Abstract
To establish whether allergic asthma could be induced experimentally in a nonhuman primate using a common human allergen, three female rhesus monkeys (Macaca mulatta) were sensitized with house dust mite (Dermatophagoides farinae) allergen (HDMA) by subcutaneous injection, followed by four intranasal sensitizations, and exposure to allergen aerosol 3 hours per day, 3 days per week for up to 13 weeks. Before aerosol challenge, all three monkeys skin-tested positive for HDMA. During aerosol challenge with HDMA, sensitized monkeys exhibited cough and rapid shallow breathing and increased airway resistance, which was reversed by albuterol aerosol treatment. Compared to nonsensitized monkeys, there was a fourfold reduction in the dose of histamine aerosol necessary to produce a 150% increase in airway resistance in sensitized monkeys. After aerosol challenge, serum levels of histamine were elevated in sensitized monkeys. Sensitized monkeys exhibited increased levels of HDMA-specific IgE in serum, numbers of eosinophils and exfoliated cells within lavage, and elevated CD25 expression on circulating CD4+ lymphocytes. Intrapulmonary bronchi of sensitized monkeys had focal mucus cell hyperplasia, interstitial infiltrates of eosinophils, and thickening of the basement membrane zone. We conclude that a model of allergic asthma can be induced in rhesus monkeys using a protocol consisting of subcutaneous injection, intranasal instillation, and aerosol challenge with HDMA.
The prevalence of asthma, especially in children, has been increasing in industrialized countries throughout the last 30 years with ∼10% of the population currently being affected. 1 To better understand the mechanisms involved in the pathogenesis and pathophysiology of allergic asthma, several species of laboratory animals have been used as models. Although animal models of asthma have been critical to the understanding of immunological mechanisms, the investigation of pathophysiological mechanisms of asthma has been hindered by the lack of an experimental animal model that closely replicates the main features of the disease found in human asthmatics, including: immunological alterations, paroxysmal bronchoconstriction after allergen challenge, variable but persistent airway hyperresponsiveness, and airways remodeling. Although not used extensively as a model of allergic asthma, the rhesus monkey (Macaca mulatta) would seem to be well suited for this purpose for two reasons: 1) the immune system of nonhuman primates is similar to that of humans, 2-6 and 2) all of the components of the intrapulmonary conducting airways that are altered in human asthmatics are found throughout the intrapulmonary airway tree of adult rhesus monkeys and, as in humans, undergo extensive postnatal development and maturation. 7-10 To establish whether allergic asthma could be induced experimentally using a known human allergen, we used a sensitization protocol similar to that described by Yasue et al. 6 We sensitized adult rhesus monkeys to house-dust mite (Dermatophagoides farinae) allergen by subcutaneous injection. We followed this sensitization with a series of intranasal and aerosolized house-dust mite allergen (HDMA) challenges. When compared to nonsensitized, nonexposed monkeys, the HDMA-sensitized monkeys developed an allergic response with the characteristics used to define allergic asthma in humans. 1
Materials and Methods
Animals and Experimental Protocol
Young adult rhesus monkeys (4.9 to 7.0 kg body weight) were used in this study. Three adult female monkeys were sensitized using an expanded protocol consisting of subcutaneous injection and intranasal placement of allergen as described in Table 1 ▶ . The animals were subsequently exposed to HDMA as an aerosol (see Table 2 ▶ ) at 17 weeks after the initial injection and weekly beginning on week 33 until time of necropsy (week 42 or 45 after the initial injection). The allergen was commercially available house-dust mite extract (Greer Laboratories, Inc., Lenoir, NC) diluted in phosphate buffered saline and nebulized with a high-flow rate nebulizer (HEART; Westmed, Inc., Tucson, AZ) immersed in an ice-water bath during operation to reduce water evaporation from the solution. From the nebulizer, polydispersed droplets with a volume median diameter of ∼2 μm were produced in an air stream of 19.9 liters per minute. The electrostatic charge on the droplets was reduced to the Boltzman equilibrium by conveyance through a krypton-85 discharging column. 11 The aerosol was then mixed with the inlet air stream of a 4.2-m 3 volume exposure chamber 12 to yield an aerosol of dry particles composed of allergen and salt residues in the chamber. During exposure, air samples were drawn from the animal breathing zone of the chamber to characterize this aerosol. Mass concentrations were measured by weighing samples collected on preweighed Teflon-coated glass fiber filters (Pallflex Emfab; Pall Gelman Sciences, Ann Arbor, MI). Selected mass concentration samples were also submitted to the University of California–Davis Molecular Structure Facility to determine the protein content of the aerosol by amino acid analysis (System 6300, System Gold software; Beckman Coulter, Inc., Fullerton, CA). 13 Aerodynamic size distributions were determined using a seven-stage Mercer-type cascade impactor (ARIES, Inc., Davis, CA). 14 The content of chloride anion derived from saline residue in the particles was measured on each impactor stage and the after-filter by ion chromatography (Model DX-120; Dionex Corp., Sunnyvale, CA). A log-normal distribution was fitted to each sample set of data, and the values reported are the mass median aerodynamic diameter and the geometric SD (ςg) of the fitted distributions. Additional adult rhesus monkeys from the colony, all of whom had negative skin tests for HDMA, served as controls.
Table 1.
House-Dust Mite Allergen Sensitization, Exposure, and Procedure Schedule in Adult Rhesus Monkeys
| Time (days) | Procedure |
|---|---|
| 0 | 12.5 mg HDMA in 10 mg A1OH, SQ and 2.5× 1011 killed Boardetella pertussis cell i.m. as adjuvant |
| 14,15,16 | 94 mg HDMA intranasally under anesthesia |
| 30 | 12.5 mg HDMA in 10 mg A1OH, SQ |
| 46 | 94 mg HDMA intranasally under anesthesia |
| 74 | 12.5 mg HDMA in 10 mg A1OH, SQ |
| 105 | Skin testing for HDMA |
| 125 | HDMA aerosol given via facemask |
| 138 | HDMA aerosol challenge |
| 165 | HDMA aerosol challenge |
| 172 | Methacholine aerosol challenge |
| 227–312 | 2–3 hour HDMA aerosol, chamber exposure, approximately every 3 days |
| 243 | Methacholine aerosol challenge |
| 256 | Methacholine aerosol challenge |
| 262 | HDMA aerosol challenge |
| 282 | Methacholine aerosol challenge |
| 289–312 | Necropsy |
Table 2.
