Abstract
Isoprostanes (IsoP) are produced exclusively from free radical damage to arachidonic acid, a fatty acid that is evenly distributed throughout white matter and gray matter, whereas neuroprostanes (NPs) are generated analogously from docosahexaenoic acid (DHA), a fatty acid enriched in gray matter where it is concentrated in neurons. IsoP and NPs derive from endoperoxide intermediates that isomerize to D/E-ring forms or that are reduced to F-ring compounds. We quantified F-ring and D/E-ring IsoP and NPs in temporal and parietal cortex, hippocampus, and cerebellum of nine definite Alzheimer’s disease (AD) patients and 11 age-matched controls. Total NP levels (F-ring plus D/E-ring), but not total IsoP, were significantly greater in AD than controls (P < 0.0001); only cerebral regions in AD patients had NPs greater than controls (P < 0.05). The F-ring to D/E-ring ratio for NPs, but not IsoP, was 40 to 70% lower in all brain regions of AD patients compared to controls (P < 0.005). These data extend results from in situ techniques, that have localized reactive products of lipid peroxidation primarily to neurons, by quantifying significantly greater free radical damage to the DHA-containing compartments in cerebrum in AD patients than controls, and suggest that one mechanism of increased oxidative stress may be diminished reducing capacity in DHA-containing compartments.
Numerous in vitro, cell culture, and animal studies have implicated lipid peroxidation in the pathogenesis of Alzheimer’s disease (AD). 1,2 Cellular localization and quantification of lipid peroxidation to brain in AD are important goals because this information will help refine hypotheses about disease pathogenesis, and will aid in the development and evaluation of therapeutics. Tissue homogenates from post mortem human brain have been used to measure levels of some products of lipid peroxidation; however, most of the techniques used are not quantitative in vivo and this experimental approach does not provide information on cellular localization. 3,4 Immunohistochemical and histochemical techniques that localize proteins modified by lipid peroxidation products have been used by others and us to complement tissue homogenate studies of AD brain. 5-10 However, these earlier in situ results also are not quantitative for free radical damage because levels of protein adducts detected in tissue are influenced by many factors including rate of production, metabolism, and turnover of the modified proteins.
Isoprostanes (IsoPs) are exclusive products of free radical damage to arachidonic acid (AA) (C20:4ω6) that are formed esterified to lipid (bound) and then are hydrolyzed (free). 11 Measurement of the major class of IsoPs, F2-IsoPs, has been used widely to quantify free radical damage in vivo. 12 Compared to controls, F2-IsoP levels are elevated in cerebrospinal fluid from probable AD patients early in the course of dementia, and from definite AD patients where F2-IsoP levels correlate with pathological measures of AD severity. 13-15 Bound F2-IsoP levels in the frontal cortex obtained with short post mortem intervals from definite AD patients also are significantly greater than age-matched controls. 16 However, in another study, bound F2-IsoP concentrations in occipital, temporal, and parietal cortex were not different between definite AD patients and controls, 17 raising the intriguing possibility that F2-IsoP generation is limited to the frontal lobe in AD.
Unlike AA that is evenly distributed in gray and white matter, docosahexaenoic acid (DHA) (C22:6ω3) is enriched in gray matter of the central nervous system, where it is synthesized in astrocytes and then transported and concentrated in neurons. 18,19 Previously, we described the formation of neuroprostanes (NPs) from free radical catalyzed peroxidation of DHA via reactions analogous to IsoP generation. 20 We proposed that NPs may provide more specific information on free radical damage in DHA-containing compartments, ie, neurons, and that NP’s may be more sensitive markers of free radical damage because DHA is more labile to peroxidation than is AA. Free F4-NPs are increased in cerebrospinal fluid of definite AD patients compared to controls, and the levels of cerebrospinal fluid F4-NPs are greater than F2-IsoPs. 20 Interestingly, others demonstrated that bound F4-NPs (called F4-IsoP by these authors) are increased in occipital and temporal cortex, but not parietal cortex, of AD patients compared to controls. 17 This regional pattern does not correspond to the distribution of pathological changes in AD, and suggests that lipid peroxidation may be more widespread in AD brain than are histopathological changes.
