Abstract
Transforming growth factor (TGF)-β1 plays an essential role in cell growth and differentiation. It is also considered as a gatekeeper of immune homeostasis with gene disruption leading to autoimmune and inflammatory diseases. TGF-β1 is produced as an inactive precursor polypeptide that can be efficiently secreted but correct proteolytic cleavage is an essential step for its activation. Assessment of the cleavage site has revealed a unique R-H-R-R sequence reminiscent of proprotein convertase (PC) recognition motifs and has previously demonstrated that this PC-like cleavage site is correctly cleaved by furin, a member of the PC family. Here we report that among PC members, furin more closely satisfies the requirements needed to fulfill the role of a genuine TGF-β1 convertase. Even though six members of the PC family have the ability to cleave TGF-β1, ectopic expression of α1-antitrypsin Portland (α1-AT-PDX), a potent furin inhibitor, blocked 80% of TGF-β1 processing mediated by endogenous enzymes as demonstrated in an in vitro digestion assay. Genetic complementation of a furin-deficient LoVo cell line with the wild-type gene restores the production of mature and bioactivable TGF-β1. Moreover, both furin and TGF-β are coordinately expressed and regulated in vitro and in vivo in the hematopoietic and immune system, an important tissue target. These results demonstrate for the first time that furin is an authentic and adaptive TGF-β1-converting enzyme whereas other members of the PC family might substitute or supplement furin activity. Our study advances our comprehension of the complexity of the TGF-β system and should facilitate the development of therapeutically useful TGF-β inhibitors.
Transforming growth factor (TGF)-β is defined as a 25,000 kd molecular weight homodimer with a unique N-terminal amino acid sequence. 1-3 The genes coding for TGF-β and other closely related genes have been cloned and sequenced, providing evidence for a structurally related family. 4 In mammals, there are three distinct molecular forms of TGF-β designated TGF-β1, TGF-β2, and TGF-β3. 5-7 This family of polypeptide growth factors are potent regulators of cell growth and differentiation. TGF-β1, the most extensively studied isoform, is increasingly recognized as a natural suppressor of immune and inflammatory reactions. 8-9 It is produced by every leukocyte lineage and its expression serves to control the differentiation, proliferation, and state of activation of the immune system. 10 Convincing evidence for the immunosuppressive and anti-inflammatory roles of TGF-β1 has been obtained from TGF-β1-deficient mice, which develop a multiple-organ inflammatory disease that is lethal by 3 to 5 weeks of age as well as the appearance of autoimmune responses. 11,12 In contrast, increased production of active TGF-β1 has been linked to immune defects associated with malignancy, susceptibility to opportunistic infections, and to the fibrosis associated with chronic inflammatory conditions. 9 Because of its broad spectrum of injurious effects, this cytokine is an important target for therapeutic interventions.
All three mammalian TGF-β isoforms are first synthesized as larger biologically inactive precursors which are proteolytically processed to yield a 25-kd homodimer. 5,13 The biosynthesis of the β1 isoform is the most extensively studied and generally regarded as the prototype of the TGF-β family. TGF-β1 is initially synthesized as a 390 amino-acid precursor molecule. 5 Studies of the molecular events in the processing of this precursor dictate that important proteolytic sites are present and contribute in the formation of the final product. After synthesis, the signal peptide cleavage occurs at the Gly-29-Leu-30 peptide bond of the preproTGF-β1, yielding proTGF-β1 (amino acids 30 to 390). Proteolytic processing of the precursor to yield the mature TGF-β takes place at a cluster of basic amino acids (R-H-R-R) immediately preceding Ala-279. 13 Interestingly, this processing site is a consensus cleavage motif for the mammalian convertase furin and we have provided evidence that the TGF-β1 precursor is effectively processed in vitro and in vivo by human furin releasing a mature TGF-β1 moiety homologous to the natural product. 14 Such observation shed new light on the process of TGF-β maturation events but further experiments were needed to verify if furin is a genuine TGF-β1-converting enzyme.
Furin, a calcium-dependent serine protease, belongs to a family of mammalian processing enzymes called proprotein convertases (PCs). 15,16 Up to seven members of this family have been identified to date. These are Ca+2-dependent serine proteases that share overlapping cleavage site specificity and tissue distribution. They have been shown to cleave mostly C-terminal to R-R or K-R pairs of basic amino acids. Furin, the first PC member to be extensively characterized, has been shown to process many pro-proteins including BMP-4 17,18 pro-β-NGF, 19 the insulin receptor, 20 the Notch1 receptor 21 the HIV-1 glycoprotein gp160, 22 as well as several metalloproteases 23-26 among others. The fur gene, which encodes furin, seems to be ubiquitously expressed in all tissues and cell types examined to date but in variable amounts among them. 27-29 Furin is mostly concentrated in the trans-Golgi network 30,31 and can recycle from the cell membrane to endosomes. 31 Substrate specificity studies have revealed that furin requires a R-X-X-R motif for cleavage whereas the R-X-K/R-R sequence provides an optimum processing site. 32 Apart from furin, the other members of the PC family include PC1/PC3, PC2, PC4, PACE-4, PC5/PC5A/PC6A, PC5/PC5B/PC6B (a splice variant of PC5), and PC7/PC8/LPC. According to their tissue distribution, the PCs can be classified into distinct subgroups where furin and PC7/PC8 are ubiquitously distributed, PACE-4, PC5/PC6A, and PC5/PC6B are expressed to varying degrees in many tissues and whereas the other convertases PC1, PC2, and PC4 are restricted to specific tissues such as neural and endocrine ones (PC1, PC2) and testicular spermatogenic cells (PC4).
Unlike disruption of PC2 33 or PC4, 34 silencing of the expression of mouse furin results in embryonic lethality between days 10.5 and 11.5. 35 This is presumably because of hemodynamic insufficiency associated with several developmental defects including failure of the heart tube to fuse and undergo looping morphogenesis and failure of the embryo to undergo axial rotation. These findings are consistent with a role of furin in the maturation/activation of several members of the TGF-β family including TGF-β1, BMPs, nodal, dorsalin, and lefty-1 and -2 and suggest that they are candidate physiological furin substrates.
Given the similarity of cleavage site specificity between all PCs and their overlapping expression in different tissues, it is often difficult to assign cleavage of a given precursor to a particular convertase. However, processing within the constitutive secretory pathway is probably achieved by either furin, PACE-4, PC5A and PC5B, or PC7. Because these proteases all have specificity toward multiple (clusters) of basic amino acids, it is possible one or more PCs are involved in the endoproteolytic cleavage of the TGF-β precursor. Among the processing competent PCs, an authentic protease responsible for TGF-β activation would have to fulfill several indispensable requirements. These include correct cleavage in vitro and in vivo at the naturally occurring cleavage site as well as coordinated expression and regulation of the precursor and the enzyme. Other essential proofs are provided from specific inhibition studies whereas inactivation or genetic alteration of the enzyme should invariably prevent processing of the precursor in the intact cell. This study was designed to define if furin meets the requirement of an authentic TGF-β-converting enzyme and if other members of the PC class of proteases also expresses TGF-β convertase activity. So far, our results indicate that furin fulfills the essential requirements needed for an authentic TGF-β1-converting enzyme whereas other members of the PC family might supplement or substitute in part furin activity. Possible involvement of these findings in embryogenesis as well as TGF-β-related biological and pathological conditions are discussed.
Materials and Methods
Vaccinia Recombinants and Co-Infections
The vaccinia virus wild type (VV:WT) and the VV recombinant engineered to express the proopiomelanocortin (POMC) polypeptide (VV:POMC) are used as control vaccinia virus and control recombinant vaccinia virus, respectively. Full-length hTGF-β1 cDNA (ATCC, Rockville, MD) was cloned into the vaccinia insertion plasmid pJM602 and homologous recombination performed according to established procedures. 36 Recombinant vaccinia viruses expressing each PC (mPC1/PC3, mPC2, hPACE-4, mPC5A, mPC5B, hPC7, and hfurin) have been constructed and isolated as previously reported. 37-40 VV:α1-AT was a generous gift from Dr. Gary Thomas (Vollum Institute, Oregon Health Science University, Portland, OR). BSC 40 or LoVo cells were infected with different recombinant vaccinia viruses according to previously published protocols 36 with the exception that supernatants were collected 18 hours after infection.
