Abstract
Nitric oxide produced from nitric oxide synthase(s) is an important cell signaling molecule in physiology and pathophysiology. In the present study, we describe a very sensitive and convenient analytical method to measure NOx (nitrite plus nitrate) in culture media by employing an ultra-sensitive nitric oxide-selective electrochemical sensor which became commercially available recently. An aliquot of conditioned culture media was first treated with nitrate reductase/NADPH/glucose-6-phosphate dehydrogenase/glucose-6-phosphate to convert nitrate to nitrite quantitatively. The nitrite (that is present originally plus the reduced nitrate) was then reduced to equimolar NO in an acidic iodide bath while NO was being detected by the sensor. This analytical method appears to be very useful to assess basal and stimulated NO release from cultured cells.
Keywords: Endothelial cells, eNOS, nitric oxide, assay
INTRODUCTION
Nitric Oxide (NO) produced from the endothelium plays critical roles in normal vascular biology and pathophysiology [1]. It regulates vasodilation and inhibits platelet aggregation, leukocyte adherence, and vascular muscle cell proliferation. NO production is stimulated by shear stress and humoral factors including vascular endothelial growth factor, acetylcholine and bradykinin [2,3]. The loss or attenuation of bioavailable NO production in the endothelium is a biochemical marker of endothelial dysfunction found in many cardiovascular diseases such as hypertension and atherosclerosis [4].
For the study of NO biology and associated mechanisms, numerous methods have been generated to detect NO production in biological systems. NO is very unstable with a half-life of 2–30 s and rapidly reacts with molecular oxygen to form nitrite [5]. In the presence of oxidizing species such as oxyhemoproteins, nitrite can be further oxidized to nitrate [6]. Nitrite or nitrate (collectively referred to as NOx) accumulate in extracellular fluids such as sera, urine and cell culture media, and thus detection of NOx has been a useful tool to measure NO production from endothelial cells .
The Griess reaction detects nitrite based on the reaction with sulphanilic acid to form the diazonium ion [7,8]. This ion is then coupled to N-(1-naphthyl)ethylenediamine to form a chromophoric azo derivative, detectable spectrophotometrically. The detection limit of this method is 0.1–1.0 μM and its utility is hindered by low sensitivity. A fluorometric detection of nitrite using 2,3-diaminonaphthalene (DAN) has an advantage in terms of sensitivity. This method is based on the acid-catalyzed ring closure of DAN with nitrite to form highly fluorescent 2,3-naphthotriazole [7,9]. The DAN assay is barely sensitive enough to measure NO production from endothelial cells stimulated by shear stress or some agonists [10,11].
The chemiluminescence technique provides highly sensitive and specific detection of NOx [12]. With this technique, NOx is reduced back to NO in a reflux chamber which contains vanadium (III) chloride at 95°C. NO in the gas phase then reacts with ozone to form NO2* at an excited state which emits chemiluminescence proportional to the NOx concentration. Because the instrumentation is expensive and requires significant maintenance, the chemiluminescence technique has only been successfully used in a limited number of labs [13].
Electron spin resonance is another commonly used method that measures bioactive NO. ESR, also called electron paramagnetic resonance, is a technique that detects species that have unpaired electrons by measuring the transition of the unpaired electrons in an applied magnetic field [14,15]. This method is the most direct but because of NO’s short half-life, NO must be measured indirectly through the use of spin traps that react with NO such as colloid iron diethyl dithiocarbanate, FE(DETC)2 [15]. ESR requires expensive and highly technical equipment making it not available to all laboratories.
NO can be detected amperometrically using an NO specific electrode, typically carbon or platinum coated, where the voltage of the electrode is held constant above the oxidation potential of NO (+900mV) and the current generated is a linear function of NO concentration at the electrode surface [15–18]. Recently, Berkels et al. reported a method to measure NOx by converting them to NO which is then detected by an NO-selective electrochemical sensor [19]. This method is very attractive because it provides a rapid and easy assay. Furthermore, instruments for this technique are inexpensive enough to be affordable in many laboratory settings. The application of this technique however has been limited by the quality of the sensor. In the present study, we attempted to update this technique by using a new version of electrode which has become commercially available recently. The current method appeared to be very sensitive and convenient to assess basal and stimulated NO production from cultured endothelial cells.
METHODS
NO-selective sensor
The amperometric NO-selective sensor (AmiNO700) was purchased from Innovative Instruments, Inc. (Tampa, FL). This sensor is very specific to NO and 100 times more sensitive than the previous version [19] . The detection limit of the electrode is about 0.1 nM which generates a current of 7 pA or more.
Reagents
‘Acidic iodide bath’ (20 mM potassium iodide in 0.1 N sulfuric acid) was prepared fresh daily. Standard solutions of sodium nitrite and sodium nitrate were prepared fresh in de-ionized water. The ‘nitrate reductor (NR)’ consisted of 0.2 units/ml nitrate reductase, 0.4 units/ml glucose 6-phosphate dehydrogenase, 0.25 mM glucose 6-phosphate, and 0.2 μM NADPH in 14 mM sodium phosphate buffer (pH 7.4) [20]. For convenience, stock solutions of each component were prepared in 14 mM sodium phosphate buffer (pH 7.4) and kept frozen at −80°C: Nitrate reductase (Sigma, N7265), 1 unit/60 μl; Glucose-6-phosphate dehydrogenase (Sigma, G4134), 2 units/12 μl; Glucose-6-phosphate, 3.8 mg/50 μl; NADPH, 9 μg/10 μl. Just prior to use, all of these four components were diluted in the appropriate volume of buffer to make up 5 ml of ‘NR’.