Characterization of Dermatophagoides farinae Allergen Aerosol in Chamber during Exposure of Rhesus Monkeys
| Aerosol concentrations from filter samples | Number of samples | ||
|---|---|---|---|
| Amino acid | 0.437 + 0.069 | 2 | |
| analysis | |||
| mg/m3,±SD | |||
| protein | |||
| Total mass | 7.26 + 0.57 | 40 | |
| mg/m3, ±SD | |||
| Aerodynamic size from cascade impactor samples* | |||
| MMAD† | 1.03–0.12 | 3 | |
| μm,±SD | |||
| ςg‡, ±SD | 2.44–0.07 | 3 | |
*Log-normal distribution fitted to chloride content of stages and after-filter.
†Mass median aerodynamic diameter.
‡Geometric standard deviation.
Intradermal Skin Testing
Monkeys were anesthetized with ketamine hydrochloride and hair was clipped from an area of the thorax. Three separate intradermal injections (0.1 ml each) of phosphate-buffered saline (PBS) containing either HDMA (1:1000 w/v), histamine (1:1,000), or diluent alone were made at the site. Injection sites were observed for 30 minutes and wheal diameters were measured after 20 minutes (Table 3) ▶ .
Table 3.
Comparison of Skin Reactivity to HDMA in Sensitized and Control Rhesus Monkeys
| Group | Monkey ID no. | Wheal size (mm) | ||
|---|---|---|---|---|
| Histamine | Diluent | HDMA | ||
| Control | 29644 | 16 | 9 | 10 |
| Control | 28911 | 15 | 6 | 6 |
| Control | 30229 | 11 | 1 | 0 |
| Control | 30250 | 18 | 11 | 9 |
| Control | 29841 | 15 | 7 | 7 |
| Control | 29686 | 12 | 6 | 6 |
| Sensitized | 27796 | 11 | 9 | 13 |
| Sensitized | 28213 | 13 | 8 | 15 |
| Sensitized | 28354 | 13 | 9 | 15 |
Sedation and Anesthesia
Monkeys were sedated using Telazol (8 mg/kg, i.m., Fort Dodge Labs, Fort Dodge, IA) and maintained (if necessary) with ketamine hydrochloride (5 to 10 mg/kg, i.m.). For evaluation of airway responsiveness and bronchoscopy, the monkeys were anesthetized using Diprivan (0.1 to 0.2 mg/kg/minutes, i.v., Astra Zeneca Pharmaceuticals, Wilmington, DE) with the dose adjusted as deemed necessary by the attending veterinarian, intubated with a cuffed endotracheal tube (4.5 to5.0 mm), and placed in a head-out body plethysmograph with the intubation tube or facemask attached to a pneumatic four-way valve/pneumotachograph assembly. For necropsy, sedated animals were deeply anesthetized with intravenous sodium pentobarbital, the trachea intubated, and the animal maintained on positive pressure respiration (15.0 ml/kg, 20.0 bpm, 10 to 20 minutes) until killed by exsanguination via the systemic aorta.
Fluorometric Determination of Histamine Concentration
Histamine was measured in plasma using a previously published fluorometric method. 15 Histamine concentrations in samples were determined by comparison to a standard curve using known concentrations of histamine (range, 0.005 to 0.5 μg/ml).
Bronchoalveolar Lavage and Differential Cell Counts
Bronchoalveolar lavage specimens were obtained by bronchoscopy of sedated monkeys 24 hours after airway responsiveness testing by instillation of 10 ml of endotoxin-free PBS (Sigma, St. Louis, MO) and collected from the right caudal lobe at the time of necropsy (150 ml). For leukocyte differentials, lavage samples were cytocentrifuged, air dried, stained with a modified Wright’s stain (Diff-Quik) and the proportion of macrophages, neutrophils, eosinophils, lymphocytes, and epithelial cells determined by counting 300 cells/monkey by light microscopy (×1000).
Immunophenotyping of Leukocytes by Flow Cytometry
Peripheral blood mononuclear cells were freshly isolated using a Ficoll density gradient (Histopaque 1077; Sigma, St. Louis, MO), washed Ca/Mg-free Hanks’ balanced salt solution, counted, resuspended (1 to 2 × 10 5 cells per sample) in PBS (with 2.5% calf serum and 0.1% sodium azide) for multicolor immunophenotyping as previously described. 16 Immunostaining for lymphocyte characterization using the following antibodies: mouse monoclonal anti-human CD2 (fluorescein isothiocyanate- conjugated), CD4 (phycoerythrin) and CD25 (DAKO, Carpinteria, CA), CD3 (fluorescein isothiocyanate-conjugated) (Pharmingen, San Diego, CA), and goat F(ab′)2 anti-mouse immunoglobulins (phycoerythrin-Cy5) (Southern Biotechnology Associates, Inc., Birmingham, AL). Two- and three-color analysis was performed on a FACScan flow cytometer in list mode, acquiring 30,000 to 50,000 events per sample and analyzed with CELLQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Pulmonary Mechanics and Airways Responsiveness Testing
Pulmonary mechanics were measured using a transfer impedance method 17 with monkeys breathing spontaneously through a pneumotachograph (Fleisch no. 2) while the thorax is vibrated using a pseudo-random noise waveform encompassing frequencies of 2 to 128 Hz by two speakers mounted in the walls of the head-out plethysmograph (Pulmetrics Group, Boston, MA) and flow and pressure changes measured (4 second intervals) using a Microswitch transducer (model 743PC). Transfer impedance (Ztr) was calculated as the ratio of the Fourier transform of pressure inside the box versus the Fourier transform of airway flow at each frequency. 17 Airway resistance (Raw) was obtained by fitting the impedance data to the six-element model of the respiratory tract proposed by DuBois and colleagues 18 using a gradient optimization technique. 19 Because only five of the six elements can be uniquely estimated 20 using this technique, we restricted Cg to a constant value determined using a published regression equation relating functional residual capacity and body weight for rhesus monkeys. 21 Tidal volume and breathing frequency were recorded on a breath-by-breath basis by integrating the output of the pneumotachograph using a digital data acquisition system (Po-Ne-Mah, Inc., Valley View, OH). Arterial oxygen saturation (O2Sat%) was monitored via a pulse oximeter (MOD II) and then recorded at the beginning and ending of each data collection period.
