic and spinal bone mineral density (Figure 5) ▶ decreasing from 0.1650 ± 0.0029 g/cm 2 to 0.154 ± 0.0023 g/cm 2 (P < 0.05) and from 0.167 ± 0.0041 to 0.150 ± 0.0044 g/cm 2 (P < 0.01), respectively. Analysis of total bone mineral density of whole body was also significantly reduced decreasing from 0.223 ± 0.0022 to 0.217 ± 0.0028 g/cm 2 (P < 0.05). Analysis of 12-week-old mice showed no significant differences in bone mineral density between eNOS+/+ and eNOS−/− mice.
Figure 5.
Bone mineral density measurements of femur/pelvis (a) and spine (b) from eNOS+/+ and eNOS−/− mice. *, P < 0.05; **, P < 0.01.
Osteoblast Proliferation and Differentiation
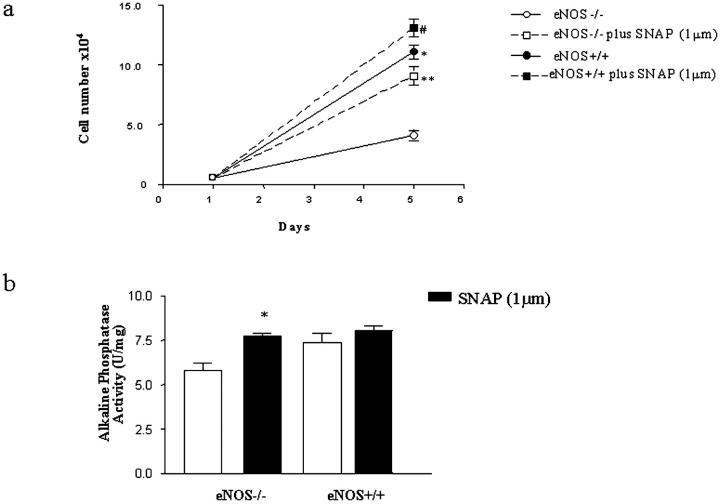
After 5 days in culture, cell counts (Figure 6a) ▶ showed that there was a significantly greater number of eNOS+/+ osteoblasts (10.87 ± 0.597 × 10 4 cells) compared to eNOS−/− osteoblasts (4.032 ± 0.368 × 10 4 cells) (P < 0.001). Analysis of alkaline phosphatase activity after 12 days in culture revealed (Figure 6b) ▶ that this was significantly lower in eNOS−/− osteoblasts (5.8 ± 0.399 U/mg protein) compared to wild type (7.9 ± 0.150 U/mg protein; P < 0.05). Osteoblasts from eNOS−/− mice formed significantly fewer mineralized bone nodules (33 ± 9) compared to eNOS+/+ (98 ± 14; P < 0.01) (Figure 7, A and B) ▶ . In all experiments the reduction in cell numbers, alkaline phosphatase activity, and bone nodule numbers seen in eNOS−/− osteoblasts could be restored to values seen in wild type by exogenous administration of SNAP (Figures 6 and 7) ▶ ▶ .
Figure 6.
Assessment of cell proliferation (a) and alkaline phosphatase activity (b) of primary calvarial osteoblasts from eNOS+/+ and eNOS−/− mice. It can been seen that retarded growth and differentiation of eNOS−/− osteoblasts is restored toward wild-type levels by SNAP. a: *, P < 0.0001 eNOS+/+ versus eNOS−/−; **, P < 0.001 eNOS−/− versus eNOS−/− plus SNAP; #, P < 0.05 eNOS+/+ versus eNOS+/+ plus SNAP. b: *, P < 0.05 eNOS−/− versus eNOS−/− plus SNAP.
Figure 7.
Assessment of formation of mineralized bone nodules (A and B) of primary calvarial osteoblasts from eNOS+/+ and eNOS−/− mice. It can be seen that there are fewer bone nodules formed in the eNOS−/− cultures but this is restored toward wild-type by SNAP. **, P <0.001 eNOS−/− versus eNOS +/+; *, P < 0.01 eNOS +/+ versus eNOS+/+ plus SNAP.