Calibrations
The top of the NO sensor was dipped in 10 ml of ‘acidic iodide bath’ in a small beaker while the bath was being stirred at room temperature. The current was continuously monitored by the device provided. When the background signal was stabilized, 100–200 μl aliquots of standard nitrite solution at various known concentrations were added to the beaker to monitor changes in the current. The peak currents were used for quantifications.
Standard nitrate solution was also used for the generation of an electrode calibration curve. An aliquot of nitrate solution was incubated with one volume of ‘NR’ or buffer only at room temperature for 1 hr prior to the measurements.
NOx measurements in conditioned media
Bovine aortic endothelial cells (BAEC) were cultured (37°C, 5% CO2) in Dulbecco’s minimum Eagle’s medium (DMEM) containing 20% fetal bovine serum (FBS, Atlanta Biologicals) without antibiotics. For the measurement of NO production, cells were washed with a low serum-containing ‘medium A’ (phenol red-free DMEM containing 0.5% FBS and 25 mM Hepes, pH 7.4), and then kept in the same medium with or without any treatments for specified periods. The conditioned media were used for NOx measurements in the same fashion as described above. Briefly, an aliquot of conditioned media was incubated with ‘NR’ and the incubated sample was added to the ‘acidic iodide bath’ while monitoring the current change due to NO generation.
Since the routine culture media contained significant amounts of nitrite or nitrate, especially when serum was included, a calibration curve was generated using a standard solution of nitrate or nitrate prepared in the same culture medium (Medium A).
Fluorescence assay using DAN
For comparative purposes, we also conducted a fluorometric assay using DAN [9]. 100 μl of standard nitrate solution was mixed with ‘NR’ or buffer only and incubated at room temperature for 1 hr. Then, 20 μl of DAN reagent (50 μg/ml in 0.62 N HCl) was added and incubated for 10 min. The reaction was stopped by adding 10 μl of 2.8 N NaOH and the fluorescence was read at 360 nm excitation wavelength and 460 nm emission wavelength.
Protein assay
Following experimental treatments, cells were washed in ice-cold phosphate buffered saline (PBS) and lysed in 0.1 ml lysis buffer (10 mM Tris-HCl, pH 7.6, 1 mM sodium vanadate and 1% SDS). The lysate was further homogenized by repeated aspiration through a 25-gauge needle. Protein content of each sample was measured using a Bio-Rad DC assay [21].
Statistical analysis
Statistical analysis was performed by Student’s t-test. A p-value of less than 0.05 based on at least 3 or more independent experiments was considered to be statistically significant.
RESULTS
Calibration with nitrite
The quantitative reduction of nitrite to NO and simultaneous detection of NO by the NO sensor were performed in ‘acidic iodide bath’. To generate the calibration curve with nitrite, the sensor was equilibrated in the 10 ml bath and then 100 μl of sodium nitrite standard solutions were added to the bath with the current change being monitored. As shown in Fig. 1A, sequential addition of standard sodium nitrite solutions increased the peak current in a dose-dependent manner. The response curve showed the linear relationship between the current peak and the amount of nitrite added (Fig. 1B). The apparent sensitivity of the sensor, 1 pA = 15 pM, was 100 times higher than the previous version [19]. The detection limit of the sensor was about 0.1 nM which generated 7 pA of current over the noise level (± 2 pA) (Fig. 2).
Fig. 1. Calibration with nitrite.
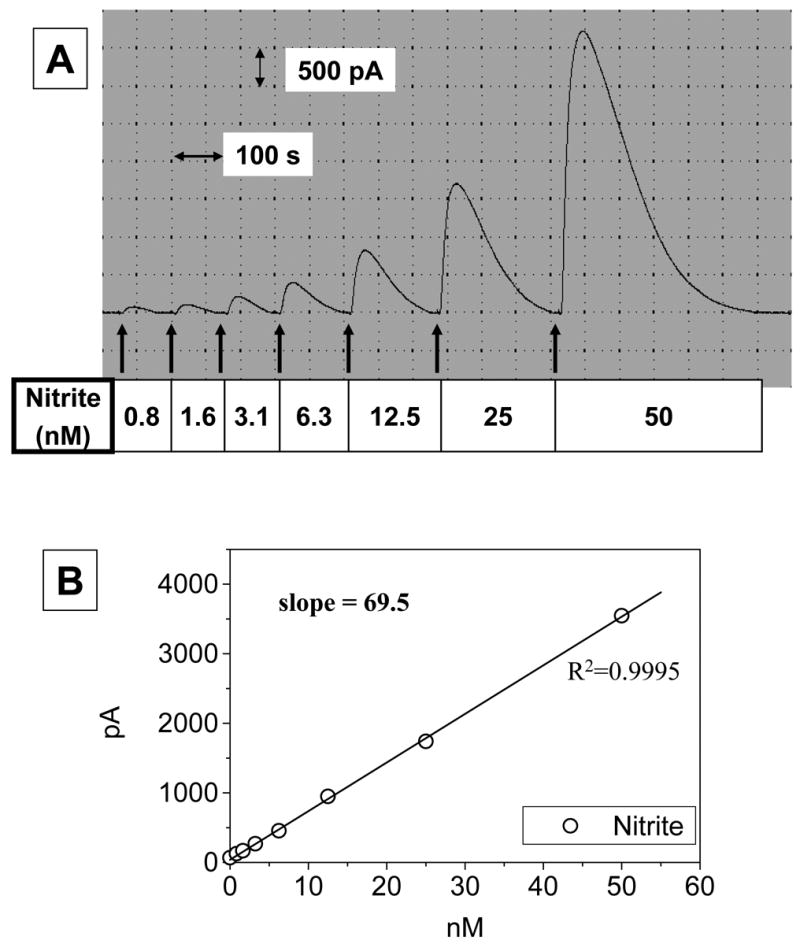
To a 10-ml ‘acidic iodide bath’ (20 mM potassium iodide in 0.1 N sulfuric acid), 100 μl of sodium nitrite solutions at varied concentrations were added successively while the current change was monitored. Panel A shows a typical recording of the signal. As shown in panel B, linear regression yielded a good correlation between nitrite added (0.8–50 nM) and the current generated.