Western Blot Analysis
After co-infections, the supernatants were dialyzed overnight against 0.2 mol/L acetic acid, lyophilized, and resolved into reducing 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. In selected experiments, the cell pellets were lysed with 1% Nonidet P-40-containing lysis buffer supplemented with a cocktail of protease inhibitors (1 mmol/L phenylmethyl sulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin). Separated proteins were then transferred onto nitrocellulose membranes, blocked, and probed overnight with affinity-purified goat anti-human LAP IgG (1:1,000; R&D Systems, Minneapolis, MN) or rabbit PAN-specific anti-TGF-β antibodies (1:1,000; R&D Systems), rabbit furin-specific antisera (1:10,000; Chiron Corp., Emeryville, CA) or rabbit α1-antitrypsin-specific antisera (1:2,000; DAKO Corp., Carpinteria, CA). The membranes were then washed and incubated 1 hour with horseradish peroxidase-labeled anti-rabbit IgG (1:5,000) or anti-goat IgG. (1:8,000) Immunoreactive bands were revealed using the enhanced chemiluminescence detection system (Amersham Canada Ltd., Oakville, Ontario, Canada).
In Vivo and in Vitro mRNA Modulation
In vitro modulation of furin and TGF-β1 mRNA was performed as previously described. 41 For in vivo modulation, mice were injected intraperitoneally with 5 μg recombinant human TGF-β1, a generous gift from Dr. Antony F. Purchio (Oncogene Corp., Seattle, WA), or 1 μg lipopolysaccharide (Sigma, Oakville, Ontario, Canada). Tissues were collected 6 hours or 24 hours after mice injection, grinded in TriReagent (Molecular Research Center, Inc., Cincinnati, OH) solution, and mRNA extracted according to the TriReagent protocol. For the tissue expression or regulation of furin and TGF-β1, 5 μg of total RNA was used for Northern blot analysis.
PCs mRNA Determination
Plasmids and Probes
The rat cRNA furin, PC1, PACE-4, PC5, and PC7 riboprobes were generated as previously described. 29,42 The rat cRNA TGF-β1 riboprobe was produced from a 985-nucleotide cDNA (ATCC) which corresponds to the coding region nucleotides 413 to 1582 of the published sequence. 43 Briefly, the cDNA insert was excised from pBluescript2KS+ by HindIII + XbaI digestion and transferred into pGEM-7Zf (Promega Corp., Nepean, Ontario, Canada). This new TGF-β1/pGEM vector was linearized with XbaI and the antisense riboprobe was transcribed with RNA polymerase SP6 as described for the other riboprobes. Radiolabeled riboprobes were prepared using [32P]UTP (800 Ci/mmol; Amersham Canada Ltd.) according to the Ambion MAXIscript in vitro transcription kit (Ambion Inc., Austin, TX). Briefly, transcription mixtures were constituted of 50 μCi of [32P]UTP, 10 mmol/L dithiothreitol, 0.5 mmol/L of ATP, CTP, and GTP, 1× transcription buffer, 12.5 U of RNase inhibitor, 1 μg of the appropriate linearized plasmid, and T7 or SP6 RNA polymerase in a total volume of 20 μl. The reactions were performed for 90 to 120 minutes at 37°C. One μl of RNase-free DNase1 was then added for 15 minutes at 37°C to remove the DNA template, and the riboprobes were purified over Sephadex G-50 (Pharmacia Fine Chemicals, Uppsala, Sweden) spin columns.
As a control of RNA loading and integrity, blots were hybridized with a 1.0-kb PstI cDNA probe of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH; American Type Culture Collection, Rockville, MD) or a 3.45 kb cDNA probe of the mouse ribosomal 18S (ATCC). The GAPDH and 18S probe were labeled with a multiprime DNA-labeling system (Amersham) by using [32P]dCTP (specific activity >3,000 Ci/mmol; Amersham).
Northern Analysis
Total RNA was extracted from cells according to the previously described Tri-Reagent protocol. Aliquots of 5 to 10 μg of total RNA were run on a horizontal gel apparatus in 1% agarose gel containing 1× MOPS and 6% formaldehyde submerged in 1× MOPS buffer (pH 7.0). The samples were transferred onto a nylon membrane Hybond N+ (Amersham) by overnight capillary action with 10× standard saline citrate (SSC). After blotting, the RNA was fixed with uv light, baked for 2 hours at 60°C, and the membranes were stained in 0.02% methylene blue in 0.3 mol/L sodium acetate (pH 5.5). The membranes were then prehybridized for 2 hours at 68°C with 1× hybridization buffer containing 120 mmol/L Tris (pH 7.4), 600 mmol/L NaCl, 8 mmol/L ethylenediaminetetraacetic acid (pH 8.0), 0.1% Na4PP, 0.2% SDS, 625 μg/ml heparin, and 10% dextran sulfate. Hybridization began with the addition of the [32P]UTP-labeled cRNA probe and performed overnight in one part 2× hybridization buffer and one part formamide. The membranes were sequentially washed in 2× SSC/1% SDS at room temperature, 2× SSC/1% SDS at 68°C, 0.1× SSC/0.2% SDS at 68°C, and 0.1× SSC/0.1% SDS at 68°C.
For the cDNA GAPDH probe, prehybridization and hybridization were performed in the same prehybridization buffer used for the cRNA riboprobe. The membranes were prehybridized for 4 hours at 68°C and hybridization was performed overnight. The membranes were then washed once at room temperature for 20 minutes in 2× SSC, once with 0.1× SSC/0.5% SDS at 68°C for 60 minutes, and were rinsed off at room temperature in 0.1× SSC. The membranes were then exposed to Kodak XAR-5 film (Eastman Kodak, Rochester, NY) with intensifying screens at −80°C for times ranging from 2 hours to 3 days. Signal intensity was quantitated by densitometry with a Pharmacia LKB Ultrascan XL (Pharmacia Biotech, Uppsala, Sweden). Densitometric values are expressed as the ratio of convertase/GAPDH densitometric quantification with control values set at 1.
Measure of Bioactive TGF-β
Bioactive TGF-β was monitored using a growth inhibition assay with Mv1Lu mink lung epithelial cells (CCL-64; ATCC) essentially as originally described by Tucker et al. 44 In brief, Mv1Lu cells were plated in 96-well flat-bottom plates at 2,500 cells/well. After 48 hours, the medium was removed and serial dilutions of the samples to assay for TGF-β activity were added. After 72 hours incubation, the cells were pulsed with [3H]thymidine for 24 hours. Cells were collected and radioactivity counted in a liquid scintillation counter. One unit of activity was defined as the amount of TGF-β required to give 50% maximal response in the assay. In selected experiments, samples were assayed for active TGF-β using a commercially available enzyme-linked immunosorbent assay kit specific for mature and bioactive TGF-β1 (R&D Systems). The limit of detection for this assay is 30 pg/ml TGF-β1.
Results
TGF-β-Converting Capacity of PCs
Co-Infection Studies in Furin-Deficient Cells
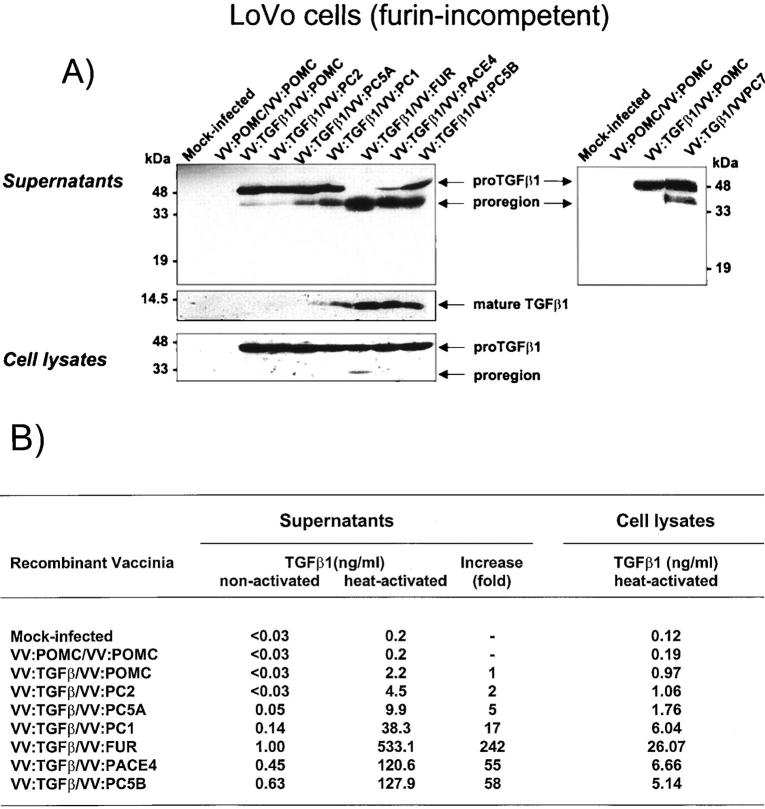
To delineate which enzyme(s) in the PC family of proteases expresses TGF-β1 convertase activity, we used vaccinia virus as an expression vector to produce both the substrate (proTGF-β1) and the enzymes in recombinant forms. In a first set of experiments, we infected LoVo cells, a furin-deficient cell line, 45 with both vaccinia recombinants for TGF-β1 precursor and each of the PCs. After co-infection, concentrated supernatants were analyzed for TGF-β processing by immunoblotting. As illustrated in Figure 1A ▶ (left), LoVo cells co-infected with vaccinia virus encoding proTGF-β1 and control recombinant virus (VV:POMC) exhibited very limited cleavage of proTGF-β1 as evidenced by the appearance of a major band with an apparent molecular weight of 50 kd that corresponds to the intact TGF-β1 precursor and a faint 40-kd band corresponding to the proregion on probing with antibodies against the TGF-β1 precursor. Efficient secretion of the TGF-β1 precursor form has already been reported. 13,14 In contrast, co-infection of VV:TGF-β1 with vaccinia virus encoding PC1, PC5B, PACE-4 or furin resulted in a significant loss of an immunoreactive 50-kd band and concomitant appearance of the 40-kd proregion. The intensity of such a shift is more pronounced with furin co-expression followed by PACE-4 and PC5B with moderate cleavage found with PC1. Although LoVo cells infected with each of the convertases express mRNA for the respective convertase equivalently (data not shown), PC2 and PC5A do not seem to occasion significant impact on TGF-β conversion. PC5A activity in LoVo cells has previously been reported. 46 Reprobing of the same blot with an antisera specific for the mature c-terminal portion of TGF-β indicated that the observed intensity of the 40-kd polypeptide corroborates with the detection of the 12.5-kd mature product. This correlated with a 242-, 58-, 55-, and 17-fold increase in biologically active TGF-β1 for furin, PC5B, PACE-4, and PC1, respectively (Figure 1B) ▶ . In separate experiments, PC7 was found to possess less converting capacity than furin with a 37-fold increase of bioactive TGF-β1 compared to 132-fold for furin (Figure 1A ▶ , right, and data not shown).
