Abstract
The relationship of apocrine metaplasia to invasive breast cancer is controversial. Different authors have reported that apocrine differentiation in proliferative lesions may be a risk factor, a precursor lesion, or have no association with malignancy. The aim of this study was to compare the genetic alterations in benign apocrine hyperplasia with apocrine ductal carcinoma in situ (DCIS) and invasive apocrine carcinomas of the breast using comparative genomic hybridization. The mean number of alterations in apocrine hyperplasia was 4.1 (n = 10) compared to 10.2 in apocrine DCIS (n = 10) and 14.8 (n = 4) in invasive carcinoma. The most common alterations in apocrine hyperplasia were gains of 2q, 13q, and 1p and losses of 1p, 17q, 22q, 2p, 10q, and 16q. Apocrine DCIS and invasive carcinomas showed gains of 1q, 2q, 1p, and losses of 1p, 22q, 17q, 12q, and 16q as their most common DNA copy number changes. Apocrine hyperplasia is considered to be a benign lesion and its relationship to invasive carcinoma remains unclear. Our data suggest that some apocrine hyperplasias may be clonal proliferations. The mean number of alterations are lower in apocrine hyperplasia, however the changes show considerable overlap with those identified in in situ and invasive apocrine carcinoma. These alterations are also commonly seen in nonapocrine breast cancer. The data are consistent with apocrine hyperplasia as a putative nonobligate precursor of apocrine carcinoma.
Apocrine epithelial cells have abundant eosinophilic cytoplasm containing finely granular, periodic acid-Schiff (PAS)-positive diastase-resistant granules, with a basally-located nucleus and a prominent nucleolus. 1,2 Apocrine sweat glands are found in the skin of the axilla, groin, anogenital region, and other sites, and are morphologically and functionally different from the cutaneous sebaceous and sweat glands. 3 A number of benign and malignant lesions in the breast contain epithelial cells which are cytologically identical to those which comprise the apocrine glands. 4 Microscopic apocrine change is common in the female breast after the age of 30, it is rarely seen in women younger than 19, and increases with age, persisting postmenopausally. 5 Mammary apocrine cells are most frequently encountered in the epithelium of tension cysts 6 where they appear as flat to cuboidal cells and usually form a single layer of cells or exhibit proliferative change resulting in isolated blunt papillae. Often more complex papillary architecture may be seen. Grossly palpable cysts lined by apocrine epithelium tend to contain a cyst fluid with a high K+/Na+ ratio, which is characteristic of the type I cyst. 7
Gross cystic disease fluid protein (GCDFP-15), a 15-kd glycoprotein which was isolated in the cystic fluid of fibrocystic breast disease, represents an immunocytochemical marker of apocrine differentiation. 8-10 The gene has been localized to chromosome 7, and is identical to that of the prolactin-inducible protein. 11 Immunohistochemical studies of benign and malignant breast lesions showing apocrine differentiation report that the cells lack estrogen and progesterone receptors, but stain positive for androgen receptor, contrasting with the normal breast epithelium. 1,12 These intriguing observations reflect the fact that apocrine cells differ from nonapocrine normal cells not only morphologically but also biologically.
The presence of apocrine cells in the breast has generally been regarded as a metaplastic process, 13,14 however this has recently been debated. Cells with biochemical characteristics of apocrine differentiation (GCDFP-15 expression) during human fetal breast development, as well as normal adult mammary gland tissue, have been reported. 2,10 Several authors now suggest that the presence of apocrine cells in the breast be termed a normal process of differentiation, and that these cells are a normal constituent of the glandular structure of the breast. 1
The proliferative capacity of ordinary apocrine cells is uncertain. The flat apocrine cells lining cysts may be an end stage of cellular differentiation, but studies of the cyst fluid indicate metabolic activity. 3 Ki67 staining of unselected apocrine epithelium seems to support this hypothesis, although a small number of cases showed continuing proliferation. 15
There have been many studies examining the possible relationship of apocrine differentiation, especially apocrine cysts, to breast carcinoma, however the data remain inconclusive. Several authors have investigated breasts with and without carcinoma and found no significant difference in the frequency of apocrine epithelium. 16-18 A number of follow-up studies, however, have suggested that apocrine epithelium may be a predictor for the subsequent development of carcinoma. 19-21 Although an unexpectedly large number of cancers have been reported after a short follow-up of women with cystic change, a relative risk of only 1.7 was reported in a long term follow-up of women with benign breast disease. A slightly increased risk of 2.4 has been reported for those lesions showing complex patterns of papillary change. 22 The relative risk of breast carcinoma in patients with type I cysts is higher than in those with type 2 cysts with cystic fluid containing a low K+/Na+ ratio, 23 although this has been disputed. 21 Histological evidence of transitions from apocrine differentiation to apocrine carcinoma based on morphological criteria have also been described. 19,24
The more complex forms of micropapillary apocrine changes in the breast are frequently seen with tension cysts. The micropapillary structures have the cytology of the ordinary benign apocrine cells (ie, granular eosinophilic cytoplasm and round to ovoid nucleus with prominent nucleolus), but their architecture is identical to the micropapillary structures seen in so-called micropapillary carcinomas. 3,25,26 Yet despite the similar architecture, the proliferation is regarded as hyperplastic on the basis of the apocrine cytology. 27
A study of apocrine differentiation in sclerosing adenosis by Wells and co-workers 28 identified c-erbB2 and p53 immunopositivity in incidental papillary apocrine metaplasia in one case, and c-erbB2 immunopositivity in unremarkable apocrine metaplasia in a further 3 of 48 cases studied. These data, coupled with previous studies demonstrating abnormal ras and c-myc expression in apocrine metaplasia, 29 suggest a possible premalignant nature for these lesions.
To date there is little molecular data on these lesions. Loss of heterozygosity studies at loci associated with invasive breast carcinomas have revealed no evidence of allelic imbalance in apocrine hyperplasias, although only a few selected loci were investigated. 30,31
In view of the architectural similarity to low-grade ductal carcinoma in situ (DCIS), the possible association with subsequent invasive carcinoma and the lack of molecular data, we have investigated the molecular cytogenetics of micropapillary apocrine hyperplasia and compared these to apocrine DCIS and invasive apocrine carcinomas.
