Abstract
Although evidence suggests that neurofibrillary tangles (NFTs) and neuronal cell loss are prominent features of Alzheimer’s disease (AD), the relationship between the two remains unknown. In the present study, the relationship between the activation of apoptotic mechanisms and NFT formation in AD was investigated using a caspase-cleavage site-directed antibody to fodrin, an abundant neuronal cytoskeleton protein. This antibody recognized cleavage products of fodrin after digestion by caspase-3, but did not recognize full-length fodrin. In vitro analysis of this fodrin caspase-cleavage product (CCP) antibody demonstrates that it is a specific probe for the detection of apoptotic but not necrotic pathways in cultured neurons. To determine whether caspases cleave fodrin in vivo, tissue sections from controls and AD were immunostained for fodrin (CCPs). Although no staining was observed in control cases, labeling of neurons was observed in the hippocampus of all AD cases, which increased as a function of disease progression. To determine a possible relationship between caspase activation and NFT formation, double-labeling experiments with fodrin CCP and PHF-1 were performed. Co-localization of these markers was observed in many neurons, and quantitative analysis showed that as the extent of NFT formation increased, there was a significant corresponding increase in fodrin CCP immunolabeling (r = 0.84). Taken together, these results provide evidence for the activation of apoptotic mechanisms in neurons in the AD brain and suggest that there is an association between NFT formation and the activation of apoptotic pathways in AD.
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by a progressive decline in cognition that influences multiple systems, including memory, language, executive functions, and visual-spatial skills. AD is diagnosed based on the extent of accumulation of senile plaques and neurofibrillary tangles (NFT), and neuron loss. 1 NFTs consist of paired helical filaments (PHF) resulting from the hyperphosphorylation of the microtubule-binding protein tau in select neuronal populations. 2 Hyperphosphorylation of tau leads to reduced microtubule-binding capacity and impaired microtubule assembly, both of which are thought to be critical early events in tangle formation. 3,4 A newly emerging feature of AD is the apparent activation of apoptotic mechanisms in neurons of the AD brain. 5-8 Apoptosis is characterized by three stages: 1) a regulation stage involving pro- and anti-apoptotic proteins such as Bax and Bcl-2, respectively; 2) an activation stage in which activation of a family of cysteine aspartate proteases known as caspases occurs; and 3) a commitment stage, characterized by plasma membrane blebbing, nuclear condensation, and DNA fragmentation. 9,10 Although both NFTs and apoptotic type mechanisms are prominent features of AD, the question of whether these pathways are causally related or independent remains an unresolved issue. Several reports have demonstrated a lack of co-localization between NFTs and specific apoptotic markers including DNA fragmentation 11-13 and activated caspase-3. 14,15 Conversely, others have shown co-localization between NFTs and DNA fragmentation 16 and Bax. 17,18
To address the question of whether there is a relationship between NFTs and neuronal apoptosis in AD, we designed a caspase-cleavage site-directed antibody to fodrin, a major constituent of the membrane cytoskeleton in neurons. 19 We chose fodrin as our marker for apoptosis because it is expressed predominantly and abundantly in neurons 19 and it is an excellent substrate for caspase-3 cleavage. 20-23 Using this novel probe, we demonstrate widespread caspase activation and accumulation of stable caspase cleavage products in AD brain, and provide support for the hypothesis that activation of apoptotic mechanisms may contribute to disease progression.
Materials and Methods
Materials
All chemicals used were of the highest grade available. Poly-d-lysine and concanavalin A (Con A) were obtained from Sigma Chemical Co. (St. Louis, MO). Staurosporine was from Calbiochem-Novabiochem (La Jolla, CA). Z-Val-Ala-Ala-Asp (OMe)-FMK (Z-VAD) was from Enzyme Systems Products (Livermore, CA). A multiple antigen peptide (MAP) containing the SVEALI sequence that was used for generation of polyclonal antibodies was synthesized by Research Genetics, Inc. (Huntsville, AL). Apopain/YAMA/CPP32/caspase-3-agarose was from Upstate Biotechnology (Lake Placid, NY). The sulfolink kit used to affinity purify antibody was purchased from Pierce (Rockford, IL).
Cell Culture
Human neuroblastoma SH-SY5Y cells (SY5Y) were grown to confluence on 12-well plates (∼2 × 10 6 cells/well) in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 100 U/ml penicillin and 100 μg/ml streptomycin. Sprague-Dawley rat embryos (day 18 of gestation) were used to produce short-term cultures of cortical neurons as previously described. 24 For all morphological studies, cells were plated at a density of 1.5 × 10 5 cells/ml in 24-well poly-d-lysine-coated plates. For Western blot analysis, neurons were plated at 3.75 × 10 5 cells/ml in 12-well poly-d-lysine-coated plates. All experiments were performed on day 4 of the neuronal cultures.
Treatment Protocols
Con A was made up as a 25 μmol/L stock in Dulbecco’s modified Eagle’s medium and filter sterilized before use. Z-VAD was prepared as a 50 mmol/L stock in sterile dimethyl sulfoxide. To permit adequate cellular loading, Z-VAD was added 1 hour before insult. Cultures were used for experimentation on day 4 in vitro.
Immunocytochemistry in Cell Cultures
Immunocytochemistry studies in cortical neurons were performed as previously described. 25 The primary antibody, fodrin caspase cleavage product (CCP), was incubated overnight at 4°C at a final concentration of 0.4 to 0.5 μg/ml. Bound antibody was detected using a biotinylated anti-rabbit ABC peroxidase kit (Vector Laboratories, Burlingame, CA) followed by color development using diaminobenzidine (DAB kit; Vector Laboratories) as the chromogenic substrate. For fluorescence labeling of cells, bound antibody was detected using a biotin-SP-conjugated goat anti-rabbit IgG (1:200 for 1 hour at room temperature; Jackson ImmunoResearch, West Grove, PA), followed by Cy-3 conjugated streptavidin for 1 hour at room temperature (1:800).
Western Blot Analysis
Western blot analysis was performed as previously described. 23 Briefly, for analysis of fodrin CCPs, cortical neurons, treated under various conditions, were extracted with 2× sample buffer and subjected to sodium dodecyl sulfate electrophoresis on 7.5% gels, transferred to nitrocellulose, and blocked for 1 hour at room temperature with 5% nonfat dried milk. Blots were incubated with fodrin CCP antibody (1 to 2 μg/ml) overnight at 4°C, followed by horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:5,000) for 1 hour at room temperature. Blots were developed using an enhanced chemiluminescence system (Amersham, Arlington Heights, IL). Transferred gel slabs were silver-stained for total protein to ensure equal protein loading.
Human and Animal Subjects
Autopsy brain tissue from the hippocampus and entorhinal cortex of eight neuropathologically confirmed AD cases and two aged nondemented cases with no evidence of senile plaques or NFTs were studied. In addition, seven aged nondemented cases with early senile degenerative changes (senile plaques and NFTs), termed mild pathology controls, that did not reach numbers to be consistent with a diagnosis of AD were compared. Case demographics for these cases are presented in Table 1 ▶ . Braak and Braak staging was based on PHF-1 immunostaining (see below). 26 Age at death was not significantly different between AD (mean, 76.8 ± 2.21) and controls (mean, 74.1 ± 6.96). To determine whether fodrin CCP staining was associated only with NFT formation, immunostaining in the prefrontal cortex of an aged dog (12.2 years) was compared to that of a young dog (1.6 years). Human and canine brain tissues used in this project were provided by The Institute for Brain Aging and Dementia Tissue Repositories at the University of California, Irvine.
