Abstract
Adenocarcinoma of the esophagus (ADCE) with Barrett’s mucosa and adenocarcinoma of the cardia (ADCC) are often reported as a single pathological entity. In this study we have used strict anatomical-pathological criteria to distinguish between these two lesions and we have investigated their differences in TP53 mutations, MDM2 gene amplification, and cytokeratin expression. DNA was extracted from the tumor areas of formalin-fixed, paraffin-embedded sections in 26 ADCC and 28 ADCE patients. TP53 mutations were detected by temporal temperature gradient electrophoresis and identified by sequencing. MDM2 amplification was assessed by differential polymerase chain reaction. The expression of cytokeratins 4, 7, and 13 was examined by immunohistochemistry. In ADCC, the male to female ratio was 1.8:1, compared to 27:1 in ADCE. Five ADCC patients had a history of other neoplasms, compared to only one ADCE patient. The two types of tumor differed in the prevalence of TP53 mutations (31% in ADCC and 50% in ADCE) and of MDM2 gene amplification (19% in ADCC and 4% in ADCE), and in the pattern of expression of cytokeratin 7 (positive in 100% of ADCE and in 41% of ADCC) and cytokeratin 13 (positive in 81% of ADCE and in 36.5% of ADCC). ADCE and ADCC differ in their clinical characteristics, in the prevalence of TP53 mutations and MDM2 amplifications, and in the patterns of cytokeratin expression. These results support the notion that ADCC and ADCE are distinct pathological entities.
Throughout the past 20 years, the incidence of tumors of the esophagogastric junction has increased at a rate of 5 to 10% per year in the United States and several western European countries. 1 The reasons for this increase are primarily unknown. Tumors of the esophagogastric junction include two major types of adenocarcinoma: adenocarcinomas of the esophagus (ADCE) and adenocarcinomas of the cardia (ADCC).
ADCE occur in the distal part of the esophagus and develop from Barrett’s mucosa, a glandular metaplasia of the squamous epithelium that can vary in height from a few millimeters to a few centimeters. There is evidence that the metaplastic glandular cells are hybrid cells, expressing cytokeratins (CKs) of both squamous (CK4 and 13) and glandular (CK8 and 19) origin 2 and having ultrastructural features of both squamous and glandular cells. 3 Furthermore, they have been shown to constantly express CK7, in contrast to intestinal metaplastic cells of the cardia mucosa, which never do. 4 Barrett’s mucosa is often associated with chronic gastroesophageal acid reflux. However, it can also occur in combination with chronic biliary alkaline reflux as well as in the absence of reflux. 5 Factors predisposing to Barrett’s mucosa are not well documented. Recent evidence suggests that expression of certain polymorphic forms of glutathione-S-transferase P1 may be a genetic susceptibility factor for developing Barrett’s mucosa. 6 Barrett’s mucosa is a very common lesion that is thought to occur in >10% of the general population in the United States and is associated with a 10-fold increase in the risk of developing ADCE. 7
The cardia is the anatomical region corresponding to the transition between esophagus and stomach. It cannot be identified at the macroscopic level. At the microscopic level, the cardia is characterized by a thin mucosa with clear glandular cells, without any acid-secreting cells. It ranges in height from 1 to 5 mm, with an increase in size with age. The term “ADCC” applies to tumors located exclusively in the region of the cardia. However, it has not been established whether these tumors originate from the true cardial mucosa rather than from the neighboring upper fundic mucosa. Intestinal metaplasia can be observed within the cardia, in particular in connection with chronic inflammation. However, there is no clear evidence that this metaplasia predisposes to development of ADCC.
In many studies, it has been common to group ADCC and ADCE as “adenocarcinomas of the esophagogastric junction,” and such a grouping may have precluded the identification of specific risk factors other than reflux. The role of tobacco consumption is controversial. 8-11 Obesity and anti-hypertensive drugs relaxing the cardial sphincter are considered as indirect risk factors as they may favor chronic gastroesophageal reflux. A high intake of calories, fat, and iron has also been implicated. 12,13 In contrast, high ingestion of fiber, niacin, vitamin B6, iron, and zinc 8,10,12 has been suggested to protect against tumor development.
At the molecular level, mutation of the tumor-suppressor gene TP53 is the most frequent alteration identified in carcinomas of the esophagogastric junction. In ADCE, TP53 mutations have been detected in 58% of the cases (M. Olivier, personal communication, IARC TP53 mutation database, R4 version). The most frequent type of mutation is C to T transition at a dipyrimidine repeat (CpG) (50%). Mutations are thought to occur early during tumorigenesis, because they are sometimes detectable in Barrett’s mucosa without dysplasia. 8,14 Only three retrospective studies have analyzed TP53 mutations in ADCC. 15 Together, they show a mutation prevalence of 50%, with a high proportion of transitions at CpG (40%). 16,17 In this study, we have used strict criteria to collect at the time of surgery well-characterized cases of ADCC or ADCE. Tumors were classified as ADCE when they predominantly involved the lower part of the esophagus and/or a Barrett’s mucosa could be identified at the macroscopic or at the microscopic level on the surgical samples. 18 Tumors were classified as ADCC when they involved the esophagogastric junction, predominantly invading the gastric part, or when they developed at <2 cm from the esophagogastric junction. Our group of ADCE correspond to type I of the classification proposed by Siewert and Stein, 19 whereas the tumors we defined as ADCC regroup type II (true cardia tumors) and to type III (tumors from the subcardia region) of this same classification. All tumors were analyzed for the presence of mutations in TP53 and for amplification of MDM2, a gene that encodes a protein involved in the control of p53 function and that has been found to be amplified in some tumors in which TP53 is not mutated. The profile of expression of three CKs (4, 7, and 13), which show specific cell localization, has also been compared. We report that the two groups of patients showed differences in several individual and clinical parameters (sex ratio, associated neoplasms, tobacco smoking), in the prevalence of TP53 mutations and MDM2 amplification, and in the patterns of cytokeratin expression.
