Abstract
Rejection of renal and cardiac xenografts is initiated when natural antibodies of the recipient bind to donor endothelium, activating complement on the surface of endothelial cells. Pulmonary xenotransplants, however, reveal less evidence of antibody binding and complement activation and, in contrast to other xenografts, fare worse when the complement of the graft recipient is depleted. Accordingly, we asked whether distinct immunochemical reactions might occur after xenotransplantation of the lung and what implications such reactions might have for pulmonary pathophysiology. Analysis of serum from baboons after transplantation with porcine lungs revealed complexes containing baboon IgM and porcine von Willebrand factor. The baboon IgM in these complexes was specific for Galα1-3Gal. Immune complexes were also seen, albeit to a lesser extent, in the serum of kidney and heart xenotransplant recipients. Deposits of porcine von Willebrand factor and baboon C3 were detected in livers and spleens of transplanted baboons. These results indicate pulmonary xenotransplantation eventuates in formation of immune complexes and in the deposition of those complexes at distant sites. Immune complex formation could explain the peculiar fate of xenoreactive antibodies after pulmonary xenotransplantation and might contribute to the pathophysiology of the lung and systemic changes not previously considered a complication of xenotransplantation.
The rejection of organs transplanted between disparate species is generally thought to be initiated by the binding of natural antibodies to endothelial cells lining the blood vessels of the newly transplanted organ. 1-3 Xenoreactive natural antibodies bound to porcine kidneys or hearts transplanted into baboons activate the complement cascade by the classical pathway, leading to insertion of terminal complement complexes in donor endothelium and, thus, to the rejection of the organ. 3-5 Consistent with this concept, rejected xenografts contain deposits of recipient antibody and complement, including the membrane attack complex, along the luminal aspect of blood vessel endothelium. 3,4 Because endothelium is the evident target of the rejection process, the authors 6 and others 7 have suggested that the pathophysiology of xenograft rejection might be deduced from analysis of the biology of endothelial cells.
Although xenogeneic lung transplants undergo very rapid loss of function, 8,9 xenotransplantation of the lung may present a different type of rejection process than described above. The immunopathology of pulmonary xenografts reveals relatively little antibody and complement deposited on donor microvasculature. 8,10 Treatment of the recipient with cobra venom factor, which consumes complement and prevents the hyperacute rejection and early dysfunction of cardiac and renal xenografts, 11-13 does not prevent injury to pulmonary xenografts and may impair early lung function. 14 Similarly, depletion of xenoreactive antibodies may not prevent early dysfunction of xenogeneic lungs. 9
One potential explanation for the difference between pulmonary and renal and cardiac xenografts is that porcine pulmonary microvascular endothelial cells might express a lower level of antigen or different antigen than the porcine heart or kidney microvasculature. However, we recently determined that porcine lung endothelium contains similar types of glycoprotein antigens that express equivalent amounts of Galα1-3Gal, 15 the major epitope recognized by xenoreactive natural antibodies that trigger hyperacute rejection of porcine cardiac and kidney xenografts. 16-18 Another possible explanation is that immunochemical detection of bound antibody is more difficult because of distribution of antigen throughout the larger vascular area of the lung. Still another possibility is that after binding to lung endothelium, xenoreactive antibodies undergo endocytosis or shedding, which are processes that might be facilitated by formation of the membrane attack complexes, 19 or hypoxia, which is due to of pretransplant manipulation. 20 We explored the fate of xenoreactive antibodies after transplantation of porcine organs into baboons. Our studies revealed that antibody-antigen complexes are shed from the newly transplanted lungs and, to a lesser extent, newly transplanted kidneys and hearts, and that the complexes are deposited at remote locations in the recipient. The results have implications for the fundamental mechanisms by which pulmonary xenografts, and perhaps allografts, undergo immune-mediated injury and warn of potential systemic complications of xenotransplantation.