Materials and Methods
Cases
Paraffin blocks from 24 patients showing breasts with apocrine lesions were selected from the Section of Anatomical Pathology of the University of Bologna, Italy. A total of 24 lesions were obtained from these 21 patients. Ten lesions of apocrine cysts with micropapillary hyperplasia, 10 cases of apocrine DCIS, and four cases of invasive apocrine carcinoma were diagnosed from these individuals. A summary of clinicopathological data are shown in Table 1 ▶ . The lesions selected as micropapillary hyperplasia showed typical benign apocrine cytology that lined intracystic micropapillary projections (Figure 1) ▶ . DCIS were defined according to Holland et al 32 (Figure 2 ▶ and Figure 3 ▶ ). Invasive ductal carcinomas were graded according to Elston and Ellis. 33
Table 1.
Clinicopathological Data
| Case | Sex/age | Site | Surgery/clinical presentation | Main diagnosis | Follow-up | Lesion examined by CGH |
|---|---|---|---|---|---|---|
| 1 | F /52 | L-UOQ | Lumpectomy for microcalcifications | W DCIS | A&W 3 yrs | PH |
| 2 | F /50 | R- | Quadrantectomy for mammographically suspicious nodule | IDC, G1 Cribriform type | 1 yr later recurrent DCIS in subareolar region | PH |
| 3 | F /75 | R- | Radical mastectomy+ axillary dissection | IDC, G, III, 13 LN free of tumour | A&W 6 yrs | PH |
| 4 | F /41 | R- | Nodulectomy for nodule with well defined borders (? fibroadenoma) | Intraparenchymal lymph node Epithelial hyperplasia Apocrine changes | A&W 5 yrs | PH |
| 5 | F /40 | Nodulectomy for microcalcifications | Sclerosing adenosis | A&W 4 yrs | PH | |
| 6 | F /51 | R- | Radical mastectomy+ axillary dissection | IDC, G III, 2 met/24 LN | Lung met DOD 6 yrs | PH |
| 7 | F /42 | L-UOQ | Lumpectomy for microcalcifications | Nodular sclerosing adenosis with apocrine changes | A&W 6 yrs | PH |
| 8 | F /45 | R-LOQ | Lumpectomy for microcalcifications | Epithelial hyperplasia and sclerosing adenosis | A&W 3 yrs | PH |
| 9 | F /43 | L-UOQ | Lumpectomy for microcalcifications | Radial scar and epithelial hyperplasia | A&W 3 yrs | PH |
| 10 | F /52 | R-UOQ | Quadrantectomy followed by radical mastectomy (no residual tumor) | WDCIS | Lost | PH |
| 11 | F /58 | L- | Nodulectomy followed by quadrantectomy (no residual tumor) | IDCIS in ductal adenoma | A&W 5 yrs | Apocrine IDCIS |
| 12 | F /69 | R-subareolar | Nodulectomy for microcalcifications+ subsequent radical mastectomy | WDCIS | A&W 5 yrs | Apocrine WDCIS |
| 13 | F /46 | L-UOQ | Quadrantectomy | Intracystic ductal carcinoma (probably in situ) | A&W 6 yrs | Apocrine WDCIS |
| 14 | F /43 | R-LIQ | Nodulectomy | PDCIS in fibroadenoma | A&W 3 yrs | Apocrine PDCIS |
| 15 | F /41 | L- | Lumpectomy for microcalcifications | WDCIS | A&W 3 yrs | Apocrine WDCIS |
| 16 | F /42 | R- | Mastectomy+ axillary dissection | PDCIS+ multiple foci of microinvasion 2 met/16 LN | Subsequent chemotherapy A&W 3 yrs | Apocrine PDCIS |
| 17 | F /52 | R- | Mastectomy | WDCIS in ductal adenoma | A&W 6 yrs | Apocrine WDCIS |
| 18 | F /58 | R-UOQ | Quadrantectomy+ axillary dissection | IDC, G III+ PDCIS 3 met/29 LN | 3 yrs recurrence in breast+ met in cervical nodes | Apocrine PDCIS Apocrine IDC G III |
| 19 | F /37 | R- | Radical mastectomy for recurrent tumor (previous quadrantectomy) | IDC, G III+ PDCIS | A&W 4 yrs | Apocrine PDCIS Apocrine IDC G III |
| 20 | F /62 | L-UOQ | Mastectomy | IDC, G III+ PDCIS | Breast recurrence 3 yrs | Apocrine PDCIS Apocrine IDC G III |
| 21 | F /60 | L- | Mastectomy+ axillary dissection | IDC G III 4 met/14 LN | DOD 6 yrs | Apocrine IDC G III |
Figure 1.
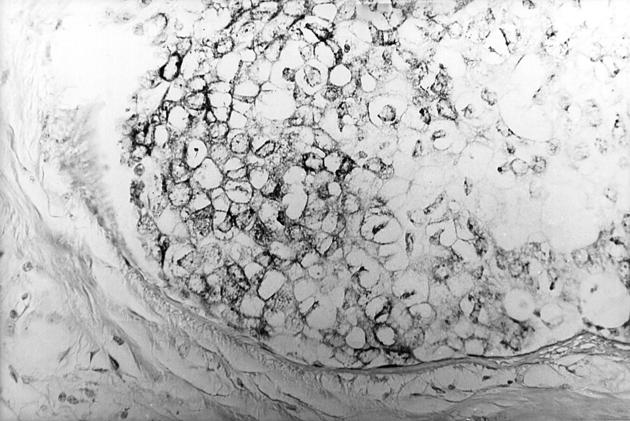
Micropapillary hyperplasia showing benign apocrine cytology. H&E stain; original magnification, ×250.
Figure 2.