Materials and Methods
Patients and Tumors
Tumor tissues were collected at the time of surgery from patients recruited at the E. Herriot Hospital (Lyon, France). The criteria for inclusion in the study were: 1) the presence of a primary adenocarcinoma of the esophagogastric junction; 2) no primary treatment; and 3) signature of an informed consent form. Most tissue samples were obtained at surgery. However, for ADCE, biopsies were also included in the study after endoscopic detection of tumors of the lower part of the esophagus within a Barrett’s mucosa. ADCC was defined as a tumor centered close to the esophagogastric junction (<2 cm), predominantly invading the stomach without any evidence of a long or a short segment of Barrett’s. Clinical records were reviewed to collect information on the patients’ past medical histories and their tobacco smoking habits. The tissue and data collection protocol was approved by the local and institutional ethical committees.
DNA Extraction
DNA was isolated from microdissected tissue. Tissues were fixed in 10% buffered formalin and embedded in paraffin. Serial 4-μm-thick sections were cut from paraffin blocks. For each sample, morphological assessment was performed on one 4-μm tissue section stained with hematoxylin and eosin. The sections after this one were used for DNA extraction. Briefly, the slides were rehydrated. The areas of interest were selected by analysis of the stained slide and then scraped from the slide using a scalpel blade. The scraped material was transferred into an Eppendorf tube containing 50 μl of extraction buffer (10 mmol/L of Tris-HCl, pH 9, 20 mg/ml of proteinase K, 0.1% Nonidet P-40). Samples were incubated for 3 days at 56°C, with addition of 2 μl of proteinase K (20 mg/ml) twice a day. Proteinase K was then inhibited by incubation at 95°C for 10 minutes.
Immunohistochemistry
Tissue sections were deparaffinized using standard protocols. After inactivation of endogenous peroxidases (30 minutes in 0.3% H2O2/methanol), slides were rehydrated, incubated with a blocking solution (phosphate-buffered saline containing 5% nonfat milk powder) and exposed overnight at 4°C to the primary antibody. For CK and mdm2 protein labeling, this sequence of treatments was preceded by an antigen-unmasking procedure (3 × 5 minutes in a microwave oven for CK4, 7, and 13, and 10 minutes in a pressure cooker for mdm2; Vector Laboratories Inc., Biosys S.A., Compiègne, France). The following primary antibodies were used: CM1 (purified rabbit IgG anti-human p53, 1/500; Novocastra Laboratories Ltd., Newcastle, UK), mdm2(Ab-1) (monoclonal antibody against human mdm2, clone IF2, 1/100; Calbiochem, San Diego, CA), CK4 (monoclonal antibody, clone 6B10, 1/100; Novocastra Laboratories Ltd.), CK7 (monoclonal antibody, clone AE1/AE3, 1/100; DAKO, Copenhagen, Denmark), and CK13 (monoclonal antibody, clone KS-1A3, 1/50; Novocastra Laboratories Ltd.). Incubation with the relevant secondary antibodies (either anti-mouse or an anti-rabbit biotinylated IgG, 1/200, Vectastain Elite-ABC kit; Vector Laboratories Inc.) for 30 minutes at room temperature was followed by streptavidin-peroxidase (1/50, 30 minutes at 37°C). Peroxidase activity was detected with a diaminobenzidine-based detection kit (Vector Laboratories, Inc.) and sections were counterstained with Mayer’s hematoxylin before dehydration and mounting.