Materials and Methods
Sources of Blood
Serum samples were prepared from blood taken from adult baboons before and after orthotopic transplantation with porcine lungs, porcine kidneys, or porcine hearts, 14,21 or baboon blood before and after ex vivo perfusion of a porcine kidney, heart, or lung. 22,23 Human serum was prepared from blood obtained from healthy volunteers. Serum samples were stored at −80°C until needed.
Cell Cultures
Microvascular endothelial cells were isolated from porcine lungs, as previously described. 15 The endothelial cells were grown in Dulbecco’s modified Eagle’s medium containing l-glutamine (2.0 mmol/L), penicillin (100 U/ml), streptomycin (100 μg/ml) (Life Technologies, Grand Island, NY), 10% fetal calf serum (Hyclone, Logan, UT), 90 μg/ml heparin, and 75 μg/ml endothelial cell growth supplement (Sigma Chemical Co., St. Louis, MO). The microvascular cells took up acetylated, low-density lipoprotein and did not react with antibodies specific for smooth muscle α-actin (Accurate Chemical and Scientific Corp., Westbury, NY). 24
Electron Microscopy
Porcine lung tissue samples, from before and after transplantation into baboons, were fixed in 1% glutaraldehyde and 4% formaldehyde in 0.1 mol/L phosphate buffer, pH 7.2, rinsed in phosphate-buffered saline (PBS), pH 7.2 (Life Technologies, Inc,) and postfixed in phosphate buffered 1% osmium tetroxide for 1 hour. Samples were rinsed in distilled water and stained en bloc with 2% uranyl acetate for 30 minutes at 60°C. Samples were rinsed in distilled water, dehydrated in progressively increasing fractions of ethanol in propylene oxide and embedded in Spurr’s resin. Sections of 90 nm were cut and stained with lead citrate.
Isolation and Characterization of Porcine Antigens
Immune complexes in baboon blood after orthotopic porcine lung transplantation, or perfusion of the blood through porcine lungs, kidneys, or hearts were isolated as follows. Serum prepared from baboon and human blood was diluted to 50% in PBS, treated with 10 mmol/L of dl-dithiothreitol (Sigma Chemical Co.) and incubated at 37°C for 15 minutes to depolymerize IgM. To remove the dl-dithiothreitol after the reduction reaction, serum was dialyzed against three changes of PBS in Slide-A-Lyzer cassettes, 10,000 molecular weight cutoff (Pierce, Rockford, IL) at 4°C. Serum was then diluted to a final concentration of 20% with 12% polyethylene glycol (PEG) 8000 and 60 mmol/L of ethylenediaminetetraacetic acid (EDTA) in veronal buffered saline (0.145 mol/L sodium chloride, 2 mmol/L sodium barbital, and 3 mmol/L barbital) for 16 hours at 4°C. The serum/PEG solution was centrifuged at 2,000 × g for 20 minutes at 4°C to pellet immune complexes. Precipitates were resuspended in PBS, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and visualized by Western blotting, as described below.