All allergen and histamine challenges were administered as aerosols at a set tidal volume and breathing frequency (15.0 ml/kg and 20.0 bpm) using a compressed air nebulizer (Vortran, Inc.) in series with a positive pressure ventilator (Bird Mark 7A respirator). Allergen challenges used a set HDMA concentration (0.02 mg protein/ml) delivered at repeated 5-minute intervals separated by 60-second data collection periods and were terminated when Raw doubled. Data were expressed as the cumulative dose of allergen (mass concentration of allergen in mg protein/ml × tidal volume in ml × number of breaths) that doubles Raw (CDA200Raw). Histamine challenges used repeated 30-second challenge periods separated by 240-second data collection periods, beginning with saline followed by doubling concentrations starting at 0.0625 mg/ml and ending at the concentration that doubles Raw. Data were expressed as the concentration that increases Raw by 150% (EC150Raw). The CDA200Raw and EC150Raw were determined by linear interpolation on the log-log plot of the dose-response curve with the response expressed as the percent of baseline Raw.
HDMA-Specific IgE Concentrations in Serum
Allergen-specific IgE was measured in serum samples by enzyme-linked immunosorbent assay. Briefly, 96-well microtiter plates were sensitized with 3 μg/well HDMA in carbonate/bicarbonate buffer. Samples were precipitated with 35% saturated ammonium sulfate to remove interfering IgG, and supernatants were used as sample without further dilution. Binding of IgE was detected with a goat anti-human IgE-horseradish peroxidase conjugated antibody (Bethyl Laboratories, Inc., Montgomery, TX) Positive controls consisted of pooled human HDMA IgE-positive sera (RAST tested high level). Negative controls consisted of PBS and serum from 18 HDMA skin test-negative monkeys. Data are presented as ratios of sample to negative monkey serum.
Necropsy and Histopathology
After exsanguination, the thorax was opened by midline incision and the entire mediastinal contents removed en bloc. The trachea and extrapulmonary bronchi were exposed and the lobar bronchi cannulated. Separate lobes were fixed by airway infusion with 1% paraformaldehyde (left cranial lobe), 4% paraformaldehyde (right cranial lobe), or glutaraldehyde/paraformaldehyde (1%/1%) (right middle lobe) at 30-cm hydrostatic pressure. A cross-section of the trachea was also fixed in each fixative. The airway tree of the caudal segment of the left cranial lobe was exposed by microdissection and stored in PBS for whole mount histochemistry. 22 The other fixed lobes were sliced (5 to 10 mm thick) perpendicular to the long axis of the axial intrapulmonary airway and all slices embedded in paraffin (left and right cranial) or Araldite (right middle). 22 Whole mounts of the airways exposed by microdissection were evaluated for the presence of major basic protein (antibody no. RDI-CBL 419; Research Diagnostics,Inc., Flanders, NJ) by whole mount immunoperoxidase 22 or for mucus glycoproteins by periodic acid-Schiff (PAS)/Alcian blue. 23 After evaluation by stereomicroscopy, portions of the whole mounts were embedded in methacrylate and sectioned at 1 μm. Araldite sections (1 μm) were stained with toluidine blue or methylene blue/azure II.
Immunohistochemical Detection of IgE-Producing Cells in Monkey Lung Sections
Paraffin sections of paraformaldehyde-fixed lung from airway generations 2, 3, and 4 were examined for the presence of IgE-producing cells by immunoperoxidase staining with anti-human IgE (Bethyl Laboratories, Inc.) using a standard protocol. 24 Two sections were examined for each airway generation of lung from the three sensitized and three nonsensitized monkeys.
Statistical Analysis
All data are reported as mean ± SD. Groups were compared using Student’s t-test with the significance level set at P < 0.05.
Results
Intradermal Skin Tests
As presented in Table 3 ▶ , all three sensitized monkeys developed positive intradermal skin tests to HDMA on day 105 after initiation of the sensitization protocol. In sensitized monkeys, wheal size was greater than either the positive histamine control or the negative diluent. This was not the case for control monkeys from the colony.
Airway Responsiveness to Allergen and Histamine
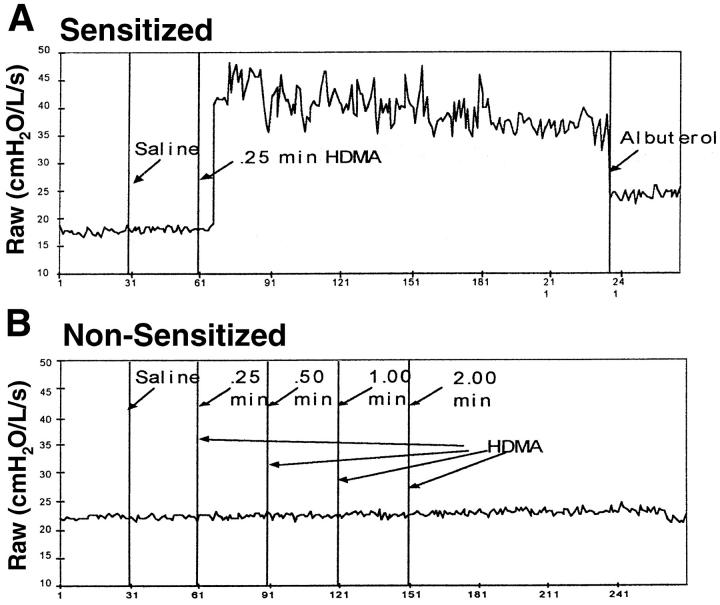
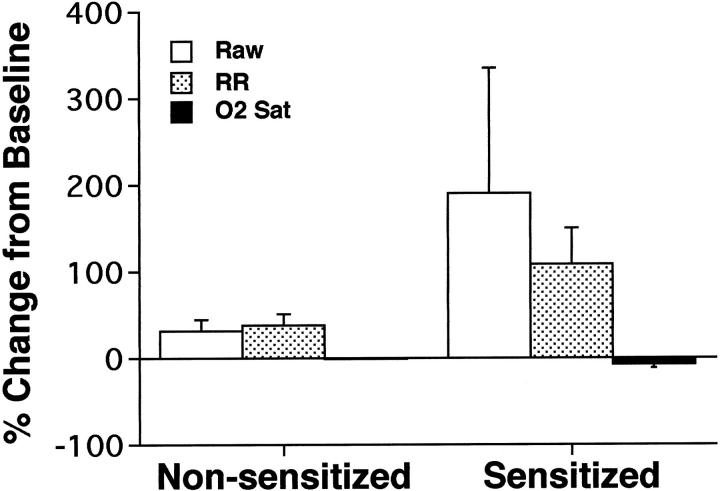
As previously shown by Madwed and Jackson, 17 the DuBois six element model of the respiratory tract 18 provided an excellent fit to the Ztr data collected in rhesus monkeys in both control and obstructed conditions. All of the HDMA-sensitized monkeys developed marked airway obstruction after inhalation of a HDMA aerosol compared to nonsensitized monkeys that show no obstructive response (Figures 1 and 2) ▶ ▶ . Two of the monkeys (MMU4 and MMU6) demonstrated an obstructive response to HDMA aerosol with their first HDMA challenge whereas one did not develop this response until after multiple HDMA challenges (MMU5). The obstructive response in HDMA-sensitized monkeys was associated with a marked tachypnea and a decrease in arterial oxygen saturation (Figure 2) ▶ . The tachypnea was often associated with apnea, bradycardia, and hypotension. Forty-eight to 72 hours after a HDMA aerosol challenge inhalation of doubling doses of histamine resulted in a progressive increase in Raw in all monkeys studied (Figure 3) ▶ . The concentration of histamine aerosol required to produce a 150% increase in Raw was significantly less (P < 0.03) in HDMA-sensitized monkeys than that required in nonsensitized monkeys (Figure 3B) ▶ .