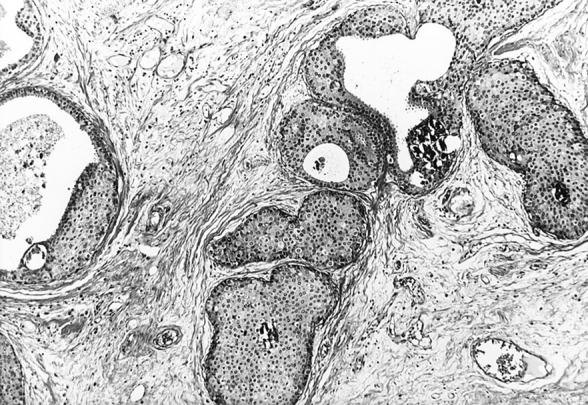
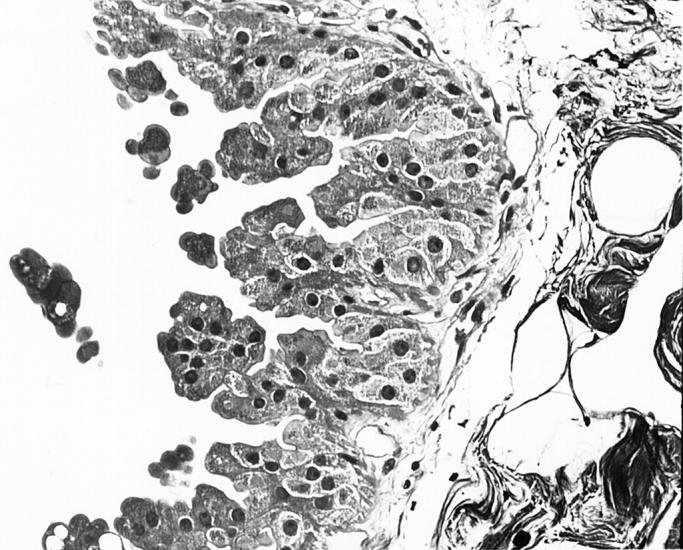
Well- (top, Figure 2 ▶ ) and poorly (bottom, Figure 3 ▶ ) differentiated apocrine DCIS. H&E stain. Original magnifications, ×125 (Figure 2) ▶ and ×200 (Figure 3) ▶ .
Figure 3.
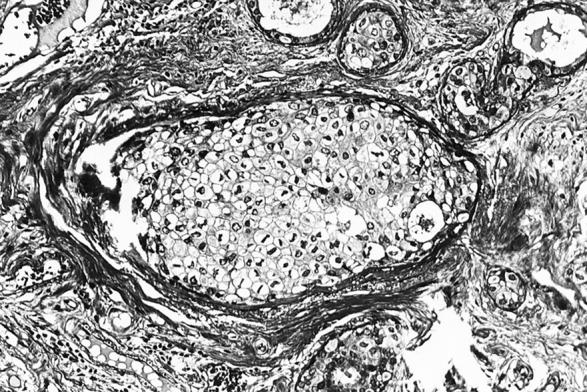
Well- (top, Figure 2 ▶ ) and poorly (bottom, Figure 3 ▶ ) differentiated apocrine DCIS. H&E stain. Original magnifications, ×125 (Figure 2) ▶ and ×200 (Figure 3) ▶ .
Immunohistochemistry and in Situ Hybridization
The avidin-biotin-peroxidase method was used for immunohistochemistry. 34 The antisera used are outlined in Table 2 ▶ . Negative and positive controls were included with each batch of slides tested.
Table 2.
Antisera Used for Immunohistochemistry
| Antigen | Manufacturer and clone | Dilution and antigen retrieval |
|---|---|---|
| GCDFP-15 (gross cystic disease fluid protein 15) | Signet Lab, D6 | 1:500, nd |
| Estrogen receptor | DAKO, 1D5 | 1:100, pc |
| Progesterone receptor | Ylem, PgR 636 | 1:50, pc |
| Androgen receptor | Biogenex, F39.4.1 | 1:30, pc |
| bcl2 | DAKO, 124 | 1:10, pc |
A 600-bp cDNA encoding human prolactin-inducible protein cloned into the pV21 bluescript vector (pPIP-8–3 cDNA clone supplied by Dr. R. Shiu and Y. Myal, Winnipeg, Manitoba, Canada) was used for in situ hybridization. The riboprobe was cleaved with the restriction enzyme Xbal, and antisense RNA molecules were obtained using a T7 DNA polymerase and digoxigenin-labeled nucleotide mixture (DIG RNA labeling kit; Boehringer Mannheim, Mannheim, Germany). A detailed protocol for in situ hybridization using radioactively labeled c-RNA probes has been described previously, 35 and the same procedure used here with digoxigenin-labeled riboprobe with some modifications has also been published. 9 Appropriate positive and negative controls were used. Hybridization with nonspecific riboprobes, such as that used for somatostatin, produced negative results under identical hybridization conditions. RNase digestion of tissue sections before the hybridization step abolished the specific staining.
Microdissection and DNA Extraction
Lesions were microdissected using the PixCell II Laser Capture Microdissection system (Arcturus, Mountain View, CA), using 5-μm thick sections cut from formalin-fixed, paraffin-embedded tissue. Normal tissue from the same patient was dissected for use as control reference DNA. The samples varied in size, the smallest lesion dissected was a cyst from which ∼100 to 200 cells were obtained for subsequent molecular analysis. The lesions were microdissected onto the CapSure Transfer Film (Arcturus), and the DNA extracted overnight in a humidified chamber at 55°C in 20 μl of extraction buffer (0.5 μg/μl proteinase K in 50 mmol/L KCl, 10 mmol/L Tris-HCl, pH 8.0, 2.5 mmol/L MgCl2, 0.1 mg/ml gelatin, 0.45% Nonidet P-40, 0.45% Tween 20). Before polymerase chain reaction, proteinase K was inactivated at 95°C for 10 minutes.