Table 1.
Case Demographics and Neuron Counts
| Group | Age (yrs) | Sex | PMI | MMSE | Clinical diagnosis | Neuro-pathological diagnosis | Braak and Braak stage | NFT* | Fodrin CCP* | Co-localization* |
|---|---|---|---|---|---|---|---|---|---|---|
| AD | 74 | M | 2.75 | 0 | Probable AD | AD | V | 417 (72.8) | 100 (17.5) | 56 (9.8) |
| AD | 69 | F | 3.75 | 0 | Probable AD | AD | VI | N/A | N/A | N/A |
| AD | 97 | F | 3.0 | N/A | Probable AD | AD | V | N/A | N/A | N/A |
| AD | 86 | M | 5.25 | 4 | Probable AD | AD | V | 99 (53.8) | 58 (31.5) | 27 (14.7) |
| AD | 73 | M | 4.33 | 7 | Probable AD | AD | V | N/A | N/A | N/A |
| AD | 71 | M | 3.75 | 9 | Probable AD | AD | VI | 281 (53.5) | 195 (37.1) | 49 (9.3) |
| AD | 79 | F | 4.75 | 17 | Probable AD | AD | V | 553 (67.0) | 140 (17.0) | 133 (16.1) |
| AD | 78 | M | 3.58 | 24 | Probable AD | AD | V | 331 (50.8) | 178 (27.3) | 142 (21.8) |
| Ctl | 68 | F | 6.00 | N/A | N/A | MPC | II | N/A | N/A | N/A |
| Ctl | 75 | F | 2.75 | 30 | Normal | MPC | II | 12 (42.9) | 9 (32.1) | 7 (25.0) |
| Ctl | 78 | M | 6.00 | N/A | N/A | MPC | N/A | N/A | N/A | N/A |
| Ctl | 84 | F | 7.10 | N/A | N/A | MPC | II | 31 (79.5) | 4 (10.3) | 4 (10.3) |
| Ctl | 92 | F | 6.50 | 25 | Normal | MPC | 0 | 0 (0) | 0 (0) | 0 (0) |
| Ctl | 93 | F | 8.00 | N/A | N/A | MPC | I | N/A | N/A | N/A |
| Ctl | 92 | F | 4.25 | 28 | Normal | MPC | II | 34 (94.4) | 1 (2.8) | 1 (2.8) |
| Ctl | 69 | M | 6.60 | N/A | N/A | Normal | 0 | 38 (100) | 0 (0) | 0 (0) |
| Ctl | 39 | M | 10.50 | N/A | N/A | Normal | 0 | 0 | 0 (0) | 0 (0) |
PMI, postmortem interval in hours; NFT, neurofibrillary tangles; CCP, caspase cleavage product; MPC, mild pathological changes consistent with Braak and Braak Stage I/II; MMSE, mini mental state examination; Ctl, control; N/A, not available.
*Total number of neurons counted (proportion of total neurons counted).
Immunohistochemistry in Human and Canine Brain
Free-floating 50-μm-thick serial sections from the hippocampus with entorhinal cortex were used for immunocytochemical studies as previously described. 27 For single-labeling experiments, sections were incubated overnight in either anti-fodrin CCP (1:100), anti-PHF-1 (1:1,000; kindly provided by Dr. P. Davies, Albert Einstein College of Medicine, Bronx, NY) or anti-phosphorylated tau at serine 202 (AT8, 1:30,000; Innogenetics, Ghent, Belgium) at room temperature. Controls were performed to rule out nonspecific immunostaining by eliminating the primary or secondary antibody. To determine whether cross-reactivity to reagents was a factor in double-labeling experiments, experiments were replicated with the antibodies reversed. All controls were negative.
Immunofluorescence and Confocal Microscopy
For fodrin CCP and PHF-1 co-localization studies, sections were incubated with anti-fodrin CCP (0.4 to 0.5 μg/ml) overnight at room temperature. Subsequently, sections were incubated for 1 hour at room temperature in biotin-SP-conjugated goat anti-rabbit IgG (1:200; Jackson ImmunoResearch) followed by Cy-3 conjugated streptavidin for 1 hour at room temperature (1:250). For the second label, sections were incubated with anti-PHF-1 (1:1,000) followed by 1-hour incubations in succession with biotin-SP-conjugated goat anti-mouse IgG and Cy-2-conjugated streptavidin (1:200).
Confocal images were collected on an Olympus IX70 inverted microscope using both a ×20 and ×40 objective for image analysis and barrier filters at 510 and 605 nm. Channel 1 was used to acquire Cy2 fluorescence and channel 2 was used to collect Cy-3 immunofluorescence. A z-series at 1-μm intervals was captured to determine the spatial co-localization characteristics of fodrin CCP and NFTs staining within individual neurons.
Quantification
The number of PHF-1, and fodrin CCP-positive-only neurons were counted using a ×10 objective while excluding double-labeled structures in five regions of double-immunostained sections: area CA1, hilus, and subiculum of the hippocampus and the superficial and deep layers of entorhinal cortex. Raw counts for each region and for each immunostain were summed and analyzed using SPSS for Windows (SPSS, Chicago, IL) with an α of 0.05 followed by calculation of either a Pearson or Spearman rank correlation coefficient. Similarly, a multiple linear regression analysis was used to determine the relationship between fodrin CCP, dementia severity (Mini Mental State Examination scores), postmortem interval, and age at death for total counts.
Results
Development and Characterization of the Fodrin CCP Antibody Using a Cell-Free System
The goal of the present study was to develop a neuron selective marker for apoptosis. Because caspases are only activated during apoptosis, we took the approach of designing a cleavage site-directed antibody against caspase-mediated cleavage products of fodrin. Our analysis of the amino acid sequence for the α-subunit of fodrin yielded a number of potential caspase cleavage sites. As an initial approach, we chose the six-amino acid sequence, SVEALI, to immunize rabbits using the general guidelines established for calpain cleavage sites in proteins. 28 The rationale for picking this sequence was based on the fact that this site represents the newly generated N-terminal neoepitope generated after active caspase cleavage and not the C-terminal fragment containing the caspase consensus recognition site. Thus, this approach reduces the potential of an antibody to cross-react with other caspase cleavage fragments. In addition, this site, when cleaved by caspases, would give predicted CCPs of 120 and 55 kd, which are the major CCPs of fodrin cleaved by caspase-3 that have been characterized in the literature. 20-22
The initial screening of the antibodies was performed by enzyme-linked immunosorbent assay using the MAP peptides adsorbed to the enzyme-linked immunosorbent assay plate. Once a sufficient titer of antibody was obtained, the antibody was purified using a sulfolink column (Pierce) coupled with the peptide SVEALIC. Purified antibody showed strong immunoreactivity at dilutions up to 1:4,000 in enzyme-linked immunosorbent assays (data not shown). To validate this antibody as a specific probe for apoptosis, a series of in vitro experiments was undertaken, beginning with testing the ability of this antibody to recognize CCPs of fodrin after digestion with caspase-3 in a cell-free system. Purified fodrin, prepared as previously described, 29 or rat cortical neuron extracts were incubated with or without caspase-3 for 24 hours at room temperature. As shown in Figure 1A ▶ , the CCP antibody recognized two primary CCPs of fodrin corresponding to molecular weights of 120 and 55 kd, but did not recognize full-length fodrin, which runs at 240 kd, thus illustrating the specificity of the fodrin CCP antibody for caspase-3 cleaved fragments. As shown in Figure 1B ▶ , purified fodrin when probed with a full-length anti-fodrin antibody gave two bands corresponding to 240 and 150 kd, respectively. The 150-kd band most likely represents a breakdown product of fodrin as a result of calpain activation during the purification process. The fodrin CCP antibody did not label this band either in the control or after digestion with caspase-3 (Figure 2A) ▶ . Figure 1B ▶ also shows the numerous fragments generated, including the 120- and 55-kd fragments, after digestion of purified fodrin with caspase-3. This result is in accordance with the multiple predicted caspase cleavage sites in fodrin.