Figure 1.
A: Processing of TGF-β1 precursor by PCs in LoVo cells. LoVo cells were infected with vaccinia recombinants for both the TGF-β1 precursor (VV: TGF-β) or an unrelated control vaccinia recombinant (VV: POMC) and one of the PCs (PC1/PC3, VV: PC1; PC2, VV: PC2; PC5A, VV: PC5A; furin, VV: FUR, PACE-4, VV: PACE-4; PC5B, VV: PC5B; PC7, VV: PC7) at a multiplicity of infection of 5. Eighteen hours after infection, supernates or cell lysates were electrophoresed on 12% SDS-PAGE gels under reducing conditions. Immunoblots were performed with an anti-human LAP IgG (revealing an ∼55-kd proTGF-β1 and a 40-kd proregion forms) or PAN-specific anti-TGF-β antibodies (revealing an ∼12-kd mature TGF-β1 form). A representative experiment out of four performed is shown. B: Measure of TGF-β1 in cell supernatants or cell lysates. Eighteen hours after cell infection, LoVo cell supernates were collected, heat-activated (80°C, 5 minutes), and used to measure bioactive TGF-β1. A representative experiment out of four performed is shown.
We have previously reported that cleavage of proTGF-β1 by furin occurs at the predicted and physiologically used R-H-R-R site. 14 Because all TGF-β1-competent convertases depicted similar migration patterns of the cleavage products when compared to furin, it is likely that in each case proTGF-β1 is processed at the same R-H-R-R cleavage site. In addition, the absence of contaminating bands that may have occurred after cleavage at pairs of basic amino acids found within the proTGF-β1 molecule 5 provides additional evidence for the specificity of cleavage of TGF-β1 precursor by each of the convertases (Figure 1A ▶ , left and right panels).
Intracellular Processing of TGF-β1 Precursor
Because several indications revealed that proTGF-β1 to TGF-β1 conversion occurs intracellularly, likely within the trans-Golgi network, we assessed the presence of proteolytic products of TGF-β1 in cell lysates. LoVo cells were therefore co-infected with TGF-β1 precursor and one of the different PCs. Eighteen hours after infection, cell lysates were analyzed for the presence of different TGF-β cleavage products by immunoblotting on reducing SDS-PAGE gels. As illustrated in Figure 1A ▶ , co-infection of VV:TGF-β1 and VV:FUR resulted in the production of the 40-kd pro-region species as revealed using an anti-proTGF-β1. As previously described, 47 TGF-β cleavage products are less abundant in cell lysates compared to cell supernates presumably because of the localization of furin in the trans-Golgi. Measure of bioactive TGF-β indicated that among the convertases tested, furin co-infection resulted in a higher production of intracellular mature TGF-β1 (Figure 1, A ▶ , left, and B). The reported accumulation of furin in the trans-Golgi compartment might explain, in part, its efficiency in intracellular conversion of TGF-β1 precursor. 30,31
Co-Infection Studies in BSC-40 Cells
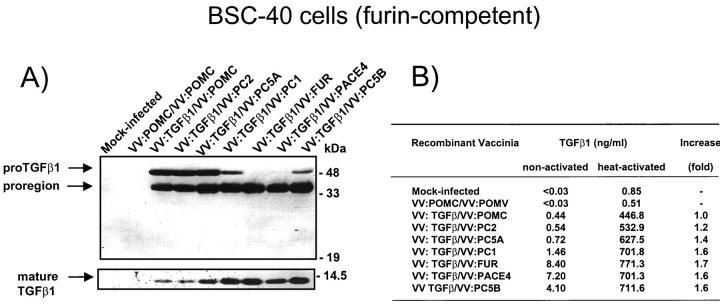
It was reported that endogenous furin may participate in the activation (removal of the proregion) of the proprotein convertase PC2. 48 We therefore wished to confirm the lack of TGF-β1-maturating potential of the convertase PC2 obtained with LoVo cells using this time a furin-positive cell line (BSC-40). As shown in Figure 2A ▶ , BSC-40 cells infected with vaccinia encoding proTGF-β1 produces ∼50% of processed TGF-β1. Co-expression of furin, PACE-4, PC5B, and PC1 resulted in an increase in TGF-β1 maturation with full or near-complete processing observed with furin and PACE-4, PC5B, and PC1. As observed in LoVo cells, no processing (greater than baseline level) was observed with the convertase PC2. The observed increase in TGF-β1 processing correlated with an increase in bioactivity as measured in the conditioned medium (Figure 2B) ▶ .
Figure 2.
A: Processing of TGF-β1 precursor by PCs in BSC-40 cells. Cells were infected with vaccinia recombinants for both the TGF-β1 precursor (VV: TGF-β) or an unrelated control vaccinia recombinant (VV: POMC) and one of the PCs (PC1/PC3, VV: PC1; PC2, VV: PC2; PC5A, VV: PC5A; furin, VV: FUR, PACE-4, VV: PACE-4; PC5B, VV: PC5B) at a multiplicity of infection of 5. Eighteen hours after infection, supernates were collected, concentrated, and electrophoresed on 12% SDS-PAGE gels under reducing conditions. Immunoblots were performed with an anti-human LAP IgG (revealing an ∼55-kd proTGF-β1 and an ∼40-kd proregion forms) or PAN-specific anti-TGF-β antibodies (revealing an ∼12-kd mature TGF-β1 form). A representative experiment out of three performed is shown. B: Measure of TGF-β1 in cell supernates. Eighteen hours after cell infection, cell supernates were collected, heat-activated (80°C, 5 minutes), and used to measure bioactive TGF-β1. A representative experiment out of three performed is shown.