Comparative Genomic Hybridization (CGH) Analysis
Amplification and fluorescent labeling of the DNA from microdissected tissue was performed by degenerate oligonucleotide primed-polymerase chain reaction in two rounds as previously published. 36 Normal male metaphase spreads (Vysis UK Ltd., Richmond, UK) were denatured at 75°C for 5 minutes in 70% formamide, 2× standard saline citrate and dehydrated through a series of alcohols. Approximately 500 ng each of test (fluorescein-labeled) and reference (rhodamine-labeled) DNA samples from the DOP-polymerase chain reaction were co-precipitated with 30 μg of human Cot-1 DNA (Gibco Life Technologies, Paisley, UK) and 10 μg of salmon sperm DNA, and resuspended in 10 μl of hybridization buffer (50% deionized formamide, 20% w/v dextran sulfate, 2× standard saline citrate, 0.1 mmol/L ethylenediaminetetraacetic acid, pH 8.0, 0.2 mmol/L Tris-HCl, pH 7.6). The denatured probes were then hybridized to the metaphases under a coverslip for 2 to 3 days at 37°C. After hybridization, the slides were washed in 50% formamide, 2× standard saline citrate (3 × 10 minutes at 45°C), 2× standard saline citrate (2 × 10 minutes at 45°C, 1 × 10 minutes at room temperature), TNT buffer (10 mmol/L Tris-HCl, pH 8.0, 0.15 mol/L NaCl, 0.05% Tween; 10 minutes) and ddH2O (10 minutes), before dehydration through the alcohol series. Finally the slides were mounted in an anti-fade medium (Vector Laboratories, Burlingame, CA) containing 4,6-diamino-2-phenylindole as a counterstain. Metaphase chromosome preparations were captured using a Zeiss Axioskop microscope (Welwyn Garden City, UK), Photometrics KAF1400 CCD camera (Photometrics, Tucson, AZ) and Vysis SmartCapture software (Vysis). Image analysis was performed using Vysis Quips CGH software (Vysis). Between five and 10 representative images of high quality hybridizations were analyzed, and the results combined to produce an average fluorescence ratio for each chromosome. Control experiments were performed using normal:normal co-hybridizations, whose average red:green ratio levels and 95% confidence intervals were used to set the lower and upper limits for scoring losses and gains of genetic material as 0.80 to 1.20.
Results
Clinicopathological and Immunohistochemical Data
A summary of the immunohistochemical data are given in Table 3 ▶ . Patients ranged from 37 to 75 years old (n = 21). Ten of the selected cases contained apocrine papillary hyperplasia. Five of these presented a carcinoma (either in situ or invasive) in another area of the same breast, whereas in the other five a benign condition only was present. In 10 cases an apocrine DCIS was selected for study of which four were well, one intermediate, and five poorly differentiated DCIS. Three of the latter cases also contained apocrine invasive ductal carcinomas 37 grade III which were also taken into consideration for the present study. All lesions diagnosed as containing apocrine differentiation were immunohistochemically positive with anti-GCDFP-15, and were also positive with the in situ hybridization method, most with strong staining (Figures 4, 5, and 6) ▶ ▶ ▶ . All apocrine lesions stained were androgen receptor-positive. All apocrine cysts with papillary hyperplasia were estrogen- and progesterone-negative, 8 of 14 apocrine DCIS or invasive apocrine carcinomas showed estrogen receptor and progesterone receptor positivity.
Table 3.
Immunohistochemical and Molecular Cytogenetic Results
| Case | Lesion | GCDFP-15 | PIP | Estrogen receptor | Progesterone receptor | Androgen receptor | BCL2 | CGH | |
|---|---|---|---|---|---|---|---|---|---|
| Gains | Losses | ||||||||
| 1 | PH | +20% | +50% | Negative | Negative | +80% | Negative | 18p | 6q,10q,17p |
| 2 | PH | +10% | +30% | Negative | Negative | +70% | Negative | 1p,1q,2q | 2p,10q,12q,17q |
| 3 | PH | +40% | +100% | Negative | Negative | +100% | Negative | 13q | 1p,16q,17q |
| 4 | PH | +20% | +90% | Negative | Negative | +100% | Negative | 1p,2q | 1p,2p,10p,11p,11q,22q |
| 5 | PH | +30% | +40% | n.d. | n.d. | +100% | n.d. | 2q,13q | 1p,17q,22q |
| 6 | PH | +80% | +70% | Negative | Negative | +100% | Negative | 2q,13q | 1p,16p,16q,19q,22q |
| 7 | PH | +10% | +10% | Negative | Negative | +100% | Negative | None | None |
| 8 | PH | +100% | +30% | Negative | Negative | +100% | Negative | 6q(2),12p,12q,13q | 2q |
| 9 | PH | +80% | +100% | Negative | Negative | +100% | Negative | None | None |
| 10 | PH | +50% | +50% | Negative | Negative | n.d. | Negative | None | None |
| 11 | Apocrine IDCIS | +20% | +70% | +80% | +50% | +90% | +5% | 1p,1q,2q,7q,9p | 2p,2q,3p(2),4p,5p,6p,8q,9q,11q,12p,12q,21q,22q |
| 12 | Apocrine WDCIS | +50% | +50% | +100% | +100% | n.d. | +100% | 5q,6q,7p,11p | 1p,6p,13q,16q, 5q17q,19q,22q |
| 13 | Apocrine WDCIS | +10% | +5% | +80% | +20% | n.d. | +100% | 1p,1q,2q,3q,4q,5q,7q,13q | 1p,2p,2q,11p,16q,17q |
| 14 | Apocrine PDCIS | +30% | + rare | Negative | Negative | +30% | Negative | 1p,1q,2q,3q,4q | 1p,9q,10p,10q,12q,16q,17q |
| 15 | Apocrine WDCIS | +50% | +100% | Negative | Negative | +100% | Negative | 2q | 1p,8q,22q |
| 16 | Apocrine PDCIS | +60% | +90% | Negative | Negative | +50% | Negative | None | 1p,17q,22q |
| 17 | Apocrine WDCIS | +5% | +40% | +75% | +100% | +80% | +100% | 3q,4q,8q | 1p,12q,22q |
| 18 | Apocrine PDCIS | +5% | +10% | +40% | +5% | +70% | +70% | 1p,1q,2q,6q,7q | 2p,3p,3q,4p,5p,8q,9q,10q,12p,12q,13q,14q,17q |
| 18 | Apocrine IDC GIII | +5% | +10% | +40% | +5% | +70% | +70% | 2q,3p,3q,4p,4q,5q,7q(2),13q,Xq | 2p,5q(2),8q,9q,11q,12p,15q,16q,17q,22q,Xp,Xq |
| 19 | Apocrine PDCIS | +60% | +60% | Negative | Negative | +90% | +100% | 2q,3p,5q,6q,12p,13q,15q | 1p,2q,14q,16q,17p,17q,19q,22q |
| 19 | Apocrine IDC GIII | +2% | +20 | Negative | Negative | +70% | +50% | 3p,3q,6,12q | 1p,2q,5q(2),8p,8q,9q,11q,13q(2) |
| 20 | Apocrine PDCIS | +80% | +90% | +90% | +15% | +50% | Negative | 1q,3p | None |
| 20 | Apocrine IDC GIII | +5% | +5% | +90% | +15% | +50% | +50% | 3p,15q,18q | None |
| 21 | Apocrine IDC GIII | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2q,3q,5q,6p,6q,9p,10p,12q,13q,16q,18q | 1p,2p,2q,4p,9q(2),12q,17q |
Figure 4.