TP53 Mutation Analysis
TP53 exons 4 to 9 were analyzed by temporal temperature gradient electrophoresis using the DGene system (BioRad, Richmond, CA) and the primers described by Hamelin and colleagues 20 (exons 5, 7, and 8) and Guldberg and colleagues 21 (exons 4, 6, and 9). DNA was amplified in a DNA thermocycler (Perkin Elmer, Norwalk, CT) in a 50-μl reaction mixture containing 5 μl of genomic DNA, 20 pmol of sense and anti-sense primers, 200 μmol/L of each dNTP, 1× amplification buffer, 1 X Q solution and 0.5 μl (2.5 U) of Taq polymerase (HotStarTaq DNA polymerase; Qiagen, Hilden, Germany). Polymerase chain reaction (PCR) conditions were: 15 minutes at 95°C followed by 35 cycles at 95°C (1 minute), 56°C (exons 5 proximal, 8, and 9) or 62°C (exons 4a, 4b, 5 distal, 6, and 7) (1 minute), 72°C (90 seconds). The reaction was ended by a 10-minutes extension at 72°C. Heteroduplex formation was induced by denaturation for 10 minutes at 98°C, followed by 30 minutes at the respective annealing temperature (56°C or 62°C). Temporal temperature gradient electrophoresis was run at 130V at temperatures optimized for each DNA fragment (exon 4p, 4d, 6, and 9: 58 to 70°C; exon 5p: 56 to 70°C; exon 5d: 63 to 70°C; exon 7: 59 to 70°C; exon 8: 53 to 67°C). A negative control (wild-type sample) and positive control (known mutant) were included in each analysis. Samples that showed additional and/or abnormal bands were re-amplified from genomic DNA and a second temporal temperature gradient electrophoresis was performed. If confirmed, mutant alleles were cut from this second gel, re-amplified using the same primers, and analyzed by direct sequencing after asymmetric PCR amplifications as previously described. 20,22 Two cases with positive p53 immunostaining (>50%) did not show reproducible patterns of abnormal bands in temporal temperature gradient electrophoresis (case 12, Table 1 ▶ , and case 3, Table 2 ▶ ). In these two cases, mRNA was isolated from frozen biopsies, and cDNA was prepared and tested using the yeast functional assay as described by Flaman et al. 23 Positive colonies were sequenced using the ABI PRISM 310 Genetic analyzer (Perkin Elmer Biosystems, Foster City, CA).
Table 1.
Clinical and Molecular Characteristics of Patients with Adenocarcinoma of the Esophagus
| Case | Sex | Age (yrs) | Size (cm) | Stage/Lauren’s* | p53 IHC (% cells) | TP53 mutation† | mdm2 IHC (% cells)‡ | MDM2 amplification§ |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 69 | 7.5 | T1N0/INT | <10 | 135 | >50 | − |
| 2 | M | 62 | 1 | T1N0/INT | na | 220 | Na | − |
| 3 | M | 69 | 5 | T1N0/INT | 0 | − | >50 | − |
| 4 | M | 80 | 7 | T1N0/INT | 0 | − | 20 | − |
| 5 | M | 50 | 2 | T2N0/INT | >50 | 230 | 0 | − |
| 6 | M | 82 | 5 | T2N0/INT | 0 | − | 0 | − |
| 7 | M | 70 | 4 | T2N0/INT | 0 | − | 10−20 | − |
| 8 | M | 67 | 8 | T3N0/DIF | 0 | − | 0 | − |
| 9 | M | 53 | 4 | T3N0/INT | >50 | 282 | 0 | − |
| 10 | M | 71 | 2.5 | T3N0/INT | na | − | 20−50 | − |
| 11 | M | 82 | 2 | T3N0/INT | 0 | − | 0 | − |
| 12 | M | 72 | 3.5 | T3N0/INT | >50 | 272 | 0− | |
| 283 | ||||||||
| 13 | M | 74 | 4 | T3N0/INT | <10 | − | 0 | − |
| 14 | M | 49 | 4.5 | T3N0/INT | 0 | − | >50 | − |
| 15 | M | 69 | 5 | T3N1/INT | >50 | 157 | Na | − |
| 16 | M | 68 | 3.5 | T3N1/INT | 20–50 | 159 | 0 | − |
| 17‡ | M | 76 | 3 | T3N1/INT | 0 | 175 | 0 | − |
| 18 | M | 72 | 3.5 | T3N1/INT | 0 | 234 | 0 | − |
| 19 | F | 73 | 6 | T3N1/INT | 0 | 279 | >50 | − |
| 20 | M | 66 | 9 | T3N1/INT | 0 | − | 0 | − |