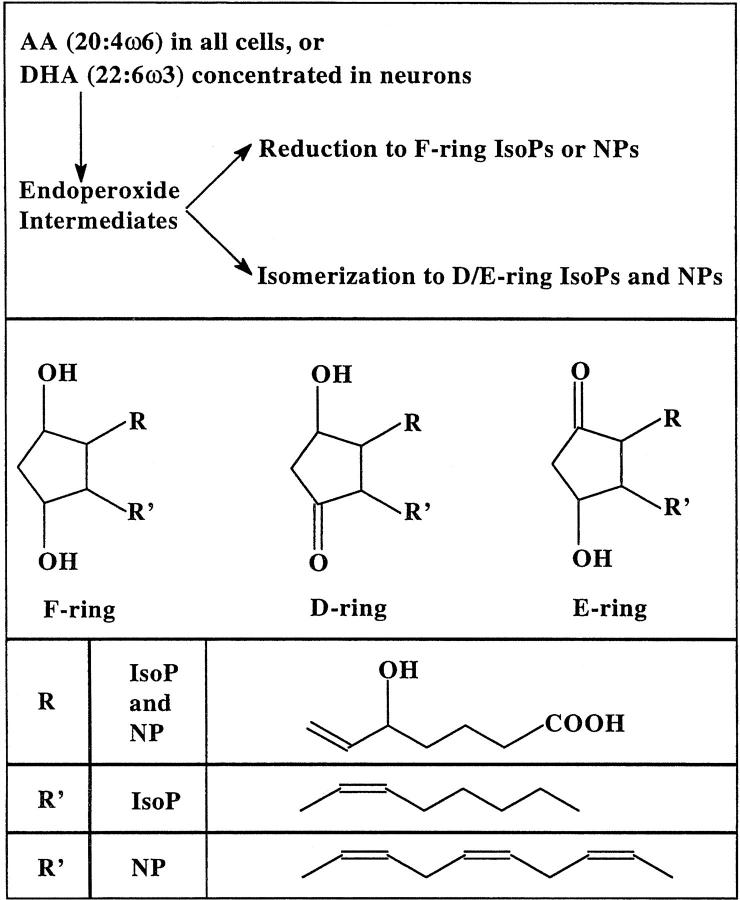
IsoP and NP formation proceeds through bicyclic endoperoxide intermediates that are reduced to F-ring compounds or undergo rearrangement to D/E-ring compounds (Figure 1) ▶ . Ex vivo oxidation of rat hepatic microsomes produces higher levels of D/E-ring than F-ring IsoPs, the reverse of what is observed after oxidation of liver in vivo. 21 Ex vivo oxidation of rat brain synaptosomes similarly yields increasing levels of D/E-ring and F-ring NPs at a ratio of 8:1, respectively. 22 In vitro, increasing the concentration of cellular reductants, such as glutathione, favors reduction of the endoperoxide intermediates resulting in greater amounts of F-ring compounds and lower amounts of D/E ring compounds. 21 Therefore, calculation of the F-ring to D/E-ring ratio supplies information on the reducing environment in which lipid peroxidation occurred.
Figure 1.
Diagram of reaction pathways and chemical structures for F-ring and D/E-ring IsoPs and NPs.
Levels of D/E-ring IsoP or NPs have not been reported in human brain. Moreover, the two studies published on F-ring IsoPs and the one study on F-ring NPs raise the intriguing possibility that the distribution of these products in brain does not correspond to the distribution of damage in AD. Therefore, we have undertaken the first comprehensive quantification of F2-IsoP, D2/E2-IsoP, F4-NPs, and D4/E4-NPs in four different brain regions from clinically and pathologically characterized definite AD patients and age-matched controls. Finally, we determined F-ring to D/E-ring ratios of IsoPs and NPs, the first time this has been done in tissue, as a reflection of reducing capacity in specific lipid microenvironments in brain.