Stimulation of wild-type osteoblasts by 17-β-estradiol increased cell numbers significantly (10.34 ± 0.710 × 10 4 cells to 12.25 ± 0.667 × 10 4 cells, P < 0.05) but did not have any mitogenic effects on eNOS−/− osteoblasts (Figure 8) ▶ .
Figure 8.
Addition of 17-β estradiol (10−7 mol/L) induced an increase in cell number in eNOS+/+ osteoblasts but had no effect on eNOS−/− cells. *, P < 0.05 eNOS +/+ versus eNOS+/+ plus 17-β estradiol.
Chemotaxis Assay
In response to a transforming growth factor-β gradient eNOS+/+ there was a significant increase in the proportion of cells that had migrated across the polycarbonate filter toward increasing concentrations of the cytokine. This response was attenuated in eNOS−/− osteoblasts and there was no evidence of significant numbers of cells that had migrated across the filter (Figure 9) ▶ .
Figure 9.
Chemotaxis response of primary calavarial explant osteoblasts to transforming growth factor-β. It can be seen that although eNOS+/+ migrated toward the cytokine this effect was attenuated in the eNOS−/− cells. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Discussion
NO synthesized by eNOS 17-24 and also iNOS 9-16 is strongly implicated in the regulation of bone metabolism exerting powerful effects on cells of both the osteoblast and osteoclast lineage. However, the specific functional roles of these enzymes in these cells and their effects on bone turnover are not clearly defined. This relates, in part, to the fact that many of the compounds that have been used previously to investigate the roles of eNOS and iNOS are not sufficiently selective in their action to be able to discriminate unequivocally between the different isoforms. 4,37
In this study we have used an established eNOS gene-deficient mouse model and their wild-type counterparts 30 to investigate parameters of bone remodeling and skeletal integrity under normal physiological conditions. Initially we used bone histomorphometry to show that eNOS gene-deficient mice had marked bone abnormalities with significant reductions in bone formation rates and bone volume confirming that eNOS is fundamental to the physiological regulation of bone turnover. The histomorphometry data also implicated osteoblast dysfunction as a significant cause of these abnormalities. This was suggested by marked differences in surface estimates of new bone matrix synthesis, mineralizing activity, and accretion rate of new bone, which were all significantly lower in the eNOS−/− mice compared to eNOS+/+ mice. In part, this was found to be related to there being significantly fewer osteoblasts lining trabecular bone surfaces with a 44% reduction in surface coverage in eNOS−/− mice suggesting impaired recruitment and/or attachment to bone resorption sites. Concordant alterations in tissue-level parameters of bone remodeling were also evident. In particular, bone formation rate was decreased by ∼52 and 41% in eNOS−/− mice, compared to eNOS+/+ mice at 6 and 9 weeks of age, respectively. Consequently, with less bone being laid down femoral bone volume was reduced by 28 and 45% in 6- and 9-week-old eNOS gene knockout mice, respectively.
This altered pattern of bone remodeling was found to be age-dependent and by 12–18 weeks of age, which corresponds to the period of peak bone mass, bone histomorphometry parameters were primarily restored to wild-type levels. This suggests that the consequences of eNOS gene deficiency are most pronounced in the rapidly growing, neonatal and adolescent skeleton. Regardless of eNOS expression the histomorphometry data show significantly higher bone turnover and higher bone remodeling activity in young adult mice compared to older animals. Similar findings have also been reported in rats and humans demonstrating that remodeling activity is related to skeletal maturation. 33 In addition, we have previously shown that eNOS expression in rat femur is developmentally regulated and is most abundant in the bones of neonates and young adults and decreases markedly in older animals. From these data it is possible to suggest that eNOS expression is more abundant in younger animals and correlates to the higher bone metabolism and bone cell activity at these stages. Therefore as demonstrated in our histomorphometry studies lack of eNOS gene produced a higher impact in bone remodeling and turnover in pubescent young adult stages rather than in older adult mice.