Figure 1.
Raw response of a sensitized (A) and a nonsensitized (B) rhesus monkey to house-dust mite allergen aerosol.
Figure 2.
Comparison of physiological responses to house-dust mite allergen aerosol in sensitized and nonsensitized rhesus monkeys. RR, respiratory rate; O2 Sat, arterial blood oxygen saturation.
Figure 3.
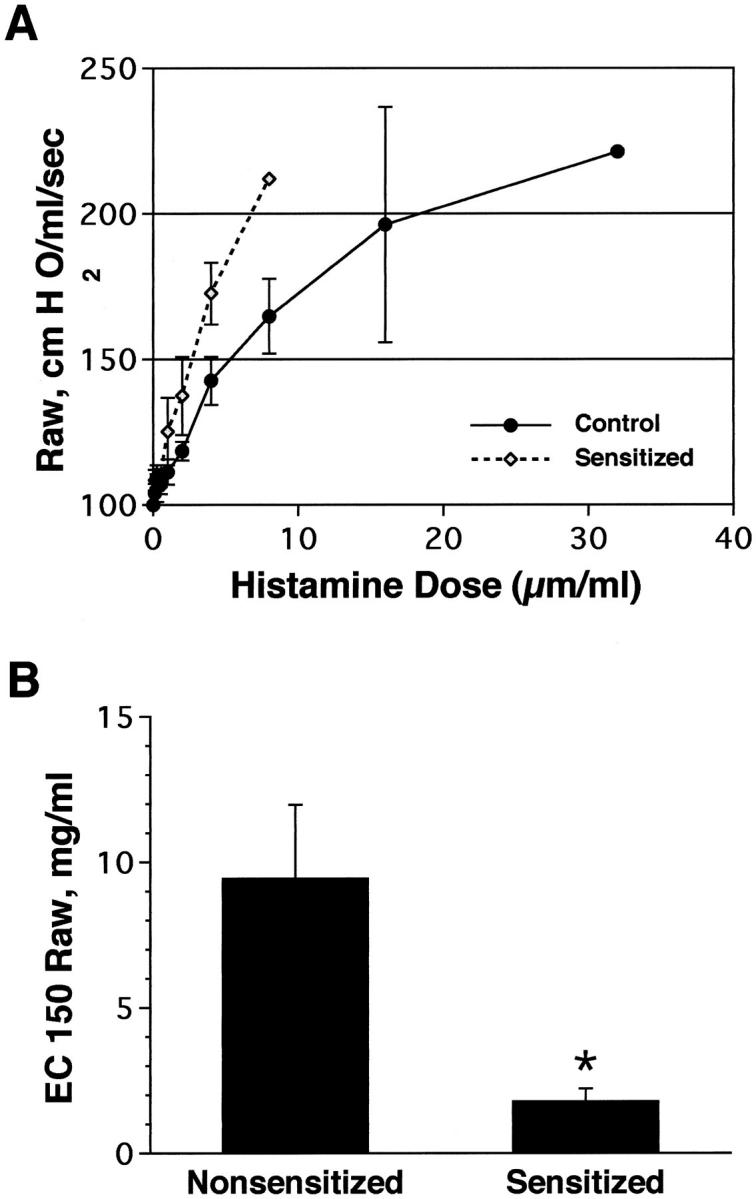
A: Comparison of percentage change in Raw as a function of increasing inhaled histamine concentrations (mg/ml) in sensitized and nonsensitized (control) rhesus monkeys. B: Comparison of effective concentration of histamine to produce a 150% (EC150) increase in Raw in sensitized and nonsensitized (control) rhesus monkeys.
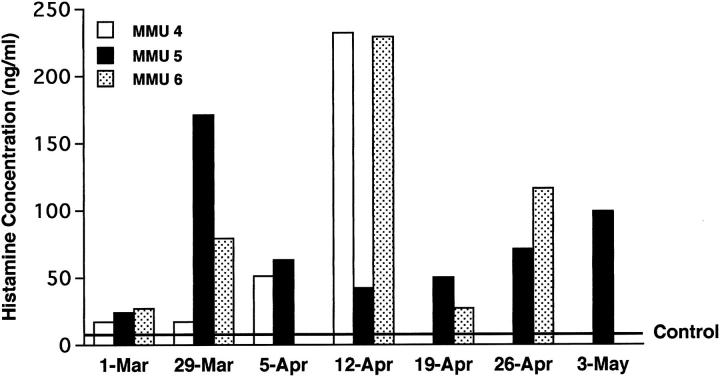
Histamine in Plasma
Plasma concentrations of histamine in all three sensitized monkeys were elevated above levels in plasma of nonsensitized monkeys within 30 minutes after completion of an aerosol challenge, although the responses did not always occur at the same time points in the exposure protocol (Figure 4) ▶ .
Figure 4.
Concentration of histamine in plasma of sensitized rhesus monkeys (MMU 4, 5, 6) after aerosol exposure to HDMA. Histamine concentrations in plasma of nonsensitized (control) monkeys, marked as a line, were 7.83 ± 1.33 (mean ± 1 SD).