Materials and Methods
After appropriate consent was obtained, all individuals included in this study underwent post mortem examination as part of a rapid autopsy program at the Alzheimer’s Disease Research Center at the Sanders-Brown Center on Aging, University of Kentucky. No patient had a post mortem interval longer than 4 hours. All AD patients were diagnosed with probable AD during life and were shown by neuropathological examination to meet the criteria for definite AD without neuropathological evidence of Lewy bodies or complications of cerebrovascular disease. 23-25 Controls were age- and gender-matched individuals without clinical evidence of dementia or other neurological disease. Each control individual had annual mental status testing with all scores in the normal range. Neuropathological examination of controls showed only age-associated changes. Braak staging was performed on all cases. 26 Neuritic plaques and neurofibrillary tangles (NFT) were counted in histological sections according to previously published methods 27 from inferior parietal lobule (IPL), superior and middle temporal gyri, and hippocampus. APOE was determined post mortem in all cases. 28
All tissue sections were dissected at the time of autopsy and kept frozen at −80°C until used. Lipids from specimens of hippocampus at the level of the lateral geniculate nucleus, superior and middle temporal gyri, IPL, and cerebellar cortex were extracted by the method of Folch. 15 D2/E2-IsoPs and D4/E4-NPs esterified in tissue were converted to O-methyloxime derivatives in Folch solution. IsoPs and NPs were hydrolyzed by chemical saponification, extracted using C-18 and silica Sep-Pak cartridges, purified by thin layer chromatography, converted to pentaflurobenzyl ester trimethylsilyl ether derivatives, and quantified by stable isotope dilution techniques using gas chromatography/negative ion chemical ionization/mass spectrometry using [2H4]-8-iso-PGF2α and [2H4]-PGE2 as internal standards as previously described. 22 The derivatized D/E-ring IsoPs or NPs co-migrate on silica thin layer chromatography plates and GC and had identical masses, therefore the levels of these isomers are reported as combined values. AA and DHA concentrations were determined as previously described. 29 Briefly, a 1-ml aliquot of Folch extract from each tissue sample was transmethylated and the total fatty acid composition quantified using gas chromatography with flame ionization detection.
Statistical analyses were preformed using GraphPad Prism software (San Diego, CA). Student’s t-tests was used for paired comparisons. All two-way analyses of variance (ANOVA) were used for data stratified by AD versus control and by four brain regions (1 × 3 degrees of freedom). One-way ANOVA with Bonferroni’s repeated comparison correction was used for post hoc analysis. Spearman’s ranked correlation was used for discontinuous data such as Braak stage and APOE genotype.
Results
Table 1 ▶ presents information on the 20 individuals included in this study. Age, gender, and post mortem intervals were not significantly different between the two groups. Control individuals had an APOE ε4 allele frequency that is similar to the general population. AD patients had an APOE ε4 allele frequency that was increased compared to controls, but within the expected range for AD patients. 30 AD patients had characteristic average disease duration, as well as significantly lower brain weights than controls (P < 0.05).
Table 1.
Characterization of AD Patients and Controls
| n | Age (yr) | M:F | Disease duration (yr) | Postmortem interval (hr) | Brain weight (g) | % of APOE alleles as ε4 | |
|---|---|---|---|---|---|---|---|
| AD | 9 | 78.1 ± 2.7 | 4:5 | 8.1± 1.1 | 2.6 ± 0.2 | 1091 ± 41* | 61% |
| Control | 11 | 80.7 ± 2.5 | 5:6 | NA | 2.7 ± 0.2 | 1220 ± 40 | 18% |
*P < 0.05 compared with controls.
AA and DHA levels were quantified in Folch extracts of frozen superior and middle temporal gyri, hippocampus, IPL, and cerebellar cortex from the AD patients and controls described in Table 1 ▶ . Overall, AA and DHA were 7.7 ± 0.4% and 14.3 ± 0.4%, respectively, of total fatty acids. Two-way ANOVA showed no effect of disease or brain region on AA concentrations. Two-way ANOVA for DHA tissue levels also showed no significant variance with disease, but there was significant variance with brain region (P < 0.01). The hippocampus had the lowest concentrations of DHA (12.0 ± 0.2% of total fatty acids) whereas the IPL had the highest (15.5 ± 0.4% of total fatty acids).
