Abstract
Although several genes/genetic loci involved in the etiology of Wilms’ tumor have been identified, little is known of the molecular changes associated with relapse. We therefore undertook an analysis by comparative genomic hybridization (CGH) of 58 tumor samples of favorable histology Wilms’ tumor taken at initial diagnosis and/or relapse. Tumors with anaplastic histology were excluded as this is known to be associated with p53 mutation and a poor prognosis. A control group of 21 Wilms’ tumors that did not relapse was also analyzed. The overall frequency of gains or losses of genetic material detected by CGH was similar in both groups (77% in relapsing tumors and 70% in the nonrelapse group) as was the median number of changes per tumor (relapse group: n = 4, range, 1 to 19; nonrelapse group: n = 3, range, 1 to 8). However, gain of 1q was significantly more frequent in the relapse series [27 of 46 (59%) versus 5 of 21 (24%), P = 0.019]. In 12 matched tumor pairs, the CGH profiles, including 1q gain, were similar at diagnosis and relapse, with little evidence for further copy number changes being involved in clonal evolution. The results suggest that 1q gain at diagnosis could be used to identify patients with favorable histology Wilms’ tumor at increased risk of relapse who might benefit from early treatment intensification.
Wilms’ tumor or nephroblastoma, is one of the success stories of pediatric oncology, with overall long-term survival rates in excess of 85%. However, there remains a small group of patients whose tumors progress or relapse unexpectedly. In these cases, the chance of successful retreatment is much poorer, despite intensive second line therapy. 1 Furthermore, survivors risk compromised long-term renal function through use of potentially nephrotoxic chemotherapeutic agents administered to a uninephric patient. Therefore, selection of patients at diagnosis who might benefit from intensification of initial chemotherapy is important. The prognostic factors used currently by the two major international Wilms’ tumor groups for risk-adapted stratification of therapy are tumor stage and histological subtype. Unfavorable histology Wilms’ tumor is defined by the presence of anaplasia, which can be focal or diffuse, the latter subtype having survival rates of <50%. Anaplasia is associated with somatic p53 mutation that can be confined to areas of focal anaplasia, implying its involvement in clonal evolution. 2 However, the majority of Wilms’ tumors that relapse do not show anaplasia, implying that other factors must be involved in treatment failure.
Several genes are known to be involved in Wilms’ tumor development, including the WT1 gene at 11p13, one or more genes at the Beckwith-Wiedemann syndrome locus at 11p15, at least two familial Wilms’ tumor genes at 17q and 19q, plus other loci defined by allele loss and/or rare translocation breakpoints. 3-6 WT1 is mutated in ∼10% of sporadic Wilms’ tumors, where its prognostic significance is not defined, although WT1 mutation has been associated with a poor outcome in acute myeloid leukemia. 7 Extensive allele loss studies have suggested that loss of heterozygosity (LOH) for 1p, 16q, and possibly 22q are adverse prognostic features in Wilms’ tumor. 8-10 These are now being tested prospectively in the current National Wilms’ Tumor Study Group trial, NWTS-5. Expression of the multidrug resistance gene, MDR1, is not common in Wilms’ tumor. 11 More recently, other molecular factors such as expression of p53 and high levels of telomerase activity have been associated with increased risk of relapse in Wilms’ tumor. 12,13 In this study, we have screened for genomic imbalances using comparative genomic hybridization (CGH) in favorable histology Wilms’ tumors that relapsed and compared the results with cases that did not.
Materials and Methods
Tumors that were snap-frozen after surgery were received from the National Wilms’ Tumor Study Group, various United Kingdom Children’s Cancer Study Group centers, and Germany. There were 58 relapsed Wilms’ tumors (12 matched tumor pairs sampled at diagnosis and at relapse, 29 at diagnosis only, and five at relapse only). A nonrelapse control group consisting of 21 Wilms’ tumors from patients with a minimum of 2 years of follow-up without relapse was also analyzed, with observer blinding to clinical outcome data. The distribution of tumor stage in the two groups is shown in Table 1 ▶ . Tumors with anaplastic histology were deliberately excluded, as these are associated with p53 mutations and seem to be a distinct biological entity.
Table 1.
