Abstract
Abnormalities in nuclear morphology are frequently observed in malignant tissues but the mechanisms behind these phenomena are still poorly understood. In this study, the relation between abnormal nuclear shape and chromosomal instability was explored in short-term tumor cell cultures. Mitotically unstable ring and dicentric chromosomes were identified by fluorescence in situ hybridization at metaphase and subsequently localized in interphase nuclei from five malignant soft tissue tumors. The vast majority (71 to 86%) of nuclear blebs, chromatin strings, and micronuclei contained material from the unstable chromosomes, whereas few (<11%) were positive for stable chromosomes. Nuclear morphology was also evaluated in fibroblasts and an osteosarcoma cell line exposed to irradiation. A linear correlation was found between the frequency of abnormalities in nuclear shape, on one hand, and cells with unstable chromosomes (r = 0.87) and anaphase bridge configurations (r = 0.98), on the other hand. The relation between nuclear shape and karyotypic pattern was investigated further in cultures from 58 tumors of bone, soft tissue, and epithelium. Blebs, strings, and micronuclei were significantly more frequent in tumors that contained rings, dicentrics, or telomeric associations than in those exhibiting only stable aberrations (P < 0.001) and a positive correlation (r = 0.78) was found between the frequency of such nuclear abnormalities and the intratumor heterogeneity of structural chromosome aberrations. These results indicate that the formation of nuclear blebs, chromatin strings, and micronuclei in malignant tissues is closely related to the breakage-fusion-bridge type of mitotic disturbances. Abnormalities in nuclear shape may thus primarily be regarded as an indicator of genetic instability and intratumor heterogeneity, independent of cytogenetic complexity and the grade of malignancy.
Malignant tissues frequently exhibit abnormal nuclear morphology including variability in nuclear size, abnormal chromatin structure, and irregularities in nuclear shape. 1-4 In some tumor types, such as endometrial, ovarian, and mammary adenocarcinomas, the presence of nuclear atypia is associated with an unfavorable prognosis. 5-7 In other neoplasms, however, such as soft tissue sarcomas, the presence of nuclear irregularities has little prognostic impact. 8,9 Some investigations have indicated that nuclear irregularities may be associated with chromosomal aberrations. 10 In experimental models of chemically induced neoplasia, atypia has been reported to increase with the degree of aneuploidy, 11 and a correlation between atypia and nuclear DNA content has been reported in ovarian and mammary carcinomas. 5 Recently, the development of DNA fluorescence in situ hybridization (FISH) has allowed more detailed investigations of the relation between chromosome abnormalities and alterations of nuclear morphology. In neuroblastomas and mesenchymal tumors, amplified DNA sequences have been shown to preferentially aggregate in micronuclei, nuclear strings, or protrusions of the nuclear membrane, so-called nuclear blebs. 12-15 In mesenchymal neoplasms, the amplified material is frequently carried in mitotically unstable dicentric, multicentric, 16,17 or ring-shaped chromosomes. 12,18 At anaphase, such rearranged chromosomes frequently fail to segregate in an orderly manner, instead forming bridges between the spindle poles. At the anaphase-telophase transition, these bridges may subsequently break, resulting in novel chromosomal variants in the daughter cells. 19-21
In this study, we further explored the relationship between such bridge-breakage instability and nuclear membrane irregularities in short-term cultures from musculoskeletal and epithelial tumors. FISH was used to identify mitotically unstable chromosome aberrations in metaphase cells from soft tissue tumors and to determine the positions of these chromosomes in abnormal interphase nuclei. The correlation between anaphase bridging and abnormalities in nuclear shape was evaluated at different levels of chromosomal instability in irradiated fibroblasts and tumor cells. To delineate the relationship between nuclear abnormalities and the pattern of chromosomal aberrations in several histopathological entities, we also analyzed 58 tumors from ovary, lung, bone, and soft tissue.
Materials and Methods
Tumor Cell Culture, Chromosome Preparation, and Scoring of Abnormal Nuclei
Tumor material was obtained from the University Hospital, Lund, Sweden; the Karolinska Hospital, Stockholm, Sweden; and the Center for Human Genetics, Leuven, Belgium. The osteosarcoma cell line (OSA) has been previously described. 22 The fibroblast lines GM498B and GM3349B were obtained from the National Institute of General Medical Sciences, Human Cell Repository, Camden, NJ. Culture, harvest, and chromosome preparation were according to standard procedures. 23 Cultured cells on chamber slides were treated with hypotonic 0.06 mol/L KCl for 30 minutes and then fixed in methanol:acetic acid, 3:1. Metaphase chromosomes and interphase nuclei were stained with Wright’s stain and at least 25 metaphase cells and 1,000 interphase nuclei were analyzed per case. The total rate of abnormal nuclear structures (ANS) in each case was determined as the sum of the frequency of nuclei showing at least one nuclear bleb or nuclear string, and the frequency of micronuclei (MN).