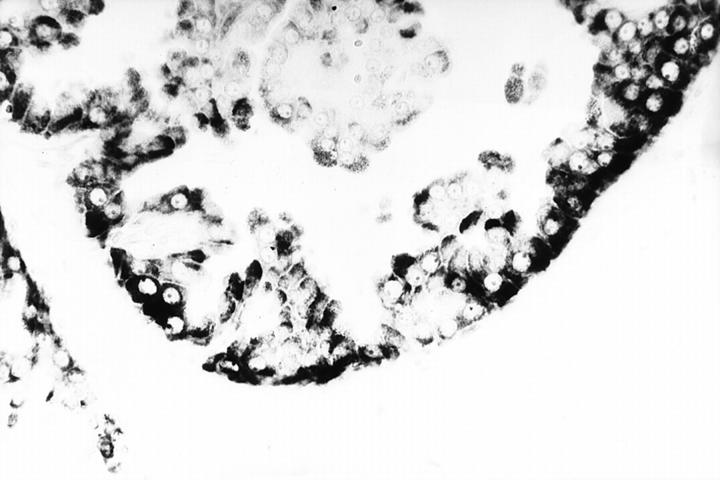
In situ hybridization of prolactin-inducible protein in showing (top, Figure 4 ▶ ) and poorly-differentiated apocrine DICS (bottom, Figure 5 ▶ ). Original magnification, ×300.
Figure 5.
In situ hybridization of prolactin-inducible protein in showing (top, Figure 4 ▶ ) and poorly-differentiated apocrine DICS (bottom, Figure 5 ▶ ). Original magnification, ×300.
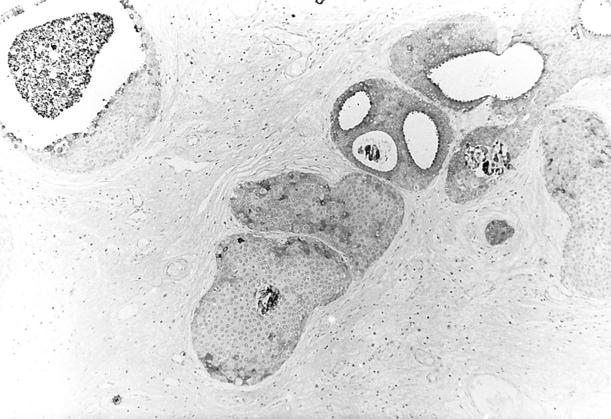
Figure 6.
Immunohistochemcial localization of CDFP-15 in well-differentiated apocrine DCIS. Original magnification, ×275.
CGH
Summary karyograms of the CGH data are presented in Figure 7 ▶ . Three of the 10 cases of apocrine cyst with papillary hyperplasia showed no genetic alterations as detected by CGH. The other 10 cases showed between four and eight regions of copy number change. The mean number of alterations for this lesion was 4.1. The most common areas of DNA copy number change were losses at 1p (4 of 10 cases), 17q, 22q (both 3 of 10), 2p, 10q, and 16q (all 2 of 10), and gains at 2q, 13q (both 4 of 10 cases), and 1p (2 of 10). All of the cases of apocrine DCIS showed genetic alterations, ranging from two up to a maximum of 19. The mean number of alterations was 10.4. The most common losses were at 1p (7 of 10 cases), 22q (6 of 10), 17q (5 of 10), 12q, 16q (both 4 of 10), 2p, 2q, 8q, and 9q (all 3 of 10); the most common gains were at 1q, 2q (both 5 of 10), 1p (4 of 10), 3q, 4q, 5q, and 7q (all 3 of 10). A further 27 chromosomal loci showed a copy number change in at least one case. The invasive apocrine carcinomas showed between three and 23 alterations. The mean number of DNA copy number changes was 14.8. A summary of the CGH data is given in Table 3 ▶ .
Figure 7.