Figure 1.
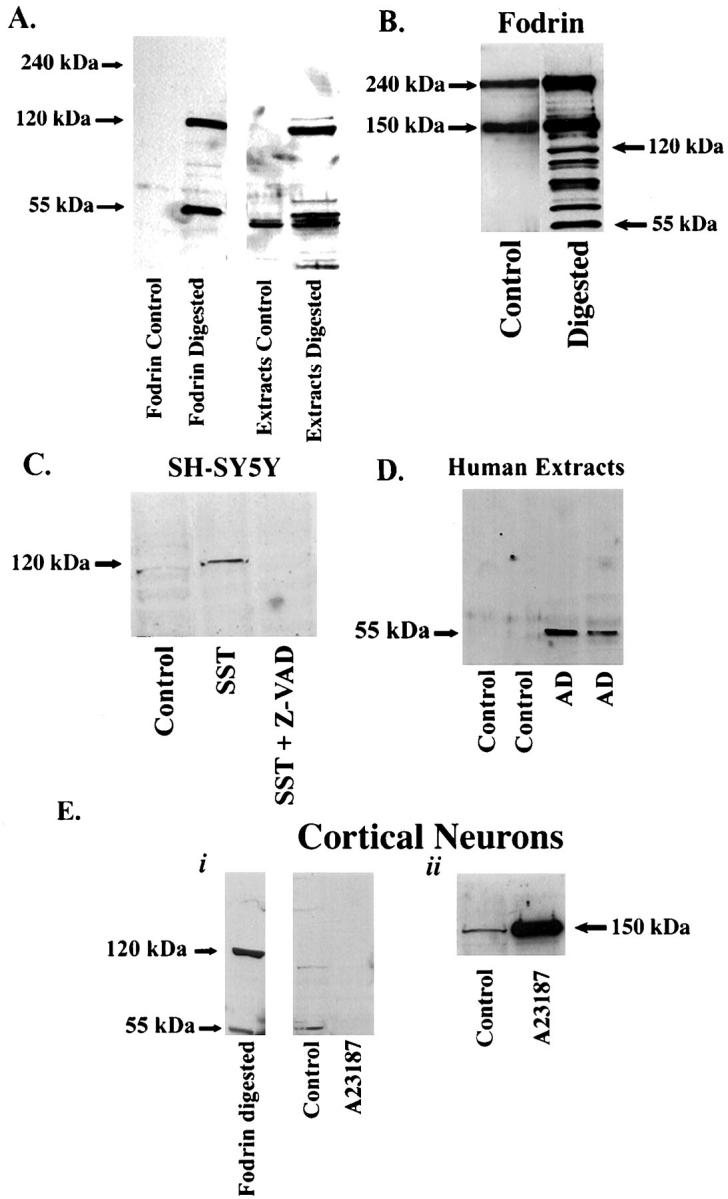
Characterization of fodrin CCP by Western blot analysis in either a cell-free system, or in a model system of apoptosis consisting of SY5Y cells or primary cultures of neurons. A: Purified fodrin or extracts from rat cortical neurons were digested with 0.015 μg/ml of caspase-3 overnight at room temperature. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with purified fodrin CCP antibody (0.4 μg/ml). B: Purified fodrin under control conditions or after digestion with caspase-3 were probed with an antibody against full-length fodrin. C: Extracts from SY5Y cells treated with 500 nmol/L staurosporine for 24 hours were run on 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels for Western analysis and were probed with purified fodrin CCP. A prominent band at 120 kd was labeled that was absent after pretreatment of SY5Y cells with the caspase inhibitor, Z-VAD. D: Human extracts from control or AD cases were analyzed by Western blot analysis as described above using fodrin CCP. A predominant band at 55 kd was labeled with fodrin CCP only in AD extracts. E: Primary cortical neurons were treated with the necrotic insult, A23187 for 2 hours and extracts were analyzed by Western blot analysis using fodrin CCP and compared with purified fodrin digested with caspase-3 (i). Cortical neuronal extracts in i were immunoblotted with an antibody that specifically recognizes the calpain-mediated cleavage fragment of fodrin but not full-length fodrin (ii).
Figure 2.
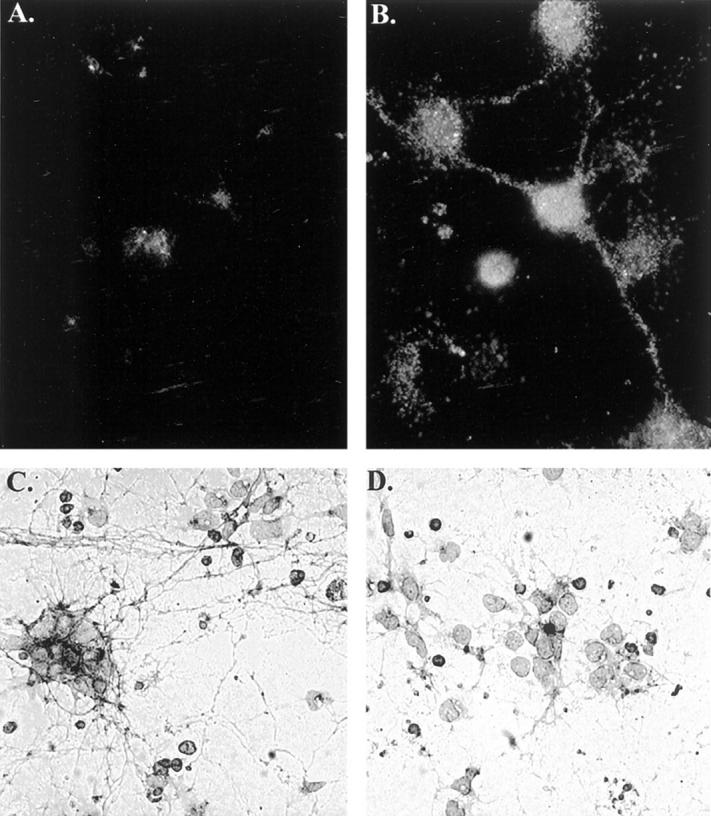
Extensive neurite labeling with fodrin CCP after treatment of rat cortical neurons with an apoptotic insult. Control (A) and Con A-treated (B) cortical neurons, respectively, were immunolabeled with fodrin CCP antibody and bound fodrin CCP was detected by immunofluorescence (see Materials and Methods). C: Five-hour treatment with 500 nmol/L Con A showing extensive neurite staining. D: Identical experimental conditions as C except neurons were pretreated for 1 hour with the caspase inhibitor, Z-VAD. Data are representative of three independent experiments.
Characterization of Fodrin CCP in a Model System of Apoptosis
When rat cortical neuronal extracts were digested with caspase-3, major bands corresponding to 120 and 55 kd were labeled by fodrin CCP, whereas no band corresponding to full-length fodrin (240 kd) was observed (Figure 1A) ▶ . Although the antibody recognized the major 55- and 120-kd CCPs, other caspase-3 cleavage products of fodrin were also labeled. These results suggest that fodrin CCP identifies the major 120- and 55-kd CCPs of fodrin after caspase-3 cleavage that have been characterized in the literature, and therefore, this antibody may serve as a useful apoptotic marker for certain neurodegenerative diseases.