How eNOS deficiency leads to these bone abnormalities is not altogether certain but on the basis of the histomorphometry data osteoblast dysfunction is implicated as a primary cause. We therefore sought to investigate the possible mechanism of impaired osteoblast function using a well-established in vitro model of osteoblast growth and differentiation. 35,38 Primary cultures of neonatal calvarial osteoblasts revealed marked disruption to the control of osteoblast proliferation and differentiation with both parameters being retarded significantly compared to wild-type osteoblasts. Importantly, such studies also showed that impaired osteoblast proliferation and differentiation could be reversed by the NO donor SNAP indicating that the disrupted function was related directly to loss of NO-dependent signaling and not a generalized effect. The importance of the eNOS-NO pathway was also demonstrated by the finding that eNOS−/− osteoblasts were unresponsive to the mitogenic effects of 17β-estradiol serving to confirm that NO-dependent signaling is important in mediating the effects of osteogenic factors such as estrogen. 18,19 We also found that eNOS−/− osteoblasts had an attenuated chemotaxis response and failed to migrate along a transforming growth factor-β gradient, which is known to be a potent cytokine in recruiting osteoblasts to remodeling sites. 36
Taken together, these data indicate that distinct components of the osteoblast phase of the bone remodeling cycle are altered in the eNOS−/− mice. The replacement of resorbed bone and the quality of the bone laid down during the remodeling process depend on a number of parameters. These include the spatially and temporally co-ordinated proliferation, recruitment, and differentiation of osteoblasts at resorption sites, the subsequent synthesis and maturation of bone matrix proteins and also by the overall life span of the osteoblast. 1-3 Our data demonstrate that NO-dependent signaling via eNOS is important in regulating several aspects of osteoblast biology including growth, differentiation, recruitment, and extracellular matrix synthesis. Although not specifically addressed in the present study the effects of eNOS gene deficiency will probably also impact on other important aspects of osteoblast biology. In particular, the eNOS-NO pathway is involved in mediating anabolic processes associated with mechanical loading and shear flow. 20-24 Other cellular signaling pathways that are likely to be directly affected by loss of an intact eNOS-NO pathway include those involving cGMP and cGMP-dependent protein kinase II that are known to be associated with the control of osteoblast replication 39 and bone growth. 40 Integrin expression and function are also known to regulated by eNOS/NO 41 and this may also affect osteoblasts in eNOS−/− mice, as integrins are crucial to osteoblast recruitment and differentiation 42 and are also involved in mediating mechanical loading signals. 43
The eNOS−/− mice were also found to have a slight (1 to 2 mm) reduction in femur length compared to wild-type mice. Although gross examination indicated there were no obvious differences in overall size or abnormalities in growth plate development (data not shown) between wild-type and knockout mice it is possible, based on the femur length that eNOS is associated with the control of skeletal growth. This would be in keeping with our recent work demonstrating that eNOS expression in rat bones is most abundant during the period of rapid growth from the neonatal to juvenile/young adult stages. 44 Moreover, Gregg and colleagues, 45 have described, in a separate eNOS knockout mouse model, a 10% incidence of limb reduction defects, although this was attributed more to malformation of limb capillaries.
The nature of the bone abnormalities observed using histomorphometry was also corroborated by DEXA analyses. These revealed significant reductions in regional bone mineral density including femur/pelvis and spine and also in total, whole-body bone mineral density. The DEXA analysis was performed specifically on female mice as a means to corroborate the bone abnormalities seen in males and to confirm that there were no marked differences associated with gender. Although a full comparative histomorphometric analysis of both male and female mice has yet to be performed the DEXA studies do at least suggest that there are no gross differences associated with gender in generating the abnormal bone phenotype expressed by eNOS−/− mice.