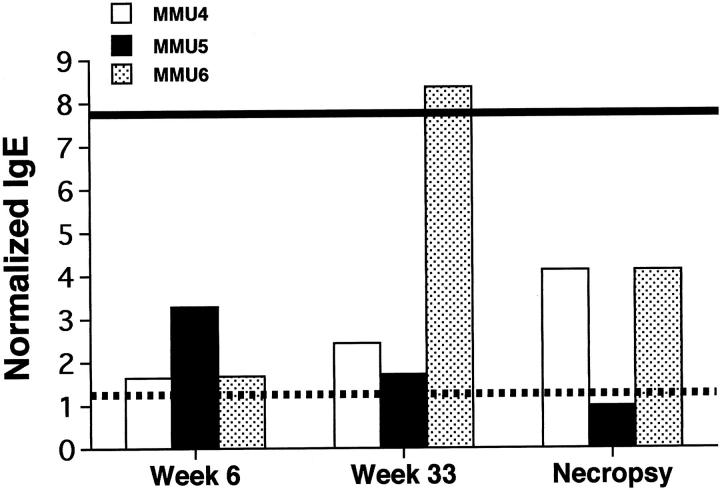
HDMA-Specific IgE in Serum
Concentrations of specific IgE in serum were significantly (P < 0.05) greater than negative control after week 6 of sensitization for all three sensitized monkeys (Figure 5) ▶ . At week 33 after aerosol challenge, the serum level of HDMA-specific IgE in MMU6 surpassed the mean value of the positive control. At necropsy MMU4 and MMU6 showed markedly elevated IgE serum concentrations.
Figure 5.
Comparison of serum levels of HDMA-specific IgE at three time points during the HDMA aerosol exposure protocol in three sensitized rhesus monkeys. Values are normalized to the mean HDMA-specific IgE level obtained from 18 rhesus monkeys that had negative skin tests for HDMA. The dashed line is the 95% confidence interval obtained from the nonsensitized monkeys. The solid line is the serum HDMA-specific IgE level in a pool of samples obtained from human patients with class 5 allergy to HDMA according to RAST (source: University of California Davis Medical Center).
Contents of Bronchoalveolar Lavage and Airway Exudates
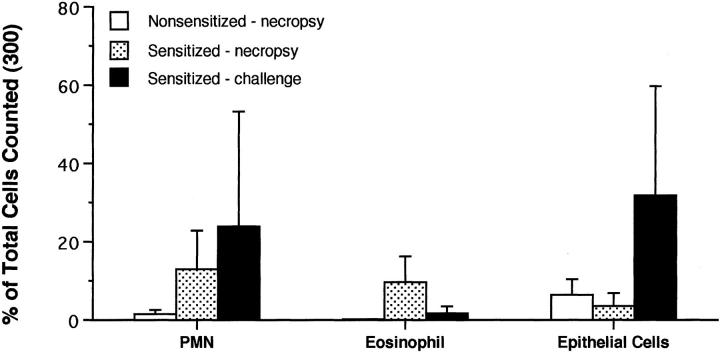
At necropsy, all three allergen-sensitized monkeys exhibited an increase in eosinophils within lavage specimens, as compared with control animals (Figure 6) ▶ . Within lavage obtained at ∼6 months after initial sensitization with HDMA, there was an increase in number of epithelial cells, predominantly large patches of ciliated columnar epithelium, from two out of three dust mite-sensitized monkeys, as compared with control animals. There were no differences in overall numbers of lymphocytes and granulocytes within blood samples among all monkeys studied, regardless of the time point during the exposure protocol when specimens were taken for evaluation.
Figure 6.
Comparison of cell composition of bronchoalveolar lavage from sensitized and nonsensitized rhesus monkeys. Cells were categorized as neutrophils (PMN), eosinophils (Eos), or epithelial cells (Epi). The values are mean ±1 SD.
Immunophenotyping of T Lymphocytes
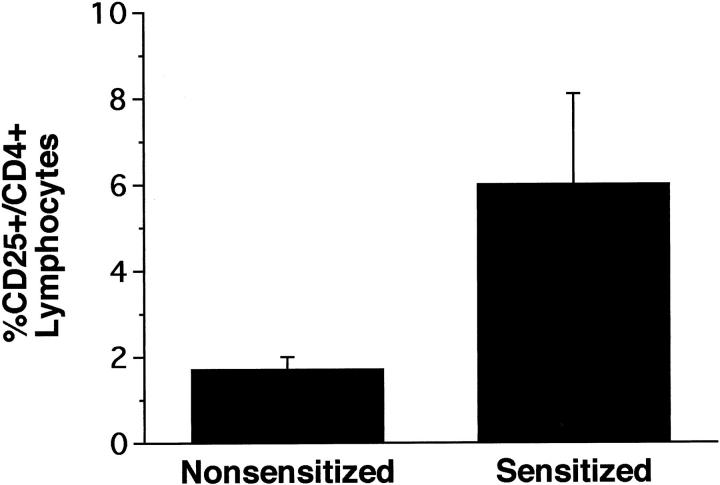
At necropsy, the proportion of the CD4+ lymphocyte population in peripheral blood of sensitized monkeys that were CD25+ (IL-2 receptor) increased over that observed in nonsensitized monkeys (Figure 7) ▶ . This functional shift in the expression of CD25 on CD4-positive cells within blood was variable among the three sensitized monkeys, with two of the monkeys having threefold to fourfold increases and the other being at the upper range for nonsensitized monkeys. However, this latter monkey had an increase in CD2+/CD25+ cells that were not either a CD4 or CD8 phenotype.
Figure 7.
Comparison of the percentage of CD25+/CD4+ T lymphocytes in peripheral blood of sensitized and nonsensitized rhesus monkeys taken at necropsy.