Disease Stage and Its Association with 1q+
| Tumor status | Tumor Stage | |||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Relapse | 1q+ | 3 | 4 | 11 | 7 | 2 |
| 1q− | 7 | 6 | 4 | 2 | 0 | |
| Nonrelapse | 1q+ | 1 | 1 | 1 | 2 | 0 |
| 1q− | 4 | 3 | 8 | 1 | 0 | |
Tumors with gain of 1q material were more likely to be of advanced stage (defined as stage III/IV) (21 of 30 versus 15 of 35, P = 0.05).
CGH was performed as described previously. 14 Briefly, 1 μg of both tumor and sex-matched reference (from healthy normal individuals) DNA was directly labeled by nick translation with either fluorescein-12-dUTP or rhodamine-12-dUTP (fluorored or fluorogreen; Amersham International, Amersham, Buckinghamshire, UK). Labeled DNA was assessed on a 1% agarose gel with an optimal size of 500 to 2,000 bp. Between 500 to 750 ng of each DNA and 25 to 40 μg of Cot 1 DNA (Life Technologies, Inc., Rockville, MD) was co-hybridized to normal denatured metaphase slides (Vysis Inc., Downer’s Grove, IL) for 72 hours at 37°C. After hybridization the slides were washed and mounted in Citifluor antifade (Vector Laboratories Inc., Burlingame, CA) with 0.1 μg/ml 4′,6-diamidino-2-phenylindole as a counterstain. Images were captured using a cooled charge-coupled device camera (Photometrics, Tuscon, AZ) and the QUIPS-CGH software package (Vysis Inc.) was used for analysis. The prevalences of chromosomal imbalances between the relapse and nonrelapse groups were compared by the two-tailed Fisher’s exact test.
Results
At least five representative metaphases were combined to produce a mean fluorescence ratio ±1 SD. The average ratios of fluorescence intensity and their SDs were determined in control CGH experiments using differentially labeled normal DNA and did not exceed 1.0 ± 0.15. A copy number change in a sample was indicated when the average fluorescence ratio from at least 10 chromosomes lay outside this range (0.85 to 1.15). High copy number gain was scored when the average ratios at a chromosomal location exceeded 1.5.
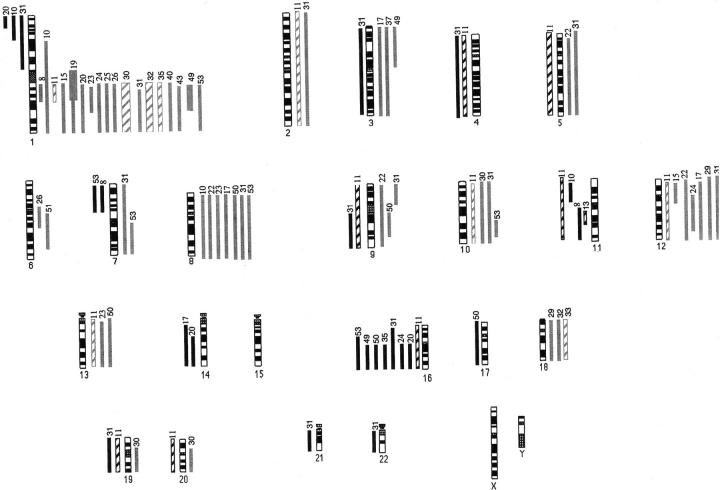
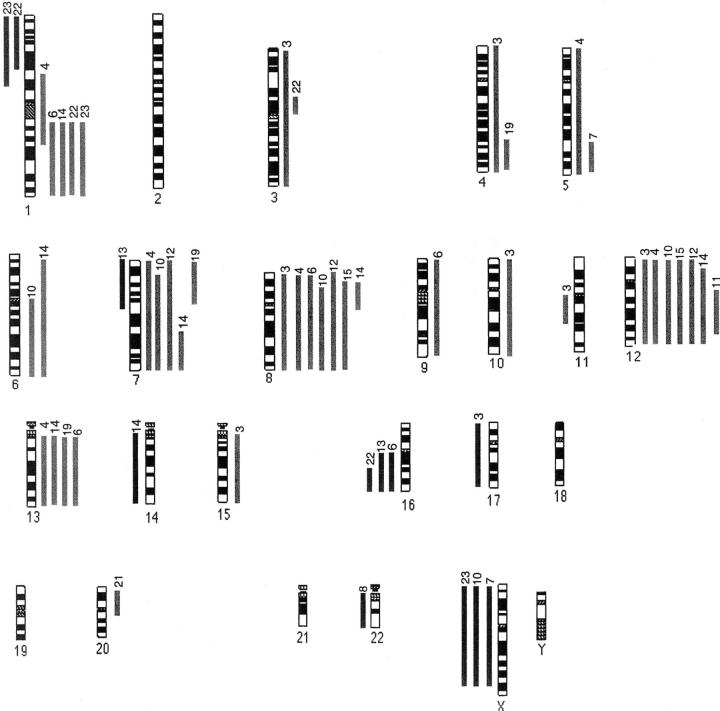
Overall, CGH analysis revealed genomic copy number changes in >70% of Wilms’ tumors in both the relapse and nonrelapse groups, with gains being more frequently observed than losses (Figures 1, 2, and 3) ▶ ▶ ▶ . Gain of 1q material was the most frequently observed change in the relapse group and this was significantly higher than the rate in the nonrelapse group [27 of 46 (59%) versus five of 21 (24%), P = 0.019, Fisher’s exact test]. Most of those tumors showed gain of the whole of chromosome 1q, but in six tumors the gain was partial and allowed definition of a smallest region of common gain spanning 1q21-25. In three cases, 1q gain was the sole CGH abnormality, including one