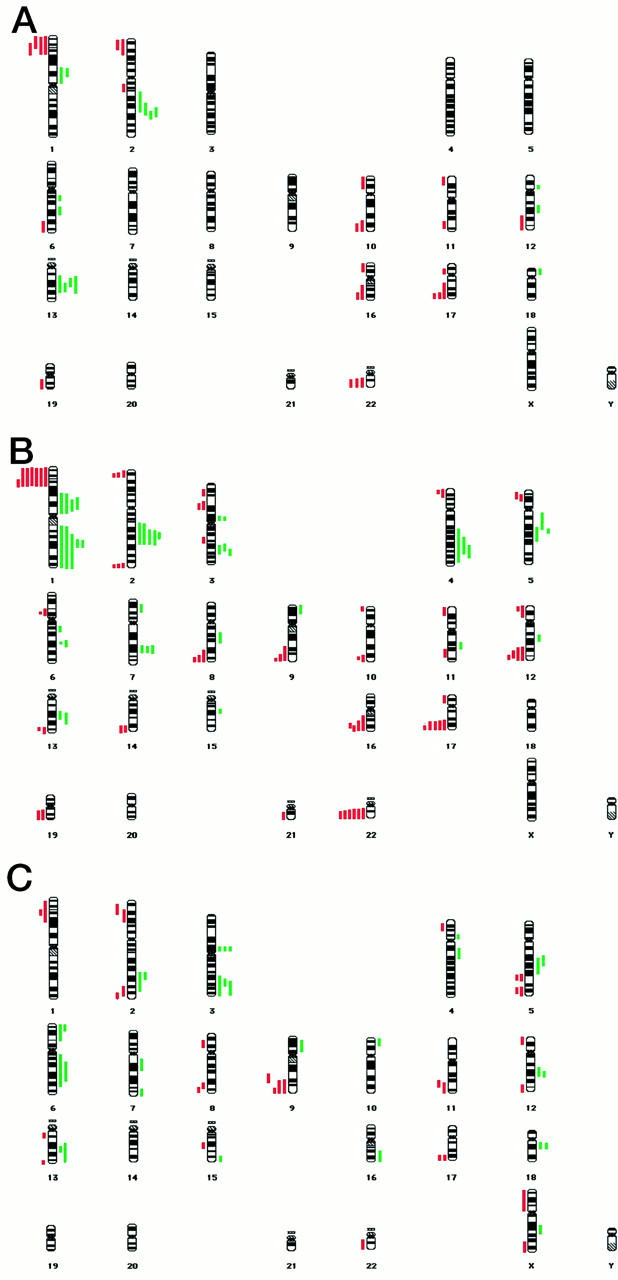
Summary karyograms showing regions of DNA copy number change for 10 cases of apocrine micropapillary hyperplasia (A); 10 cases of apocrine DCIS (B); and four cases of invasive apocrine carcinoma analyzed by CGH (C). Gains and losses are shown by the solid green bars to the right and red bars to the left of the chromosome, respectively.
Discussion
Apocrine cells are considered to be the progenitors of cystic breast disease. 14 The apocrine epithelium associated with secretion accumulation may favor the progressive unfolding of lobules, formation of microcysts, and finally the appearance of macrocysts. 14 The relationship between apocrine change and breast carcinoma, despite numerous studies, remains controversial. A number of conflicting reports using a variety of approaches have been published that have resulted in a confused picture regarding the pathogenic nature of these lesions in the breast. Some hypotheses regarding a possible relationship between apocrine epithelium and carcinoma have been proposed. 38 The apocrine epithelium may be a precursor of malignant transformation; it may reflect a response to the same stimulus which promotes carcinoma or it could indicate an instability of the breast epithelium, which causes the development of alterations with a higher propensity for cancer.
Cytological features of apocrine differentiation are important factors pushing the pathologist to err toward a benign diagnosis. Despite the architectural similarity of low-grade DCIS (nonapocrine) to apocrine papillary hyperplasia, few if any pathologists would regard the latter as a type of DCIS. We therefore wished to investigate the molecular cytogenetics of the apocrine lesions of the breast to gain some insights into the nature of these controversial entities.
All of the selected lesions were distinctly positive for the apocrine markers both at immunohistochemistry and in situ hybridization levels, indicating a definite apocrine differentiation. 4,9,10 The cases followed the characteristic staining pattern for apocrine differentiation, exhibiting estrogen receptor, progesterone receptor, and Bcl2 negativity while staining positively for androgen receptor in benign lesions. This had led some authors to postulate a possible role for androgens in the stimulation of breast epithelium and the development of apocrine cells. 39
The CGH data shows that the apocrine cysts with papillary hyperplasia exhibit a relatively large number of genetic alterations (mean, 4.1). The technique of CGH analysis has implication for the clonal nature of the sample because normal tissue contamination will mask the changes in DNA copy number. Because it is less probable that many cells acquire the same alteration independently, finding an amplification or deletion suggests that the sample is likely to be clonal. Seven of the 10 cases of this lesion showed some DNA copy number changes, indicating that at least a proportion of these apocrine metaplastic/hyperplastic lesions are likely to be clonal, neoplastic proliferations. The level of genetic alterations seen in these apocrine papillary lesions is equivalent to those described in well- and intermediately-differentiated DCIS. 40 This is perhaps not surprising in view of the architectural similarity to low-grade DCIS. It could be argued that from these observations and our data that these papillary lesions ought to be considered as part of the spectrum of low-grade DCIS. Further genetic investigations of these lesions are required and warranted. Epidemiological studies on apocrine papillary lesions have hinted at an increased risk of breast carcinoma compared to the cysts showing a single layer of epithelial cells. 22
The mean number of alterations in the DCIS group was larger than that in the hyperplasia group. The number of alterations in the two groups differs on average, but there is a fair degree of overlap in the values in the two groups. The histological grade of the DCIS lesions varied, although there were no differences in copy number changes between the grades observed in these small numbers. The mean number of genetic alterations for the invasive apocrine carcinomas was higher still than the DCIS group, showing a general trend, which would need to be tested with further case data, of increasing alterations from the hyperplastic lesions, through DCIS and up to invasive apocrine carcinoma. There were no obvious correlations between immunohistochemical characteristics of the selected lesions and genetic alterations detected by CGH, although the sample size prevented formal statistical calculations.
There was considerable overlap in the pattern of genetic alterations seen between the papillary lesions, the DCIS and the invasive carcinomas. Losses at 1p, 16q, and 17q, and gains at 2q and 13q were all seen in multiple cases of all three lesions studied. If, as has been suggested, there is a progression from the hyperplastic lesions through in situ and invasive carcinoma, alterations at these chromosomal loci may be early events in apocrine breast carcinogenesis. There were a number of regions, such as gains at 1q and 3p, and loss at 9q, which exhibited copy number changes in the DCIS and invasive carcinomas which we did not detect in the papillary lesions, suggesting that alterations at these loci may be later events.