In addition to the osteoblast, NO is known to influence the function of the osteoclast. 9-16 In this study, eroded surface, which is an indicator of osteoclast activity, 31-33 was decreased by up to 50% in femora of 6-week-old eNOS−/− mice. In the absence of significant differences in osteoclast numbers between eNOS+/+ and eNOS−/− mice, this might indicate that eNOS is involved in the control of osteoclast activity and is supported by studies showing that osteoclasts contain eNOS and constitutive (eNOS) activity might be required to stimulate osteoclast activity. 9,16 However, it would seem that by comparison to the effects on osteoblast function and bone formation the overall impact of eNOS gene deficiency on the osteoclast and bone resorption is less important. Indeed, by 9 and 18 weeks of age there were no significant differences in eroded surface or osteoclast number. Such a phenomenon would also be in general agreement with the study of Corral and colleagues 46 demonstrating that bone resorption can proceed normally even after ablation of mature osteoblasts and in the absence of any new bone formation. Moreover, most studies have suggested that iNOS is probably more important in the control of osteoclast function. 9-16 This is also strongly supported by observations on iNOS knockout mice showing that control of osteoclast function and bone resorption in response to stimulation with cytokines is significantly disrupted. 47
Endothelial NOS gene knockout mice are also known to be hypertensive with an approximate 30% elevation in mean arterial blood pressure 30 that could also have an affect on bone turnover. Studies in rats and humans have demonstrated a correlation between the incidence of high blood pressure and increased risk of osteoporosis 48,49 and is thus possible that hypertension associated with eNOS deficiency might be a contributory factor to the genesis of bone abnormalities. Although this needs to be investigated in more detail there is good evidence to suggest that the major underlying cause of disrupted control of bone turnover is in fact related directly to loss of eNOS in the local bone environment rather than to a more global affect on blood pressure. For example, there is a substantial body of evidence to indicate that eNOS is expressed by osteoblasts/osteocytes 23,24,50 and is significant in mediating the local, anabolic actions of estrogen 18,19 as well as transducing the osteogenic effects associated with mechanical loading and shear flow. 20-24 The present study also provides in vivo and in vitro evidence of distinct perturbations to osteoblast growth, differentiation, and function in eNOS−/− mice. Furthermore, chronic administration of certain NOS inhibitors in adult rats significantly diminishes bone formation, independent of any marked affect on blood pressure. 12,29 Although such studies have tended to suggest that this was attributable more to inhibition of iNOS 12,29 than eNOS the compounds used are not sufficiently selective in their action to be able to state unequivocally which isoform(s) are involved. 37
Previous studies on NOS gene knockout mice have indicated that in certain tissues other NOS isoforms can substitute for the function of the disrupted one. 51 This is based on the absence of expected disrupted function within a particular cell or tissue of a NOS gene knockout animal, which can be subsequently revealed after administration of a NOS inhibitor. The use of NOS inhibitors has not been investigated in the study. Although this could reveal the potential contributions made by other NOS isoforms the fact that eNOS−/− mice do exhibit marked bone abnormalities suggests any contribution from either iNOS or nNOS is minimal. As such, these data support the consensus that eNOS represents the predominant NOS activity in control of local bone remodeling and in the development and maintenance of skeletal mass.