case analyzed at diagnosis, one at relapse, and a matched pair. In eight cases, 1p loss coexisted with 1q gain, suggesting the existence of an isochromosome 1q or an unbalanced translocation. Other consistent changes, all found in one third or less of cases, included gains of material from chromosomes 8 and 12 and loss involving 1p, 11p, 16q, and 22q. These changes occurred at similar frequencies in both groups and were not significantly associated with adverse outcome (Table 2) ▶ . For the majority of imbalances, the average fluorescence ratios were just outside the cut-off limits. This would be consistent with cellular heterogeneity of copy number changes within tumors.
Figure 1.
Ideogram representing 34 relapsing Wilms’ tumors taken at diagnosis or relapse. Solid gray lines on the right represent gain at diagnosis and hashed lines represent gain at relapse. Solid black lines on the left represent loss at diagnosis and hashed lines represent loss at relapse. Thick solid or hashed lines represent higher levels of gain (fluorescence ratio >1.5). Case numbers are shown at the top of each vertical line.
Figure 2.
Ideogram representing 12 matched tumor pairs taken at diagnosis and relapse.
Figure 3.
Ideogram representing 21 nonrelapsing Wilms’ tumors.
Table 2.
Frequencies of the Most Common Chromosomal Imbalances Found in Relapse and Nonrelapse Wilms Tumors
| Chromosomal imbalance | Relapse n = 46 | Nonrelapse n = 21 | Fisher’s exact test, P value |
|---|---|---|---|
| +1q | 27 (59%) | 5 (24%) | 0.01* |
| −1p | 7 (15%) | 2 (10%) | 0.91 |
| +12 | 15 (33%) | 7 (33%) | 1.00 |
| −16q | 10 (22%) | 3 (14%) | 0.74 |
| −11p | 5 (13%) | 0 (0%) | 0.17 |
| −22q | 3 (7%) | 1 (5%) | 1.00 |
| +8 | 11 (24%) | 7 (33%) | 0.55 |
| −X | 4 (9%) | 3 (14%) | 0.67 |
*Significant difference. Relative risk of relapse for 1q+ v. 1q− = 4.5 (1.4–14, 95% confidence intervals).
Gain of material on 1q was associated with more advanced disease at first diagnosis: 70% of tumors with 1q gain were stage III or IV at diagnosis compared with only 43% of tumors lacking 1q gain (P = 0.05) (Table 1) ▶ .
CGH analysis of 12 paired tumor samples from both diagnosis and relapse did not reveal any evidence for clonal evolution (Figure 2) ▶ . Eleven of the 12 matched tumor pairs had abnormalities detected by CGH; the median number of changes was identical at diagnosis (median, 5; range, 2 to 19) and relapse (median, 5; range, 2 to 17). Regions of gain and loss observed at diagnosis were generally still present at relapse with occasional additional changes. Nine cases had gain of 1q and this was detectable at diagnosis in all but one case. The tumor lacking CGH changes had an identical WT1 mutation detectable in both diagnosis and relapse specimens (homozygous nonsense mutation, TCG→TAG = Ser313→STOP).