Histopathology and Immunohistochemistry
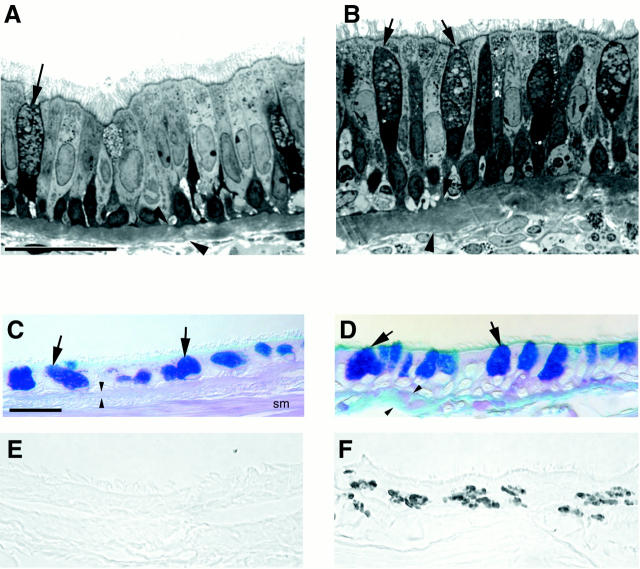
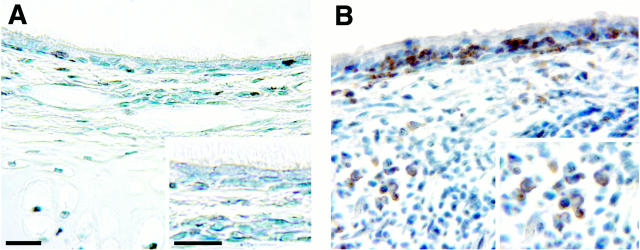
Compared to nonsensitized control animals (Figure 8A) ▶ , there was mucous goblet cell hyperplasia, thickening of the basement membrane zone, and hypercellularity in the epithelium and subepithelial connective tissue in the trachea and in proximal and distal intrapulmonary bronchi of allergen-sensitized rhesus monkeys (Figure 8B) ▶ . These changes were focal and varied greatly within various portions of the airway wall in the same airway generation, between airway generations in an individual sensitized and challenged animal, and from animal to animal in the sensitized and challenged group. Based on staining with PAS/Alcian blue, mucous goblet cells in the intrapulmonary airways of sensitized monkeys (Figure 8D) ▶ were not only more abundant, but contained more stored secretory product, than did mucous cells in nonsensitized monkeys (Figure 8C) ▶ . In areas of the airway walls with elevated cellular infiltrates, many of the infiltrating cells had multilobed nuclei and were positive for immunodetectable major basic protein (Figure 8F) ▶ . In areas of the airway wall of sensitized animals where inflammatory cells were abundant, the basement membrane zone was markedly reduced compared to control animals. In contrast, the basement membrane zone appeared to be thickened in many areas that were free of inflammatory cells. Immunoreactive IgE was detected within mononuclear cells present in airway epithelium and in accumulations of lymphoid cells within peribronchial interstitial spaces of HDMA-sensitized monkeys (Figure 9) ▶ .
Figure 8.
Histopathological comparison of wall composition in the intrapulmonary bronchi of sensitized (B, D, and F) and nonsensitized (A, C, and E) rhesus monkeys. A and B: High resolution histopathological comparison of a control monkey (A) and an area of heavy inflammatory cell infiltration with epithelial hypertrophy and mucous goblet cell (arrows) hyperplasia of sensitized monkey (B) (scale bar, 20 μm). C and D: Differences in PAS/Alcian blue-positive mucous substance in the mucous goblet cells (arrows) of nonsensitized (C) and sensitized (D) rhesus monkeys (scale bar, 20 μm). E and F: Distribution of major basic protein in the epithelium of nonsensitized (E) and sensitized (F) rhesus monkeys (scale bar, 20 μm). Arrowheads indicate the basement membrane zone. Smooth muscle (SM).
Figure 9.
Immunohistochemical comparison of the distribution of IgE-positive cells of intrapulmonary bronchi in sensitized (B) and nonsensitized rhesus monkeys (A). The majority of the immunoreactive immunoglobulin was in the cytoplasm of mononuclear cells within peribronchial interstitial lymphoid accumulations and within adjacent epithelium (scale bar, 20 μm).
Discussion
This study demonstrates that allergic asthma can be produced experimentally in a nonhuman primate, the rhesus monkey, using a recognized human allergen, house dust mite (Dermatophagoides farinae). Sensitized animals exhibit the hallmarks of allergic asthma defined for humans by the 1997 National Heart, Lung, and Blood Institute National Asthma Education and Prevention Program Clinical Practice Guidelines 1 as:
“A chronic inflammatory disorder of the airways in which many cells and cellular elements play a role, in particular, mast cells, eosinophils, T lymphocytes, macrophages, neutrophils, and epithelial cells. In susceptible individuals, this inflammation causes recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, particularly at night or in the early morning. These episodes are usually associated with widespread but variable airflow obstruction that is often reversible either spontaneously or with treatment. The inflammation also causes an associated increase in the existing bronchial hyperresponsiveness to a variety of stimuli. Moreover, recent evidence indicates that remodeling of the airway may occur in some patients with asthma and that these changes contribute to persistent abnormalities in lung function.”
Based on this definition, we established eight formal physiological, immunological, and pathological criteria for evaluating the presence of an asthma-like condition in allergen-exposed rhesus monkeys. First, the sensitized monkeys developed a positive skin test for HDMA. In addition, we found elevated levels of IgE in serum of these animals and IgE-positive cells within the tracheobronchial airway walls. Second, the animals developed airway obstruction after the inhalation of aerosolized allergen that was associated with cough, rapid shallow breathing, and decreased arterial oxygen saturation. The latter was reversible by treatment with aerosolized albuterol. In addition, elevations in serum histamine concentrations could be detected in sensitized monkeys after allergen aerosol exposures. Third, shed epithelial cells were detected in the exudates and bronchoalveolar lavage of the sensitized monkeys immediately after allergen aerosol challenge. As with human asthmatics, the majority of the cells were ciliated cells. Fourth, there was a marked increase in the abundance of immune cells, specifically eosinophils, in airway exudates and bronchoalveolar lavage. This was accompanied by elevations in CD25 expression on CD4+ lymphocytes in both lavage and serum. Fifth, the animals developed nonspecific airways responsiveness, which was characterized as a fourfold reduction in the dose of histamine aerosol required too produce a 150% increase in airway resistance. Sixth, histopathological evaluation of intra- and extra-pulmonary conducting airways found marked mucous cell hyperplasia accompanied by general epithelial hypertrophy. Seventh, these same intrapulmonary bronchi had thickening of the basement membrane zone. Eighth, there were marked accumulations of eosinophils in the epithelial and subepithelial matrix compartments of the airway walls. All three of these histopathological features were focal and distributed throughout the conducting airway trees of the three sensitized monkeys studied.