Fig. 2. Sensitivity of the NO sensor.
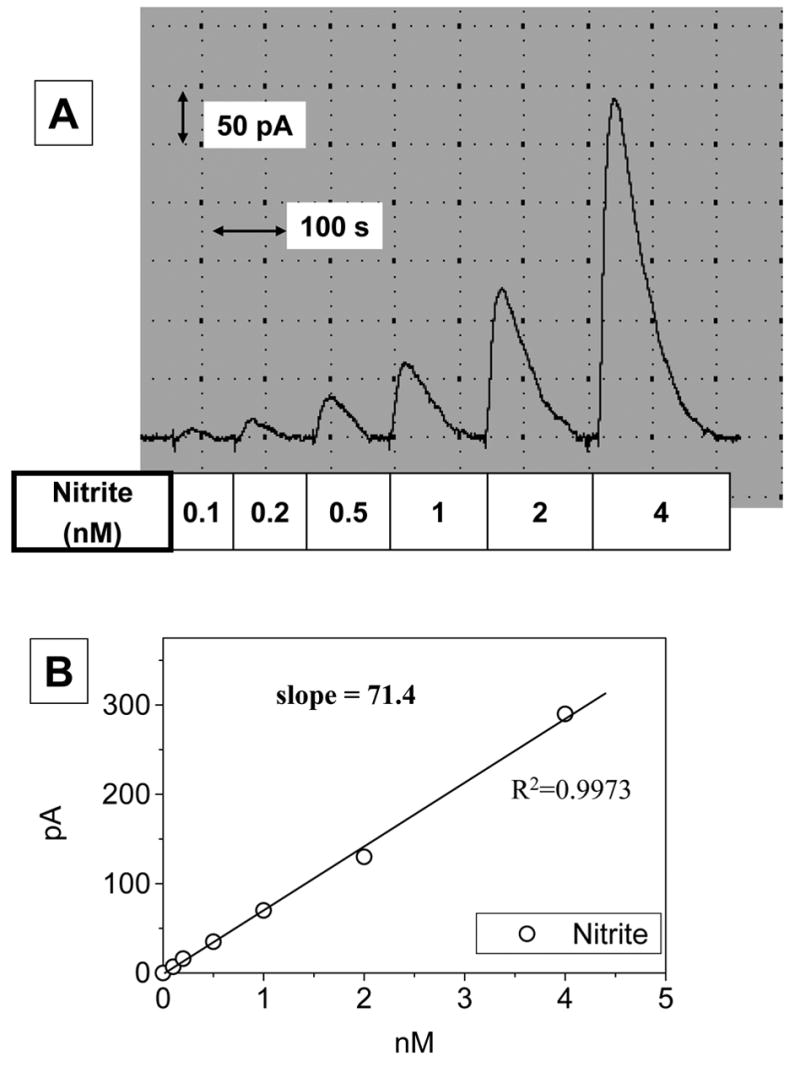
To a 10-ml ‘acidic iodide bath’, 100 μl of sodium nitrite solutions at varied concentrations were added successively while current change was monitored, as in Fig. 1. Panel A shows a typical original recording and the signal generated by 0.1 nM which is evidently distinguishable from the background level. As also shown in panel B, a linear correlation between nitrite added and the current generated was found even when the nitrite level is 0.1–4 nM.
Calibration with nitrate
Nitrate was not converted to NO directly in an acidic iodide solution unless it was reduced to nitrite beforehand as shown in Fig. 3A. Nitrate could be reduced to nitrite by ‘NR’ which consisted of nitrate reductase, NADPH, glucose-6-phosphate dehydrogenase and glucose-6-phosphate. The coupled enzyme reactions appeared to catalyze the reduction of nitrate to nitrite very efficiently. As shown in Fig. 3B, the response curve of nitrate with ‘NR’ was essentially identical with that of nitrite in terms of slope, indicating quantitative conversion of nitrate to equimolar nitrite.