The α1-Antitrypsin Portland Mutant Inhibits Processing of TGF-β by Endogenous Convertases
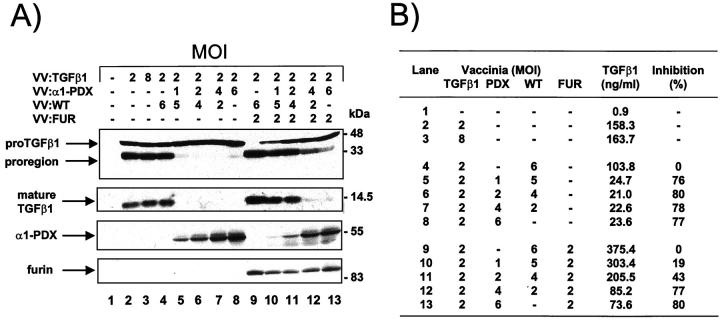
We next tested the possibility that TGF-β1 is proteolytically activated by endogenous furin-like endoproteases, by overexpression of α1-antitrypsin Portland (α1-PDX), a modified serpin with potent furin inhibitory activity. 17,49 For this, the furin-positive BSC-40 cells were co-infected with vaccinia recombinants encoding for the substrate and/or the α1-PDX inhibitor. As demonstrated in Figure 3A ▶ , lane 2 (also shown in Figure 2A ▶ ), BSC-40 cells infected with vaccinia encoding proTGF-β1 produced ∼50% of processed TGF-β as seen by the relative intensity of the pro-region and proTGF-β1 bands. Co-infection of cells with 1 to 6 multiplicity of infection of α1-PDX vaccinia abrogated proTGF-β1 proteolytic processing mediated by endogenous cellular enzyme(s) whereas co-expression with a wild-type virus did not affect basal level of TGF-β proteolytic processing (Figure 3A ▶ , lanes 5 to 8). In parallel, the amounts of active TGF-β1 released in cell culture medium were diminished by the expression of α1-PDX (Figure 3B) ▶ with an average of 78% inhibition observed at an multiplicity of infection of 1 to 6 of VV:PDX. As a control, co-infection of the BSC-40 cells with vaccinia encoding furin resulted in complete processing of TGF-β1 precursor which is also inhibited by α1-PDX co-expression in a dose-related manner (Figure 3 ▶ , lanes 9 to 13). Theses results suggest that most of the TGF-β1-converting activity found in BSC-40 cells is related to the furin protease. Because complete inhibition of proTGF-β1 processing by α1-PDX could not be achieved with a plateau observed at all of the multiplicity of infection tested, our results also suggest that other convertase(s) present in BSC-40 cells and not inhibited by the serpin could be accessory in this process.
Figure 3.
A: Inhibition of TGF-β1 processing by α1-PDX. BSC-40 cells were infected with vaccinia recombinants for TGF-β1 precursor (VV: TGF-β), control vaccinia virus (VV: WT), furin-encoding vaccinia virus (VV: FUR) and α1-PDX-encoding vaccinia virus (VV: α1-PDX) at the indicated multiplicity of infection. Eighteen hours after cell infection, cell supernates were collected and electrophoresed on 12% SDS-PAGE gels under reducing conditions. Immunoblots were performed with an anti-human LAP, PAN-specific anti-TGF-β antibodies, furin-specific antisera (revealing an ∼95-kd form) and α1-antitrypsin-specific antisera (revealing an ∼55-kd α1-PDX form). B: Measure of TGF-β1 in cell supernates. Eighteen hours after cell infection, cell supernates were collected, heat-activated (80°C, 5 minutes), and used to quantitate bioactive TGF-β1 as described in Material and Methods. A representative experiment out of two performed is shown.
Co-Expression of Furin and TGF-β1 in Cell Lines and Tissues
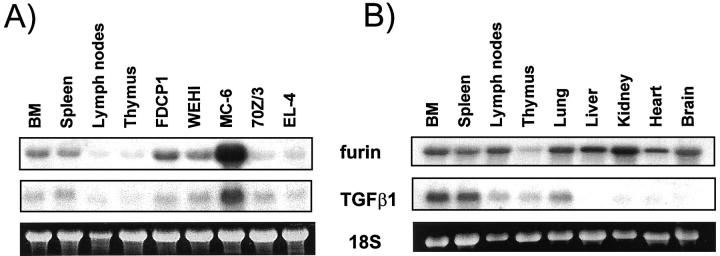
To determine whether the pattern of expression of furin is appropriate for a TGF-β1 convertase, total mRNA from mice tissues and cell lines was probed for both furin and TGF-β1 expression. Because TGF-β1 has been reported to be an important natural suppressor of the hematopoietic and immune system, we first investigated hematopoietic/immune cells and tissues. Northern blot analysis of TGF-β1 and furin mRNA, using the same mRNA sample, indicated that mRNA levels for both the PC and the substrate are coordinately expressed among the hematopoietic and immune tissues and cell lines examined (Figure 4A) ▶ . Highest expression of both mRNAs was observed in the promastocyte cell line MC-6 followed by the myeloid progenitor cell lines FDCP1 and WEHI, bone marrow, spleen, and lymph nodes (see Figure 4B ▶ ). Lower expression was found in thymus as well as the proB cell line 70Z/3 and T cell line EL-4.
Figure 4.
A: Furin and TGF-β1 mRNA expression in hematopoietic/immune cells and tissues. B: Furin and TGF-β1 mRNA expression in mice tissues. Northern blot analysis used total mRNA (5 μg/lane) and a rat TGF-β1 or a rat furin riboprobe. Ethidium bromide staining of 18S is shown as a control for mRNA integrity.
Further comparison with nonhematopoietic/lymphoid tissues indicated that whereas furin mRNA expression is abundant in lung, liver, kidney, heart, and brain, a significant amount of TGF-β1 mRNA is co-expressed only in the lung (Figure 4B) ▶ . The presence of TGF-β1 in the lung could be because of high content of cells from hematopoietic origin such as alveolar macrophages which are known to express this particular TGF-β isoform. 50 Our findings are consistent with previous reports that the TGF-β1 isoform is predominant in immune/hematopoietic cells 8,51 and that furin is ubiquitously expressed among tissues and cell lines. 27-29 Therefore, endogenous furin, particularly within the hematopoietic/immune system, is expressed in appropriate amounts and cellular/tissue location to efficiently cleave TGF-β1 precursor.
In Vitro and in Vivo Co-Regulation of Furin and TGF-β1
Regulation of PC Gene Expression by Recombinant TGF-β
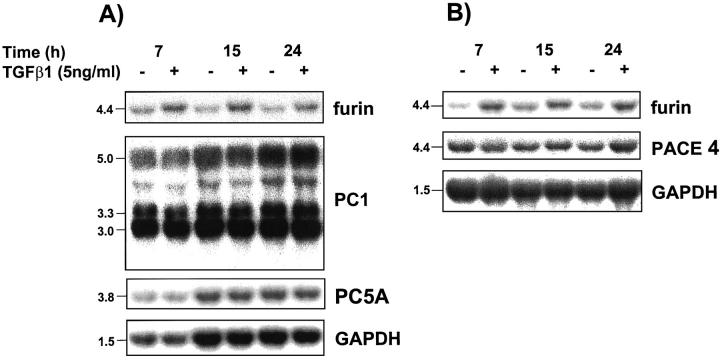
In synoviocytes and fibroblastic cells, TGF-β1 was shown to increase furin gene expression creating an autoregulation pathway generated by the proteolysis product. 41 We therefore determined whether the bioactive cleavage product could also regulate cellular levels of other PC mRNAs. For this, we performed Northern blot analysis of total cellular RNA obtained from the fibroblastic NRK-49F cells and the insulinoma Rin m5F cells cultured for various time periods in the presence or absence of 5 ng/ml of TGF-β1. As reported, Rin m5F and NRK-49F cells express a 4.4-kb signal on probing with a rat furin riboprobe. 29 Treatment of these cells with 5 ng/ml of TGF-β1 resulted in a sustained increase in furin steady-state mRNA levels (Figure 5, A and B) ▶ . This did not represent a general increase in cellular gene expression because the levels of mRNA for GAPDH were unaffected. Conversely, corrected levels of PACE-4, PC1 (3.0-kb, 3.3-kb, and 5.0-kb transcripts), PC5A and PC7 (Figure 5, A and B ▶ , and Figure 6 ▶ ) were unchanged. Note that under our hybridization conditions, mRNA for the convertase PC5B was not detectable in these cell lines. These results indicate that among the PCs analyzed, only furin is modulated by TGF-β.
Figure 5.
PC mRNA regulation by TG F-β1. The rat insulinoma cell line (Rin m5F) (A) or rat fibroblastic kidney cell line (NRK-49F) (B) were incubated for 7, 15, and 24 hours in the presence or absence of 5 ng of recombinant human TGF-β1. Total mRNAs (10 μg/lane) were probed with rat riboprobes specific for furin, PC1, PC5A/PC5B, PACE4, or GAPDH cDNA.
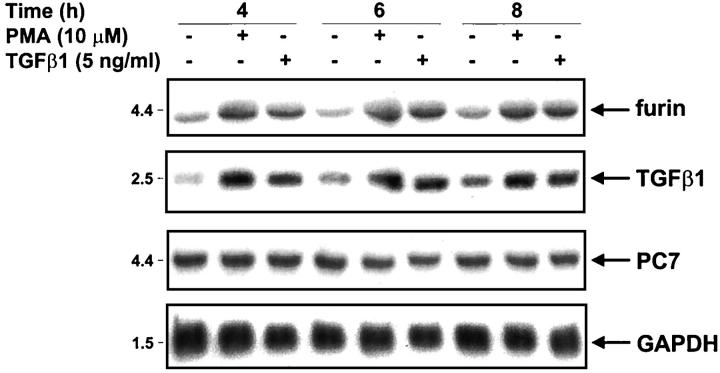
Figure 6.