In human patients with allergic asthma, inhalation of allergen induces an immediate bronchoconstriction that is followed in ∼50% of patients by a delayed bronchoconstriction 4 to 8 hours later. 25,26 Mast cell degranulation and the release of bronchoconstrictive mediators, including histamine, are thought to be important in the immediate bronchoconstriction. 27,28 The HDMA-sensitized monkeys in the present study demonstrated a similar immediate bronchoconstrictive response to inhaled allergen (Figure 1 and 2) ▶ ▶ that was associated with increased serum histamine levels (Figure 4) ▶ . During early stages of asthma exacerbation in human patients alveolar ventilation is maintained and arterial CO2 may be reduced secondary to onset of tachypnea. 29 However, as breathing pattern becomes more rapid and shallow and bronchoconstriction intensifies, mismatches of ventilation and perfusion develop and hypoxemia and respiratory insufficiency ensue. 29 Inhalation of HDMA resulted in marked tachypnea that was associated with the development of mild hypoxemia (Figure 2) ▶ . As in human asthmatics, 25,26 we observed in our HDMA-sensitized monkeys that a delay of 48 to 72 hours after HDMA aerosol challenge was characterized by the development of airway hyperresponsiveness to histamine, a nonspecific stimuli. An important similarity between humans and rhesus monkeys is that they both produce histamine as the primary early mediator of type 1 hypersensitivity, whereas, the mouse and rat produce serotonin instead of histamine. 30 Elevated levels of histamine in plasma of monkeys after HDMA aerosol substantiate the importance of this mediator in allergic asthma of primates.
In addition to the aforementioned physiological features of the respiratory system, the molecular nature and distribution of inflammatory cells within the rhesus monkey closely parallel that of humans. Phylogenetic conservation of leukocyte cell surface proteins between rhesus monkeys and humans is evident by the high degree of cross-reactivity to monoclonal antibodies generated against human antigens (range of 45 to 53% reactivity of antibodies tested). 4,31 Further, T helper and T cytotoxic lymphocyte subsets in peripheral blood and lymphoid tissue from normal monkeys are similar to values obtained in humans; this feature is important for evaluating immunological mechanisms for analogous allergic responses. 4 Our observation of elevated levels of CD25+ T helper cells in the circulation of allergen-sensitized monkeys is consistent with previous reports of activated T lymphocytes in the peripheral blood of individuals with severe acute asthma. 32,33 Moreover, the development of HDMA-specific IgE in serum coupled with the increased numbers of IgE-producing cells within the walls of tracheobronchial airways of sensitized monkeys, parallels the development of circulating allergen-specific IgE in human patients with allergic asthma. The presence of IgE-producing cells within tracheobronchial airways combined with the elevated serum histamine levels after HDMA inhalation in the sensitized monkeys is supportive of the importance of local IgE production for the initiation of type 1 hypersensitivity-mediated reactivity to inhaled allergen in rhesus monkeys. These findings cumulatively suggest that an allergen-mediated immune response within the microenvironment of the rhesus lung is comparable to that of humans.
An additional feature of allergic asthma in humans is dramatic remodeling of the walls of the conducting airways. Mucous goblet cells and submucosal glands, normally present in human bronchi, undergo extensive hyperplasia and hypersecretion. We found the same changes in sensitized monkeys. Basal cells, which play a role in the epithelial sloughing observed in human asthmatics, are present and altered throughout the airways in both humans and monkeys. Mesenchymal components of the intrapulmonary conducting airways, including a distinct subepithelial basement membrane, an extensive layer of attenuated fibroblasts and myofibroblasts beneath the basement membrane, and large bands of smooth muscle interspersed with cartilage, are modified in human asthmatics. These airway components are also present in tracheobronchial airways of healthy rhesus monkeys and underwent extensive thickening and hyperplasia in the allergen-sensitized and -challenged monkeys in this study.
This study confirms our initial premise that nonhuman primates would be appropriate models of allergic asthma because they share with humans characteristics of the immune and respiratory systems that seem critical for the development of allergic asthma. This model will permit definition of the roles of all of the components of the conducting airway wall and their interaction with physiological and immunological responses that are characteristic of human allergic asthma as the allergic response develops and the role that exposure to environmental pollutants could play in the exacerbation of allergic asthma. Further, application of this model to infant monkeys has the potential for establishing the mechanisms by which allergen exposure of young children during postnatal lung development modulates cellular differentiation and morphogenesis to create allergic asthma in children.
Acknowledgments
Development of this model was the product of the interactions of all of the faculty and staff members of the Respiratory Diseases Unit at the California Regional Primate Research Center, whose members, in addition to those listed above, include J. Bric, A. M. Chang, T. R. Duval, K. Kott, D. Morin, S. J. Nishio, N. K. Tyler, and A. J. Weir. The support of Primate Services at the California Regional Primate Research Center for animal handling, care, and coordination and Veterinary Care, especially the efforts of Laurie Crignolo, D.V.M., Kari Christe, D.V.M, Sarah Davis, and Bruce Rodello, were critical to this study and gratefully acknowledged.
Footnotes
Address reprint requests to Prof. Edward S. Schelegle, Department of Anatomy, Physiology and Cell Biology, School of Veterinary Medicine, 1 Shields Ave., University of California, Davis, CA 95616. E-mail: esschelegle@ucdavis.edu.
Supported in part by National Institute of Environmental Health and Safety grant P01 ES00628, and NCRR grant RR00169, and the NIEHS Center for Environmental Health Sciences (ES 05707).