Fig. 3. Efficiency of nitrate reductor.
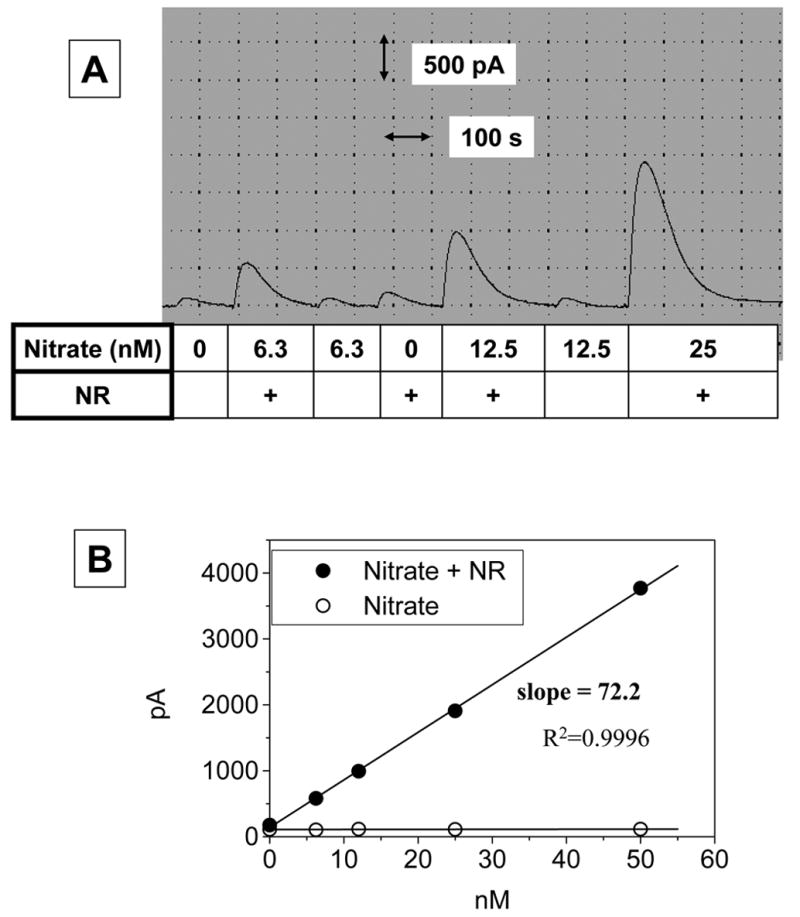
Sodium nitrate solutions were incubated with ‘nitrate redactor (NR)’ which consisted of 0.2 units/ml nitrate reductase, 0.4 units/ml glucose 6-phosphate dehydrogenase, 0.25 mM glucose 6-phosphate, 0.2 μM NADPH in 14 mM Na-PO4 buffer (pH 7.4). The sample solutions, ie. sodium nitrate incubated with or without ‘NR’ were added to a 10-ml ‘acidic iodide bath’ while the current change was monitored, as in Fig. 1. Nitrate was not converted directly to NO as detected by the NO sensor unless it is first reduced to nitrite by ‘NR’ (Panel A). Reduction of nitrate to nitrite by ‘NR’ was found to be quantitative (Panel B)
The current analytical method using the NO sensor appeared to be significantly more sensitive than a fluorescence assay using DAN of which the detection limit was about 100 nM, as shown in Fig. 4.
Fig. 4. Limited sensitivity of DAN assay.
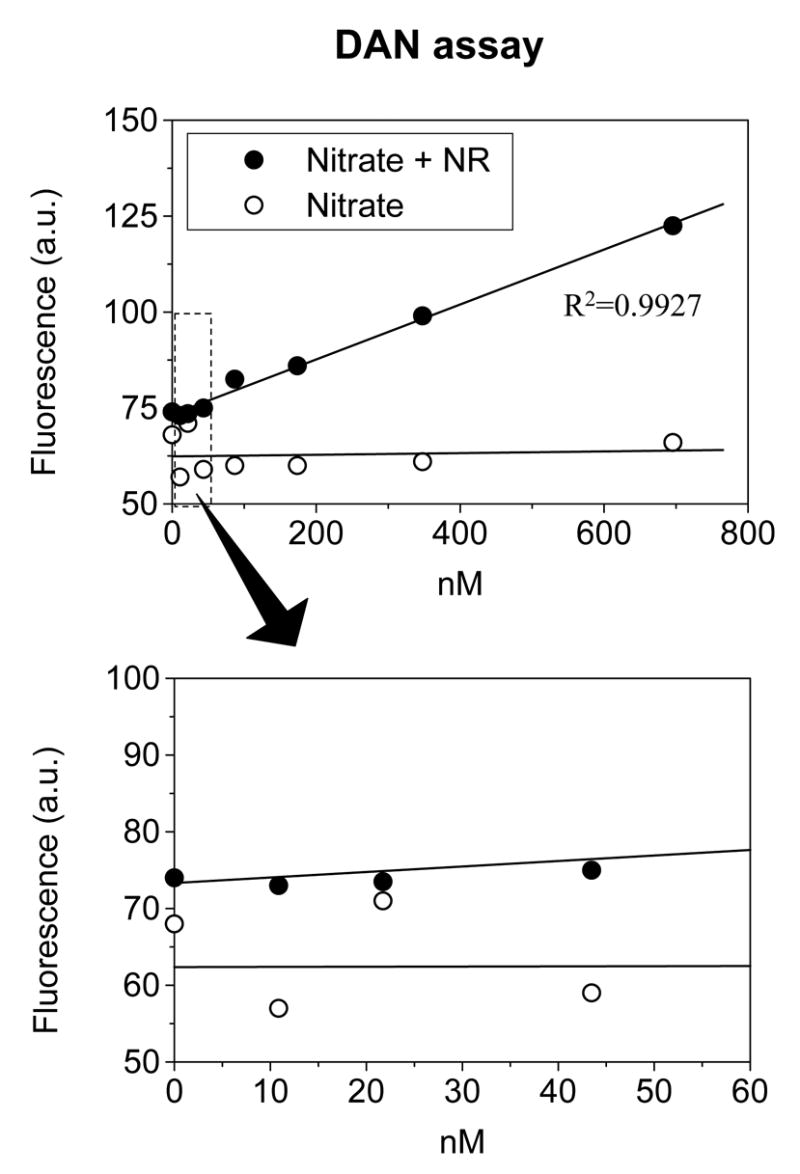
Sodium nitrate solutions of known concentrations were incubated with or without ‘nitrate redactor (NR)’ at room temperature for 1 hr. The NOx level was then measured by the DAN assay as described in METHODS. The linear correlation between the nitrite level and fluorescence was evident only when NOx was over 100 nM.
NOx in serum