Discussion
This is the first CGH study to focus on relapsed Wilms’ tumor and demonstrates a significant association between gain of 1q genomic material detected at original diagnosis and risk of tumor recurrence, with a 4.5-fold increase in the relative risk of relapse. The region of common gain is large, spanning 1q21-q25. This region has also been associated with resistant disease in another childhood embryonal tumor, neuroblastoma. 15 Although gain of 1q is one of the commonest changes observed in both adult and pediatric solid tumors, this is usually as part of a spectrum of other changes. 16 In three cases of Wilms’ tumor studied here, 1q gain was the sole abnormality and it was the only genomic copy number alteration to show a difference between relapsed and nonrelapsed cases. This suggests that it may be possible to define a molecular marker at diagnosis to identify a poorer risk group among favorable histology Wilms’ tumors and ultimately to stratify treatment intensity.
Overall, this analysis shows a greater frequency but similar pattern of chromosomal imbalances to previous CGH studies of Wilms’ tumors sampled at diagnosis in which no clinical outcome data were presented. 17,18 These two previous analyses had found a prevalence of only 21% and 40% CGH abnormalities, respectively, in contrast to the >70% found in this study. The inclusion of a control group of nonrelapsed tumors in the current analysis suggests that this apparent difference is most likely because of differences of technique sensitivity rather than patient selection. With the exception of the excess of 1q gain, the pattern and frequency of abnormalities detected by CGH in this series of relapsed Wilms’ tumors is similar to that found cytogenetically in unselected tumors. A review of 142 Wilms’ tumor karyotypes with clonal abnormalities revealed trisomy 8 and 12 in 20 to 25% of cases and gain of 1q in 20%. Chromosome loss was less frequent, affecting mainly 11p (20% of cases). 16 These percentages are likely to be an overrepresentation of the true prevalence of these changes, because Wilms’ tumors with normal karyotypes were not included in the analysis, even though they undoubtedly exist, as shown by both cytogenetic and CGH studies. The mechanism for gain of 1q detected by CGH could reflect iso(1q) formation or unbalanced translocation with 16q as the most frequent partner. 19,20 However, in the 27 cases with 1q gain, there was corresponding loss of 1p in only seven cases and of 16q in only 10 cases. Taken together, these comparisons suggest that the association of 1q gain with increased risk of tumor recurrence in favorable histology Wilms’ tumor is a real one.
Previous allele loss studies of Wilms’ tumor have highlighted LOH at 16q, 22q, and possibly 1p as being associated with increased risk of relapse. 8-10 In those three studies, the prevalence of allele loss at 16q (13 to 17%) and at 1p (10%) was similar to the frequency of genomic loss detected by CGH in our study whereas that for 22q LOH (14%) was somewhat higher. However, there was no significant association between CGH abnormalities at these loci and relapse (Table 2) ▶ . This apparent discrepancy could either be because of allele loss occurring together with reduplication, which would not be detected by CGH, or to these regions of allele loss being associated with anaplastic Wilms’ tumor, which was not included in our study. Certainly, in only one study was the adverse effect of 16q LOH independent of unfavorable histology and in another study, both 16q and 22q were associated with anaplasia. 8,10
CGH abnormalities at the sites of other known or putative Wilms’ tumor genes were not significantly associated with relapse (Table 2) ▶ . Loss of material from 11p, the site of the WT1 gene and the more telomeric Beckwith-Wiedemann loci, was found at a much lower frequency than the 30 to 50% of Wilms’ tumors reported to show allele loss. This implies that 11p LOH commonly involves mitotic recombination or loss and reduplication. The glypican 3 gene at Xp36 is mutated in the Simpson-Golabi-Behmel syndrome of overgrowth and predisposition to Wilms’ tumor. 21 However, loss of the X chromosome was seen in only seven tumors and was not associated with relapse.