When the data from our series of malignant apocrine lesions were compared with CGH studies from the literature on nonapocrine breast tumors, a good concordance in the patterns of genetic alterations was generally seen. Losses at 1p, 3p, 16q, 17q, and 22q, and gain at 1q are common regions of copy number change reported in breast carcinomas, and were observed in a high proportion of our apocrine cases. There were, however, a number of chromosomal loci showing alterations in our apocrine lesions that have not previously been reported as playing a significant role in breast tumorigenesis. These include losses at 2p, 9q, and 1q, and gains at 2q, 3p, and 13q. These observations are not inconsistent with the hypothesis that apocrine carcinomas arise via different carcinogenic pathways from ordinary invasive ductal carcinomas. 31 Nothing is known about the underlying genetics of these regions, but these loci may provide new targets for investigation in breast cancer pathogenesis.
The apocrine cysts showing papillary hyperplasia have long been a controversial lesion, and numerous studies have investigated their association with breast carcinoma. The molecular cytogenetic data presented here showing that they can exhibit a range of genetic alterations seem to indicate that at least a proportion of these lesions may be clonal neoplasms, and that given the considerable overlap in copy number changes with the apocrine malignancies, these papillary lesions may represent nonobligate precursors of apocrine DCIS and invasive apocrine carcinoma. At this stage, the clinical significance remains uncertain and follow-up studies will be required to evaluate this issue.
Acknowledgments
We thank Drs. Janet Shipley and Peter Osin for critically reviewing the manuscript; and Professors Joy Delhanty, A. Munro Neville, Mike Waterfield, and Dr. Mike O’Hare for encouragement and support.
Footnotes
Address reprint requests to Sunil R. Lakhani, Rockefeller Building, University Street, London, WC1E 6JJ, UK. E-mail: s.lakhani@ucl.ac.uk.
Supported in part by the Ludwig Institute for Cancer Research, the Sydney and Phyllis Goldberg Memorial Charitable Trust, and Ministero dell Università e della Ricerca Scientifica e Tecnologica (60%) Rome. C. J. is supported by the University College London and Middlesex Special Trustees. D. W. is supported by a Medical Research Council Research Fellowship. The image capture equipment was funded by The Wellcome Trust (grant 039938).
References
- 1.Eusebi V, Damiani S, Losi L, Millis RR: Apocrine differentiation in breast epithelium. Adv Anat Pathol 1997, 4:139-155 [Google Scholar]
- 2.Viacava P, Naccarato AG, Bevilacqua G: Apocrine epithelium of the breast: does it result from metaplasia? Virchows Arch 1997, 431:205-209 [DOI] [PubMed] [Google Scholar]
- 3.Rosen PP: Benign papillary tumours. Rosen’s Breast Pathology. 1997, :pp 67-104 Lippincott-Raven, Philadelphia [Google Scholar]
- 4.Losi L, Lorenzini R, Eusebi V, Bussolati G: Apocrine differentiation in invasive carcinoma of the breast. Appl Immunohistochem 1995, 3:91-98 [Google Scholar]
- 5.Wellings SR, Alpers CE: Apocrine cystic metaplasia: subgross pathology and prevalence in cancer-associated versus random autopsy breasts. Hum Pathol 1987, 18:381-386 [DOI] [PubMed] [Google Scholar]
- 6.Azzopardi JG, Ahmed A, Millis RR: Problems in breast pathology. 1979. WB Saunders Co. London [PubMed]
- 7.Naldoni C, Massimo C, Dogliotti L, Bruzzi P, Bucchi L, Buzzi G, Torta M, Angeli A: Association of cyst type with risk factors for breast cancer and relapse rate in women with gross cystic disease of the breast. Cancer Res 1992, 52:1791-1795 [PubMed] [Google Scholar]
- 8.Haagensen DE, Mazoujian D, Dilley WG, Pedersen CE, Kisters SJ, Wells SA: Breast gross cystic disease fluid analysis. I. Isolation and radioimmunoassay for a major component protein. J Natl Cancer Inst 1979, 62:239-247 [PubMed] [Google Scholar]
- 9.Pagani A, Eusebi V, Bussolati G: Detection of PIP-GCDFP-15 gene expression in apocrine epithelium of the breast and salivary glands. Appl Immunohistochem 1994, 2:29-35 [Google Scholar]
- 10.Pagani A, Sapino A, Eusebi V, Bergnolo P, Bussolati G: PIP/GCDFP-15 gene expression and apocrine differentiation in carcinomas of the breast. Virchows Arch 1994, 425:459-465 [DOI] [PubMed] [Google Scholar]
- 11.Myal Y, Gregory C, Wang H, Hamerton JL, Shiu RP: The gene for prolactin-inducible protein (PIP), uniquely expressed in exocrine organs, maps to chromosome 7. Somat Cell Mol Genet 1989, 15:265-270 [DOI] [PubMed] [Google Scholar]
- 12.Tavassoli FA, Purcell CA, Man YG: Down regulation of bcl2, ER, PR associated with AR expression is triggered by apocrine differentiation of mammary epithelium [Abstract]. Mod Pathol 1996, 9:25A
- 13.Ahmed A: Apocrine metaplasia in cystic hyperplastic mastopathy. Histochemical and ultrastructural observations. J Pathol 1975, 115:211-214 [DOI] [PubMed] [Google Scholar]
- 14.Wellings SR, Jensen HM, Marcum RG: An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst 1975, 55:231-243 [PubMed] [Google Scholar]
- 15.Moriya T, Sakamoto K, Sasano H, Kawanaka M, Sonoo H, Manabe T, Ito J: Immunohistochemical analysis of Ki-67, p53, p21 and p27 in benign and malignant apocrine lesions of the breast: its correlation to histologic findings in 43 cases. Mod Pathol 2000, 13:13-18 [DOI] [PubMed] [Google Scholar]