A nuclear bleb was defined as a round or oval protrusion of the nuclear membrane connected to the main part of the nucleus by a thinner chromatin segment, a nuclear string as a chromatin thread connected to the membrane(s) of one or two nuclei, and a micronucleus as a rounded chromatin fragment located adjacent to a nucleus, with a diameter not exceeding one third of the diameter of that nucleus. 24 Cells exhibiting strings in connection with blebs were classified as nuclear strings. To exclude that artifacts in nuclear morphology were caused by the hypotonic treatment, cultures from a de-differentiated liposarcoma were also stained by Giemsa after direct fixation with methanol:acetic acid. No significant difference in ANS (17% compared to 18%) was detected between these preparations and those exposed to hypotonic treatment.
Cytological Staining
To separately visualize cytoplasm and chromatin, cultured cells were fixed without Colcemid treatment, stained for 30 seconds with 0.2 mg/ml Evan’s blue/phosphate-buffered saline, mounted with 1 μg/ml diamidinophenylindole/2% 1,4-diazabicyclooctane/glycerol, and analyzed by epifluorescence microscopy on a ChromoFluor System (Applied Imaging International Inc., Newcastle, UK). At least 100 chromatin bridges were evaluated in each case.
FISH
Slides were prepared for FISH according to standard procedures. 25 Biotin-labeled whole chromosome painting probes for chromosomes 9, 10, and 15, and digoxigenin-labeled probes for 3, 9, and 12, were from Cambio (Cambridge, UK) and Oncor (Gaithersburg, MD), respectively. The multicolor karyotyping of the malignant fibrous histiocytomas MFH2 and MFH3 has been described previously. 14 Morphological features of interphase nuclei were evaluated after diamidinophenylindole staining. To determine the chromosomal content of interphase chromatin, at least 100 abnormal nuclei were analyzed in each case.
Irradiation of Cell Cultures
Cell cultures from two normal fibroblast cell lines and the OSA tumor cell line were grown to confluence and then exposed to 6 Gy from 137Cs at 0.54 Gy/minute. The culture medium was changed immediately after irradiation and the first passage was made after 24 hours. At least 50 metaphase cells and 1,000 interphase nuclei per passage were analyzed after Wright’s staining. At least 50 anaphase figures were analyzed by Giemsa staining in parallel cultures.
Analysis of Tumor Cells in Primary Cultures
Archived Wright- or Giemsa-stained chromosome preparations from 70 solid tumors were selected for evaluation of nuclear morphology. Cases were compiled with the aim of achieving a high variability of karyotypic features within the sample, regarding both the number and the type of cytogenetic aberrations. After exclusion of cases with incomplete karyotypes or ambiguous diagnoses, 58 tumors remained for statistical analysis, including seven lung carcinomas, five ovarian carcinomas, one chondroblastoma, two chondromas, 11 chondrosarcomas, two aneurysmal bone cysts, six giant cell tumors of bone, nine osteosarcomas, two typical lipomas, five atypical lipomatous tumors, seven liposarcomas (two dedifferentiated, one round cell, two mixed-type, one myxoid, and one sclerosing), and one malignant fibrous histiocytoma. Karyotypes, described according to An International System for Human Cytogenetic Nomenclature, 26 were used to determine the highest number of structural and numerical aberrations in a clonal population for each case. Intratumor variability of numerical aberrations was assessed by the number of cells with chromosome counts deviating from the modal number, divided by the number of cells analyzed, whereas variability in chromosome structure was assessed by the number of structural rearrangements not included in the stemline, divided by the number of cells analyzed.