To further validate fodrin CCP as an apoptotic marker, a model system of apoptosis consisting of human neuroblastoma SH-SY5Y cells was used. Treatment of SH-SY5Y cells with the apoptotic insult, staurosporine, resulted in the generation of a 120-kd band after immunoblotting with fodrin CCP. This band was completely abolished if SH-SY5Y cells were pretreated with the caspase inhibitor, Z-VAD (Figure 1C) ▶ .
Fodrin is not only a target for certain caspases, but is also a sensitive target for calpain. 30 In contrast to the 120- and 55-kd cleavage products produced by caspase-mediated cleavage, calpain-mediated cleavage of fodrin produces a predominant 150-kd fragment. 31 To rule out the possibility that fodrin CCP may recognize calpain cleavage products of fodrin, primary cultured neurons were treated with the calcium ionophore, A23187 to induce necrosis and activate calpain. After treatment, Western blot analysis was performed and neuronal extracts were probed with fodrin CCP. As shown in Figure 1E ▶ (i), fodrin CCP did not recognize a 150-kd fragment, or any other fragments, after treatment of neurons with A23187 (lane marked A23187). To ensure that the 150-kd calpain cleavage product was generated under these experimental conditions, we probed the same neuronal extracts with a site-directed antibody that specifically recognizes the 150-kd calpain cleavage product of fodrin but not full-length fodrin. 28 Accordingly, Figure 1E ▶ (ii) shows a significant increase in the 150-kd band recognized by this antibody after treatment of neurons with A23187. Thus, taken together, these results demonstrate that fodrin CCP recognizes cleavage products generated after the activation of apoptotic, but not calpain-mediated or necrotic, pathways.
Experiments were also undertaken to characterize the fodrin CCP antibody by immunocytochemistry using rat cortical neurons as our model apoptotic system. In this case, we used the lectin toxin, Con A, which has previously been shown to be an effective apoptotic insult in this model system. 25 As previously demonstrated, treatment of neurons with Con A caused a majority of the neurons to show morphological characteristics of apoptosis including neurite degeneration, nuclear condensation, and internucleosomal DNA cleavage 25 (data not shown). To examine fodrin CCP antibody staining in situ, cortical neurons were treated with Con A, fixed in 4% paraformaldehyde, and immunolabeled with fodrin CCP. Although very little immunoreactivity was observed in control cells, extensive punctate process and perikaryal labeling was observed in Con A-treated neurons (Figure 2, A and B) ▶ . The pronounced neurite staining seen after treatment with Con A is consistent with reports demonstrating that fodrin is localized in both the neuronal processes as well as in neuronal cell bodies. 32 This neurite staining was examined further by low-field magnification. Extensive neurite staining was observed after treatment of cortical neurons with Con A (Figure 2C) ▶ . No staining was observed in untreated neurons or neurons treated with Z-VAD only (data not shown). Importantly, treatment with the caspase inhibitor Z-VAD under identical experimental conditions primarily abolished fodrin CCP neurite labeling (Figure 2D) ▶ , indicating the involvement of caspases in this neurodegenerative process. Although these data support a role for caspase activation in fodrin cleavage, it is possible that one or more downstream proteases may also result in fodrin cleavage, because Z-VAD has also been shown to block a variety of cysteine proteases including members of the cathepsin family at concentrations used to inhibit caspase activity in cell cultures. 33 Taken together, these results support the conclusion that fodrin CCP recognizes CCPs of fodrin after cleavage by caspases, and therefore, may be a useful marker for following the activation of apoptotic pathways in certain neurodegenerative diseases.
Fodrin CCP as a Marker for Apoptosis in AD
We next examined whether this novel antibody probe could provide insights into a possible relationship between the activation of neuronal apoptotic pathways and NFTs in the AD brain. Tissue sections from the hippocampus of a severe AD case immunostained with fodrin CCP showed extensive labeling of neurons throughout the hippocampus (Figure 3, B and D) ▶ . No staining was observed when preimmune serum was used in place of the primary antibody, illustrating the specificity of the fodrin CCP antibody (Figure 3C) ▶ . In addition, staining with fodrin CCP was prevented after preabsorption with free peptide (data not shown). Moreover, no staining was observed in aged-matched nondemented controls without neuropathology after incubation of hippocampal sections with anti-fodrin CCP (Figure 3A) ▶ . These results were confirmed after Western blot analysis using brain extracts from representative control and AD cases. As shown in Figure 1D ▶ , fodrin CCP recognized a predominant band at 55 kd in two representative AD cases that was absent in controls. Also note that full-length fodrin (240 kd) was not recognized by the anti-fodrin CCP providing additional evidence of the specificity for caspase-cleaved fragments. In some instances, we also were able to detect a faint band at 120 kd in extracts from severe AD (data not shown). Evidently, the 55-kd band is perhaps the most stable cleavage product of fodrin in vivo. Therefore, results from both immunohistochemistry and Western blot analysis support the conclusion that activation of apoptotic pathways occurs in a subpopulation of neurons in the AD brain but not in normal aged-matched controls.
Figure 3.
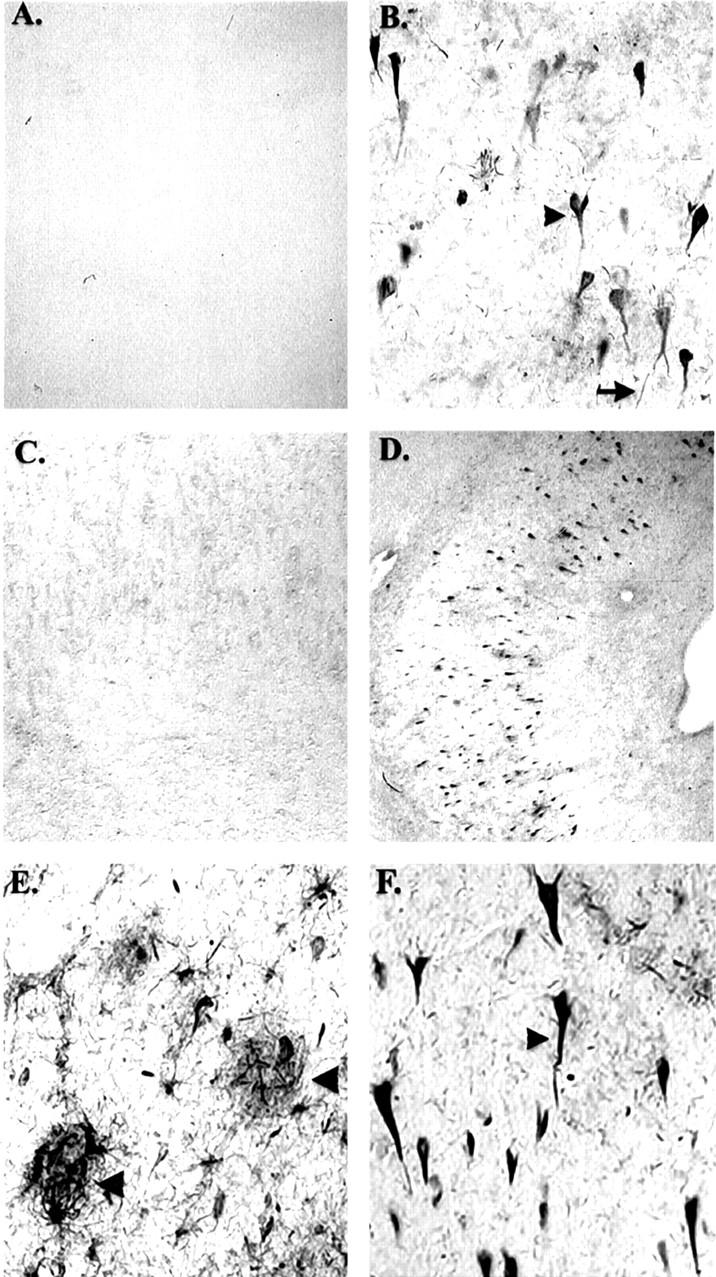
Fodrin CCP immunoreactivity in Alzheimer’s disease. A: Representative immunohistochemical staining with fodrin CCP in a control case showing complete absence of immunoreactivity. B: Serial hippocampal tissue sections from a severe AD case showing extensive neuronal labeling using fodrin CCP, which is absent after staining with preimmune serum (C). D-F: Low (4×) and high (40×) magnification of fodrin CCP labeling in the hippocampus in severe AD cases. Arrowheads denote neuronal labeling within plaque-rich regions (E) or a neuron with apparent NFT morphology (F).