Given the sensitive analytical method available, we examined the level of nitrite and nitrate in FBS which we commonly use for cell culture. As shown in Fig. 5, the addition of serum to the acidic iodide bath caused only a small increase in current, indicating nitrite level in serum is low. However, the serum treated with ‘NR’ increased the peak current about 100-fold, indicating the abundance of nitrate in the serum. The total NOx representing nitrite plus nitrate in the serum was estimated to be about 20 μM.
Fig. 5. Nitrate accumulation in serum.
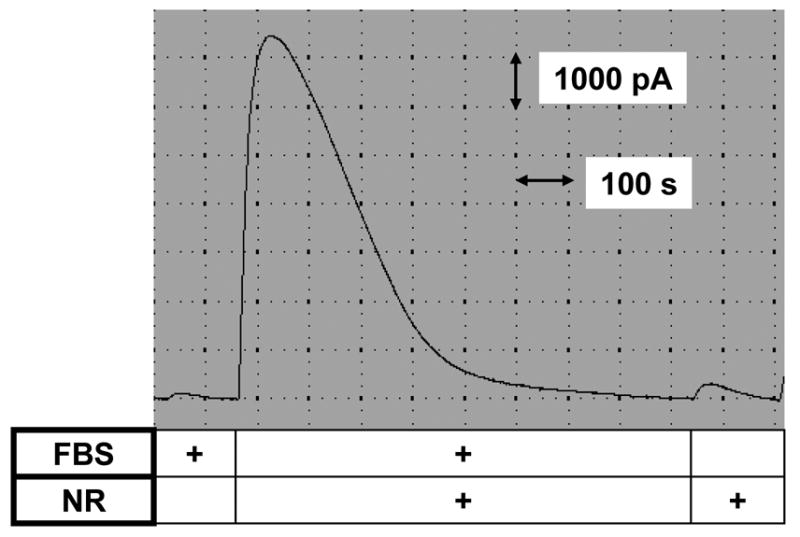
Aliquots of fetal bovine serum (FBS) were incubated with or without ‘nitrate redactor (NR)’. The samples were added to an ‘acidic iodide bath’ while monitoring NO with the NO sensor, as in Fig. 3. These measurements indicate nitrate, but not nitrite, is the major form accumulated in serum.
Calibration curve for NOx in culture media
Due to the high sensitivity of the electrode, most cell culture media including serum-free media were found to contain a little but detectable amount of NOx. The use of culture medium with high background NOx contamination should therefore be avoided for accurate NOx determination. If the background NOx contamination is not too significant, the interference from contaminating NOx can be corrected by generating a calibration curve with nitrite or nitrate prepared in the same culture medium. The calibration curve shown in Fig. 6 was generated in ‘medium A’ (phenol red-free DMEM containing 0.5% FBS and 25 mM Hepes, pH 7.4).
Fig. 6. Calibration curve for the determination of NOx in culture media.