| 21 | M | 76 | 2.5 | T3N1/INT | 0 | − | 0 | − |
| 22 | M | 60 | 4 | T3N1/INT | 0 | − | 0 | − |
| 23 | M | 65 | n.d∥ | n.d/INT | 20–50 | 213 | Na | − |
| 24 | M | 61 | n.d | n.d/INT | >50 | 245 | 20−50 | − |
| 25 | M | 73 | n.d | n.d/INT | 0 | 38 | >50 | − |
| 26 | M | 70 | n.d | n.d/INT | >50 | 237 | Na | − |
| 27 | M | 70 | n.d | n.d/INT | 10–20 | − | <10 | − |
| 28 | M | 65 | n.d | n.d/INT | 0 | − | >50 | + |
| 29¶ | M | 58 | 12 | T1N0/INT | 0 | − | 20 | + |
| 30 | F | 25 | 2.5 | T2N0/INT | 0 | 213 | 0 | − |
| 31 | F | 59 | 5.5 | T3N0/DIF | 0 | − | >50 | − |
| 32 | M | 45 | 7 | T3N0/INT | 0 | 163 | 0 | − |
| 33¶ | F | 70 | 1.2 | T3N0/INT | >50 | 220 | 0 | − |
| 34 | F | 72 | 9.5 | T3N0/INT | 0 | − | 0 | − |
| 35 | M | 41 | 2 | T3N1/DIF | 0 | − | 0 | − |
| 36 | M | 56 | 7 | T3N1/DIF | 0 | − | 0 | − |
| 37 | M | 50 | 8 | T3N1/DIF | 0 | − | >50 | − |
| 38¶ | M | 82 | 10 | T3N1/INT | 0 | 175 | 0 | − |
| 39 | M | 77 | 6 | T3N1/INT | 0 | 213 | Atypical | − |
| 40 | M | 70 | 5 | T3N1/INT | >50 | 220 | 0 | − |
| 41 | M | 61 | 2.5 | T3N1/INT | 10–20 | 273 | 0 | − |
| 42¶ | F | 69 | 7 | T3N1/INT | 20–50 | 282 | >50 | − |
| 43 | M | 63 | 7 | T3N1/INT | 0 | − | 0 | − |
| 44 | M | 64 | 2.5 | T3N1/INT | 0 | − | 0 | − |
| 45 | F | 60 | 4 | T3N1/INT | 10–20 | − | >50 | + |
| 46¶ | F | 56 | 6 | T3N1/INT | 0 | − | >50 | + |
| 47 | F | 80 | 2.5 | T3N1/INT | 0 | − | 20–50 | − |
| 48 | M | 62 | 4.5 | T3N1/INT | 0 | − | 0 | − |
| 49 | M | 70 | 5.5 | T3N1/INT | 0 | − | 0 | − |
| 50 | M | 58 | 6 | T3N1/INT | 0 | − | >50 | − |
| 51 | M | 70 | 4 | T3N1/INT | 0 | − | >50 | + |
| 52 | M | 79 | 7 | T3N1/INT | 0 | − | 10–20 | − |
| 53 | F | 74 | 2.5 | T3N1/INT | 0 | − | >50 | + |
| 54 | M | 63 | 7 | T3N1/INT | 0 | − | >50 | − |
*Lauren’s classification: Int, intestinal type; Dif, diffuse type.
†Mutations were identified by temporal temperature gradient electrophoresis/sequencing, except for cases 12 and 33 (yeast functional assay/sequencing).
‡IHC, immunohistochemistry.
§+, amplification of MDM2, as detected by a MDM2/DRD2 signal ratio ≥2.5.
¶Additional tumors: squamous cell carcinoma of head and neck (n = 17); pleomorphic adenoma of the parotid (n = 29); adenocarcinoma of the breast (n = 33); adenocarcinoma of the intestine (n = 38); adenocarcinoma of the breast (n = 42); malignant melanoma and adenocarcinoma of the breast (n = 46).
∥n.d; not determined.
Table 2.
TP53 Mutations in Tumors of the Esophagogastric Junction
| Case | Codon | Base change | Amino acid change |
|---|---|---|---|
| ADCE* | |||
| 1 | 135 | TGC→TTC | Cys-Ser |
| 2 | 220 | TAT→TGT | Tyr-Cys |
| 5 | 230 | ACC→CCC | Thr-Pro |
| 9 | 282 | CGG→TGG (CpG)† | Arg-Trp |
| 12 | 272 | GTG→ATG | Val-Met |
| 283 | CGC→CAC (CpG) | Arg-His | |
| 15 | 157 | GTC→TTC | Val-Phe |
| 16 | 159 | GCC→GTC | Ala-Val |
| 239 | Deletion 13 bp | ||
| 17 | 175 | CGC→CAC (CpG) | Arg-His |
| 18 | 234 | TAC→TAA | Tyr-Stop |
| 19 | 279 | GGG→GGA | Gly-Glu |
| 23 | 213 | CGA→CAA (CpG) | Arg-Glu |
| 24 | 245 | GGC→AGC (CpG)§ | Gly-Ser |
| 25 | 38 | CAA→TAA | Gln-Stop |
| 26 | 237 | ATG→ATA | Tyr-Cys |
| ADCC‡ | |||
| 30 | 213 | CGA→TGA (CpG) | Arg-Stop |
| 32 | 163 | TAC→TGC | Tyr-Cys |
| 33 | 220 | TAT→TGT | Tyr-Cys |
| 38 | 175 | CGC→CAC (CpG) | Arg-His |
| 39 | 213 | CGA→TGA (CpG) | Arg-His |
| 40 | 220 | TAT→TGT | Tyr-Cys |
| 41 | 273 | CGT→TGT (CpG) | Arg-Cys |
| 42 | 282 | CGG→TGG (CpG) | Arg-Trp |
*Adenocarcinoma of the esophagus.
†CpG, pyrimidine dinucleotide repeats.
‡Adenocarcinoma of the cardia.
§CpG site in between codons 244 and 245.