Results
The Chromosomal Content of ANS
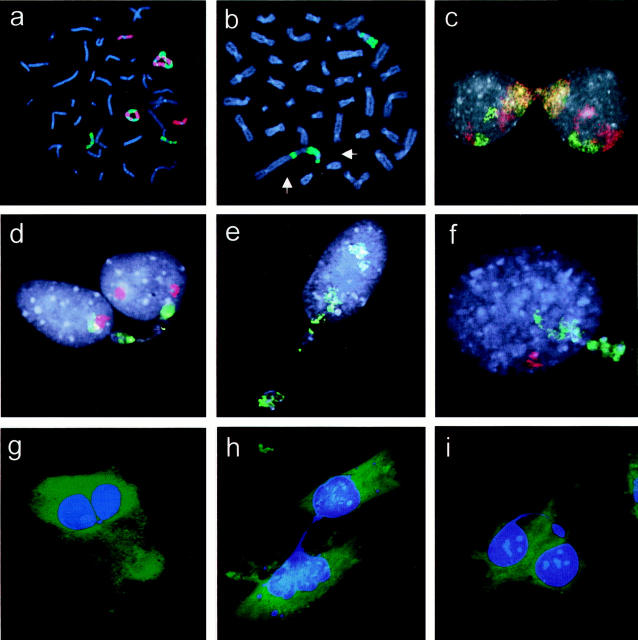
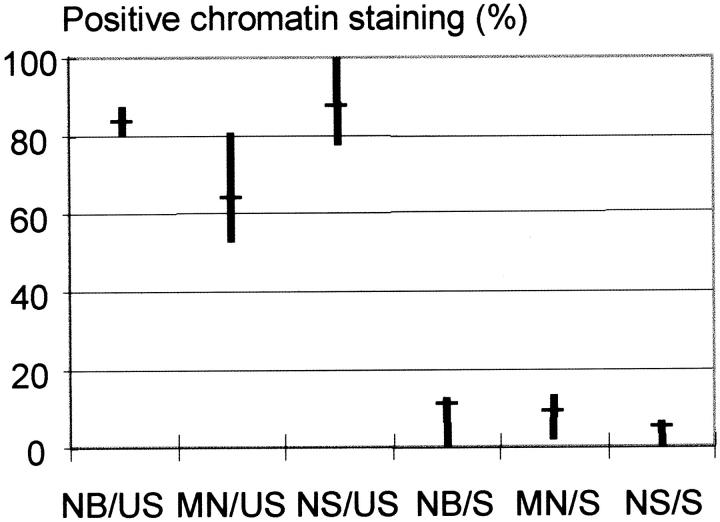
FISH analysis was performed to identify mitotically unstable chromosomes in five soft tissue tumors with abnormal nuclear morphology. Two atypical lipomatous tumors (ALT1 and ALT2) and a low-grade malignant fibrous histiocytoma (MFH1) exhibited simple karyotypic changes including supernumerary ring chromosomes, shown by whole chromosome painting to contain sequences from chromosome 12; the rings in MFH1 also carried material from chromosome 9 (Figure 1a) ▶ . The two other tumors (MFH2 and MFH3) were both high-grade lesions and had complex karyotypes including ring and dicentric chromosomes. Multicolor analysis showed that the majority of rings and dicentrics contained material from chromosomes 9 (Figure 1b) ▶ and 3, respectively. At analysis of interphase nuclei performed with whole chromosome paint for the unstable chromosomes, 71 to 86% of the blebs, strings, and micronuclei stained positive (Figures 1, c–f, and 2) ▶ ▶ . As a control, whole chromosome painting was also performed for chromosomes not involved in mitotically unstable aberrations according to G-band analysis: chromosome 9 in ALT1 and ALT2, chromosomes 3 and 15 in MFH1, chromosome 10 in MFH2, and chromosome 1 in MFH 3. The proportion of ANS that stained positive for these chromosomes was 0 to 11%.
Figure 1.
Whole chromosome painting. a: MFH1. Two ring chromosomes composed of material from chromosomes 9 (red) and 12 (green). b: MFH2. Two abnormal chromosomes, of which one is dicentric (arrows), are positive for chromosome 9 (green). c: MFH1. A chromatin bridge between nuclei is positive for chromosomes 9 (red) and 12 (green), resulting in a mixed red-yellow-green fluorescence; the chromatin of the normal homologues is also stained. d–f: MFH2. A chromatin string, micronuclei, and a nuclear bleb, respectively, containing sequences from chromosome 9 (green) but not from chromosome 10 (red). g–i: ALT1. Chromatin string connecting two lobes of a nucleus, connecting nuclei in two adjacent cells, and hanging freely in the cytoplasm, respectively.
Figure 2.
Results of FISH analysis of nuclear blebs (NB), micronuclei (MN), and nuclear strings (NS) in five soft tissue tumors. Whole chromosome painting for the mitotically unstable (US) chromosomes stained the majority of abnormal structures in all cases, whereas only a minor proportion stained positive for the selected stable (S) chromosomes used as a control. The fraction of abnormal nuclear structures with signals for the tested chromosome is indicated by median values (horizontal bars) and ranges (vertical bars).
The Cellular Organization of Chromatin Strings
The arrangement of chromatin strings was studied further by epifluorescence microscopy in MFH1 and ALT1. Three different configurations were observed: chromatin bridges connecting two nuclear lobes within the same cell (41% of bridges in ALT1 and 20% in MFH1; Figure 1g ▶ ); chromatin bridges connecting the nuclei of neighboring cells (35% in ALT1 and 37% in MFH1; Figure 1h ▶ ); and chromatin strings protruding freely from a nucleus into the cytoplasm (24% in ALT1 and 43% in MFH1; Figure 1i ▶ ). The strings connecting nuclei of different cells were invariably sheathed by cytoplasm.