Immunohistology
Porcine organs (lungs, kidneys, and hearts) transplanted into baboons, and baboon livers and spleens were biopsied at various time points. Tissues were covered with OCT compound (Sakura, Torrance, CA), snap-frozen by immersion in precooled isopentane, and stored at −80°C. Tissue sections (4-μm thick) were mounted on positively charged microscope slides (Superfrost Plus, Fisher Scientific, Pittsburgh, PA), briefly heat-fixed, and stored at −80°C in an air-tight slide container until needed. Before staining, slides were briefly air-dried at room temperature, fixed for 10 minutes in cold acetone, and air-dried for an additional 10 minutes. Sections were postfixed for 2 minutes in 100 mmol/L Tris-buffered 1% paraformaldehyde containing 1 mmol/L EDTA, pH 7.2, and rinsed with three changes of PBS, pH 7.2. Porcine von Willebrand factor (vWF) was detected using murine antibodies specific for porcine vWF (clone W1-8, kindly supplied by Dr. David Fass of Mayo Foundation, Rochester, MN). 25 Baboon IgG and IgM were detected using goat anti-human IgG [fluorescein isothiocyanate (FITC)-conjugated; Kirkegaard & Perry Laboratories, Gaithersburg, MD] and goat anti-human IgM (FITC-conjugated, Kirkegaard & Perry Laboratories), respectively. Baboon C3 was detected using goat anti-human C3 (FITC-conjugated; Cappel/Organon Teknika, Durham, NC). Galα1-3Gal was detected based on binding of Griffonia (Bandeiraea) simplicifolia lectin I-isolectin B4 (GSL I-B4) (FITC-conjugated; Vector Laboratories, Burlingame, CA). The antibodies listed above and GSL I-B4 were diluted in PBS containing 5% bovine serum albumin (Sigma Chemical Co.), applied to tissue sections for 30 minutes, and rinsed with PBS. Binding of mouse anti-porcine vWF to the sections was detected using affinity purified goat F(ab′)2 fragments against mouse IgG (FITC-conjugated; Organon Teknika, Durham, NC) that had been pre-absorbed with porcine and human serum. All sections were coverslipped with a 1:8 dilution of Vectashield-DAPI (4,6-diamidino-2-phenylindole) in PBS (Vector Laboratories) and evaluated using an epifluorescence microscope.
Quantitation of IgM Binding to Porcine Pulmonary Microvascular Endothelial Cells
The amount of baboon and human IgM that bound to porcine pulmonary microvascular endothelial cells was measured, as follows, by enzyme-linked immunosorbent assay (ELISA). Porcine pulmonary microvascular endothelial cells were grown to confluence in 96-well plates, washed with PBS, and fixed with cold 0.1% glutaraldehyde in PBS for 5 minutes. The fixed cells were blocked with 1% bovine serum albumin in PBS for 1 hour at room temperature and incubated with baboon serum diluted in PBS. Cells were washed and IgM binding was detected using alkaline phosphatase-conjugated goat anti-human IgM (μ-chain-specific) (Sigma Chemical Corp.), p-nitrophenylphosphate and diethanolamine, as described previously. 26
Western Blotting
Baboon serum proteins were precipitated with PEG, or isolated by Galα1-3Gal affinity columns (described below), and subjected to SDS-PAGE on 7.5% polyacrylamide gels. Proteins were transferred to Immun-Blot polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA), as described previously. 26 Membranes were blocked with 0.3% Tween-20 (Sigma Chemical Co.) in Tris-buffered saline, pH 7.2. The reactivity of blotted proteins with human IgM was tested as follows. Western blots were incubated with 5% human serum in PBS for 3 hours at 4°C and washed with 0.3% Tween-20 in Tris-buffered saline. IgM binding was detected with alkaline phosphatase-conjugated goat anti-human IgM (μ-chain-specific) and developed with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Promega Corp., Madison, WI), as described previously. 26
Porcine vWF was identified in Western blots using a mouse antibody specific for porcine vWF (described above). Binding of the mouse antibodies was detected using alkaline phosphatase-conjugated goat anti-mouse IgG (γ-chain-specific), then developed as described previously, 26 or horseradish peroxidase-conjugated donkey anti-mouse IgG, then detected by enhanced chemiluminescence (Amersham International, Arlington Heights, IL).
Baboon vWF was identified in Western blots using a rabbit antibody for human vWF (Sigma Chemical Co.), which binds to baboon vWF but not porcine vWF. Binding of rabbit antibody was detected using alkaline phosphatase-conjugated mouse anti-rabbit IgG (γ-chain-specific) and developed as described previously. 26
Digestion of Immune Complexes with α-Galactosidase
Immune complexes were precipitated with PEG (described above) from the serum of baboons with pulmonary xenografts and resuspended in digestion buffer (100 mmol/L NaCl and 50 mmol/L sodium acetate, pH 5.0) containing 1 U/ml α-galactosidase (green coffee bean; Boehringer Mannheim Corp, Indianapolis, IN), or in digestion buffer alone, as a control. The samples were incubated for 5 hours at 37°C with gentle agitation and precipitated overnight at −80°C with ethanol for evaluation by SDS-PAGE and Western blotting, as described above.