Co-regulation of furin and TGF-β1 mRNAs. NRK-49F cells were incubated for 4, 6, or 8 hours in the presence or absence of 10 μmol/L PMA or 5 ng/ml human recombinant TGF-β1. Total mRNAs (10 μg/lane) were probed with rat riboprobe specific for furin, TGF-β1, PC7, and a GAPDH cDNA.
In Vitro and in Vivo Co-Modulation of Furin and TGF-β1 mRNAs
To determine whether the furin convertase is coordinately regulated with TGF-β1, the NRK cells were incubated in the presence of 5 ng/ml of TGF-β1 or 10 μmol/L PMA and mRNA analyzed for specific TGF-β1 and furin messages. These stimuli were chosen because they are well established TGF-β1 inducers. 52,53 With either TGF-β1 or PMA stimulation, furin and TGF-β1 are up-regulated with an increase in both messages seen after 4 hours stimulation with sustained increase observed until the end of cell stimulation (8 hours) (Figure 6) ▶ . In the same experiments, the levels of PC7 remained unchanged.
To determine whether similar co-regulation could be observed in vivo, adult BALB/c mice were injected intraperitoneally with either 5 μg of TGF-β1 or 1 μg of lipopolysaccharide. Six hours and 24 hours after, mRNA was extracted from various tissues and analyzed for furin and TGF-β1 messages. Interestingly, a single intraperitoneal injection of TGF-β1 or lipopolysaccharide, a known in vivo TGF-β inducer, 54 resulted in an important increase in furin mRNA in all tissues examined except for the brain tissue. The most important modulation was detected in the heart, thymus, and lung (Table 1) ▶ . Reblotting with TGF-β1-specific riboprobe indicated that the TGF-β1 message is co-modulated with furin in the spleen, lymph node, thymus, and lung. As described above, the expression of TGF-β1 mRNA in liver, kidney, heart, and brain was too low to be accurately quantitated by densitometric analysis. These results indicate that on in vivo challenge with inflammatory agents, furin and TGF-β1 are coordinately modulated in lymphoid/hematopoietic tissues. The exact reason for the lack of furin message modulation in the brain is currently unclear.
Table 1.
In Vivo Modulation of Furin and TGF-β1 mRNA
| Tissues (densiometric values)* | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modulator/time (hours) | Spleen | Lymph nodes | Thymus | Lung | Liver | Kidney | Heart | Brain | ||||||||
| Fur | TGF-β | Fur | TGF-β | Fur | TGF-β | Fur | TGF-β | Fur | TGF-β | Fur | TGF-β | Fur | TGF-β | Fur | TGF-β | |
| PBS | ||||||||||||||||
| 6 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | —§ | 1.0 | — | 1.0 | — | 1.0 | — |
| TGF↠| ||||||||||||||||
| 6 | 1.4 | 1.4 | 1.8 | 1.6 | 5.3 | 2.8 | 3.4 | 2.9 | 2.9 | — | 2.3 | — | 8.0 | — | 1.1 | — |
| 24 | 0.5 | 0.6 | 1.1 | 0.9 | 2.0 | 0.6 | 2.6 | 2.2 | 2.2 | — | 1.0 | — | 6.5 | — | 0.5 | — |
| LPS‡ | ||||||||||||||||
| 6 | 4.2 | 2.4 | 0.7 | 0.8 | 4.7 | 1.6 | 4.5 | 2.4 | 2.4 | — | 2.6 | — | 11.2 | — | 0.8 | — |
| 24 | 0.6 | 0.5 | 1.1 | 2.1 | 1.7 | 0.8 | 1.1 | 1.5 | 1.5 | — | 1.4 | — | 4.9 | — | 0.7 | — |
Fur, furin; PBS, phosphate-buffered saline; LPS, lipopolysaccharide.
*Densiometric values expressed as the ratios furin/18S and TGF-β1/18S densiometric quantification with control (PBS) values set at 1.
†Single i.p. injection of 5-μg purified human TGF-β1 in PBS.
‡Single i.p. injection of 1-μg LPS in PBS.
§mRNA expression too low for densitometric quantification.
Discussion
In vitro evidence has been provided that furin, the prototypical PC can correctly cleave TGF-β1 precursor at the furin RHRR↓ sequence. 14 Because this processing site could also be recognized by other PCs, it is possible that one or more members of the PC family are responsible for such a maturation process. In fact, the results herein indicate that the convertases expressed through the constitutive secretory pathway, furin, PC5B, PACE-4, and to a lesser extent PC7, all have the capacity to correctly cleave proTGF-β1, except PC5A which is a soluble splice variant of PC5B. In contrast, the convertases targeted to the regulated secretory pathway PC1 and PC2 have moderate to poor capacity to process this precursor. Our findings are consistent with earlier findings from Gentry and others, 13 which indicate that TGF-β, as most growth factor precursors, follows a constitutive route of secretion and mature within the Golgi apparatus. 55 The inability of PC2 to process TGF-β in the furin-competent BSC-40 cells would likely be because of the lack of PC2 chaperone (7B2) expression in nonendocrine cells. Even though the isoforms PC5A and PC5B have been shown to contain identical catalytic domains, 56 only the C-terminal extended and membrane-bound form efficiently cleaves proTGF-β1 whereas PC5A, which is a soluble form of the enzyme, expresses poor activity. This raises the possibility that in addition to enzyme-specific variations in affinity for the RHRR recognition sequence, immobilization of the enzymes and/or compartimentation could enhance the efficiency of cleavage of proTGF-β1. Among the tested enzymes, furin and PC5B contain localization motifs in their cytoplasmic tails that enable them to remain in the Golgi stacks, but in separate compartments. 31,56,57 Moreover, comparative studies in cells indicate that the membrane-anchored enzymes furin and PC5B are often the most potent converting enzymes for proproteins that transit through the secretory pathway including the neurotrophins proNGF, proBDNF, and proNTF. 58,59 Even though PC7 also possess a membrane-bound segment, this enzyme is not phosphorylated and does not contain the same localization motifs as furin and PC5B within its cytoplasmic tail suggesting differential subcellular localization. In fact, recent studies indicate that the localization of PC7 is distinct from furin and is rather concentrated in trans-Golgi network-derived vesicles instead of the TGN. 60 The poor capacity of PC7 to process TGF-β precursor might also come from subcellular localization constraints. Even though both furin and PC7 show an absolute requirement for substrate having an arginine residue at the P-1 position, an arginine in position P-6, not found in the TGF-β1 cleavage site, seems to be more important for PC7 processing of substrates. 37,61,62
In an effort to identify the endogenous convertase involved in TGF-β1 processing we took advantage of the availability of two specific tools. First, we tested the impact of the inhibitor α1-antitrypsin Portland (α1-PDX) on TGF-β1 maturation. The α1-PDX is an engineered variant of the endogenous elastase inhibitor which now mimics the minimum consensus sequence (R-X-X-R) required for furin recognition and has been shown to inhibit processing of precursors mediated by PCs primarily within the constitutive secretory pathway. 63,64 Recent studies using purified enzymes and either a fluorogenic synthetic substrate or the natural substrate BMP-4 have demonstrated that α1-PDX is a potent inhibitor of furin with a Ki of 0.6 nmol/L. 17,48 Our data indicates that this serpin significantly blocks the maturation of TGF-β1 precursor in BSC-40 cells due to the endogenous converting enzyme resulting in the accumulation in supernates of the inactive (cannot be activated by heat or acid) precursor form. These cells are known to express furin as well as PACE-4, PC5A,and PC5B. 29,64 Therefore, in this system, the endogenous TGF-β1 convertase is clearly a member of the PC family.
Second, we overexpressed TGF-β1 precursor in a furin-deficient cell line, the LoVo cells. These are colon carcinoma cells which have a point mutation in both alleles of the fur gene leading to production of a defective enzyme. 45 These furin knockout cells have been extensively used to study the contribution of furin in a cellular context. Data presented in this study indicate that in contrast with the furin-positive BSC-40 cells, LoVo cells are deficient in TGF-β maturation resulting in the release of the precursor form with <5% conversion into mature product. In addition to defective furin, LoVo cells express high levels of the 4.4-kb mRNA for PACE-4, 29,65 moderate levels of PC5A/PC5B, 65 and some PC7. 42,66 Although these proteases properly cleave TGF-β1 precursor in the overexpression system, the lack of TGF-β conversion in these cells indicates that they do not play an important role as endogenous TGF-β1 convertases. This is supported by recent data from our laboratory indicating that LoVo cells do not produce detectable amounts of endogenous TGF-β1 ligand in their conditioned medium but genetic complementation with wild-type furin resulted in the production of 1 to 2 ng/ml of bioactivatable TGF-β1 (unpublished observation). Clearly, these observations indicate that furin is a predominant endogenous TGF-β1 convertase. It is interesting to point out that mutations in other components of the TGF-β pathway, ie, the type I and type II receptors as well as members of the TGF-β transducers SMADs have been described in various colon carcinoma cell lines and tissues. The role of these mutations in malignant transformation and tumor development is now established. 67,68 In this context, mutation in the TGF-β-converting enzyme might represent another mechanism of inactivation of the TGF-β growth regulation pathway. Efforts are underway to verify this possibility.