F4-NPs were by far the most abundant of the compounds measured, having an overall average (all individuals, all regions) level of 13.7 ± 0.8 ng/g. The corresponding overall average level of F2-IsoPs was 4.9 ± 0.3 ng/g, 2.8-fold less than F4-NPs. Levels of D/E-ring IsoPs and NPs were the lowest, averaging 1.5 ± 0.1 ng/g and 1.4 ± 0.2 ng/g, respectively. Tissue levels of F2-IsoPs did not correlate with F4-NPs concentrations. In addition, tissue levels of F-ring compounds did not correlate with the concentration of the corresponding D/E-ring compounds.
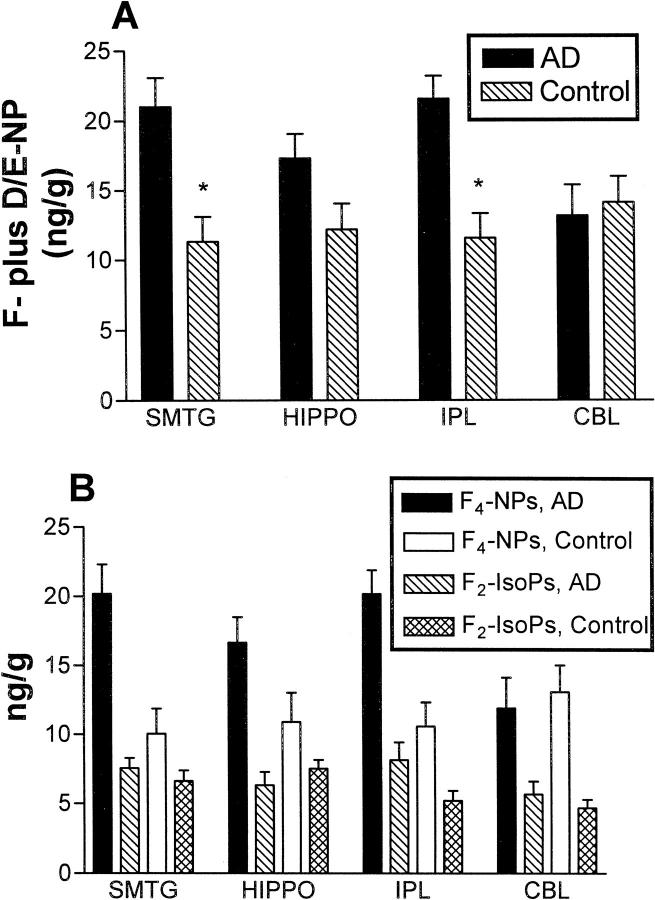
Levels of F-ring plus D/E-ring compounds were determined to assess the magnitude of free radical damage to AA and DHA. Two-way ANOVA for tissue levels of NPs was performed after stratifying data by the presence of AD and by brain region (Figure 2A) ▶ . Tissue levels of F- plus D/E-NPs were significantly higher in AD patients versus controls (P < 0.0001), and were significantly associated with brain region (P < 0.05), a consequence of NPs being higher in cerebral cortical regions than in cerebellum of AD patients. An analogous two-way ANOVA for tissue levels of F-ring plus D/E-ring IsoPs was not significant for presence of AD or brain region (P > 0.05). Two-way ANOVA’s for only F-ring compounds were significant for AD versus control for both F4-NPs (P < 0001) and F2-IsoPs (P < 0.05); however, only the F4-NPs were significantly associated with brain region (Figure 2B) ▶ . Although D4/E4-NP tended to be greater in AD patients than in controls, two-way ANOVA for D4/E4-NP was not significant for AD or brain region. Similarly, D2/E2-IsoPs were not statistically significant different with respect to AD or brain region.
Figure 2.