Mitotically Unstable Chromosomes and Nuclear Abnormalities in Irradiated Cells
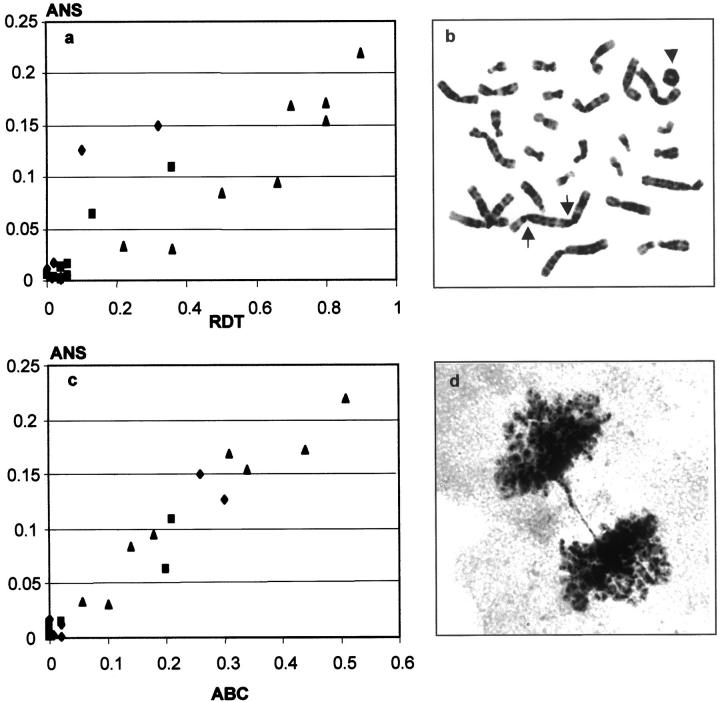
Nuclear morphology, metaphase chromosomes, and anaphase division figures were monitored during at least six passages after exposure to radiation. The results of the metaphase and anaphase analyses have been partly described previously. 16 Before irradiation, the fibroblast cultures and OSA had ANS frequencies of 0.3% and 3%, respectively. In exposed cultures, the proportion of cells containing at least one ring chromosome, dicentric chromosome, or telomeric association (RDT), the frequency of anaphase bridge configurations (ABC), and ANS were determined after each passage. All cultures showed elevated RDT, ABC, and ANS levels at the first passage after irradiation. These parameters subsequently decreased in a parallel manner and reached the level of normal, nonirradiated cells at passages 3 to 4 in the fibroblasts and passage 7 in OSA. When RDT was compared to ANS at each passage, a linear correlation was observed (r = 0.87; Pearson correlation, P < 0.001, two-tailed; Figure 3, a and b ▶ ). An even stronger correlation was found between ANS and ABC (r = 0.98; P < 0.001; Figure 3, c and d ▶ ). Similar results were obtained when nuclear blebs, NS, and MN were separately compared to RDT (r = 0.88, 0.82, and 0.84, respectively; P < 0.001) and ABC (r = 0.94, 0.93, and 0.98, respectively; P < 0.001). There was no significant correlation between MN and the frequency of metaphase cells with acentric fragments (r = 0.22; P = 0.31). The frequency of nuclear abnormalities was thus proportional to the mitotic instability of chromosomes in both fibroblasts and osteosarcoma cells.
Figure 3.
The relation of mitotic chromosomal instability and abnormal nuclear structures (ANS) in irradiated cultures of fibroblasts GM498B (squares) and GM3349B (diamonds), and OSA osteosarcoma cells (triangles). a: Positive correlation (r = 0.87) between the proportion of metaphase cells carrying unstable chromosomes (RDT) and ANS. b: A dicentric (arrows) and a ring (arrowhead) chromosome in an irradiated OSA cell. c: Positive correlation (r = 0.98) between the frequency of anaphase bridge configurations (ABC) and ANS. d: Anaphase bridge in an irradiated OSA culture.