Figure 3, D–F ▶ , shows the low- and high-magnification characteristics, respectively, of fodrin CCP staining in the hippocampus of a representative AD case. Immunolabeling of neurons was not restricted to the hippocampus: significant neuronal labeling was also observed in the subiculum, entorhinal cortex, and frontal cortex of AD. To assess whether the number of fodrin CCP-positive neurons was greater in the AD cases (mean = 134.2, SEM = ±25.1, n = 5) than in nondemented controls (both mild and no pathology, mean = 2.0, SEM = ±1.29, n = 7), an independent t-test was used to indicate that there were significantly more fodrin CCP-positive neurons in AD cases (t(10) = 6.34, P < 0.0004). No significant correlation with postmortem interval was found. In addition to neuronal cell soma being labeled, numerous neuropil threads and processes of various lengths were fodrin CCP-positive in the hippocampus (data not shown), including areas with extensive plaque formation (Figure 3E ▶ , arrowheads). It is noteworthy that the antibody against fodrin CCPs was sensitive to tissue fixation; more extensive neuronal immunolabeling was observed in 4% paraformaldehyde-fixed than 10% formalin-fixed tissues.
Relationship between Caspase Activation and NFT Formation in AD
Figure 3F ▶ illustrates a high magnification image of typical fodrin CCP staining in the hippocampus from a severe AD case. Of interest was that the appearance of neurons labeled with fodrin CCP was similar to that of NFTs (Figure 3F ▶ , arrowhead). To examine a possible relationship between NFT-bearing neurons and caspase activation, double-labeling experiments were undertaken using anti-fodrin CCP and AT8/PHF-1 as markers for NFT formation. As shown in Figure 4, A and B ▶ , a subset of neurons was fodrin CCP-positive and could be clearly differentiated from NFT-containing neurons. In addition, co-localization of PHF-1 and fodrin CCP was evident within individual neurons (Figure 4B ▶ , arrow). Figure 4, C and D ▶ , provides further evidence that not all fodrin CCP-positive neurons contain NFT pathology (arrowheads). Taken together, these results suggest that caspase activation may occur in NFT-bearing neurons.
Figure 4.
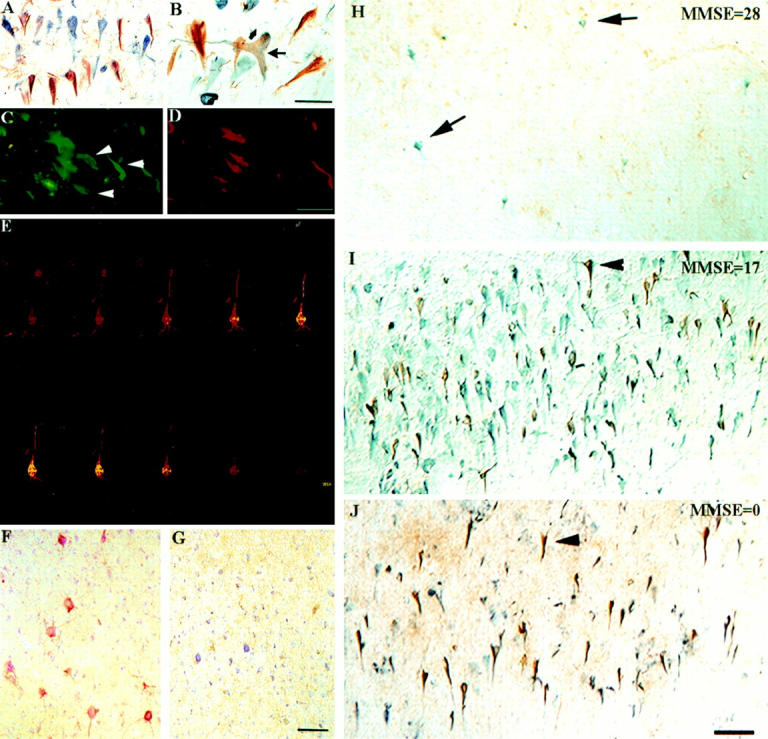
Fodrin CCP labeling of NFT-bearing hippocampal neurons in AD. A: Double-labeling immunohistochemical analysis for fodrin CCP and PHF-1 illustrates a subset of neurons are fodrin CCP-positive (brown) and can be clearly differentiated from NFT-containing neurons (blue). B: Co-localization of fodrin CCP and PHF-1 within individual neurons (arrow). C and D: Double-immunofluorescence overlap image of a hippocampal tissue section from a severe AD case showing double-labeling of NFT (AT8/PHF-1, green) and fodrin CCP (red). Although three neurons show co-localization, three other neurons are labeled with only AT8/PHF-1 (C, white arrowheads). E: Confocal imaging of a hippocampal, pyramidal neuron double-labeled with PHF-1 (yellow) and fodrin CCP (red). The z-scan shows labeling of fodrin CCP is primarily localized to the plasma membrane, whereas that of PHF-1 is more cytoplasmic. F and G: Serial prefrontal tissue sections counterstained with cresyl violet from an aged canine with no NFT formation (G) but with deep layer pyramidal neurons being positive for fodrin CCP (F). H–J: Series of double-label immunohistochemistry studies for fodrin CCP (brown) and NFTs (blue) taken from area CA1 of the hippocampus in three cases ranging in dementia severity (Mini Mental State Examination). Arrows indicate NFT-bearing neurons and arrowheads fodrin CCP-positive neurons. Note that NFT formation seems to precede the appearance of caspase-cleavage products of fodrin. The number of fodrin CCP-positive neurons increases with disease severity. In the severe case (J) note the extensive ghost NFT formation. Scale bars: 50 μm (A–D); 20 μm (E); and 100 μm (F–J).
To examine in further detail the spatial co-localization of fodrin CCP and NFTs, confocal imaging was used. Figure 4E ▶ shows a single pyramidal neuron from a severe AD case with co-localization of both fodrin CCP (red) and PHF-1 (yellow). A series of scans were taken at 1-μm intervals and clearly demonstrates that fodrin CCP labeling is primarily localized to or near the plasma membrane whereas that of PHF-1 is cytoplasmic. This profile is predicted based on the localization of each structure in neurons: fodrin is a membrane-associated protein, whereas NFTs are predominantly localized intracellularly in association with microtubules.