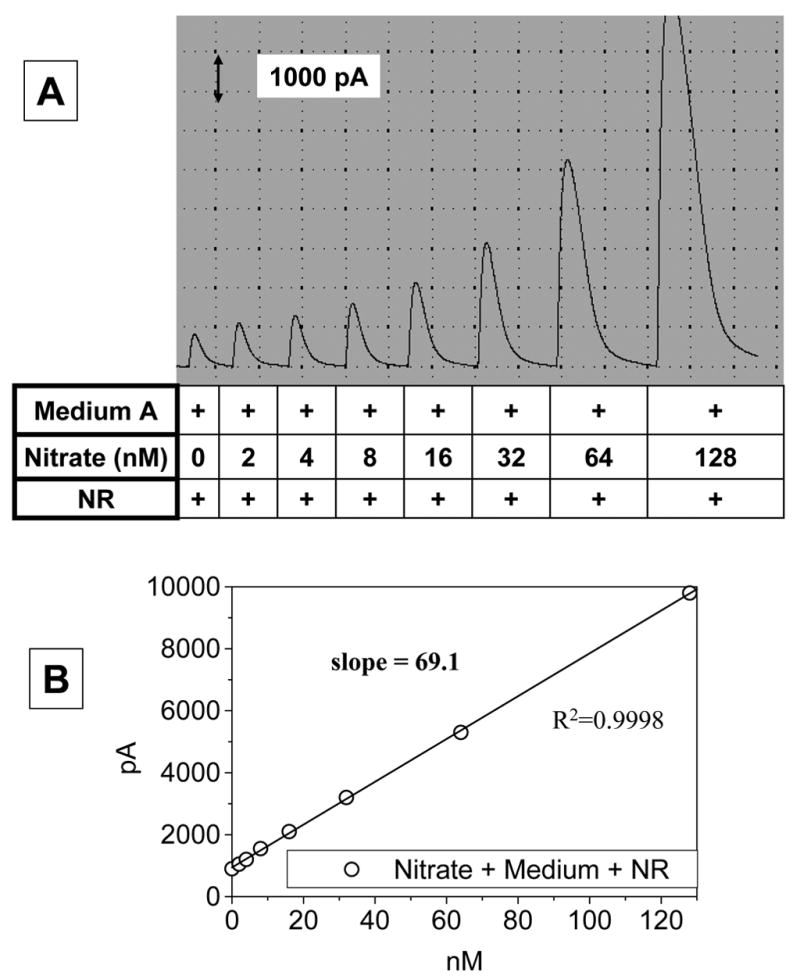
Standard sodium nitrate solutions were prepared in a culture medium to correct for the background level of NOx contamination usually found in ordinary culture media. In this study we used a low serum-containing ‘medium A’ (phenol red-free DMEM containing 0.5% FBS and 25 mM Hepes, pH 7.4). Aliquots of the standard solutions were treated with ‘nitrate redactor (NR)’ and added to the ‘acidic iodide bath’ in order to measure the NOx level as in Fig. 3. Panel A shows a typical original recording while panel B shows a calibration curve.
Determination of NO production from cultured cells
NO produced from NO-producing cells is oxidized to nitrite rapidly, and then to nitrate slowly. In this sense, the accumulation of nitrite plus nitrate (NOx) is a good indication of NO production if it is inhibited by a nitric oxide synthase inhibitor.
We measured basal NO production from cultured BAEC by employing the current method. The confluent cultured cells were washed with a low serum-containing ‘medium A’ and incubated for 20 hr in the absence or presence of 1 mM NG-nitro-L-arginine methyl ester (L-NAME), a nitric oxide synthase inhibitor. After the incubation, an aliquot of the conditioned medium was treated with ‘NR’ and added to the ‘acidic iodide bath’ while monitoring the NO-dependent current change. The typical current changes are shown in Fig. 7A. The data were normalized for total protein amount as shown in Fig. 7B. The NOx level in the conditioned medium from BAEC was much higher than that in the medium itself, and the NOx accumulation was blocked by treating the cells with L-NAME. This result indicates that the NOx that accumulated in the conditioned medium was derived from NO produced by the cells.
Fig. 7. Basal NO production from cultured endothelial cells.
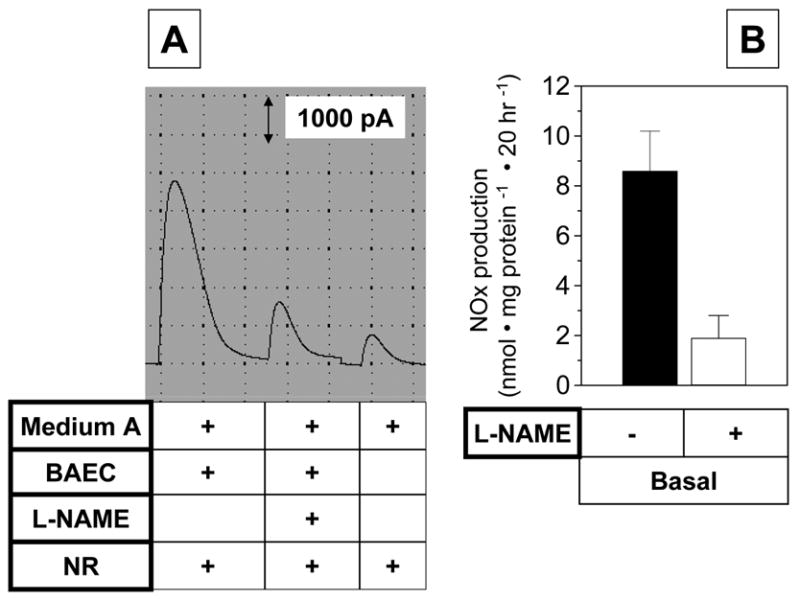
Confluent bovine aortic endothelial cells (BAEC) were washed with a low serum-containing ‘medium A’ (phenol red-free DMEM containing 0.5% FBS and 25 mM Hepes, pH 7.4), and then kept in the same medium with or without 1 mM L-NAME for 20 hr. The conditioned media were used for NOx measurements in the same fashion as described in Fig. 6. Panel A shows a typical original recording while panel B shows the quantification. Data represent mean ± SEM (n=3).