On the basis of nature of their bone abnormalities eNOS−/− mice might also prove to be a useful model to investigate mechanisms of metabolic bone turnover and the development of diseases such as osteoporosis in which NO is known to be involved. 18,52 However, by comparison to other osteoporosis-like phenotype models eNOS−/− mice are distinct. For example, osteoprotegerin-gene-deficient mice demonstrate an early onset osteoporosis-like phenotype, 53 similar to eNOS−/− mice but is because of increased osteoclast numbers and resorption rather than attenuated osteoblast activity. Moreover, studies in mice overexpressing transforming growth factor-β are known to express an osteoporotic phenotype primarily because of perturbation of osteoblast activity, but unlike eNOS−/− mice this phenotype is not expressed by adolescent mice but develops with increasing age. 54 The finding that eNOS−/− mice recover from the early onset osteoporosis-like phenotype after 3 to 4 months represents another relevant difference between this and other osteoporosis-like phenotype models. As discussed above, eNOS gene-deficiency seems to specifically retard osteoblastic activity and the consequences of this become most manifest during periods of rapid bone turnover as seen in the postnatal development of the skeleton. The consequences of pathological disruption to bone turnover such as estrogen-deplete osteoporosis have not been investigated in the present study but it would be reasonable to suggest that because of the reduced responsiveness of their osteoblasts eNOS−/− mice are likely to be rendered more sensitive to such endocrine imbalance. This may also result in defective or protracted bone repair mechanisms.
In summary, we have investigated the impact of eNOS gene deletion on the murine skeleton under normal physiological conditions. The data show that eNOS is involved in the postnatal regulation of bone mass and lack of eNOS gene results in reduced bone volume and bone formation. The data also suggest that these bone abnormalities are attributable primarily to the impaired osteoblast function and identify eNOS as a significant regulator of local bone formation and turnover.
Acknowledgments
We thank Professor Peter Revell (Department of Histopathology, Royal Free Hospital, London, UK) for advice on bone histomorphometry; and Dr. John Wharton and Dr. Anne Bishop (Department of Histochemistry, Imperial College School of Medicine, Hammersmith Campus, London, UK) for critical review of the manuscript.
Footnotes
Address reprint requests to Dr. Lee D. K. Buttery, Department of Histochemistry, Imperial College School of Medicine, Hammersmith Campus, Du Cane Rd., London W12 0NN, United Kingdom. E-mail: l.buttery@ic.ac.uk.
Supported by project grants awarded by The Wellcome Trust, the Dunhill Medical Trust, and by generous donations from the Golden Charitable Trust.
J. A. and L. B. contributed equally to this work.
References
- 1.Bab A, Einhorn T: Polypeptide factors regulating osteogenesis and bone marrow repair. J Cell Biochem 1994, 55:358-365 [DOI] [PubMed] [Google Scholar]
- 2.Parfitt A: Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem 1994, 55:273-286 [DOI] [PubMed] [Google Scholar]
- 3.Russel RGG, Croucher P, Oyajobi B, Rahman S, Rogers M, Scott A: Bone biology and pathophysiological mechanisms of bone disease. Hukkanen MVJ Polak JM Hughes SPF eds. Nitric Oxide in Bone and Joint Disease. 1998, :pp 21-38 Cambridge University Press, Cambridge [Google Scholar]
- 4.Moncada S, Higgs A: The L-arginine-nitric oxide pathway. N Engl J Med 1993, 329:2002-2012 [DOI] [PubMed] [Google Scholar]