To examine a possible association between caspase activation and NFT pathology, a statistical analysis was performed by counting neurons with positive immunostaining for PHF-1 and fodrin CCP after double-labeling experiments (Table 1) ▶ . The number of neurons with NFT (r = 0.801, n = 12, P < 0.002), the number of neurons with fodrin CCP (r = 0.935, n = 12, P < 0.0001), and the number of neurons where these two markers were co-localized (r = 0.879, n = 12, P < 0.001) were significantly associated with Braak and Braak stage. Moreover, as the extent of NFT formation (number of neurons with NFT) increased, there was a significant corresponding increase in the number of neurons positive for fodrin CCP (r = 0.840, n = 12, P < 0.001). The number of neurons exhibiting both fodrin CCP and PHF-1 immunolabeling in AD cases (mean = 81.4, SEM = ±23.4, n = 5) was significantly higher than in the control cases (mean = 1.7, SEM =±1.0, n = 7) [t(10) = 4.097, P < 0.002]. In addition, a multiple linear regression analysis using a stepwise procedure that included extent of NFT formation, postmortem interval, and disease severity (Mini Mental State Examination) indicates that the best predictor of the extent of fodrin CCP neuronal labeling was the extent of NFT formation [F(1,7) = 9.941, P < 0.022]. Therefore, there does seem to be a significant association between NFT formation and caspase activation in AD.
In the next set of experiments, we assessed the temporal relationship between NFT formation and caspase activation in hippocampal sections from seven cases of AD with increasing dementia severity as determined by Mini Mental State Examination scores (ranging from 0 to 30). NFT formation, identified by using anti-PHF-1 immunostaining (blue), was evident in mild to severe cases of AD in a greater frequency than that of fodrin CCP (brown) staining (Figure 4, H and I) ▶ . In contrast, although the number of fodrin CCP-positive neurons was considerable in more severe cases of AD (Figure 4J) ▶ , staining was absent in nondemented control cases and infrequent in nondemented mild pathology cases (Figure 4H) ▶ , and extensive in moderate AD cases (Figure 4I ▶ and Table 1 ▶ ). These results suggest that NFT formation may precede the activation of neuronal apoptotic pathways in a subpopulation of neurons in AD.
To confirm and extend these findings, we also explored whether the generation of fodrin CCP was dependent on NFT formation by studying the canine model of brain aging. In the canine model, animals show extensive Aβ deposition as a function of age, but do not exhibit any NFT pathology. 34,35 Figure 4, F and G ▶ , illustrates the results of experiments in an aged canine. Numerous fodrin CCP-positive neurons were present in the prefrontal cortex of an aged canine that was not present in young dogs. Deep-layer pyramidal neurons were fodrin CCP-positive in the prefrontal cortex sections (Figure 4F) ▶ , which was completely devoid of NFTs (Figure 4G) ▶ . These results suggest that PHF formation may be one, but not the only factor, involved in the activation of an apoptotic program in AD brain. In addition, postmortem intervals were no longer than 30 minutes and allowed us to further rule out the possibility of confounding postmortem artifacts.
Discussion
Recently, the α-subunit of fodrin has been found to be a substrate for the caspase family of proteases. 20,22 Caspase cleavage of critical cellular proteins is believed to be responsible for the morphological and functional changes that occur when cells undergo apoptosis. 36 Fodrin is one of the first substrates to be cleaved by caspases during the initiation phase of apoptosis. 37,38 In addition, accumulation of abnormal deposits of fodrin in neurons in AD 31,39 and an increase in fodrin proteolysis in fibroblasts from aged and AD donors has been reported, 40 indicating that there is a widespread alteration in the proteolytic processing of this protein that occurs with aging. Because fodrin caspase cleavage products (CCPs) accumulate as stable intracellular deposits, they represent death products with novel antigenic epitopes that provide a signature of the contribution of caspases to neurodegeneration. In this study, we designed a site-directed antibody based on the unique antigenic epitopes that are generated by caspase cleavage of fodrin. This strategy has been successfully used for the cytoskeleton protein, actin, to demonstrate apoptosis-like mechanisms in plaque-associated neurons and microglia in AD. 7
Our antibody, which we term fodrin CCP, specifically recognized the major 120- and 55-kd caspase cleavage products of fodrin characterized in the literature, but did not recognize full-length fodrin. Detailed characterization of this antibody in vitro suggested that this antibody may provide a diagnostic tool to better assess the contribution of apoptotic mechanisms (caspase activation and accumulation of cleavage products) in neurodegenerative diseases. Fodrin CCP was present within numerous neurons throughout the hippocampus and entorhinal cortex in AD brain. Neuronal labeling by fodrin CCP supports the conclusion that widespread activation of neuronal apoptotic-like mechanisms occurs in AD, and that this is likely to be a significant cell death pathway active during disease progression.
Fodrin is a major constituent of the cytoskeleton of neurons 19,41 and has been implicated in transport of synaptic vesicles and organelles within the axon, 42 and coupling membrane-spanning cell surface proteins to cytoplasmic elements. 43 Therefore, the cleavage of fodrin by caspase would be expected to have dire consequences for the neuron, disrupting these important functions of fodrin as well as altering cell morphology. Altering cell morphology, in turn, may either activate a death signal or disrupt the survival signal necessary to suppress the intrinsic cell death signal. 44
Although unequivocal evidence has not been presented in the current study demonstrating that caspase-3 is the caspase responsible for the cleavage of fodrin-producing cleavage products that are recognized by the fodrin CCP antibody, we hypothesize that this is indeed the effector caspase involved. First, digestion of fodrin by caspase-3 generated cleavage products that were strongly immunoreactive with the fodrin CCP antibody. Second, a recent study has demonstrated that caspase-3 is required for the cleavage of fodrin but dispensable for the cleavage of other substrates. 22 Finally, in a study by Wang et al 20 the cleavage of fodrin by caspase-3 was examined in detail. By using a series of recombinant GST-fodrin fusion peptides, they were able to map the consensus caspase cleavage site responsible for producing an ∼50- and 120-kd products they observed after digestion with caspase-3. They determined this sequence to be SVEALI, the sequence we chose to immunize rabbits to generate the fodrin CCP antibody. Thus, the results of the current study suggest that caspase-3 is the effector caspase responsible for the generation of cleavage products immunolabeled with fodrin CCP antibody in AD. Consistent with this is other evidence for caspase activation in AD detected by an antibody against amyloid precursor protein fragments cleaved predominantly by caspase-3. 5 Therefore, pharmacological blockade of caspase-3 may be an effective therapeutic strategy for the treatment of AD.
Interestingly, the majority of fodrin CCP-positive neurons were closely associated with NFT formation. Furthermore, as the extent of NFT formation increased, there was a corresponding increase in fodrin CCP immunolabeling (r = 0.840). Thus, there does seem to be a significant correlation between NFT formation and the activation of neuronal apoptotic mechanisms associated with AD. In addition, a multiple regression analysis indicates that NFT formation is the best predictor of the presence of fodrin CCP. The results of the current study contrasts with other studies by Stadelmann et al 15 and Selznick et al. 14 These authors did not find an association between activated caspase-3 with NFT but rather with granulovacuolar degeneration. 14,15 One possible difference in these studies and the current study is the use of thicker, free-floating sections as compared with paraffin-embedded sections. Further, we hypothesize that fodrin-CCP serves as a marker that is able to accumulate over a period of time after cleavage by caspase-3.
It should be emphasized that even though we have demonstrated that NFT formation and fodrin CCP are frequently present within the same neurons and are correlated, this does not imply that they are causally interrelated events. Indeed, we were also able to detect neurons that labeled only with fodrin CCP or with NFT. These may, therefore, represent two independent processes. However, our results do not rule out the possibility of a causal relationship.