Chromatography Affinity Purification
Serum from pigs or from baboons with pulmonary xenografts was incubated with Galα1-3Galβ (Bdi)-PAA immobilized on Sepharose CL6B (GlycoTech, Rockville, MD) overnight at 4°C with agitation. The beads were washed with 20 column volumes of cold PBS and boiled for 5 minutes to release bound material. The released material was evaluated by SDS-PAGE and Western blotting, as described above.
Results
The Fate of Xenoreactive Antibodies after Xenotransplantation
The rejection of porcine organs transplanted into baboons is thought to be initiated by the binding of xenoreactive natural antibodies to donor organ endothelium. 1-3,6 As a result of antibody deposition in the transplanted organ, the concentration of xenoreactive antibodies in the blood decreases precipitously, and complement deposits along endothelial surfaces. 3
However, consistent with our previous observations, porcine lungs transplanted into baboons exhibited little Ig and complement deposition along the microvasculature, in contrast to porcine kidneys (Figure 1) ▶ . 8,10 To determine whether xenoreactive antibodies were, in fact, absorbed from the blood of xenograft recipients, the amount of xenoreactive natural antibody remaining in the blood of baboons was measured by ELISA at various times after transplantation with a porcine kidney or a porcine lung, using porcine pulmonary microvascular endothelial cells as targets. As Figure 2 ▶ shows, the levels of xenoreactive IgM decreased rapidly after kidney (73% decrease) and lung transplantation (86% decrease), suggesting the xenoreactive antibodies were depleted from the circulation.
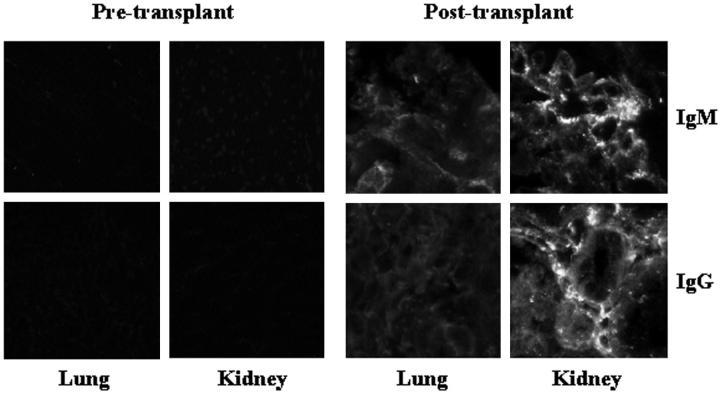
Figure 1.
Detection of xenoreactive natural antibodies in pulmonary and renal xenografts. Tissues obtained from porcine lungs and kidneys before and 2 hours after transplantation into baboons were stained for IgG and IgM. The renal xenografts contain prominent deposits of IgM and IgG; the pulmonary xenografts contain very little antibody of the recipient.
Figure 2.