Overall, this study of a large series of relapsed Wilms’ tumors highlights only a single genomic region, namely gain of 1q21-25, as being associated with tumor recurrence. This change is also associated with more advanced disease at first diagnosis. Although the region of gain detected here is large, it would span an amplicon at 1q21-22 found commonly in sarcomas. 22 It is also of interest that regulatory sequences of an unknown putative target gene for WT1 map to 1q21-22. 23 Of clinical relevance, we find no evidence for instability of genomic copy number changes with tumor recurrence. This suggests that whatever the molecular abnormality contained within the 1q gain, it is active from first presentation of the tumor. Further analyses are underway to refine the common region of gain/amplification and to identify overexpressed genes within this region. In this way, we aim to identify a molecular marker(s) that may discriminate apoorer risk group among favorable histology Wilms’ tumors and that would be amenable to testing in a much larger series of Wilms’ tumors in a prospective clinical trial.
Acknowledgments
We thank the National Wilms’ Tumor Study Group, the Cooperative Human Tissue Network (Columbus, OH), which is funded by the National Cancer Institute, the German (GPOH) nephroblastoma study, and the United Kingdom Children’s Cancer Study Group for access to specimens; and Dr. I. Jeffrey (St George’s Hospital, London, UK), Dr. A. Kelsey (Children’s Hospital, Manchester, UK), Dr. S. Variend (Children’s Hospital, Sheffield, UK), and Dr. K. Brown (University of Bristol, Bristol, UK); Roger A’hern (Royal Marsden Hospital Sutton, Surrey), for statistical advice; and Miss Elizabeth Parr for secretarial assistance.
Footnotes
Address reprint requests to Dr. Kathy Pritchard-Jones, Section of Paediatric Oncology, Institute of Cancer Research/Royal Marsden, NHS Trust, Downs Rd., Sutton, Surrey SM2 5PT, UK. E-mail: kpj@icr.ac.uk.
Supported by the Cancer Research Campaign (to K. P. J., L. K. U., and Y. J. U.), and the Children’s Cancer Unit Fund, Royal Marsden Hospital (to S. H.).
References
- 1.Pinkerton CR, Groot-Loonen JJ, Morris-Jones PH, Pritchard J: Response rates in relapsed Wilms’ tumor. Cancer 1990, 67:567-571 [DOI] [PubMed] [Google Scholar]
- 2.Bardessey N, Beckwith B, Pelletier J: Clonal expansion and attenuated apoptosis in Wilms’ tumors are associated with p53 gene mutations. Cancer Res 1995, 55:215-219 [PubMed] [Google Scholar]
- 3.Hastie ND: The genetics of Wilms’ tumour—a case of disrupted development. Annu Rev Genet 1994, 28:523-558 [DOI] [PubMed] [Google Scholar]
- 4.Wilmore HP, White GF, Howell RT, Brown KW: Germline and somatic abnormalities of chromosome 7 in Wilms’ tumour. Cancer Genet Cytogenet 1994, 77:93-98 [DOI] [PubMed] [Google Scholar]
- 5.Rahman N, Arbour L, Tonin P, Renshaw J, Pelletier J, Baruchel S, Pritchard-Jones K, Stratton MR, Narod SA: Evidence for a familial Wilms’ tumour gene (FWT1) on chromosome 17q12–q21. Nat Genet 1996, 13:461-463 [DOI] [PubMed] [Google Scholar]
- 6.McDonald JM, Douglass EC, Fisher R, Geiser CF, Krill CE, Strong LC, Virshup D, Huff V: Linkage of familial Wilms’ tumour predisposition to chromosome 19 and a two-locus model for the etiology of familial tumours. Cancer Res 1998, 58:1387-1390 [PubMed] [Google Scholar]
- 7.King-Underwood L, Pritchard-Jones K: Wilms’ tumor (WT1) gene mutations occur mainly in acute myeloid leukemia and may confer drug resistance. Blood 1998, 91:2961-2968 [PubMed] [Google Scholar]