- 16.Dawson EK: Sweat carcinoma of the breast. Edinburgh Med J 1932, 39:409-438 [PMC free article] [PubMed] [Google Scholar]
- 17.Foote FW, Jr, Stewart FW: Comparative studies of cancerous versus non-cancerous breasts. Ann Surg 1945, 12:6-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tóth J, Számel I, Svastics E, deSombre ER: Significance of apocrine metaplasia in mammary carcinogenesis. A preliminary morphological and immunohistochemical study. Ann NY Acad Sci 1990, 586:238-251 [DOI] [PubMed] [Google Scholar]
- 19.Haagensen CD, Bodian C, Haagensen DE: Apocrine epithelium. Breast carcinoma. 1981, :pp 83-105 WB Saunders, Risk and Detection. Philadelphia [Google Scholar]
- 20.Page DL, Van der Zwaag R, Rogers LW, Williams LT, Walker WE, Hartmann WH: Relation between component parts of fibrocystic disease complex and breast cancer. J Natl Cancer Inst 1978, 61:1055-1063 [PubMed] [Google Scholar]
- 21.Dixon JM, McDonald C, Elton RA, Miller WR: Risk of breast cancer in women with palpable breast cysts: a prospective study. Edinburgh Breast Group. Lancet 1999, 353:1742-1745 [DOI] [PubMed] [Google Scholar]
- 22.Page DL, Jensen RA, Dupont WD: Papillary apocrine change of the breast—cancer risk indicator? Cancer Epidemiol Biomarkers Prev 1996, 5:29-32 [PubMed] [Google Scholar]
- 23.Bruzzi P, Dogliotti L, Naldoni C, Bucchi L, Constantini M, Cicognani A, Torta M, Bussi GF, Angeli A: Cohort study of association of risk of breast cancer with cyst type in women with gross cystic disease of the breast. BMJ 1997, 315:925-928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yates AJ, Ahmed A: Apocrine carcinoma and apocrine metaplasia. Histopathology 1988, 13:228-231 [DOI] [PubMed] [Google Scholar]
- 25.De Potter CR, Foschini MP, Schelfhout AM, Schroeter C, Eusebi V: Immunohistochemical study of neu protein overexpression in clinging in situ duct carcinoma of the breast. Virchows Archiv A Pathol Anat Hisopathol 1993, 422:375-380 [DOI] [PubMed] [Google Scholar]
- 26.Eusebi V, Feudale E, Foschini MP, Micheli A, Conti A, Riva C, Di Palma S, Rilke F: Long term follow up of in situ carcinoma of the breast. Semin Diagn Pathol 1994, 11:223-235 [PubMed] [Google Scholar]
- 27.O’Malley FP, Page DL, Nelson EH, Dupont WD: Ductal carcinoma in situ of the breast with apocrine cytology: definition of a borderline category. Hum Pathol 1994, 25:164-168 [DOI] [PubMed] [Google Scholar]
- 28.Wells CA, McGregor IL, Makunura CN, Yeomans P, Davies JD: Apocrine adenosis: a precursor of aggressive breast cancer? J Clin Pathol 1995, 48:737-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agnantis NJ, Mahera H, Maounis N, Spandidos DA: Immunohistochemical study of ras and myc oncoproteins in apocrine breast lesions with and without papillomatosis. Eur J Gynaeocol Oncol 1992, 13:309-315 [PubMed] [Google Scholar]
- 30.Lakhani SR, Slack DN, Hamoudi RA, Collins N, Stratton MR, Sloane JP: Detection of allelic imbalance indicates that a proportion of mammary hyperplasia of usual type are clonal, neoplastic proliferations. Lab Invest 1996, 74:129-135 [PubMed] [Google Scholar]
- 31.Lininger RA, Zhuang Z, Man Y, Park WS, Emmert-Buck M, Tavassoli FA: Loss of heterozygosity is detected at chromosomes 1p35-36 (NB), 3p25 (VHL), 16p13 (TSC2/PKD1), and 17p13 (TP53) in microdissected apocrine carcinomas of the breast. Mod Pathol 1999, 12:1083-1089 [PubMed] [Google Scholar]
- 32.Holland R, Peterse JL, Millis RR, Eusebi V, Faverly D, Van de Vijevr MJ, Zafrani B: Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol 1994, 11:167-180 [PubMed] [Google Scholar]
- 33.Elston CW, Ellis IO: Pathological prognostic factors in breast cancer. 1. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991, 19:403-410 [DOI] [PubMed] [Google Scholar]
- 34.Hsu SM, Raine L, Fanger H: The use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase technique. J Histochem Cytochem 1981, 29:577-580 [DOI] [PubMed] [Google Scholar]
- 35.Hoefler H, Childers H, Montminy MR, Lechan RM, Goodman RH, Wolfe HJ: In situ hybridization methods for the detection of somatostatin mRNA in tissue sections using antisense RNA probes. Histochem J 1986, 18:597-604 [DOI] [PubMed] [Google Scholar]
- 36.Wells D, Sherlock JK, Handyside AH, Delhanty JDA: Detailed chromosomal and molecular genetic analysis of single cells by whole genome amplification and comparative genomic hybridization. Nucleic Acids Res 1999, 27:1214-1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eusebi V, Millis RR, Cattani MG, Bussolati G, Azzopardi JG: Apocrine carcinoma of the breast. Am J Pathol 1986, 123:532-541 [PMC free article] [PubMed] [Google Scholar]
- 38.Haagensen DE: Is cystic disease related to breast cancer? Am J Surg Pathol 1991, 15:687-694 [DOI] [PubMed] [Google Scholar]
- 39.Selim A-GA, Wells CA: Immunohistochemical localisation of androgen receptor in apocrine metaplasia and apocrine adenosis of the breast: relation to oestrogen and progesterone receptors. J Clin Pathol 1999, 52:838-841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buerger H, Otterbach F, Simon R, Poremba C, Diallo R, Decker T, Riethdorf L, Brinkschmidt C, Dockhorn-Dworniczak B, Boecker W: Comparative genomic hybridization of ductal carcinoma in situ of the breast-evidence of multiple genetic pathways. J Pathol 1999, 187:396-402 [DOI] [PubMed] [Google Scholar]