In another experiment, we also measured NO production in BAEC stimulated with the calcium ionophore A23187 for one hour in the absence or presence of L-NAME. As shown in Fig. 8, NO production was increased about 8-fold by the calcium ionophore treatment and it was completely blocked by L-NAME.
Fig. 8. Stimulated NO production from cultured endothelial cells.
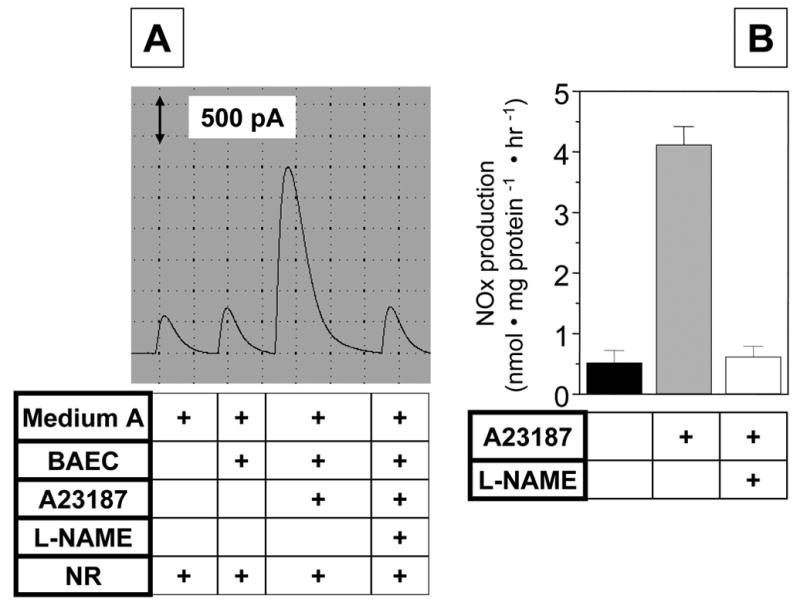
Confluent bovine aortic endothelial cells (BAEC) were washed with a low serum-containing ‘medium A’ (phenol red-free DMEM containing 0.5% FBS and 25 mM Hepes, pH 7.4). Then the medium was changed to fresh ‘medium A’ containing vehicle or 1 mM L-NAME. After preincubation with L-NAME for 30 min, the cells were treated with 1 μM A23187 for one hr. The conditioned media were collected to measure NOx in the same fashion as described in Fig. 6. Panel A shows a typical original recording while panel B shows the quantification. Data represent mean ± SEM (n=3).
DISCUSSION
The discovery and growing interest in NO as a biomolecule with critical roles in normal vascular biology and pathophysiology have warranted the need for analytical methods for its quantification. There are many different analytical methods to determine NO production directly or indirectly. Each method may have its own advantages as well as limitations. The objective of this work was the improvement of a method to determine nitrate and nitrite in biological fluid employing an NO-selective electrochemical sensor, previously described by Berkels et al. [19]. The current method is very sensitive, with a detection limit of 0.1 nM, which means that, in our system, a sample of 100 μl with a nitrite content of 10 nM would be detected. The volume of the sample may be increased up to 1 ml if the nitrite level in the sample is 1–10 nM. The detection limit of the current method was significantly lower than the DAN assay.
One of the major improvements was achieved by using a new sensor. The sensor used in this study generated 100 times higher current than the old version, providing far more stable signals (Fig. 1 and 2). The sensors employ a gas permeable membrane for selectivity assurance, coupled with a controlled instrumental parameter. The sensor is very specific to NO because 1) the gas membrane eliminates all ions and other compounds except gases, and 2) the applied electrical potential and electrode material eliminate interferences from other gases such as oxygen, carbon monoxide, carbon dioxide, etc.
The analytical method is based on the conversion of nitrite to NO in acidic iodide solutions, which can be detected by the sensor. The conversion of nitrate to nitrite is necessary to measure the total amount of NO formed by the cells. Without a conversion, NO was not produced in an acidic iodide solution (Fig. 3). Quantitative conversion of nitrate to nitrite was achieved by using coupled enzyme reactions of nitrate reductase and glucose-6-phosphate dehydrogenase (Fig. 3). The coupled enzyme system was so efficient that re-oxidation of nitrite to nitrate was prevented during analysis.
The current analytical method was designed to determine NO production from cultured cells incubated for a fixed time period but not to monitor the NO release online at a defined time. A big advantage of this method is that the total (basal and agonist-induced) NO release of cells in culture can be measured because the stable degradation products of NO (nitrate/nitrite) accumulate in the incubation media. Due to the sensitivity of the current analytical method, we could determine the background nitrite/nitrate level in culture media. Therefore further improvement of this method is expected to be achieved by developing nitrate/nitrite-free culture media because the sensitivity of the sensor is already high enough. In summary, we presented a highly sensitive and convenient method of determining NO production from cultured endothelial cells.