Analysis of MDM2 Gene Amplification
Differential PCR was performed as previously described 24 with the following modifications: 5 μl of template DNA was amplified in 50 μl of a reaction mixture containing 20 pmol each of sense and anti-sense primers for MDM2 and for the dopamine D2 receptor gene DRD2 (used as a reference), 200 μmol/L of each dNTP, 1× amplification buffer, 1 X Q solution and 0.5 μl (2.5 U) of HotStarTaq DNA Polymerase (Qiagen). PCR conditions were: 15 minutes at 95°C, followed by 27 cycles at 95°C for 45 seconds, 55°C for 45 seconds, and 72°C for 1 minute with a final extension at 72°C for 5 minutes. The primers were as follows: 5′-GAGGGCTTTGATGTTCCTGA-3′ (sense) and 5′-GCTACTAGAA GTTGATGGC-3′ (anti-sense) for MDM2, and 5′-CCACTGAATCTGTCCTGGTATG-3′ (sense) and 5′-GTGTGGCATAGTAGTTGTAGTGG-3′ (anti-sense) for human DRD2. PCR products were electrophoresed on 7.5% polyacrylamide gels stained with ethidium bromide, photographed, and the films were analyzed by scanning densitometry (GS-670; BioRad, Hercules, CA). An MDM2/DRD2 ratio of 2.5 or above was regarded as indicative of MDM2 amplification and a ratio between 2 and 2.5 was regarded as compatible with MDM2 amplification.
Statistical Evaluations
Frequency tables of independent variables were evaluated for statistical significance by Pearson’s chi-square test.
Results
Clinical and Individual Characteristics of the Patients
Twenty-six cases of ADCC and 28 cases of ADCE were collected between 1995 and 1999 (Tables 1 and 2) ▶ ▶ . In one case, a tumor was classified as ADCE on the basis of the previous diagnosis on biopsy of a Barrett’s mucosa that was no longer detectable at surgery. None of the patients had received chemotherapy or radiotherapy before biopsy or surgery.
The mean age of patients was 62.1 ± 13.6 years (range, 25 to 82 years) for ADCC and 68.1 ± 9 years (range, 50 to 82 years) for ADCE. The group of ADCC patients investigated included 17 men and nine women (male/female ratio of 0.65), whereas the ADCE patients were almost exclusively males (27 males and one female; male/female ratio 0.97). Despite the short follow-up period for some of the patients, medical records revealed that five of the ADCC patients had additional tumors. Three women developed a breast adenocarcinoma either before (19 years, patient 42; 2 years, patient 46) or after (3 years, patient 33) diagnosis of ADCC. One of these patients (patient 46) also developed a malignant melanoma 10 years before ADCC. One man (patient 38) had an adenocarcinoma of the intestine 10 years before ADCC and another (patient 29) presented a pleomorphic adenoma of the parotid 5 years before ADCC. Among the ADCE patients, only one patient had a history of a previous cancer (a squamous cell carcinoma of head and neck in a male patient who was a heavy smoker and alcohol drinker). The available data do not suggest the existence of a familial history of cancer in these patients.
Reliable information on tobacco smoking was available for 15 patients with ADCC and for 14 patients with ADCE. Six of these 15 ADCC patients (40%) and 10 of the 14 ADCE patients (71.5%) were regular smokers (>5 packs-year).
The mean size of the 22 resected ADCE (4.5 cm; range, 1 to 8 cm), was not significantly different from that of the ADCC (5.5 cm; range 1.2 to 12 cm). However, the tumors in the two groups differed in their stage distribution with more frequent lymph-node involvement in the ADCC group than in the ADCE group. According to the TNM classification, four ADCE were stage T1N0 (18%), three were T2N0 (13.5%), seven were T3N0 (32%), and eight were T3N1 (36.5%). In comparison, one ADCC was stage T1N0 (4%), one was T2N0 (4%), four were T3N0 (15.5%), and 20 were T3N1 (77%).
TP53 Mutations
TP53 mutations were detected in 8 of 26 (31%) ADCC and in 14 of 28 (50%) ADCE (Tables 1 and 2) ▶ ▶ . In two cases of ADCE, two mutations were detected. The mutation spectrum showed that 5 of 8 (62.5%) of the ADCC mutations and 5 of 16 (31%) of the ADCE mutations were C to T transitions at CpG sites (Table 3) ▶ . The concordance between TP53 mutation status and p53 overexpression, as detected by immunohistochemistry using the CM-1 antibody, was 92% in both tumor types (Tables 1 and 2) ▶ ▶ .
Table 3.
Percentage of Tumor Cells Expressing Cytokeratin (CK) 4, 7, or 13 in Tumors of the Esophagogastric Junction
| CK4* (%) | CK7* (%) | CK13* (%) | |
|---|---|---|---|
| ADCC† | 100* | 41 | 36.5 |
| ADCE‡ | 83.5 | 100 | 81 |
*Only tumors expressing the antigen in more than 10% of the cells were scored as positive.
†Adenocarcinoma of the cardia.
‡Adenocarcinoma of the esophagus.