References
- 1.Guidelines for the Diagnosis and Management of Asthma, ed 2. Bethesda, National Institutes of Health, 1997, pp 1–153
- 2.Gundel RH, Kinkade P, Torcellini CA, Clarke CC, Watrous J, Desai S, Homon CA, Farina PR, Wegner CD: Antigen-induced mediator release in primates. Am Rev Respir Dis 1991, 144:76-82 [DOI] [PubMed] [Google Scholar]
- 3.Miadonna A, Tedeschi A, Brasca C, Folco G, Sala A, Murphy RC: Mediator release after endobronchial antigen challenge in patients with respiratory allergen. J Allergy Clin Immunol 1990, 85:906-913 [DOI] [PubMed] [Google Scholar]
- 4.Reimann KA, Waite BCD, Lee-Parritiz DE, Lin W, Uchanska-Zeigler B, O’Connell MJ, Letvin NL: Use of human leukocyte-specific monoclonal antibodies for clinically immunophenotyping lymphocytes of rhesus monkeys. Cytometry 1994, 17:102-108 [DOI] [PubMed] [Google Scholar]
- 5.Wells E, Harper ST, Jackson CG, Mann J, Eady RP: Characterization of primate bronchoalveolar mast cells. I: IgE-dependent release of histamine, leukotrienes, and prostaglandins. J Immunol 1986, 137:3933-3940 [PubMed] [Google Scholar]
- 6.Yasue M, Nakamura S, Yokota T, Okudaira H, Okumara Y: Experimental monkey model sensitized with mite antigen. Int Arch Allergy Immunol 1998, 115:303-311 [DOI] [PubMed] [Google Scholar]
- 7.Plopper CG, Heidsiek JG, Weir AJ, St. George JA, Hyde DM: Tracheobronchial epithelium in the adult rhesus monkey: quantitative histochemical and ultrastructural study. Am J Anat 1989, 184:31-80 [DOI] [PubMed] [Google Scholar]
- 8.Plopper CG, Alley JL, Weir AJ: Differentiation of tracheal epithelium during fetal lung maturation in the rhesus monkey Macaca mulatta. Am J Anat 1986, 175:59-71 [DOI] [PubMed] [Google Scholar]
- 9.Plopper CG, Weir AJ, Nishio SJ, Cranz DL, St. George JA: Tracheal submucosa gland development in the rhesus monkey, Macaca mulatta: ultrastructure and histochemistry. Anat Embryol 1986, 174:167-178 [DOI] [PubMed] [Google Scholar]
- 10.Plopper CG, St. George JA, Cardoso W, Wu R, Pinkerton K, Buckpitt A: Development of airway epithelium. Patterns of expression for markers of differentiation. Chest 1992, 101:2-5 [PubMed] [Google Scholar]
- 11.Teague SV, Yeh HC, Newton GJ: Fabrication and use of krypton-85 aerosol discharge devices. Health Phys 1978, 35:392-395 [PubMed] [Google Scholar]
- 12.Hinners RG, Buckhart JK, Punte CL: Animal inhalation exposure chambers. Arch Environ Health 1968, 16:194-206 [DOI] [PubMed] [Google Scholar]
- 13.Ozols J: Amino acid analysis. Guide to protein purification. Methods Enzymol 1990, 182:587-601 [DOI] [PubMed] [Google Scholar]
- 14.Mercer TT, Tillary MI, Newton GJ: Multistage low flow rate cascade impactor. Aerosol Science 1970, 1:9-15 [Google Scholar]
- 15.Shore PA, Burkhalter A, Cohn VH: A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther 1959, 127:182-186 [PubMed] [Google Scholar]
- 16.Miller LA, Butcher EC: Human airway epithelial monolayers promote selective transmigration of memory T cells: a transepithelial model of lymphocyte migration into the airways. Am J Respir Cell Mol Biol 1998, 19:892-900 [DOI] [PubMed] [Google Scholar]
- 17.Madwed JB, Jackson AC: Determination of airway and tissue resistance after antigen and methacholine in nonhuman primates. J Appl Physiol 1997, 83:1690-1696 [DOI] [PubMed] [Google Scholar]
- 18.Dubois AB, Brody AW, Lewis DH, Burgess BF: Oscillation mechanics of lungs and chest in man. J Appl Physiol 1956, 8:587-594 [DOI] [PubMed] [Google Scholar]
- 19.Csendes T: Nonlinear parameter estimation by global optimization efficiency and reliability. Acta Cybernetica 1988, 8:361-370 [Google Scholar]
- 20.Peslin R, Duvivier C, Gallina C: Total respiratory input and transfer impedances in humans. J Appl Physiol 1985, 59:492-501 [DOI] [PubMed] [Google Scholar]
- 21.Pare PD, Michoud MC, Hogg JC: Lung mechanics following antigen challenge of ascaris suum-sensitive rhesus monkeys. J Appl Physiol 1976, 41:668-676 [DOI] [PubMed] [Google Scholar]
- 22.Plopper CG: Structural methods for studying bronchiolar epithelial cells. Gil J eds. Models of Lung Disease Microscopy and Structural Methods. 1990, :pp 537-559 Marcel Dekker, Inc., New York [Google Scholar]
- 23.St. George JA, Nishio SJ, Cranz DL, Plopper CG: Carbohydrate cytochemistry of rhesus monkey tracheal submucosal glands. Anat Rec 1986, 216:60-67 [DOI] [PubMed] [Google Scholar]
- 24.Gershwin LJ, Olsen CL: Application of image analysis for quantitative immunoperoxidase detection of IgE containing cells in lymphoid tissues. J Immunol Methods 1985, 76:39-46 [DOI] [PubMed] [Google Scholar]
- 25.Cartier A, Thompson NC, Frith PA, Roberts R, Tech M, Hargreave FE: Allergen-induced increase in bronchial responsiveness to histamine: relationship to late asthmatic response and change in airway caliber. J Allergy Clin Immunol 1982, 70:170-177 [DOI] [PubMed] [Google Scholar]
- 26.Cockcroft DW: Airway hyperresponsiveness: therapeutic implications. Ann Allergy 1987, 59:405-412 [PubMed] [Google Scholar]
- 27.Liu MC, Bleecker ER, Lichtenstein LM, Kagey-Sobotka A, Niv Y, Mclemore TL, Permutt S, Proud D, Hubbard WC: Evidence for elevated levels of histamine, prostaglandin D2, and other bronchoconstricting prostaglandins in the airways of subjects with mild asthma. Am Rev Respir Dis 1990, 142:126-132 [DOI] [PubMed] [Google Scholar]
- 28.Bleecker ER: Cholinergic and neurogenic mechanisms in obstructive airway disease. Am J Med 1986, 81:93-102 [DOI] [PubMed] [Google Scholar]
- 29.McFadden ER, Lyons HA: Arterial blood gas tension in asthma. N Engl J Med 1968, 278:1027-1032 [DOI] [PubMed] [Google Scholar]
- 30.Dvorak AM, Dvorak HF, Galli SJ: Ultrastructural criteria for identification of mast cells and basophils in humans, guinea pigs, and mice. Am Rev Respir Dis 1983, 128:49-52 [DOI] [PubMed] [Google Scholar]
- 31.Ozwara H, Niphuis H, Buijs L, Jonker M, Heeney JL, Bambra CS, Thomas AW, Langermans JA: Flow cytometric analysis on reactivity of human T lymphocyte-specific and cytokine-receptor-specific antibodies with peripheral blood mononuclear cells of chimpanzee (Pan troglodytes), rhesus macaque (Macaca mulatta), and squirrel monkey (Saimiri sciureus). J Med Primatol 1997, 3:164-171 [DOI] [PubMed] [Google Scholar]
- 32.Corrigan CJ, Kay AB: CD4 T-lymphocyte activation in acute severe asthma. Relationship to disease severity and atopic status. Am Rev Respir Dis 1990, 141:970-977 [DOI] [PubMed] [Google Scholar]
- 33.Corrigan CJ, Hartnell A, Kay AB: T lymphocyte activation in acute severe asthma. Lancet 1988, 1:1129-1132 [DOI] [PubMed] [Google Scholar]