Nuclear Aberrations and Karyotypic Patterns
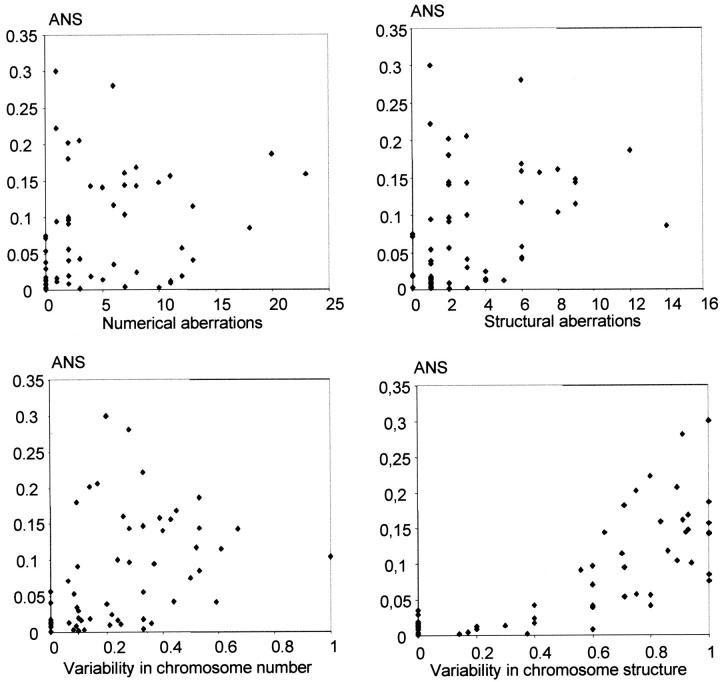
Preparations from short-term cultures of 12 epithelial and 46 mesenchymal tumors were used for parallel investigation of the chromosomal aberration pattern and nuclear abnormalities. An extensive variation was observed in the frequency of nuclear aberrations within each tumor subgroup. The malignant tumors exhibited ANS between 0 and 30%, whereas the benign lesions all had ANS <2%. Tumors of borderline malignancy, including giant cell tumors of bone and atypical lipomatous tumors, had ANS between 4 and 20%. At metaphase analysis, 30 of the tumors contained ring chromosomes, dicentric chromosomes, or telomeric associations. ANS was significantly higher (14% versus 1.7%; P < 0.001, two-sided Z-test) in these tumors than in those with only stable types of chromosome aberrations, predominantly including gains and losses of chromosomes, translocations, inversions, deletions, insertions, and additions. ANS was found to correlate well to the intratumor variability of structural chromosome aberrations (r = 0.78; Pearson correlation, P < 0.001, two-tailed), but poorly to the variability in numerical aberrations (r = 0.39; P = 0.002), as well as to the total number of structural (r = 0.37; P = 0.004) and numerical (r = 0.24; P = 0.075) aberrations (Figure 4) ▶ .
Figure 4.
Nuclear abnormalities in relation to the karyotypic pattern. There were poor correlations (r = 0.24 to 0.39) between ANS and the number of both numerical and structural chromosome aberrations as well as between the intratumor variability in chromosome number, but a stronger correlation (r = 0.78; P < 0.001) between ANS and the variability in chromosome structure.
Discussion
Previous investigations of tumor cells have indicated that there might be an association between genetic aberrations and abnormal nuclear morphology. 5,10,11 In the present analysis, we used cytogenetic and molecular cytogenetic techniques to study the relation between chromosomal instability at mitosis and nuclear shape. In the five soft tissue tumors analyzed by FISH, the vast majority (71 to 86%) of blebs, strings, and micronuclei contained material from mitotically unstable, potentially bridge-forming, ring, or dicentric chromosomes, whereas only a small proportion (0 to 11%) of the abnormal structures contained material from the stable chromosomes used as controls. Moreover, in irradiated cells, a strong correlation was found between the frequencies of nuclear irregularities, on the one hand, and the proportion of cells exhibiting mitotically unstable chromosomes and anaphase bridges, on the other. Finally, in preparations from short-term cultures of primary tumor material, cases with unstable chromosomes showed significantly higher frequencies of ANS than cases in which only stable aberrations were found. These results indicate a close association between the abnormalities in nuclear shape and chromosomal bridge-breakage events.
According to studies in several organisms, chromosomes participating in anaphase bridges may rupture and the broken ends will subsequently reunite to form novel structural aberrations in the daughter cells. 19,20 Some of these newly formed abnormalities may also be dicentric or ring-shaped and, in their turn, participate in the formation of anaphase bridges. Such breakage-fusion-bridge cycles may lead to a considerable intercellular heterogeneity in the pattern of, in particular, structural chromosome aberrations. 16,18 In this study, the frequency of nuclear abnormalities correlated strongly to intratumor variability of structural chromosome aberrations, whereas the correlation to numerical variability and the total number of chromosome aberrations was weak. It should be noted that most of the tumor preparations from short-term cultures most probably contained fibroblasts or normal epithelial cells that could not be distinguished from the neoplastic cells at interphase analysis. Neither could the relative population sizes be determined with accuracy by cytogenetic analysis because a disproportionate mitotic activity among stromal and parenchymal tumor cells might give a false estimation. Cases with large proportions of normal cells might therefore exhibit lower ANS than what would have been obtained from pure tumor cell populations. However, the correlation between ANS and structural variability was still fairly strong (r = 0.78; P < 0.001) and all cases with ANS >10% exhibited >50% variability in structural aberrations, ie, more than half of the aberrant cells had rearrangements outside the stem line.