MDM2 Expression and Gene Amplification
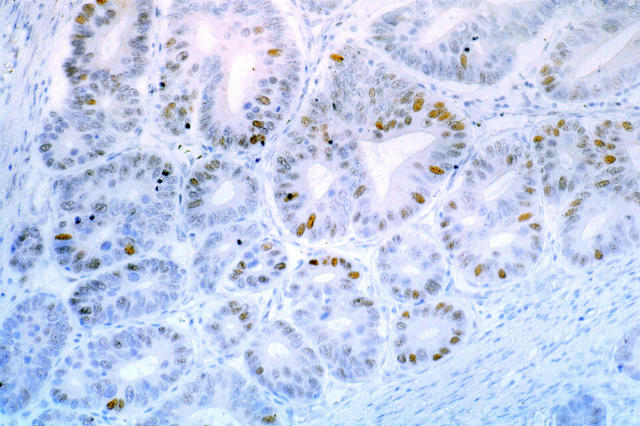
In ADCC, expression of the mdm2 protein (detectable by immunohistochemistry in at least 10% of the tumor cells) was found in 50% (13 of 26) of the tumors. In nine of these, expression was detected in >50% of the cells (Figure 1) ▶ . By differential PCR, 19% (5 of 26) of the ADCC tumors contained an amplified MDM2 gene as detected by a ratio of >2.5 between the intensities of the MDM2 band and of the DRD2 gene used as control. In addition, two further ADCC showed an MDM2/DRD2 signal ratio between 2 and 2.5 (data not shown). In ADCE, mdm2 protein was detectable by immunohistochemistry in 40% (10 of 25) of the tumors, six of them showing positive staining in >50% of the tumor cells. By differential PCR, only one ADCE showed an MDM2/DRD2 signal ratio of >2.5 (4%), and one other tumor showed a ratio of between 2 and 2.5. All ADCC and ADCE with amplified MDM2 had wild-type TP53 sequences.
Figure 1.
Example of mdm2 immunostaining in an adenocarcinoma of the cardia with amplification of the MDM2 gene (case 42). The protein is detectable in >50% of tumor cells. Original magnification, ×160.
Expression of Cytokeratins 4, 7, and 13
Precancerous and cancer lesions usually retain patterns of CK expression that characterize the cells and tissues from which they originate. 2 Table 4 shows the pattern of expression of CKs 4, 7, and 13 in ADCC and ADCE. CK4 was expressed in most ADCE (83.5%) and in all ADCC tested. However, the two tumor types differed in the pattern of expression of CK7 and CK13, with >50% of ADCC being negative for both markers, and >80% of ADCE being positive for the same markers. These observations further substantiate the hypothesis that ADCC and ADCE originate from distinct cell types.
Discussion
Tumors of the esophagogastric junction are a heterogeneous group of cancers, the incidence of which is increasing rapidly in many industrialized countries. In this study, we have applied strict anatomopathological criteria to classify these tumors into two groups: ADCE and ADCC. The latter designation was exclusively used to identify tumors that did not present any evidence of Barrett’s metaplasia, even after microscopic examination of the whole esophagogastric junction. These criteria are more stringent than the ones used in many other studies, in which the term “adenocarcinoma of the cardia” has been used for tumors with a short segment of Barrett’s mucosa (<3 cm), 16 or for tumors without evidence of Barrett’s mucosa detected pre-operatively or on gross examination of the surgical specimen. 15 It should be noted that 10 of the 22 tumors treated by surgery included in the present study as ADCE had short-segment Barrett’s mucosa not detectable macroscopically. Using these strict anatomopathological criteria to define the exact tumor type, differences in the clinical and molecular characteristics have been found between ADCE and ADCC.
First, the patient profiles were different in the two tumor types. Although the characteristics of our ADCE patients (mean age, sex ratio) were similar to those reported by others, 15-17 our group of ADCC patients included a higher proportion of female patients (35%, versus only 3.5% in ADCE; P < 0.002). The mean age was not significantly different between two groups. The proportion of regular smokers was lower among ADCC patients (47%, compared to 72% for ADCE). However, one of the most striking differences was the clinical history of associated tumors in patients with ADCC. Three of the nine female patients had a history of breast adenocarcinoma, occurring either before (in two cases) or after (in one case) the diagnosis of ADCC. To our knowledge, an association between ADCC and breast cancer has not been previously reported. ADCC has also not been reported in the spectrum of tumors occurring in individuals with germline mutations known to predispose to breast cancer, such as BRCA-1 or -2 carriers, or in the Li-Fraumeni syndrome. Two other individuals with ADCC developed another tumor (one adenocarcinoma of the intestine and one pleomorphic adenoma of the parotid). In contrast, in ADCE, only one patient had a history of associated squamous cell carcinoma of the head and neck, associated with a background of heavy tobacco and alcohol consumption.
Second, the tumors in the two groups showed differences in several molecular markers, including TP53 mutation, MDM2 amplification, and patterns of cytokeratin expression. The prevalence of TP53 mutations was 50% in ADCE, compared with 31% in ADCC (P = 0.09). For ADCE, our data are in agreement with the prevalence reported in the literature (117 mutations described in 205 tumors examined; 57%). However, for ADCC, our results diverge from those reported by Gleeson et al 16 (63%) and, to a lesser extent, those of Fléjou et al 15 (42%). We believe that these differences are the consequence of using different criteria to distinguish between ADCC and ADCE. The importance of the tumor definition is illustrated in a recent study by Ireland et al, 25 who used the classification of Siewert and Stein. 19 These authors noted that intestinal metaplasia was detectable in 89% of tumors of the esophagus, 58% of tumors of the cardia, and 33% of tumors of the subcardia. The TP53 mutation prevalence was 53%, 58%, and 17%, in the three subgroups, respectively. It is interesting to note that, in addition to the low prevalence of TP53 mutations, the subcardia group of Ireland et al 25 resembles our ADCC group in several clinical features, including the sex ratio of the patients and the stage of the tumors.