So far, the expression of the fur gene, which encodes furin seems to be ubiquitous in all tissues and cell types examined to date but in variable amounts among them. This and other studies indicate that the TGF-β1 isoform is abundantly expressed in hematopoietic/immune cells and tissues where this cytokine exerts profound suppressive effects on immune functions. 8,51 The critical immunosuppressive role of TGF-β1 is underlined in mouse models in which the TGF-β1 gene has been inactivated. These mice died by 2 to 3 weeks of age because of the development of a wasting syndrome caused by massive leukocyte infiltration into multiple organs. 11,12 Autoimmunity also accompanies the inflammatory disease as illustrated by the presence of multiple autoantibodies in the serum and glomerular Ig deposits in their kidney. Because TGF-β1 is established as a gatekeeper of the immune system, one could envision that aberrant expression of furin would impact the normal homeostasis of this system leading to inflammation/autoimmune diseases. A loss of function mutation has been generated at the furin locus but mice embryos lacking furin die during embryonic development before the establishment of the immune system. 35 Therefore, tissue-targeted DNA or a protein-based approach to silence the expression of furin in immune cells might help to define the exact role of furin in this system.
One interesting particularity of the TGF-β system is its ability to regulate the effectiveness of several important gears of its machinery. For example, TGF-β modulates gene transcription of the plasminogen activator/plasminogen activator-inhibitor system involved in the dissociation of the latent TGF-β complex 69 and is a potent inducer of its own expression through induction of the (SMAD) AP-1 complex. 70,71 Results from this and a previous study indicate that TGF-β is also a regulator of furin expression through transcriptional activation of the fur gene. 41 Such an increase in convertase expression translated into increased processing of the precursor molecule and adds to the complexity of the TGF-β system. In this study, we observed that among the known PCs, only furin is regulated by its cleavage product TGF-β1, which results in coordinated increase in transcripts for the TGF-β1 precursor and the furin convertase. Such a co-regulation adds weight to the physiological coupling of furin and the TGF-β1 precursor. Coordinated variations of both partners could influence the extent of TGF-β1 production in physiological processes such as embryonic development as well as TGF-β-related physiological and pathological conditions whereas the other PCs might not possess the plasticity needed to permit such variations. Supporting this, among the PCs, furin is co-expressed with TGF-β1 in the developing heart, bones, and fetal liver. 72-74 Interestingly, by e12 and midgestational stage (e13 to e-16), there is striking temporal correlation in the expression of furin and TGF-β1 in developing liver. 72-74 This corresponds to the establishment of hematopoiesis in this tissue, in particular the platelet-producers megakaryocytes. 73,74 It is well known that megakaryocytes and platelets are a rich source of the TGF-β1 isoform 75,76 that is thought to be essential for the initiation of the repair process and it was suggested that this isoform plays a critical role in lipoprotein deposition and vascular smooth muscle cell proliferation characteristic of atherosclerosis lesions. 76,77
Physiological levels of TGF-β are necessary for tissue repair and maintenance of organ functions. Overexpression of TGF-β is closely linked to certain diseases including tissue fibrosis. A body of literature has reported the contribution of TGF-β to renal and hepatic fibrogenesis. 78-81 In glomerulonephritis, for example, increased TGF-β results in excessive matrix accumulation, inhibits matrix-degrading proteinases, and up-regulates proteinase inhibitors. In the liver, TGF-β increases proliferation and collagen synthesis in the mesenchymal cells (by an autocrine mechanism) and this correlates with the degree of fibrosis. A recent study indicates that blockage of TGF-β signaling prevents liver fibrosis and dysfunction in the rat. 82 In this context, our findings that furin is an authentic TGF-β-converting enzyme that is regulated by the substrate may contribute to the delineation of a new target for the interruption of these diseases.
It is particularly difficult to assign a specific enzyme to a given substrate. This is especially true, in the case of PCs, where redundancy in the ability of the convertases to cleave various substrates has been clearly documented. Nevertheless, much knowledge could be obtained through the use of combined approaches including co-expression of the convertases and the substrates and the utilization of specific inhibitors or gene knockouts. The expression and regulation pattern of converting competent convertases will also dictate which of the enzymes is predominantly used in a particular tissue and cellular context. Using these criteria we provide herein evidence that furin meets the criteria for an authentic and adaptive TGF-β1-converting enzyme. Such demonstration adds to our understanding of the complexity of the TGF-β system and may help define a new target for future investigations and interventions of pathologies in which this growth factor is closely linked. These findings may also be extended to many members of the large TGF-β family, which also possess similar furin consensus motif at the junction of the pro-region and the mature polypeptide. Unlike disruption of the neuroendocrine PC2 gene, inactivation of the furin gene in the mouse results in embryonic lethality associated with several developmental defects including failure of the heart tube to fuse and undergo looping morphogenesis and failure of the embryo to undergo axial rotation. 35 These finding suggest that furin in a genuine maturation/activation enzyme of several other members of the TGF-β family including TGF-β1, BMPs, nodal, and lefty -1 and -2.
Acknowledgments
We thank Dr. R. Day for his help in riboprobe technology, and Nathalie Corriveau for secretarial assistance.
Footnotes
Address reprint requests to Claire M. Dubois, Ph.D., Immunology Division, Faculty of Medicine, Université de Sherbrooke, Sherbrooke, Quebec, Canada J1H 5N4. E-mail: cmdubois@courrier.usherb.ca.
Supported by the Canadian Arthritis Society (grant no. 91058) and the Medical Research Council of Canada (grant nos. MT13222, MT14461 to C. M. D. and GP11474 to N. G. S.). C. M. D. is scholar of the Fonds de la Recherche en Santé du Québec.
References
- 1.Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB: Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem 1983, 258:7155-7160 [PubMed] [Google Scholar]
- 2.Roberts AB, Flanders KC, Kondaiah P, Thompson NL, Van Obberghen-Schilling E, Wakefield L, Rossi P, de Crombrugghe B, Heine U, Sporn MB: Transforming growth factor β: biochemistry and roles in embryogenesis, tissue repair and remodeling, and carcinogenesis. (Review). Recent Prog Horm Res 1988, 44:157-197 [DOI] [PubMed] [Google Scholar]
- 3.Roberts AB, Anzano MA, Lamb LC, Smith JM, Sporn MB: New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci USA 1981, 78:5339-5343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kingsley DM: The TGF-β superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev 1994, 8:133-146 [DOI] [PubMed] [Google Scholar]
- 5.Derynck R, Jarrett JA, Chen EY, Eaton DH, Bell JR, Assoian RK, Roberts AB, Sporn MB, Goeddel DV: Human transforming growth factor-β complementary DNA sequence and expression in normal and transformed cells. Nature 1985, 316:701-705 [DOI] [PubMed] [Google Scholar]
- 6.Madisen L, Webb NR, Rose TM, Marquardt H, Ikeda T, Twardzik D, Seyedin S, Purchio AF: Transforming growth factor-beta 2: cDNA cloning and sequence analysis. DNA 1988, 7:1-8 [DOI] [PubMed] [Google Scholar]
- 7.Derynck R, Lindquist PB, Lee A, Wen A, Tamm J, Graycar JL, Rhee L, Masson AJ, Miller DA, Coffey RJ, Moses HL, Chen EY: A new type of transforming growth factor-beta, TGF-beta 3. EMBO J 1988, 7:3737-3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letterio JJ, Roberts AB: Regulation of immune responses by TGF-beta. Annu Rev Immunol 1998, 16:137-161 [DOI] [PubMed] [Google Scholar]
- 9.Clark DA, Coker R: Transforming growth factor-beta (TGF-beta). Int J Biochem Cell Biol 1998, 30:293-298 [DOI] [PubMed] [Google Scholar]