Little is known about the mechanisms leading to aberrant nuclear structure in pathological conditions. Some knowledge has been gained from the effects of mutagenic agents, such as radiation, on nuclear morphology. Irradiated fibroblasts are characterized by large atypical nuclei 27 and the frequency of micronuclei in exposed cells has long been used as a measure of the clastogenic insult. 28 It has been suggested that micronuclei may originate from acentric chromosome fragments, either resulting from double-strand DNA damage before cell division, or after the breakage of anaphase bridges. 29 In the irradiated cell populations in this study, an association was found between the frequency of micronuclei and chromosomal instability. MN showed a strong correlation to both RDT and ABC (r = 0.84 and 0.98, respectively; P < 0.001), whereas there was no correlation to the frequency of acentric fragments. However, in the tumors studied by FISH, the proportion of micronuclei containing material from mitotically unstable chromosomes was lower than the corresponding values for blebs and strings. In the ALTs and MFH1 60 to 80% of MN were positive for sequences from unstable chromosomes, but in the highly malignant MFH2 and MFH3, the corresponding values were only 52 and 54%, respectively (Figure 2) ▶ . This indicates that, although the formation of micronuclei in irradiated cells and many tumors may frequently be associated with anaphase bridging, other processes may also contribute. This is in accordance with previous studies, showing that the origin of micronuclei may vary with the type of cytotoxic exposure. Substances affecting spindle function, such as colchicine, induce micronuclei containing whole chromosomes, whereas clastogenic agents, such as X-rays, mainly lead to the formation of micronuclei with acentric fragments. 30
In neoplastic disease, the relation between nuclear morphology and chromosomal aberrations has particularly been studied in tumors exhibiting genomic amplification. In neuroblastomas, micronuclei and nuclear blebs have been shown to carry amplified copies of the MYCN gene, 13 and in low-grade mesenchymal tumors, amplified material from chromosome 12 frequently aggregate in nuclear blebs and strings. 12,14,15 It has been suggested that nuclear irregularities are part of a mechanism by which amplified sequences may be eliminated from the main nucleus at interphase. 13,31 Alternatively, the overrepresentation of amplified sequences in blebs, strings, and micronuclei could merely reflect their relative abundance in the genome, increasing the likelihood of detection at any location in the cell nucleus. However, in this study, a high ANS frequency was observed in several cell types where genomic amplification was not observed, including irradiated fibroblasts and three giant cell tumors of bone in which telomeric associations were the only cytogenetic abnormalities. Furthermore, aggregation of material from unstable chromosomes in ANS were seen also in the absence of intrachromosomal amplification, eg, in MFH2 and MFH3. 16 Our data thus indicate a mechanistic link primarily between certain types of nuclear abnormalities at interphase, a heterogeneous pattern of chromosome aberrations at metaphase, and chromosome bridging at anaphase. This may be explained by a scenario in which chromosomes participating in anaphase bridges decondense into aberrant nuclear structures (Figure 5) ▶ . According to this model, intact bridges would form chromatin strings, whereas fragments from broken bridges would form nuclear blebs or micronuclei. In malignant tissues, these ANS should thus be viewed primarily as an indicator of ongoing genomic reorganization. The correlation between disturbances of the chromosome segregation at mitosis and morphological parameters seems applicable to a number of different tumor types, mesenchymal as well as epithelial, regardless of the level of cytogenetic complexity and the grade of malignancy. The observation that chromatin strings frequently connect neighboring cells through cytoplasmic channels offers intriguing possibilities for intercellular communication in malignant tissues and should be explored further.
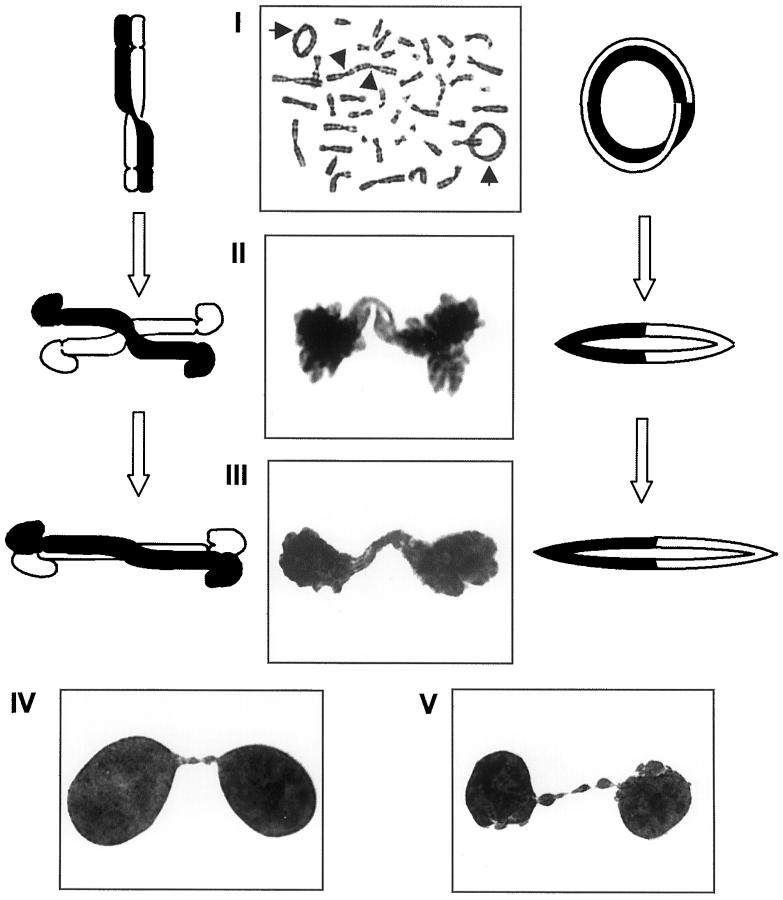
Figure 5.