- 5.Bredt D, Snyder S: Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA 1990, 87:682-685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollock J, Forstermann U, Mitchell J, Warner T, Murad F: Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci USA 1991, 88:10480-10484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Q, Cho H, Calaycay J, Munford R, Swiderek K, Lee T, Ding A, Troso T, Nathan C: Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 1992, 256:225-228 [DOI] [PubMed] [Google Scholar]
- 8.Gross S, Wolin M: Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol 1995, 57:737-769 [DOI] [PubMed] [Google Scholar]
- 9.Brandi M, Hakkanen M, Umeda T, Moradi-Bidhendi N, Bianchi S, Gross SS, Polak JM, MacIntyre I: Bidirectional regulation of osteoclast function by nitric oxide synthase isoforms. Proc Nat. Acad Sci USA 1995, 92:2954-2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collin-Osdoby P, Nickols A, Osdoby P: Bone cell function, regulation, and communication: a role for nitric oxide. J Cell Biochem 1995, 57:399-408 [DOI] [PubMed] [Google Scholar]
- 11.Helfrich M, Evans D, Grabowski P, Pollock JS, Ohshima H, Ralston SH: Expression of nitric oxide synthase isoforms in bone and bone cell cultures J Bone Miner Res 1997, 12:1108-1115 [DOI] [PubMed] [Google Scholar]
- 12.Kasten TP, Collin-Osdoby P, Patel N, Osdoby P, Krukowski M, Misko TP, Settle SL, Currie MG, Nickols GA: Potentiation of osteoclast bone-resorption activity by inhibition of nitric oxide synthase. Proc Natl Acad Sci USA 1994, 91:3569-3573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ralston SH, Ho LP, Helfrich MH, Grabowski PS, Johnston PW, Benjamin N: Nitric oxide: a cytokine-induced regulator of bone resorption. J Bone Miner Res 1995, 10:1040-1049 [DOI] [PubMed] [Google Scholar]
- 14.MacIntyre I, Zaidi M, Alam ASM, Datta HK, Moonga BS, Lidbury PS, Hecker M, Vane JR: Osteoclastic inhibition: an action of nitric oxide not mediated by cyclic GMP. Proc Natl Acad Sci USA 1991, 88:2936-2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sunyer T, Rothe L, Kirsch D, Jiang X, Anderson F, Osdoby P, Collin-Osdoby P: Ca2+ or phorbol ester but not inflammatory stimuli elevate inducible nitric oxide synthase messenger ribonucleic acid and nitric oxide (NO) release in avian osteoclasts: autocrine NO mediates Ca2+-inhibited bone resorption. Endocrinology 1997, 138:2148-2162 [DOI] [PubMed] [Google Scholar]
- 16.Ralston SH, Grabowski PS: Mechanisms of cytokine induced bone resorption: role of nitric oxide, cyclic guanosine monophosphate, and prostaglandins. Bone 1996, 19:29-33 [DOI] [PubMed] [Google Scholar]
- 17.Riancho J, Salas E, Zarrabeitia M, Olmos JM, Amado JA, Fernandez-Luna JL, Gonzalez-Macais J: Expression and functional role of nitric oxide synthase in osteoblast-like cells. J Bone Min Res 1995, 10:439-446 [DOI] [PubMed] [Google Scholar]
- 18.Wimalawansa SJ, De Marco G, Gangula P, Yallampalli C: Nitric oxide donor alleviates ovariectomy-induced bone loss. Bone 1996, 18:301-304 [DOI] [PubMed] [Google Scholar]
- 19.Armour K, Ralston SH: Estrogen upregulates endothelial constitutive nitric oxide synthase expression in human osteoblast-like cells. Endocrinology 1998, 139:799-802 [DOI] [PubMed] [Google Scholar]
- 20.Pitsillides AA, Rawlinson SC, Suswillo RF, Bourrin S, Zaman G, Lanyon LE: Mechanical strain-induced NO production by bone cells: a possible role in adaptive bone (re)modeling? FASEB J 1995, 9:1614-1622 [DOI] [PubMed] [Google Scholar]
- 21.Fox S, Chambers T, Chow J: Nitric oxide is an early mediator of the increase in bone formation by mechanical stimulation. Am J Physiol 1996, 270:E955-E960 [DOI] [PubMed] [Google Scholar]