- 10.Wahl SM: Transforming growth factor beta (TGF-β) in inflammation: a cause and a cure. J Clin Immunol 1992, 12:61-74 [DOI] [PubMed] [Google Scholar]
- 11.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annuziata N, Doetschman T: Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature 1992, 359:693-699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulkarni AB, Huh C-H, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S: Transforming growth factor-β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 1993, 90:770-774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentry LE, Lioubin MN, Purchio AF, Marquardt H: Molecular events in the processing of recombinant type I pre-pro-transforming growth factor beta to the mature polypeptide. Mol Cell Biol 1988, 8:4162-4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubois CM, Laprise M-H, Blanchette F, Gentry LE, Leduc R: Processing of transforming growth factor β1 precursor by human furin convertase. J Biol Chem 1995, 270:10618-10624 [DOI] [PubMed] [Google Scholar]
- 15.Seidah NG, Chrétien M: Eukaryotic protein processing: endoproteolysis of precursor proteins. Curr Opin Biotech 1997, 8:602-607 [DOI] [PubMed] [Google Scholar]
- 16.Steiner DF: The proprotein convertases. Curr Opin Chem Biol 1999, 2:31-39 [DOI] [PubMed] [Google Scholar]
- 17.Cui Y, Jean F, Thomas G, Chrisian JL: BMP-4 is proteolytically activated by furin and/or PC6 during vertebrate embryonic development. EMBO J 1998, 17:4735-4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Constam DB, Robertson EJ: Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases. J Cell Biol 1999, 144:139-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bresnahan PA, Leduc R, Thomas L, Thorner J, Gibson HL, Brake AJ, Barr PJ, Thomas G: Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo. J Cell Biol 1990, 111:2851-2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson BJ, Moehring JM, Moehring TJ: Defective processing of the insulin receptor in an endoprotease-deficient Chinese hamster cell strain is corrected by expression of mouse furin. J Biol Chem 1993, 268:24274-24277 [PubMed] [Google Scholar]
- 21.Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, Israel A: The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci USA 1998, 95:8108-8112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallenberger S, Bosch V, Angliker H, Shaw E, Klenk HD, Garten W: Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature 1992, 360:358-361 [DOI] [PubMed] [Google Scholar]
- 23.Pei D, Weiss SJ: Furin-dependant intracellular activation of the human stromelysin-3 zymogen. Nature 1995, 375:244-247 [DOI] [PubMed] [Google Scholar]
- 24.Roghani M, Becherer JD, Moss ML, Atherton RE, Erdjument-Bromage H, Arribas J, Blackburn RK, Weskamp G, Tempst P, Blobel CP: Metalloprotease-disintegrin MDC9: intracellular maturation and catalytic activity. J Biol Chem 1999, 274:3531-3540 [DOI] [PubMed] [Google Scholar]
- 25.Lum L, Reid MS, Blobel CP: Intracellular maturation of the mouse metalloprotease disintegrin MDC15. J Biol Chem 1998, 273:26236-26247 [DOI] [PubMed] [Google Scholar]
- 26.Maquoi E, Noel A, Frankenne F, Angliker H, Murphy G, Foidart JM: Inhibition of matrix metalloproteinase 2 maturation and HT1080 invasiveness by a synthetic furin inhibitor. FEBS Lett 1998, 424:262-266 [DOI] [PubMed] [Google Scholar]
- 27.Kiefer MC, Tucker JE, Joh R, Landsberg KE, Saltman D, Barr JP: Identification of a second human subtilisin-like protease gene in the fes/fps region of chromosome 15. DNA Cell Biol 1991, 10:757-769 [DOI] [PubMed] [Google Scholar]
- 28.Hatsuzawa K, Hosaka M, Nakagawa T, Nagase M, Shoda A, Murakami K, Nakayama K: Structure and expression of mouse furin, a yeast Kex2-related protease. Lack of processing of coexpressed prorenin in GH4C1 cells. J Biol Chem 1990, 265:22075-22078 [PubMed] [Google Scholar]
- 29.Seidah NG, Chretien M, Day R: The family of subtilisin/kexin like pro-protein and pro-hormone convertases: divergent or shared functions. Biochimie 1994, 76:197-209 [DOI] [PubMed] [Google Scholar]
- 30.Molloy SS, Thomas L, VanSlyke JK, Stenberg PE, Thomas G: Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J 1994, 13:18-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molloy SS, Anderson ED, Jean F, Thomas G: Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol 1999, 9:28-35 [DOI] [PubMed] [Google Scholar]
- 32.Takahashi S, Hatsuzawa K, Watanabe T, Murakami K, Nakayama K: Sequence requirements for endoproteolytic processing of precursor proteins by furin: transfection and in vitro experiments. J Biochem (Tokyo) 1994, 116:47-52 [DOI] [PubMed] [Google Scholar]
- 33.Furuta M, Yano H, Zhou A, Rouillé Y, Holst J, Carroll J, Ravazzola M, Orci L, Furuta H, Steiner DF: Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci USA 1997, 94:6646-6651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mbikay M, Tadros H, Ishida N, Lerner CP, De Lamirande E, Chen A, El-Alfy M, Clermont Y, Seidah NG, Chrétien M, Gagnon C, Simpson EM: Impaired fertility in mice deficient for the testicular germ-cell protease PC4. Proc Natl Acad Sci USA 1997, 94:6842-6846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roebroek AJ, Umans L, Pauli IG, Robertson EJ, Van Leuven F, Van de Ven WJ, Constam DB: Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase Furin. Development 1998, 125:4863-4876 [DOI] [PubMed] [Google Scholar]
- 36.Benjannet S, Rondeau N, Day R, Chretien M, Seidah NG: PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci USA 1991, 88:3564-3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munzer JS, Basak A, Zhong M, Mamarbachi A, Hamelin J, Savaria D, Lazure C, Benjannet S, Chretien M, Seidah NG: In vitro characterization of the novel proprotein convertase PC7. J Biol Chem 1997, 272:19672-19681 [DOI] [PubMed] [Google Scholar]
- 38.Paquet L, Bergeron F, Boudreault A, Seidah NG, Chretien M, Mbikay M, Lazure C: The neuroendocrine precursor 7B2 is a sulfated protein proteolytically processed by a ubiquitous furin-like convertase. J Biol Chem 1994, 269:19279-19285 [PubMed] [Google Scholar]
- 39.Benjannet S, Savaria D, Chretien M, Seidah NG: 7B2 is a specific intracellular binding protein of the prohormone convertase PC2. J Neurochem 1995, 64:2303-2311 [DOI] [PubMed] [Google Scholar]
- 40.Vollenweider F, Benjannet S, Decroly E, Savaria D, Lazure C, Thomas G, Chretien M, Seidah NG: Comparative cellular processing of the human immunodeficiency virus (HIV-1) envelope glycoprotein gp160 by the mammalian subtilisin/kexin-like convertases. Biochem J 1996, 314:521-532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanchette F, Day R, Dong W, Laprise M-H, Dubois CM: TGFβ1 regulates gene expression of its own converting enzyme furin. J Clin Invest 1997, 99:1974-1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seidah NG, Hamelin J, Mamarbachi M, Dong W, Tardos H, Mbikay M, Chretien M, Day R: cDNA structure, tissue distribution, and chromosomal localization of rat PC7, a novel mammalian proprotein convertase closest to yeast kexin-like proteinases. Proc Natl Acad Sci USA 1996, 93:3388-3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quian SW, Kondaiah P, Roberts AB, Sporn MB: cDNA cloning by PCR of rat transforming growth factor β-1. Nucleic Acids Res 1990, 18:3059-3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tucker RF, Branum EL, Shipley GD, Ryan RJ, Moses HL: Specific binding to cultured cells of 125I-labeled type beta transforming growth factor from human platelets. Proc Natl Acad Sci USA 1984, 81:6757-6761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi S, Nakagawa T, Kasai K, Banno T, Duguay SJ, Van de Ven WJ, Murakami K, Nakayama K: A second mutant allele of furin in the processing-incompetent cell line, LoVo. Evidence for involvement of the homo B domain in autocatalytic activation. J Biol Chem 1995, 270:26565-26569 [DOI] [PubMed] [Google Scholar]
- 46.Lissitzky J-C, Luis J, Munzer JS, Benjannet S, Parat P, Marvaldi J, Chrétien M, Seidah NG: The endoproteolytic processing of integrin pro-alpha subunits involves the redundant function of furin and PC5A but not PACE4, PC5B or PC7. Biochem J 2000, 346:133-138 [PMC free article] [PubMed] [Google Scholar]
- 47.Laprise M-H, Grondin F, Dubois CM: Enhanced TGFβl maturation in high five cells coinfected with recombinant baculovirus encoding the convertase furin/pace: improved technology for the production of recombinant proproteins in insect cells. Biotech Bioeng 1998, 58:85-91 [PubMed] [Google Scholar]