To determine a possible temporal relationship between NFTs and accumulation of caspase cleavage products, we analyzed AD cases with a range of dementia severity based on Mini Mental State Examination scores (Table 1) ▶ . Double-labeling experiments using fodrin CCP and PHF-1 revealed an overall pattern for NFT pathology preceding the accumulation of caspase-mediated break down products of fodrin. These results suggest an accumulation of caspase breakdown products throughout time. However, whether activation of caspases itself precedes or follows NFT formation has yet to be determined. This is a difficult question to address using the current sample of cases because the assumption cannot be made that mild pathology controls will transition to AD. Other model systems, in which NFT pathology accumulates in an age-dependent manner, such as the development of AD pathology in individuals with Down syndrome will help to clarify this issue.
To further test the role of NFT formation in promoting pathways associated with apoptosis and also to rule out confounding postmortem artifacts, we used tissues obtained from aged canines. Aged canines develop extensive Aβ with increasing age that is associated with cognitive decline. 45,46 On the other hand, aged canines do not develop NFTs. 34,35 Thus, if NFT formation was exclusively responsible for apoptotic cell death we predicted that fodrin CCP would not be present in the brains of aged canines. However, we found that subpopulations of neurons in the prefrontal cortex of aged canines were immunopositive for fodrin CCP suggesting that NFT may serve as one stimulus, but is not the only stimulus, promoting the activation of apoptotic pathways.
An interesting aspect to the current study is the extensive accumulation of cleaved fodrin in a large number of neurons. This suggests that caspase activation in AD neurons may not immediately lead to cell death. In contrast, our evidence seems to indicate a slow, apoptotic-like degenerative process that is profoundly different from the rather rapid, classical apoptotic pathway. One interpretation of the data are that neurons implement a number of survival mechanisms, such as the up-regulation of bcl-2, to prevent engaging the full apoptotic program. 47,48 However, consideration should be given to the fact that postmortem analyses reflect a single time point and thus, provide limited information regarding the rate of accumulation of specific proteins.
In conclusion, we have developed a caspase cleavage site-directed antibody to fodrin and have demonstrated widespread neuronal activation of apoptotic-like pathways in AD. This antibody should be useful for investigating in more detail the temporal and spatial relationship between caspase activation and other events associated with AD including Aβ deposition. The accumulation of cleavage products mediated by caspases and the commitment to an apoptotic program may be a point of convergence for a number of stimuli, such as NFT or Aβ, leading to neurodegeneration. A recent report in transgenic mice expressing mutant human SOD1, which serve as a model of amyotrophic lateral sclerosis, shows that inhibiting caspase activation by ZVAD-fmk delays disease onset and mortality. 49 This study suggests that preventing caspase activation, a downstream event in an apoptotic program, regardless of the stimuli promoting apoptosis, may be one aspect of neurodegeneration amenable to therapeutic intervention. These are promising leads into the evaluation of new therapeutics for the treatment of AD.
Acknowledgments
We thank Serena Wong for technical assistance.
Footnotes
Address reprint requests to David H. Cribbs, Ph.D., Institute for Brain Aging and Dementia, University of California at Irvine, Irvine, California 92697-4540. E-mail: dhcribbs@uci.edu.
Supported in part by National Institute on Aging grants 5T32AG00096, 5R01AG13007, and AG12694.
T. T. R. and E. H. contributed equally to this work.
Present address of T. T. R.: Department of Biology, Science/Nursing Building, Room 228, Boise State University, Boise, Idaho 83725.
References
- 1.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brounlee LM, Vogel FS, Hughes JP, VanBelle G, Berg L: The consortium to establish a registry for Alzheimer’s disease (CERAD) part II. Standardization of the neuropathological assessment of Alzheimer’s disease. Neurology 1991, 41:479-486 [DOI] [PubMed] [Google Scholar]
- 2.Trojanowski JQ, Schmidt ML, Shin RW, Bramblett GT, Goedert M, Lee MY: From pathological marker to potential mediator of neuronal dysfunction and degeneration in Alzheimer’s disease. Clin Neurosci 1993, 1:184-191 [Google Scholar]
- 3.Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM: Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 1993, 10:1089-1099 [DOI] [PubMed] [Google Scholar]
- 4.Alonso AC, Grundke-Iqbal I, Iqbal K: Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med 1996, 2:783-787 [DOI] [PubMed] [Google Scholar]
- 5.Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW: Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell 1999, 97:395-406 [DOI] [PubMed] [Google Scholar]
- 6.Su JH, Anderson AJ, Cummings BJ, Cotman CW: Immunohistochemical evidence for DNA fragmentation in neurons in the AD brain. Neuroreport 1994, 5:2529-2533 [DOI] [PubMed] [Google Scholar]
- 7.Yang F, Sun X, Beech W, Teter B, Wu S, Sigel J, Vinters HV, Frautschy SA, Cole GM: Antibody to caspase-cleaved actin detects apoptosis in differentiated neuroblastoma and plaque-associated neurons and microglia in Alzheimer’s disease [see comments]. Am J Pathol 1998, 152:379-389 [PMC free article] [PubMed] [Google Scholar]
- 8.LaFerla FM, Tinkle BT, Bieberich CJ, Haudenschild CC, Jay G: The Alzheimer’s A beta peptide induces neurodegeneration and apoptotic cell death in transgenic mice. Nat Genet 1995, 9:21-30 [DOI] [PubMed] [Google Scholar]
- 9.Takahashi A: Caspase: executioner and undertaker of apoptosis. Int J Hematol 1999, 70:226-232 [PubMed] [Google Scholar]
- 10.Metzstein MM, Stanfield GM, Horvitz HR: Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet 1998, 14:410-416 [DOI] [PubMed] [Google Scholar]
- 11.Su JH, Deng G, Cotman CW: Neuronal DNA damage precedes tangle formation and is associated with up-regulation of nitrotyrosine in Alzheimer’s disease brain. Brain Res 1997, 774:193-199 [DOI] [PubMed] [Google Scholar]
- 12.Bancher C, Lassmann H, Breitschopf H, Jellinger KA: Mechanisms of cell death in Alzheimer’s disease. J Neural Transm Suppl 1997, 50:141-152 [DOI] [PubMed] [Google Scholar]
- 13.Lassmann H, Bancher C, Breitschopf H, Wegiel J, Bobinski M, Jellinger K, Wisniewski HM: Cell death in Alzheimer’s disease evaluated by DNA fragmentation in situ. Acta Neuropathol (Berl) 1995, 89:35-41 [DOI] [PubMed] [Google Scholar]
- 14.Selznick LA, Holtzman DM, Han BH, Gokden M, Srinivasan AN, Johnson EM, Jr, Roth KA: In situ immunodetection of neuronal caspase-3 activation in Alzheimer disease. J Neuropathol Exp Neurol 1999, 58:1020-1026 [DOI] [PubMed] [Google Scholar]