Tissue levels of total NPs (F-ring plus D/E-ring compounds) (A) or F4-NPs and F2-IsoPs (B) were stratified by brain region for AD patients and controls. Values are means ± SEM. A: Two-way ANOVA for total NP’s was significant for AD patients versus controls (P < 0.0001) and for brain region (P < 0.05). Repeated pairs analysis with Bonferroni’s correction showed that total NP’s were significantly greater in cerebral cortical regions in AD patients compared to controls (*, P < 0.05). B: Two-way ANOVA of tissue levels of F-ring compounds were significantly different between AD patients and controls for both F4-NPs (P < 0001) and F2-IsoPs (P < 0.05); however, only the F4-NPs were significantly associated with brain region (P < 0.05).
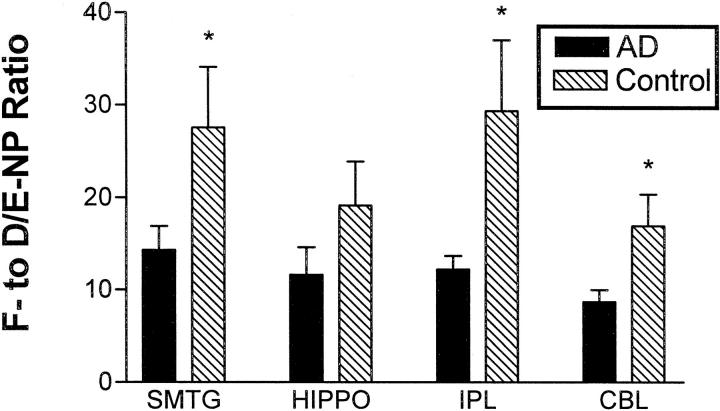
The ratios of F-ring to D/E-ring compounds were computed for IsoPs and NPs to assess the reducing environments in which free radical damage to AA and DHA occurred. Overall, the average F2- to D2/E2-IsoP ratio was 4.1 ± 0.3, a value significantly lower than the corresponding F4- to D4/E4-NP ratio of 17.5 ± 1.7 (P < 0.0001). Two-way ANOVA for the F4- to D4/E4-NP ratio was highly significant for AD (P < 0.005, Figure 3 ▶ ), but not brain region. Indeed, all four brain regions in AD patients had a lower F4- to D4/E4-NP ratio than controls; these differences were statistically significant for superior and middle temporal gyri, IPL, and cerebellar cortex, (P < 0.05) but not hippocampus. Neither AD nor brain region significantly contributed to the variance in IsoP ratios.
Figure 3.
The ratio of tissue levels of F4-NPs to D4/E4-NP’s was stratified by brain region for AD patients and controls. Values are means ± SEM. Two-way ANOVA was significantly different for AD versus control (P < 0.005), but not for brain region. Repeated pairs analysis with Bonferroni’s correction showed that the F4-NP to D4/E4-NP ratio was significantly reduced in cerebral cortical regions and cerebellum of AD patients compared to controls (*, P < 0.05).
The levels and ratios of IsoP and NPs in different brain regions did not correlate with neuritic plaque or neurofibrillary tangle density in the same brain region, Braak stage, or APOE genotype (n = 20, r < 0.4, and P > 0.1 for all comparisons).
Discussion
We tested the hypothesis that free radical damage to lipid in gray matter from AD brain is concentrated in DHA-containing rather than AA-containing compartments. In addition, we quantified F-ring to D/E-ring ratios of IsoP and NPs, a reflection of the reducing environment in which IsoPs and NPs are formed, in different regions of AD brain. Our results showed that the DHA-containing, but not AA-containing, compartments in AD cerebrum undergo significantly increased free radical damage compared to controls, and suggest that the DHA-containing compartments in AD brain may be more susceptible to free radical damage because of diminished reducing capacity. This is the first study to quantify isomers of IsoPs and NPs in tissue. This initial study concentrated on AD because it is a brain disease that derives important contributions from lipid peroxidation, and that has had lipid peroxidation localized to neurons. The methods used here also can be used to investigate other diseases of brain that are thought to derive significant contributions from lipid peroxidation.