The relatively low prevalence of TP53 mutations in ADCC prompted us to investigate the MDM2 status. We found that MDM2 was amplified in 19% of the ADCC analyzed (compared with only 4% in ADCE, P = 0.08). All tumors with amplified MDM2 had wild-type TP53 sequences. The rate of MDM2 amplification observed in ADCC is one of the highest ever reported in human carcinomas. 26 These data suggest that amplification and overexpression of MDM2 may represent an alternative mechanism for p53 protein inactivation in ADCC. Although these results are of borderline statistical significance, our results suggest that ADCC and ADCE differ at the molecular level in the prevalence of both TP53 mutations and MDM2 amplification.
Finally, we found that the two types of cancer differed in their profile of expression of CK7 and CK13. CK7 and CK13 were frequently expressed in the ADCE (100% and 85%, respectively). In contrast, less than half of the ADCC samples expressed CK7 and CK13 (41% and 37%, respectively). These differences are consistent with the results of Goldblum, 4 who reported that CK7 was constantly expressed in Barrett’s mucosa with intestinal metaplasia, but was never expressed in cardia mucosa with intestinal metaplasia. However, close examination of the upper part of the fundic mucosa revealed the presence of focal CK7 expression at the neck of the glands in a small series of normal gastric mucosa samples (data not shown; P Tanière, G Borghi-Scoazec, JF Mosnier, F Berger, P Hainaut, JY Scoazec, manuscript in preparation). This observation suggests that our detection of a subset of CK7-positive ADCC reflects a possible origin from such glandular structure rather than misclassification. In addition, our data indicate that CK4 and CK13 are not specific markers of a squamous origin of the tumor, because we detected their expression in ADCC, ADCE (Table 4),as well as in adenocarcinoma of the antrum (33% and 89% for, respectively, CK13 and CK4; data not shown).
In conclusion, our results show that ADCC differs from ADCE in the prevalence of TP53 mutations and the frequency of MDM2 amplification, and in the pattern of CK expression. Our results thus strongly support the hypothesis that ADCC and ADCE are two distinct pathological entities. This conclusion is at variance with previous reports by Gleeson et al 16 and Fléjou et al, 15 who found no major difference between the clinical, epidemiological, and molecular characteristics of these two cancers. The main reason for this discrepancy probably lies in the definition of the lesion. In the present study, the definition of ADCC was based on both localization (within 2 cm of the esophagogastric junction) and on the absence of residual Barrett’s metaplasia at the microscopic level. The presence of residual Barrett’s mucosa with a characteristic profile of CK expression seems to be an essential parameter for distinguishing ADCC from ADCE. Larger studies using precise selection criteria to distinguish between these tumors are necessary to further clarify the differences between the individual and clinical characteristics of the patients, as well as to identify the risk factors specifically associated with each tumor type.
Acknowledgments
We thank J. C. Boulez, C. Partensky, and E. Tissot (Edouard Herriot Hospital) for providing surgical specimens; J. A. Chayvialle and J. C. Saurin (Edouard Herriot Hospital) for providing biopsies; H. Ohgaki (IARC) for the gift of positive control samples for MDM2 amplification studies; N. Lyandrat and M. Laval for technical assistance with immunostaining; J. Hall and K. Mann for critical comments and helpful suggestions; and M. Olivier for information from analysis of the TP53 database.
Footnotes
Address reprint requests to P. Hainaut, Ph.D., Group of Molecular Carcinogenesis, IARC, 150 Cours Albert Thomas, 69372 Lyon Cedex 08, France. E-mail: hainaut@iarc.fr.
References
- 1.Devesa SS, Blot WJ, Fraumeni JFJ: Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998, 83:2049-2053 [PubMed] [Google Scholar]
- 2.Boch JA, Shields HM, Antonioli DA, Zwas F, Sawhney RA, Trier JS: Distribution of cytokeratin markers in Barrett’s specialized columnar epithelium. Gastroenterology 1997, 112:760-765 [DOI] [PubMed] [Google Scholar]
- 3.Shields HM, Zwas F, Antonioli DA, Doos WG, Kim S, Spechler SJ: Detection by scanning electron microscopy of a distinctive esophageal surface cell at the junction of squamous and Barrett’s epithelium. Dig Dis Sci 1993, 38:97-108 [DOI] [PubMed] [Google Scholar]
- 4.Goldblum JR: Inflammation and intestinal metaplasia of the gastric cardia: Helicobacter pylori, gastroesophageal reflux disease, or both. Dig Dis 2000, 18:14-19 [DOI] [PubMed] [Google Scholar]