- 22.Turner C, Takano Y, Owan I, Murrell C: Nitric oxide inhibitor L-NAME suppresses mechanically induced bone formation in rats. Am J Physiol 1996, 270:E634-E639 [DOI] [PubMed] [Google Scholar]
- 23.Zaman G, Pitsillides AA, Rawlinson SCF, Suswillo RFL, Mosley JR, Cheng MZ, Platts LAM, Hukkanen M, Polak JM, Lanyon LE: Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res 1999, 14:1123-1132 [DOI] [PubMed] [Google Scholar]
- 24.Klein-Nulend J, Helfisch MH, Sterck JG, MacPherson H, Jolderman M, Ralston SH, Semeins CM, Burger EH: Nitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependent. Biochem Biophys Res Commun 1998, 250:108-114 [DOI] [PubMed] [Google Scholar]
- 25.Ralston S, Todd D, Helfrich M, Benjamin N, Gragowski P: TI: human osteoblast-like cells produce nitric oxide and express inducible nitric oxide synthase. Endocrinology 1994, 135:330-336 [DOI] [PubMed] [Google Scholar]
- 26.Löwik CWGM, Nibbering PH, van de Ruit M, Papapoulos SE: Inducible production of nitric oxide in osteoblast-like cells and in fetal mouse bone explants is associated with suppression of osteoclastic bone resorption. J Clin Invest 1994, 93:1465-1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hukkanen M, Hughes FJ, Buttery LD, Gross SS, Evans TJ, Seddon S, Riveros-Moreno V, MacIntyre I, Polak JM: Cytokine-stimulated expression of inducible nitric oxide synthase by mouse, rat, and human osteoblast-like cells and its functional role in osteoblast metabolic activity. Endocrinology 1995, 136:5445-5453 [DOI] [PubMed] [Google Scholar]
- 28.Tsukahara H, Miura M, Tsuchida S, Hata I, Hata K, Yamamoto K, Ishii Y, Muramatsu I, Sudo M: Effect of nitric oxide synthase inhibitors on bone metabolism in growing rats. Am J Physiol 1996, 270:E840-E844 [DOI] [PubMed] [Google Scholar]
- 29.Turner CH, Owan I, Jacob DS, McClintock, Peacock M: Effects of nitric oxide synthase inhibitors on bone formation in rats. Bone 1997, 21:487–490 [DOI] [PubMed]
- 30.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowowitz MA, Bevan JA, Fishman MC: Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 1995, 377:239-242 [DOI] [PubMed] [Google Scholar]
- 31.Baron R, Vignery A, Neff L, Silvergate A, Santa Maria A: Processing of undecalicfied bone specimens for bone histomorphometry. Bone Histomorphometry: Techniques and Interpretation. 1983:pp 13-35 FL, CRC Press, Boca Raton
- 32.Parfitt A: A stereological basis of bone histomorphometry; theory of quantitative microscopy and reconstruction of the third dimension. Recker R eds. Bone Histomorphometry Techniques and Interpretation. 1983, :pp 143-223 FL, CRC Press, Boca Raton [Google Scholar]
- 33.Kimmel D, Jee W: Bone cell kinetics during longitudinal bone growth in the rat. Calcif Tissue Int 1980, 32:113-122 [DOI] [PubMed] [Google Scholar]
- 34.Parfitt A: Bone histomorphometry: proposed system for standardization of nomenclature, symbols and units. Calcif Tissue Int 1988, 42:284-286 [DOI] [PubMed] [Google Scholar]
- 35.Wong GL, Cohn DV: Separation of parathyroid hormone and calcitonin sensitive cells from non-responsive bone cells. Nature 1974, 252:713-715 [DOI] [PubMed] [Google Scholar]
- 36.Pfeilschifter J, Wolf O, Nautmann A, Minne HW, Mundy GR, Zeigler R: Chemotactic response of osteoblast-like cells to TGF-β. J Bone Miner Res 1990, 5:825-830 [DOI] [PubMed] [Google Scholar]
- 37.Siriwardena D, Tagori H, Thiemermann C: Nitric oxide synthase inhibitors. Evans TJ eds. Septic Shock Methods and Protocols. 2000, :pp 115-133 NJ, Humana Press, Totowa [DOI] [PubMed] [Google Scholar]