Acknowledgments
This work was supported by funding from a National Institute of Health grant HL67413 and HL075209 (HJ). This work was also supported by the Brain Korea 21 Project in 2006 (YB).
Abbreviations
- NO
Nitric Oxide
- DAN
3-diaminonaphthalene
- NR
nitrate reductor
- BAEC
bovine aortic endothelial cells
- DMEM
Dulbecco’s minimum Eagle’s medium
- FBS
fetal bovine serum
- L-NAME
NG-nitro-L-arginine methyl ester
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 2.Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol. 2003;285:C499–508. doi: 10.1152/ajpcell.00122.2003. [DOI] [PubMed] [Google Scholar]
- 3.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 4.Celermajer DS. Endothelial dysfunction: does it matter? Is it reversible? J Am Coll Cardiol. 1997;30:325–333. doi: 10.1016/s0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 5.Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci USA. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel RP, McAndrew J, Sellak H, White CR, Jo H, Freeman BA, Darley-Usmar VM. Biological aspects of reactive nitrogen species. Biochim Biophys Acta. 1999;1411:385–400. doi: 10.1016/s0005-2728(99)00028-6. [DOI] [PubMed] [Google Scholar]
- 7.Miles AM, Wink DA, Cook JC, Grisham MB. Determination of nitric oxide using fluorescence spectroscopy. Methods Enzymol. 1996;268:105–120. doi: 10.1016/s0076-6879(96)68013-6. [DOI] [PubMed] [Google Scholar]
- 8.Nagano T. Practical methods for detection of nitric oxide. Luminescence. 1999;14:283–290. doi: 10.1002/(SICI)1522-7243(199911/12)14:6<283::AID-BIO572>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 9.Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- 10.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- 11.Kleinhenz DJ, Fan X, Rubin J, Hart CM. Detection of endothelial nitric oxide release with the 2,3-diaminonapthalene assay. Free Radic Biol Med. 2003;34:856–861. doi: 10.1016/s0891-5849(02)01438-7. [DOI] [PubMed] [Google Scholar]
- 12.Cox RD, Frank CW. Determination of nitrate and nitrite in blood and urine by chemiluminescence. J Anal Toxicol. 1982;6:148–152. doi: 10.1093/jat/6.3.148. [DOI] [PubMed] [Google Scholar]
- 13.Bauer PM, Fulton D, Boo YC, Sorescu GP, Kemp BE, Jo H, Sessa WC. Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J Biol Chem. 2003;278:14841–14849. doi: 10.1074/jbc.M211926200. [DOI] [PubMed] [Google Scholar]
- 14.Dikalov S, Fink B. ESR techniques for the detection of nitric oxide in vivo and in tissues. Methods Enzymol. 2005;396:597–610. doi: 10.1016/S0076-6879(05)96052-7. [DOI] [PubMed] [Google Scholar]
- 15.Hart CM, Kleinhenz DJ, Dikalov SI, Boulden BM, Dudley SC., Jr The measurement of nitric oxide production by cultured endothelial cells. Methods Enzymol. 2005;396:502–514. doi: 10.1016/S0076-6879(05)96042-4. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Lee SI, Lee DH, Smith D, Jo H, Schellhorn HE, Boo YC. Ascorbic acid synthesis due to l-gulono-1,4-lactone oxidase expression enhances NO production in endothelial cells. Biochem Biophys Res Commun. 2006;345:1657–1662. doi: 10.1016/j.bbrc.2006.05.090. [DOI] [PubMed] [Google Scholar]
- 17.Boo YC, Kim HJ, Song H, Fulton D, Sessa W, Jo H. Coordinated regulation of endothelial nitric oxide synthase activity by phosphorylation and subcellular localization. Free Radic Biol Med. 2006 doi: 10.1016/j.freeradbiomed.2006.1003.1024.. in press. [DOI] [PubMed] [Google Scholar]
- 18.Boulden BM, Widder JD, Allen JC, Smith DA, Al-Baldawi RN, Harrison DG, Dikalov SI, Jo H, Dudley J, Samuel C. Early Determinants of H2O2-Induced Endothelial Dysfunction. Free Radic Biol Med. 2006 doi: 10.1016/j.freeradbiomed.2006.1005.1030. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkels R, Purol-Schnabel S, Roesen R. A new method to measure nitrate/nitrite with a NO-sensitive electrode. J Appl Physiol. 2001;90:317–320. doi: 10.1152/jappl.2001.90.1.317. [DOI] [PubMed] [Google Scholar]
- 20.Verdon CP, Burton BA, Prior RL. Sample pretreatment with nitrate reductase and glucose-6-phosphate dehydrogenase quantitatively reduces nitrate while avoiding interference by NADP+ when the Griess reaction is used to assay for nitrite. Anal Biochem. 1995;224:502–508. doi: 10.1006/abio.1995.1079. [DOI] [PubMed] [Google Scholar]
- 21.Jo H, Sipos K, Go YM, Law R, Rong J, McDonald JM. Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gbeta/gamma-dependent signaling pathways. J Biol Chem. 1997;272:1395–1401. doi: 10.1074/jbc.272.2.1395. [DOI] [PubMed] [Google Scholar]