- 48.Benjannet S, Rondeau N, Paquet L, Boudreault A, Lazure C, Chretien M, Seidah NG: Comparative biosynthesis, covalent post-translational modifications and efficiency of prosegment cleavage of the prohormone convertases PC1 and PC2: glycosylation, sulphation and identification of the intracellular site of prosegment cleavage of PC1 and PC2. Biochem J 1993, 294:735-743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jean F, Stella K, Thomas L, Liu G, Xiang Y, Reason AJ, Thomas G: Alpha1-Antitrypsin Portland, a bioengineered serpin highly selective for furin: application as an antipathogenic agent. Proc Natl Acad Sci USA 1998, 95:7293-7298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khalil N, Whitman C, Zuo L, Danielpour D, Greenberg A: Regulation of alveolar macrophage transforming growth factor-beta secretion by corticosteroids in bleomycin-induced pulmonary inflammation in the rat. J Clin Invest 1993, 92:1812-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fortunel NO, Halzfeld A, Halzfeld J: Transforming growth factor-β: pleiotropic role in the regulation of hematopoiesis. Blood 2000, 96:2022-2036 [PubMed] [Google Scholar]
- 52.Lucas C, Bald LN, Fendly BM, Mora-Worms M, Figari IS, Patzer EJ, Palladino MA: The autocrine production of transforming growth factor-beta 1 during lymphocyte activation. A study with a monoclonal antibody-based ELISA. J Immunol 1990, 145:1415-1422 [PubMed] [Google Scholar]
- 53.Van Obberghen-Schilling E, Roche NS, Flanders KC, Sporn MB, Roberts AB: Transforming growth factor β1 positively regulates its own expression in normal and transformed cells. J Biol Chem 1988, 263:7741-7746 [PubMed] [Google Scholar]
- 54.Assoian RK, Fleurdelys BE, Stevenson HC, Miller PJ, Madtes DK, Raines EW, Ross R, Sporn MB: Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA 1987, 84:6020-6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyazono K, Thyberg J, Heldin CH: Retention of the transforming growth factor-beta 1 precursor in the Golgi complex in a latent endoglycosidase H-sensitive form. J Biol Chem 1992, 267:5668-5675 [PubMed] [Google Scholar]
- 56.De Bie I, Marcinkiewicz M, Malide D, Lazure C, Nakayama K, Bendayan M, Seidah NG: The isoforms of proprotein convertase PC5 are sorted to different subcellular compartments. J Cell Biol 1996, 135:1261-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiang Y, Molloy SS, Thomas L, Thomas G: The PC6B cytoplasmic domain contains two acidic clusters that direct sorting to distinct trans-Golgi network/endosomal compartments. Mol Biol Cell 2000, 11:1257-1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seidah NG, Benjannet S, Pareek S, Savaria D, Hamelin J, Goulet B, Laliberte J, Lazure C, Chretien M, Murphy RA: Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem J 1996, 314:951-960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seidah NG, Benjannet S, Pareek S, Chretien M, Murphy RA: Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett 1996, 379:247-250 [DOI] [PubMed] [Google Scholar]
- 60.Wouters S, Leruth M, Decroly E, Vandenbranden M, Creemers JWM, Van de Loo J-W HP, Ruysschaert J-M, Courtoy PJ: Furin and proprotein convertase 7 (PC7)/lymphoma PC endogenously expressed in rat liver can be resolved into distinct post-Golgi compartments. Biochem J 1998, 336:311-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hosaka M, Nagahama M, Kim WS, Watanabe T, Hatsuzawa K, Ikemizu J, Murakami K, Nakayama K: Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J Biol Chem 1991, 266:12127-12130 [PubMed] [Google Scholar]
- 62.Takahashi S, Hatsuzawa K, Watanabe T, Murakami K, Nakayama K: Sequence requirements for endoproteolytic processing of precursor proteins by furin: transfection and in vitro experiments. J Biochem (Tokyo) 1994, 116:47-52 [DOI] [PubMed] [Google Scholar]
- 63.Anderson ED, Thomas L, Hayflick JS, Thomas G: Inhibition of HIV-1 gp160-dependent membrane fusion by a furin-directed alpha 1-antitrypsin variant. J Biol Chem 1993, 268:24887-24891 [PubMed] [Google Scholar]
- 64.Benjannet S, Savaria D, Laslop A, Chrétien M, Marcinkiewicz M, Seidah NG: α1-antitrypsin-Portland inhibits processing of precursors mediated by proprotein convertases primarily within the constitutive secretory pathway. J Biol Chem 1997, 272:26210-26218 [DOI] [PubMed] [Google Scholar]
- 65.Miranda L, Wolf J, Pichuantes S, Duke R, Franzusoff A: Isolation of the human PC6 gene encoding the putative host protease for HIV-1 gp160 processing in CD4+ T lymphocytes. Proc Natl Acad Sci USA 1996, 93:7695-7700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hallenberger S, Moulard M, Sordel M, Klenk H-D, Garten W: The role of eukaryotic subtilisin-like endoproteases for the activation of human immunodeficiency virus glycoproteins in natural host cells. J Virol 1997, 71:1036-1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, Brattain M, Willson JKV: Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science 1995, 268:1336-1338 [DOI] [PubMed] [Google Scholar]
- 68.Eppert K, Scherer SW, Ozcelik H, Pirone R, Hoodless P, Kim H, Tsui L-C, Bapat B, Gallinger S, Andrulis IL, Thomsen GH, Wrana JL, Attisano L: MADR2 maps to 18q21 and encodes a TGFβ-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell 1996, 86:543-552 [DOI] [PubMed] [Google Scholar]
- 69.Hua X, Liu X, Ansari DO, Lodish HF: Synergistic cooperation of TFE3 and smad proteins in TGF-beta-induced transcription of the plasminogen activator inhibitor-1 gene. Genes Dev 1998, 12:3084-3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Feng XH, Derynck R: Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature 1998, 394:909-913 [DOI] [PubMed] [Google Scholar]
- 71.Kim SJ, Angel P, Lafyatis R, Hattori K, Kim KY, Sporn MB, Karin M, Roberts AB: Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol Cell Biol 1990, 10:1492-1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng M, Streck RD, Scott REM, Seidah NG, Pintar JE: The developmental expression in rat of proteases furin, PC1, PC2, and carboxypeptidase E: implications for early maturation of proteolytic processing capacity. J Neurosci 1994, 14:4656-4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lehnert SA, Akhurst RJ: Embryonic expression pattern of TGF beta type-1 RNA suggests both paracrine and autocrine mechanisms of action. Development 1988, 104:263-273 [DOI] [PubMed] [Google Scholar]
- 74.Schmid P, Cox D, Bilbe G, Maier R, McMaster GK: Differential expression of TGF β1, β2 and β3 genes during mouse embryogenesis. Development 1991, 111:117-130 [DOI] [PubMed] [Google Scholar]
- 75.Werz O, Brungs M, Steinhilber D: Purification of transforming growth factor beta 1 from human platelets. Pharmazie 1996, 51:893-896 [PubMed] [Google Scholar]
- 76.Schini-Kerth VB, Bassus S, Fisslthaler B, Kirchmaier CM, Busse R: Aggregating human platelets stimulate the expression of thrombin receptors in cultured vascular smooth muscle cells via the release of transforming growth factor-beta1 and platelet-derived growth factor AB. Circulation 1997, 96:3888-3896 [DOI] [PubMed] [Google Scholar]
- 77.Bobik A, Agrotis A, Kanellakis P, Dilley R, Krushinsky A, Smirnov V, Tararak E, Condron M, Kostolias G: Distinct patterns of transforming growth factor-beta isoform and receptor expression in human atherosclerotic lesions. Colocalization implicates TGF-beta in fibrofatty lesion development. Circulation 1999, 99:2883-2891 [DOI] [PubMed] [Google Scholar]
- 78.Border WA, Noble NA: Cytokines in kidney disease: the role of transforming growth factor-β. Am. J Kidney Dis 1993, 22:105-113 [DOI] [PubMed] [Google Scholar]
- 79.Frishberg Y, Kelly CJ: TGF-beta and regulation of interstitial nephritis. Miner Electrolyte Metab 1998, 24:181-189 [DOI] [PubMed] [Google Scholar]
- 80.El-Youssef M, Mu Y, Huang L, Stellmach V, Crawford SE: Increased expression of transforming growth factor-beta1 and thrombospondin-1 in congenital hepatic fibrosis: possible role of the hepatic stellate cell. J Pediatr Gastroenterol Nutr 1999, 28:386-392 [DOI] [PubMed] [Google Scholar]
- 81.Arai H, Ishida A, Nakajima W, Nishinomiya F, Yamazoe A, Takada G: Immunohistochemical study on transforming growth factor-beta1 expression in liver fibrosis of Down’s syndrome with transient abnormal myelopoiesis. Hum Pathol 1999, 30:474-476 [DOI] [PubMed] [Google Scholar]
- 82.Qi Z, Atsuchi N, Ooshima A, Takeshita A, Ueno H: Blockade of type beta transforming growth factor signaling prevents liver fibrosis and dysfunction in the rat. Proc Natl Acad Sci USA 1999, 96:2345-2349 [DOI] [PMC free article] [PubMed] [Google Scholar]