- 38.Beresford JN, Graves SE, Smoothy CA: Formation of mineralised nodules by bone derived cells in vitro: a model of bone formation? Am J Med Genet 1993, 45:163-178 [DOI] [PubMed] [Google Scholar]
- 39.Mancini L, Moradi-Bidhendi M, Becherini L, Martineti V, MacIntyre I: The biphasic effects of nitric oxide in primary rat osteoblasts are cGMP dependent. Biochem Biophys Res Comm 2000, 274:477-481 [DOI] [PubMed] [Google Scholar]
- 40.Pfeifer A, Aszodi A, Seidler U, Ruth P, Hofmann F, Fassler R: Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science 1996, 274:2082-2086 [DOI] [PubMed] [Google Scholar]
- 41.Murohara T, Witzenbichler B, Spyridopoulos I, Asahara T, Ding B, Sullivan A, Losordo DW, Isner JM: Role of endothelial nitric oxide synthase in endothelial cell migration. Arterioscler Thromb Vasc Biol 1999, 19:1156-1161 [DOI] [PubMed] [Google Scholar]
- 42.Damsky CH: Extracellular matrix-integrin interactions in osteoblast function and tissue remodeling. Bone 1999, 25:95-96 [DOI] [PubMed] [Google Scholar]
- 43.Wang N, Ingber DE: Control of cytoskeletal mechanisms by extracellular matrix, cell shape and mechanical tension. Biophys J 1994, 66:2181-2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hukkanen MVJ, Platts LAM, Fernandez de Marticorena I, O’Shaughnessy M, MacIntyre I, Polak JM: Developmental regulation of nitric oxide synthase expression in rat skeletal bone. J Bone Miner Res 1999, 12:868-877 [DOI] [PubMed] [Google Scholar]
- 45.Gregg AR, Schauer A, Shi O, Liu Z, Lee CG, O’Brien WE: Limb reduction defects in endothelial nitric oxide synthase-deficient mice. Am J Physiol 1998, 275:H2319-H2324 [DOI] [PubMed] [Google Scholar]
- 46.Corral DA, Amling M, Priemel M, Loyer E, Fuchs S, Ducy P, Baron R, Karsenty D: Dissociation between bone resorption and bone formation in osteopenic transgenic mice. Proc Natl Acad Sci USA 1998, 95:3835-3840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van’t Hof RJ, Armour KJ, Liew FY, Wei X, Ralston SH: Studies in the inducible nitric oxide synthase knockout mouse reveal an essential role for nitric oxide in cytokine-induced bone resorption (abstr. T052). Bone 1998, 216
- 48.Stimpel M, Jee WS, Ma Y, Yamamoto N, Chen Y: Impact of antihypertensive therapy on postmenopausal osteoporosis: effects of the angiotensin converting enzyme inhibitor moexipril, 17beta-estradiol and their combination on the ovariectomy-induced cancellous bone loss in young rats. J Hypertens 1995, 13:1852-1856 [PubMed] [Google Scholar]
- 49.Cappuccio FP, Meilahn E, Zmuda JM, Cauley JA: High blood pressure and bone-mineral loss in elderly white women: a prospective study. Study of Osteoporotic Fractures Research Group. Lancet 1999, 354:971-975 [DOI] [PubMed] [Google Scholar]
- 50.Fox S, Chow J: Nitric oxide synthase expression in bone cells. Bone 1998, 23:1-6 [DOI] [PubMed] [Google Scholar]
- 51.Huang P, Fisherman M: Genetic analysis of nitric oxide synthase isoforms: targeted mutation in mice. J Mol Med 1996, 74:415-421 [DOI] [PubMed] [Google Scholar]
- 52.Rosselli M, Imthurn B, Keller PJ, Jackson EK, Dubey RK: Circulating nitric oxide (nitrite/nitrate) levels in postmenopausal women substituted with 17β-estradiol and norethisterone acetate. Hypertension 1995, 25:848-853 [DOI] [PubMed] [Google Scholar]
- 53.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ: Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 1998, 12:1260-1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erlebacher A, Deryck R: Increased expression of TGF-b2 in osteoblasts results in an osteoporosis-like phenotype. J Cell Biol 1996, 132:195-210 [DOI] [PMC free article] [PubMed] [Google Scholar]