Model for the formation of nuclear abnormalities illustrated by cell division figures from a low-grade malignant fibrous histiocytoma: mitotically unstable chromosome aberrations such as rings (I, arrows) and dicentrics (arrowheads) may form bridges at anaphase (II) which, after telophase (III), may remain as nuclear strings (IV), or break up into nuclear blebs and micronuclei (V).
Acknowledgments
We thank Drs. M. Johansson-Soller and T. Pejovic for providing archived metaphase preparations.
Footnotes
Address reprint requests to David Gisselsson, Department of Clinical Genetics, University Hospital, SE-221 85 Lund, Sweden. E-mail: david.gisselsson@klingen.lu.se.
Supported by the Swedish Cancer Society, the Children Cancer Fund of Sweden, the Inga Britt and Arne Lundberg Foundation, and the John and Augusta Persson Foundation for Scientific Medical Research.
References
- 1.Hansemann D: Ueber patologische Mitosen. Arch Pathol Anat Phys Klin Med 1891, 119:299-326 [Google Scholar]
- 2.Koss LG: Diagnostic Cytology, ed 4, chap 6. Philadelphia, J. B. Lippincott, 1992, pp 154–192
- 3.Erlandson RA: Diagnostic Transmission Electron Microscopy of Tumors. 1994. Raven Press, New York
- 4.Bibbo M: Comprehensive Cytopathology, ed 2 1997, W. B. Saunders Company, Philadelphia
- 5.Erhardt K, Silfverswärd C, Auer G: Nuclear DNA content and nuclear atypia. Relation to survival in patients with breast adenocarcinoma and serous ovarian tumors. Anticancer Res 1989, 9:1325-1330 [PubMed] [Google Scholar]
- 6.Vacher-Lavenu MC, Le Tourneau A, Duvillard P, Godefroy N, Pinel MC: Pathological classification and grading of primary ovarian carcinoma: experience of the ARTAC ovarian study group. Bull Cancer 1993, 80:135-141 [PubMed] [Google Scholar]
- 7.Takeshima N, Hirai Y, Hasumi K: Prognostic validity of neoplastic cells with notable nuclear atypia in endometrial cancer. Obstet Gynecol 1998, 92:119-123 [DOI] [PubMed] [Google Scholar]
- 8.Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, Goussot JF, David M, Bonichon F, Lagarde C: Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer 1984, 33:37-42 [DOI] [PubMed] [Google Scholar]
- 9.Costa J: The grading and staging of soft tissue sarcomas. Pathobiology of Soft Tissue Tumours. Edited by CDM Fletcher, PH McKee. Edinburgh, Churchill Livingstone, 1990
- 10.Atkin NB, Baker NC: Nuclear protrusions in malignant tumours with large abnormal chromosomes: observations on C-banded preparations. Experientia 1979, 35:899-901 [DOI] [PubMed] [Google Scholar]
- 11.Näslund I, Rubio CA, Auer GU: Nuclear DNA changes during pathogenesis of squamous carcinoma of the cervix in 3,4-benzopyrene-treated mice. Anal Quant Cytol Histol 1987, 9:411-418 [PubMed] [Google Scholar]
- 12.Pedeutour F, Suijkerbuijk RF, Forus A, Van Gaal J, Van de Klundert W, Coindre JM, Nicolo G, Collin F, Van Haelst U, Huffermann K, Turc-Carel C: Complex composition and co-amplification of SAS and MDM2 in ring and giant rod marker chromosomes in well-differentiated liposarcoma. Genes Chromosom Cancer 1994, 10:85-94 [DOI] [PubMed] [Google Scholar]
- 13.Ambros IM, Rumpler S, Luegmayr A, Hattinger CM, Strehl S, Kovar H, Gadner H, Ambros PF: Neuroblastoma cells can actively eliminate supernumerary MYCN gene copies by micronucleus formation—sign of tumour cell revertance? Eur J Cancer 1997, 33:2043-2049 [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki H, Ohjimi Y, Ishiguro M, Isayama T, Fujita C, Kaneko Y, Kikuchi M, Shinohara N: Supernumerary ring chromosomes and nuclear blebs in some low-grade malignant soft tissue tumours: atypical lipomatous tumours and dermatofibrosarcoma protuberans. Virchows Arch 1998, 432:521-528 [DOI] [PubMed] [Google Scholar]