The lack of difference in total IsoP levels between AD patients and age-matched controls indicated that gray matter did not experience significantly more lipid peroxidation in AD patients compared to age-matched controls. However, neurons are only one component of gray matter. Our results with total NP levels indicated that the subset of gray matter that contains DHA did experience increased levels of free radical damage in AD patients compared to controls. In combination, these results suggest that free radical damage in AD is focused in DHA-containing compartments, mostly neurons, and is not evenly distributed within gray matter. 31 Moreover, our results showed that increased free radical damage to DHA occurred in cerebral cortex and hippocampus but not cerebellar cortex. It should be noted that this regional distribution of elevated NPs, although expected if one proposes that free radical damage is an element in AD pathogenesis, is different from what was observed by others. 17
Corroborating the report of others, we observed significantly elevated F2-IsoPs in AD brain regions compared to controls. 16 Furthermore, this result is consistent with our earlier observations of significantly elevated F2-IsoPs in the cerebrospinal fluid of definite and probable AD patients compared to controls. 13-15 However, if the elevation in F2-IsoPs in AD derived exclusively from the same compartment as the more dramatically elevated NPs, one would predict a similar shift in the F-ring to D/E-ring ratio in IsoPs as was observed with NPs. Because there was no significant change in the F2-ring to D2/E2-ring ratio, it seems likely that a smaller amount of lipid peroxidation may occur in tissue elements other than neurons, eg, reactive astrocytes or activated microglia, in AD.
Reports on the concentrations of glutathione and other cellular reductants in AD have been conflicting. 1 This is an important issue to resolve because cellular reductants play an important role in anti-oxidant defenses, and would offer an accessible therapeutic target in AD. The 40 to 70% decreases in F-ring to D/E-ring NP ratio in AD patients with unchanged IsoP ratio indicated that DHA-containing compartments had significantly diminished reducing capacity in AD. In contrast, recent in situ data has demonstrated increased reductants in neuronal cytoplasm in regions of brain involved by AD. 32 One interpretation of these apparently conflicting results is that reducing capacity may vary among different microenvironments within tissue and even within neurons. Our data indicates that reducing capacity is diminished within DHA-containing microenvironments in AD brain, but cannot be extrapolated to include other subcellullar compartments, eg, neuronal cytoplasm.
It is important to note that the F-ring to D/E-ring NP ratio was lower in all AD brain regions including cerebellar cortex. However, the levels of NPs were elevated only in cerebral regions and not in cerebellar cortex. This comparison suggests that the lowered reducing capacity in DHA-containing compartments in AD brain is not necessarily a consequence of increased free radical damage. Moreover, because cerebellar cortex is not considered a site for AD pathological changes, our results raise the possibility that diminished reducing capacity in DHA-containing compartments may be a feature of patients who are vulnerable to developing AD and not an outcome of AD pathological changes.
Although our data showed that there was no significant difference in the concentration of DHA or AA in the brain regions studied between AD patients and controls, it is possible that the cellular or subcellular distribution of DHA or AA is somehow altered by AD or by reactions to injury, such as gliosis or microgliosis. If this were the case, then interpretation of our data would be complicated by differential distribution of substrate in controls and AD patients. Nevertheless, our data would still indicate that DHA, a fatty acid essential to proper neuronal function, is significantly oxidized in AD cerebrum and that this may derive, in part, from decreased reducing capacity in certain lipid microenvironments in AD. However, the possibility of significant DHA redistribution in AD seems unlikely to be a major confounding variable because our results are entirely consistent with numerous histochemical and immunohistochemical reports localizing increased accumulation of lipid peroxidation products in neurons. The advantage of our complimentary approach is that it allows an unbiased, robust quantification of these events. Such quantification will be critical to future studies that attempt to determine the efficacy of therapeutic interventions that limit lipid peroxidation to brain in AD.
Footnotes
Address reprint requests to Dr. Thomas J. Montine, Department of Pathology, Vanderbilt University Medical Center, C-3321A Medical Center North, Nashville, TN 37232. Email: tom.montine@mcmail.vanderbilt.edu.