- 15.Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Bruck W, Jellinger K, Lassmann H: Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer’s disease. Am J Pathol 1999, 155:1459-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugaya K, Reeves M, McKinney M: Topographic associations between DNA fragmentation and Alzheimer’s disease neuropathology in the hippocampus. Neurochem Int 1997, 31:275-281 [DOI] [PubMed] [Google Scholar]
- 17.MacGibbon GA, Lawlor PA, Sirimanne ES, Walton MR, Connor B, Young D, Williams C, Gluckman P, Faull RL, Hughes P, Dragunow M: Bax expression in mammalian neurons undergoing apoptosis, and in Alzheimer’s disease hippocampus. Brain Res 1997, 750:223-234 [DOI] [PubMed] [Google Scholar]
- 18.Su JH, Deng G, Cotman CW: Bax protein expression is increased in Alzheimer’s brain: correlations with DNA damage, Bcl-2 expression, and brain pathology. J Neuropathol Exp Neurol 1997, 56:86-93 [DOI] [PubMed] [Google Scholar]
- 19.Goodman SR, Zagon IS, Riederer BM: Spectrin isoforms in mammalian brain. Brain Res Bull 1987, 18:787-792 [DOI] [PubMed] [Google Scholar]
- 20.Wang KK, Posmantur R, Nath R, McGinnis K, Whitton M, Talanian RV, Glantz SB, Morrow JS: Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. J Biol Chem 1998, 273:22490-22497 [DOI] [PubMed] [Google Scholar]
- 21.Nath R, Raser KJ, Stafford D, Hajimohammadreza I, Posner A, Allen H, Talanian RV, Yuen P, Gilbertsen RB, Wang KK: Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J 1996, 319:683-690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janicke RU, Ng P, Sprengart ML, Porter AG: Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J Biol Chem 1998, 273:15540-15545 [DOI] [PubMed] [Google Scholar]
- 23.Rohn TT, Ivens KJ, Bahr BA, Cotman CW, Cribbs DH: A monoclonal antibody to amyloid precursor protein induces neuronal apoptosis. J Neurochem 2000, 74:2331-2342 [DOI] [PubMed] [Google Scholar]
- 24.Pike CJ, Cotman CW: Cultured GABA-immunoreactive neurons are resistant to toxicity induced by β-amyloid. Neuroscience 1993, 56:269-274 [DOI] [PubMed] [Google Scholar]
- 25.Cribbs DH, Kreng VM, Anderson AJ, Cotman CW: Crosslinking of membrane glycoproteins by Concanavalin A induces apoptosis in cortical neurons. Neuroscience 1996, 75:173-185 [DOI] [PubMed] [Google Scholar]
- 26.Braak H, Braak E: Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991, 82:239-259 [DOI] [PubMed] [Google Scholar]
- 27.Johnson JK, Head E, Kim R, Starr A, Cotman CW: Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol 1999, 56:1233-1239 [DOI] [PubMed] [Google Scholar]
- 28.Bahr BA, Tiriveedhi S, Park GY, Lynch G: Induction of calpain-mediated spectrin fragments by pathogenic treatments in long-term hippocampal slices. J Pharmacol Exp Ther 1995, 273:902-908 [PubMed] [Google Scholar]
- 29.Bennett V, Baines AJ, Davis J: Purification of brain analogs of red blood cell membrane skeletal proteins: ankyrin, protein 4.1 (synapsin), spectrin, and spectrin subunits. Methods Enzymol 1986, 134:55-69 [DOI] [PubMed] [Google Scholar]
- 30.del Cerro S, Arai A, Kessler M, Bahr BA, Vanderklish P, Rivera S, Lynch G: Stimulation of NMDA receptors activates calpain in cultured hippocampal slices. Neurosci Lett 1994, 167:149-152 [DOI] [PubMed] [Google Scholar]
- 31.Masliah E, Iimoto DS, Saitoh T, Hansen LA, Terry RD: Increased immunoreactivity of brain spectrin in Alzheimer disease: a marker for synapse loss? Brain Res 1990, 531:36-44 [DOI] [PubMed] [Google Scholar]
- 32.Riederer BM, Zagon IS, Goodman SR: Brain spectrin(240/235) and brain spectrin(240/235E): two distinct spectrin subtypes with different locations within mammalian neural cells. J Cell Biol 1986, 102:2088-2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schotte P, Declercq W, Van Huffel S, Vandenabeele P, Beyaert R: Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Lett 1999, 442:117-121 [DOI] [PubMed] [Google Scholar]
- 34.Cummings BJ, Head E, Ruehl W, Milgram NW, Cotman CW: The canine as an animal model of human aging and dementia. Neurobiol Aging 1996, 17:259-268 [DOI] [PubMed] [Google Scholar]
- 35.Selkoe DJ, Bell DS, Podlisny MB, Price DL, Cork LC: Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer’s disease. Science 1987, 235:873-877 [DOI] [PubMed] [Google Scholar]
- 36.Martin SJ, Green DR: Protease activation during apoptosis: death by a thousand cuts? Cell 1995, 82:349-352 [DOI] [PubMed] [Google Scholar]
- 37.Martin SJ, O’Brien GA, Nishioka WK, McGahon AJ, Mahboubi A, Saido TC, Green DR: Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J Biol Chem 1995, 270:6425-6428 [DOI] [PubMed] [Google Scholar]
- 38.Cryns VL, Bergeron L, Zhu H, Li H, Yuan J: Specific cleavage of alpha-fodrin during Fas- and tumor necrosis factor-induced apoptosis is mediated by an interleukin-1beta-converting enzyme/Ced-3 protease distinct from the poly(ADP-ribose) polymerase protease. J Biol Chem 1996, 271:31277-31282 [DOI] [PubMed] [Google Scholar]
- 39.Masliah E, Hansen L, Mallory M, Albright T, Terry RD: Abnormal brain spectrin immunoreactivity in sprouting neurons in Alzheimer disease. Neurosci Lett 1991, 129:1-5 [DOI] [PubMed] [Google Scholar]
- 40.Peterson C, Vanderklish P, Seubert P, Cotman C, Lynch G: Increased spectrin proteolysis in fibroblasts from aged and Alzheimer donors. Neurosci Lett 1991, 121:239-243 [DOI] [PubMed] [Google Scholar]
- 41.Goodman SR, Zagon IS: The neural cell spectrin skeleton: a review. Am J Physiol 1986, 250:C347-C360 [DOI] [PubMed] [Google Scholar]
- 42.Weisenberg RC, Flynn J, Gao BC, Awodi S, Skee F, Goodman SR, Riederer BM: Microtubule gelation-contraction: essential components and relation to slow axonal transport. Science 1987, 238:1119-1122 [DOI] [PubMed] [Google Scholar]
- 43.Bennett V, Gilligan DM: The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol 1993, 9:27-66 [DOI] [PubMed] [Google Scholar]
- 44.Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD: Programmed cell death and the control of cell survival. Philos Trans R Soc Lond B Biol Sci 1994, 345:265-268 [DOI] [PubMed] [Google Scholar]
- 45.Head E, McCleary R, Hahn FF, Milgram NW, Cotman CW: Region-specific age at onset of beta-amyloid in dogs. Neurobiol Aging 2000, 21:89-96 [DOI] [PubMed] [Google Scholar]
- 46.Cummings BJ, Head E, Afagh AJ, Milgram NW, Cotman CW: Beta-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol Learn Mem 1996, 66:11-23 [DOI] [PubMed] [Google Scholar]
- 47.Satou T, Cummings BJ, Cotman CW: Immunoreactivity for BCL-2 protein within neurons in the Alzheimer’s disease brain increases with disease severity. Brain Res 1995, 697:35-43 [DOI] [PubMed] [Google Scholar]
- 48.Su JH, Satou T, Anderson AJ, Cotman CW: Up-regulation of BCL-2 is associated with neuronal DNA damage in Alzheimer’s disease. Neuroreport 1996, 7:437-440 [DOI] [PubMed] [Google Scholar]
- 49.Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee J-P, Przedborski S, Friedlander RM: Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 2000, 288:335-339 [DOI] [PubMed] [Google Scholar]