- 5.Jankowski JA, Wright NA, Meltzer SJ, Triadafilopoulos G, Geboes K, Casson AG, Kerr D, Young LS: Molecular evolution of the metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Pathol 1999, 154:965-973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Lieshout EM, Roelofs HM, Dekker S, Mulder CJ, Wobbes T, Jansen JB, Peters WH: Polymorphic expression of the glutathione S-transferase P1 gene and its susceptibility to Barrett’s esophagus and esophageal carcinoma. Cancer Res 1999, 59:586-589 [PubMed] [Google Scholar]
- 7.Pera M: Epidemiology of esophageal cancer, especially adenocarcinoma of the esophagus and esophagogastric junction. Recent Results Cancer Res 2000, 155:1-14 [DOI] [PubMed] [Google Scholar]
- 8.Spechler SJ: The role of gastric carditis in metaplasia and neoplasia at the gastroesophageal junction. Gastroenterology 1999, 117:218-228 [DOI] [PubMed] [Google Scholar]
- 9.Ye W, Ekstrom AM, Hansson LE, Bergstrom R, Nyren O: Tobacco, alcohol and the risk of gastric cancer by sub-site and histologic type. Int J Cancer 1999, 83:223-229 [DOI] [PubMed] [Google Scholar]
- 10.Gammon MD, Schoenberg JB, Ahsan H, Risch HA, Vaughan TL, Chow WH, Rotterdam H, West AB, Dubrow R, Stanford JL, Mayne ST, Farrow DC, Niwa S, Blot WJ, Fraumeni JFJ: Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia [see comments]. J Natl Cancer Inst 1997, 89:1277-1284 [DOI] [PubMed] [Google Scholar]
- 11.Zhang ZF, Kurtz RC, Sun M, Karpeh MJ, Yu GP, Gargon N, Fein JS, Georgopoulos SK, Harlap S: Adenocarcinomas of the esophagus and gastric cardia: medical conditions, tobacco, alcohol, and socioeconomic factors. Cancer Epidemiol Biomarkers Prev 1996, 5:761-768 [PubMed] [Google Scholar]
- 12.Zhang ZF, Kurtz RC, Marshall JR: Cigarette smoking and esophageal and gastric cardia adenocarcinoma [editorial; comment]. J Natl Cancer Inst 1997, 89:1247-1249 [DOI] [PubMed] [Google Scholar]
- 13.Goldstein SR, Yang GY, Chen X, Curtis SK, Yang CS: Studies of iron deposits, inducible nitric oxide synthase and nitrotyrosine in a rat model for esophageal adenocarcinoma. Carcinogenesis 1998, 19:1445-1449 [DOI] [PubMed] [Google Scholar]
- 14.Montesano R, Hainaut P: Molecular precursor lesions in oesophageal cancer. Cancer Surv 1998, 32:53-67 [PubMed] [Google Scholar]
- 15.Flejou JF, Gratio V, Muzeau F, Hamelin R: p53 abnormalities in adenocarcinoma of the gastric cardia and antrum. Mol Pathol 1999, 52:263-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gleeson CM, Sloan JM, McManus DT, Maxwell P, Arthur K, McGuigan JA, Ritchie AJ, Russell SE: Comparison of p53 and DNA content abnormalities in adenocarcinoma of the oesophagus and gastric cardia. Br J Cancer 1998, 77:277-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ireland AP, Clark GW, DeMeester TR: Barrett’s esophagus.: The significance of p53 in clinical practice. Ann Surg 1997, 225:17-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casson AG, Mukhopadhyay T, Cleary KR, Ro JY, Levin B, Roth JA: p53 gene mutations in Barrett’s epithelium and esophageal cancer. Cancer Res 1991, 51:4495-4499 [PubMed] [Google Scholar]
- 19.Siewert JR, Stein HJ: Classification of adenocarcinoma of the oesophagogastric junction [see comments]. Br J Surg 1998, 85:1457-1459 [DOI] [PubMed] [Google Scholar]
- 20.Hamelin R, Jego N, Laurent-Puig P, Vidaud M, Thomas G: Efficient screening of p53 mutations by denaturing gradient gel electrophoresis in colorectal tumors. Oncogene 1993, 8:2213-2220 [PubMed] [Google Scholar]
- 21.Guldberg P, Nedergaard T, Nielsen HJ, Olsen AC, Ahrenkiel V, Zeuthen J: Single-step DGGE-based mutation scanning of the p53 gene: application to genetic diagnosis of colorectal cancer. Hum Mutat 1997, 9:348-355 [DOI] [PubMed] [Google Scholar]
- 22.Barnas C, Martel-Planche G, Furukawa Y, Hollstein M, Montesano R, Hainaut P: Inactivation of the p53 protein in cell lines derived from human esophageal cancers. Int J Cancer 1997, 71:79-87 [DOI] [PubMed] [Google Scholar]
- 23.Flaman JM, Frebourg T, Moreau V, Charbonnier F, Martin C, Chappuis P, Sappino AP, Limacher IM, Bron L, Benhattar J: A simple p53 functional assay for screening cell lines, blood, and tumors. Proc Natl Acad Sci USA 1995, 92:3963-3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biernat W, Kleihues P, Yonekawa Y, Ohgaki H: Amplification and overexpression of MDM2 in primary (de novo) glioblastomas. J Neuropathol Exp Neurol 1997, 56:180-185 [DOI] [PubMed] [Google Scholar]
- 25.Ireland AP, Shibata DK, Chandrasoma P, Lord RV, Peters JH, DeMeester TR: Clinical significance of p53 mutations in adenocarcinoma of the esophagus and cardia. Ann Surg 2000, 231:179-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Momand J, Jung D, Wilczynski S, Niland J: The MDM2 gene amplification database. Nucleic Acids Res 1998, 26:3453-3459 [DOI] [PMC free article] [PubMed] [Google Scholar]