Supported by National Institutes of Health grants AG00774, AG16835, AG05144, AG05119, DK48831, GM15431, DK26657, and CA77839, as well as grants from the Alzheimer’s Association (to T. J. M.), the Abercrombie Foundation (to W. R. M.), and a Burroughs-Wellcome Clinical Scientist Award in Translational Research (to J. D. M.).
References
- 1.Markesbery WR, Carney JM: Oxidative alterations in Alzheimer’s disease. Brain Pathol 1999, 9:133-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry G, Castellani RJ, Hirai K, Smith MA: Reactive oxygen species mediate cellular damage in Alzheimer’s disease. J Alzheimer Dis 1998, 1:45-55 [DOI] [PubMed] [Google Scholar]
- 3.Gutteridge JMC, Halliwell B: The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci 1990, 15:129-135 [DOI] [PubMed] [Google Scholar]
- 4.Moore K, Roberts LJ: Measurement of lipid peroxidation. Free Radical Res 1998, 28:659-671 [DOI] [PubMed] [Google Scholar]
- 5.Montine KS, Olson SJ, Amarnath V, Whetsell WO, Graham DG, Montine TJ: Immunochemical detection of 4-hydroxynonenal adducts in Alzheimer’s disease is associated with APOE4. Am J Pathol 1997, 150:437-443 [PMC free article] [PubMed] [Google Scholar]
- 6.Montine KS, Kim PJ, Olson SJ, Markesbery WR, Montine TJ: 4-Hydroxy-2-nonenal pyrrole adducts in human neurodegenerative disease. J Neuropathol Exp Neurol 1997, 56:866-871 [DOI] [PubMed] [Google Scholar]
- 7.Montine K, Reich E, Olson SJ, Markesbery WR, Montine T: Distribution of reducible 4-hydroxynonenal adduct immunoreactivity in Alzheimer’s disease is associated with APOE genotype. J Neuropathol Exp Neurol 1998, 57:415-425 [DOI] [PubMed] [Google Scholar]
- 8.Sayre LM, Zelasko DA, Harris PLR, Perry G, Salomon RG, Smith MA: 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer disease. J Neurochem 1997, 68:2092-2097 [DOI] [PubMed] [Google Scholar]
- 9.Smith MA, Taneda S, Richey PL, Miyata S, Yan SD, Stern D, Sayre LM, Monnier VM, Perry G: Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci USA 1994, 91:5710-5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith MA, Sayre LM, Anderson VE, Harris PL, Beal MF, Kowall N, Perry G: Cytochemical demonstration of oxidative damage in Alzheimer’s disease by immunochemical enhancement of the carbonyl reaction with 2,4-dinitrophenylhydrazine. J Histochem Cytochem 1998, 46:731-735 [DOI] [PubMed] [Google Scholar]
- 11.Morrow JD, Roberts LJ: The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res 1997, 36:1-21 [DOI] [PubMed] [Google Scholar]
- 12.Roberts LJ, II: Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Rad Biol Med 2000, 28:505-513 [DOI] [PubMed] [Google Scholar]
- 13.Montine TJ, Markesbery WR, Morrow JD, Roberts LJ: Cerebrospinal fluid F2-isoprostanes are increased in Alzheimer’s disease. Ann Neurol 1998, 44:410-413 [DOI] [PubMed] [Google Scholar]
- 14.Montine TJ, Beal MF, Cudkowicz ME, Brown RH, O’Donnell H, Margolin RA, McFarland L, Bachrach AF, Zackert WE, Roberts LJ, Morrow JD: Increased cerebrospinal fluid F2-isoprostane concentration in probable Alzheimer’s disease. Neurology 1999, 52:562-565 [DOI] [PubMed] [Google Scholar]
- 15.Montine TJ, Markesbery WR, Zackert W, Sanchez SC, Roberts LJ, Morrow JD: The magnitude of brain lipid peroxidation correlates with the extent of degeneration but not with density of NP’s or NFT’s, or with APOE genotype in Alzheimer’s disease patients. Am J Pathol 1999, 155:863-868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pratico D, Lee VM, Trojanowski JQ, Rokach J, Fitzgerald GA: Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J 1998, 12:1777-1784 [DOI] [PubMed] [Google Scholar]