- 15.Gisselsson D, Höglund M, Mertens F, Mitelman F, Mandahl N: Chromosomal organization of amplified chromosome 12 sequences in mesenchymal tumors detected by fluorescence in situ hybridization. Genes Chromosom Cancer 1998, 23:203-212 [DOI] [PubMed] [Google Scholar]
- 16.Gisselsson D, Pettersson L, Höglund M, Heidenblad M, Gorunova L, Wiegant J, Mertens F, Dal Cin P, Mitelman F, Mandahl N: Chromosomal breakage-fusion-bridge events cause genetic intratumor heterogeneity. Proc Natl Acad Sci USA 2000, 97:5357-5363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gisselsson D, Höglund M, Mertens F, Mandahl N: Variable stability of chromosomes containing amplified alpha-satellite sequences in human mesenchymal tumours. Chromosoma 1999, 108:271-277 [DOI] [PubMed] [Google Scholar]
- 18.Gisselsson D, Höglund M, Mertens F, Johansson B, Dal Cin P, Van den Berghe H, Earnshaw WC, Mitelman F, Mandahl N: The structure and dynamics of ring chromosomes in human neoplastic and non-neoplastic cells. Hum Genet 1999, 104:315-325 [DOI] [PubMed] [Google Scholar]
- 19.McClintock B: The production of homozygous deficient tissues with mutant characteristics by means of the aberrant behavior of ring-shaped chromosomes. Genetics 1938, 23:315-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClintock B: The stability of broken ends of chromosomes in Zea mays. Genetics 1940, 26:234-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koller PC: The Role of Chromosomes in Cancer Biology. 1972. Springer Verlag, Berlin [PubMed]
- 22.Roberts WM, Douglass EC, Peiper SC, Houghton PJ, Look AT: Amplification of the gli gene in childhood sarcomas. Cancer Res 1989, 49:5407-5413 [PubMed] [Google Scholar]
- 23.Mandahl N, Heim S, Arheden K, Rydholm A, Willén H, Mitelman F: Three major cytogenetic subgroups can be identified among chromosomally abnormal solitary lipomas. Hum Genet 1988, 79:203-208 [DOI] [PubMed] [Google Scholar]
- 24.Moore LE, Titenko-Holland N, Quintana PJ, Smith MT: Novel biomarkers of genetic damage in humans: use of fluorescence in situ hybridization to detect aneuploidy and micronuclei in exfoliated cells. J Toxicol Environ Health 1993, 40:349-357 [DOI] [PubMed] [Google Scholar]
- 25.Gisselsson D, Mandahl N, Pålsson E, Gorunova L, Höglund M: Locus-specific multi-fluor FISH analysis allows physical characterization of complex chromosome aberrations in neoplasia. Genes Chromosom Cancer 2000, 28:347-352 [PubMed] [Google Scholar]
- 26.ISCN: An International System for Human Cytogenetic Nomenclature. Edited by Mitelman F. Basel, Karger, 1995
- 27.Meehan SA, LeBoit PE: An immunohistochemical analysis of radiation fibroblasts. J Cutan Pathol 1997, 24:309-313 [DOI] [PubMed] [Google Scholar]
- 28.Schmid W: The micronucleus test. Mutat Res 1975, 31:9-15 [DOI] [PubMed] [Google Scholar]
- 29.Schmid W: The micronucleus test: an in vivo bone marrow method. Hsu TC eds. Cytogenetic Assays of Environmental Mutagens. 1982, :pp 221-229 Allanheld, Osmun, Totowa [Google Scholar]
- 30.Degrassi F, Tanzarella C: Immunofluorescent staining of kinetochores in micronuclei: a new assay for the detection of aneuploidy. Mutat Res 1988, 203:339-345 [DOI] [PubMed] [Google Scholar]
- 31.Toledo F, Le Roscouet D, Buttin G, Debatisse M: Co-amplified markers alternate in megabase long chromosomal inverted repeats and cluster independently in interphase nuclei at early steps of mammalian gene amplification. EMBO J 1992, 11:2665-2673 [DOI] [PMC free article] [PubMed] [Google Scholar]