- 8.Grundy PE, Telzerow PE, Breslow N, Moksnes J, Huff V, Paterson MC: Loss of heterozygosity for chromosomes 16q and 1p in Wilms’ tumours predicts an adverse outcome. Cancer Res 1994, 54:2331-2333 [PubMed] [Google Scholar]
- 9.Grundy RG, Pritchard J, Scambler P, Cowell JK: Loss of heterozygosity on chromosome 16 in sporadic Wilms’ tumour. Br J Cancer 1998, 78:1181-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klamt B, Schulze M, Thate C, Mares J, Goetz P, Kodet R, Scheurlen W, Weirich A, Graf N, Gessler M: Allele loss in Wilms’ tumors of chromosome arms 11q, 16q, and 22q correlates with clinicopathological parameters. Genes Chromosom Cancer 1998, 22:287-294 [DOI] [PubMed] [Google Scholar]
- 11.Goldstein LJ, Galski H, Fojo A, Willingham M, Lai SL, Gazdar A, Pinker R, Green A, Crist W, Brodeur GM: Expression of multidrug resistance gene in human cancers. J Natl Cancer Inst 1989, 81:116-124 [DOI] [PubMed] [Google Scholar]
- 12.Lahoti C, Thorner P, Malkin D, Yeger H: Immunohistochemical detection of p53 in Wilms’ tumors correlates with unfavorable outcome. Am J Pathol 1996, 148:1577-1589 [PMC free article] [PubMed] [Google Scholar]
- 13.Dome JS, Chung S, Bergemann T, Umbricht CB, Saji M, Carey LA, Grundy PE, Perlman EJ, Breslow NE, Sukumar S: High telomerase reverse transcriptase (hTERT) messenger RNA level correlates with tumor recurrence in patients with favorable histology Wilms’ tumor. Cancer Res 1999, 59:4301-4307 [PubMed] [Google Scholar]
- 14.Weber-Hall S, Anderson J, McManus A, Abe S, Nojima T, Pinkerton R, Pritchard-Jones K, Shipley J: Gains, losses and amplification of genomic material in rhabdomyosarcoma analysed by comparative genomic hybridisation. Cancer Res 1996, 56:3320-3324 [PubMed] [Google Scholar]
- 15.Hirai M, Yoshida S, Kashiwagi H, Kawamura T, Ishikawa T, Kaneko M, Ohkawa H, Nakagawara A, Miwa M, Uchida K: 1q23 gain is associated with progressive neuroblastoma resistant to aggressive treatment. Genes Chromosom Cancer 1999, 25:261-269 [DOI] [PubMed] [Google Scholar]
- 16.Mertens F, Johansson B, Hoglund M, Mitelman F: Chromosomal imbalance maps of malignant solid tumors: a cytogenetic survey of 3185 neoplasms. Cancer Res 1997, 57:2765-2780 [PubMed] [Google Scholar]
- 17.Getman ME, Houseal TW, Miller GA, Grundy PE, Cowell JK, Landes GM: Comparative genomic hybridization and its application to Wilms’ tumorigenesis. Cytogenet Cell Genet 1998, 82:284-290 [DOI] [PubMed] [Google Scholar]
- 18.Steenman M, Redeker B, DeMeulemeester M, Wiesmeijer K, Voute PA, Werterveld A, Slater R, Mannens M: Comparative genomic hybridization analysis of Wilms’ tumours. Cytogenet Cell Genet 1997, 77:296-303 [DOI] [PubMed] [Google Scholar]
- 19.Slater RM, Mannens MMAM: Cytogenetics and molecular genetics of Wilms’ tumor of childhood. Cancer Genet Cytogenet 1992, 61:111-121 [DOI] [PubMed] [Google Scholar]
- 20.Mitelman F: Catalog of Chromosomes Aberrations in Cancer, ed 6 1998, Wiley Liss, New York
- 21.Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Humber R, Neri G, Cao A, Forabosco A, Schlessinger D: Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet 1996, 12:241-247 [DOI] [PubMed] [Google Scholar]
- 22.Forus A, Berner JM, Meza-Zepeda LA, Saeter G, Mischke D, Fodstad O, Myklebost O: Molecular characterization of a novel amplicon at 1q21-1q22 frequently observed in human sarcomas. Br J Cancer 1998, 78:495-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Law MH, Agar E, Little M: Allelic imbalance at chromosome 1q21 in Wilms’ tumor. Cancer Genet Cytogenet 1997, 97:54-59 [DOI] [PubMed] [Google Scholar]