- 17.Nourooz-Zadeh J, Liu EH, Yhlen B, Anggard EE, Halliwell B: F4-isoprostanes as specific marker of docosahexaenoic acid production in Alzheimer’s disease. J Neurochem 1999, 72:734-740 [DOI] [PubMed] [Google Scholar]
- 18.Moore SA, Yoder E, Murphy S, Dutton GR, Spector A: Astrocytes, not neurons, produce docosahexaenoic acid (22: 6 ω3) and arachidonic acid (20:4 ω6). J Neurochem 1991, 56:518-524 [DOI] [PubMed] [Google Scholar]
- 19.Moore SA: Cerebral endothelium and astrocytes cooperate in supplying docosahexaenoic acid to neurons. Adv Exp Med Biol 1993, 331:229-233 [DOI] [PubMed] [Google Scholar]
- 20.Roberts LJ, Montine TJ, Markesbery WR, Tapper AR, Hardy P, Chemtob S, Detbarn WD, Morrow JD: Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem 1998, 273:13605-13612 [DOI] [PubMed] [Google Scholar]
- 21.Morrow JD, Roberts LJ, Daniel VC, Awad JA, Mirochnitchenko O, Swift LL, Burk RF: Comparison of formation of D2/E2-isoprostanes and F2-isoprostanes in vitro and in vivo—effects of oxygen tension and glutathione. Arch Biochem Biophys 1998, 353:160-171 [DOI] [PubMed] [Google Scholar]
- 22.Reich EE, Zackert WE, Brame CJ, Chen Y, Roberts LJ, II, Hachey DL, Montine TJ, Morrow JD: Formation of novel D-ring and E-ring isoprostane-like compounds (D4/E4-neuroprostanes) in vivo from docosahexaenoic acid. Biochemistry 2000, 39:2376-2383 [DOI] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 1984, 34:939-944 [DOI] [PubMed] [Google Scholar]
- 24.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991, 41:479-486 [DOI] [PubMed] [Google Scholar]
- 25.Ronald and Nancy Reagan: Institute of the Alzheimer’s Association and National Institute on Aging Working Group on Biological Markers of Alzheimer’s Disease. Consensus Report on the Working Group on ‘Molecular and Biochemical Markers of Alzheimer’s Disease.’ Neurobiol Aging 1998, 19:109–116 [PubMed]
- 26.Braak H, Braak E: Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 1991, 82:239-259 [DOI] [PubMed] [Google Scholar]
- 27.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR: Brain infarction and the clinical expression of Alzheimer’s disease: the nun study. JAMA 1997, 277:813-817 [PubMed] [Google Scholar]
- 28.Saunders AM, Schmader K, Breitner JC, Benson MD, Brown WT, Goldfarb L, Goldgaber D, Manwaring MG, Szymanski MH, McCown N: Apolipoprotein E epsilon 4 allele distributions in late-onset Alzheimer’s disease and in other amyloid-forming diseases [see comments]. Lancet 1993, 342:710-711 [DOI] [PubMed] [Google Scholar]
- 29.Montine TJ, Montine KS, Swift LL: Central nervous system lipoproteins in Alzheimer’s disease. Am J Pathol 1997, 151:1571-1575 [PMC free article] [PubMed] [Google Scholar]
- 30.Strittmatter WJ, Roses AD: Apolipoprotein E and Alzheimer’s disease. Proc Natl Acad Sci USA 1995, 92:4725-4727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salem N, Kim HY, Yergey JA: Docosahexaenoic acid: membrane function and metabolism. Simopoulos AP Kifer RR Martin RE eds. Health Effects of Polyunsaturated Acids in Seafoods. 1986, :pp 263-317 Academic Press, New York [Google Scholar]
- 32.Russell RL, Siedlak SL, Raina AK, Bautista JM, Smith MA, Perry G: Increased neuronal glucose-6-phosphate dehydrogenase and sulfhydryl levels indicate reductive compensation to oxidative stress in Alzheimer’s disease. Arch Biochem Biophys 1999, 370:236-239 [DOI] [PubMed] [Google Scholar]