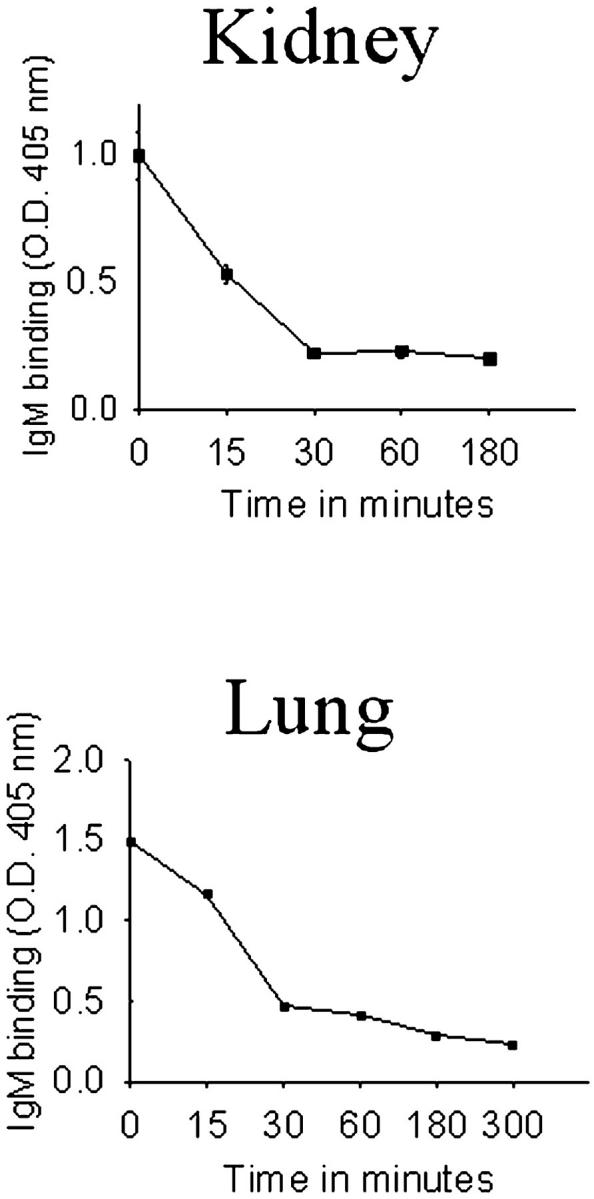
Level of xenoreactive IgM in the circulation of baboon recipients of pulmonary or renal xenografts. Blood was collected from baboons at various times after pulmonary or renal xenotransplantation and was assayed for antibody binding to cultured porcine endothelial cells by ELISA. Points of mean ±SE of triplicate determinations are shown. Standard errors are too small to be depicted.
To determine whether the absence of detectable antibody deposits might reflect distribution of bound antibody over a large vascular surface, or to other factors, we examined antibody deposits in porcine heart-lung xenografts. Figure 3 ▶ shows little IgM bound to the porcine lung, whereas abundant IgM bound to the porcine heart in the same recipient. Because the porcine heart and the lung contain similar antigenic determinants in the same configuration, 15 and because the reperfused blood passes through the lung before it passes through the heart, the absence of antibody bound to the lung is not because of dispersal of antibody over a large surface area.
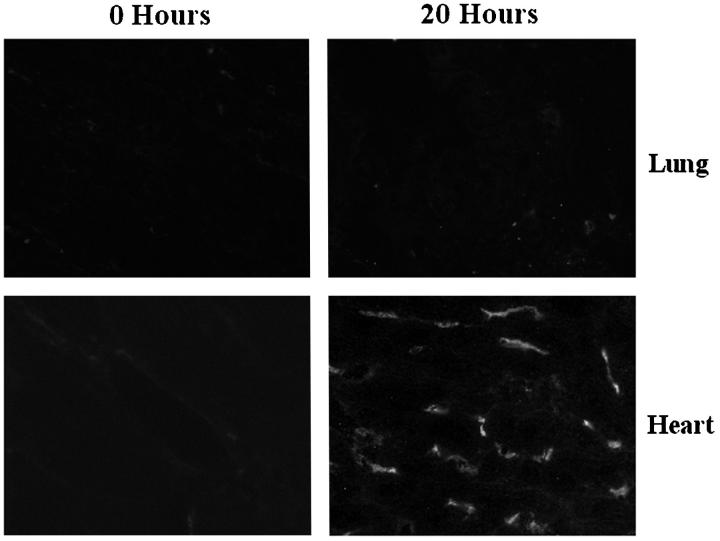
Figure 3.
Binding of xenoreactive antibodies to porcine heart and lung tissues after heart-lung xenotransplantation. Porcine hearts and lungs were biopsied 20 hours after transplantation. Frozen sections prepared from these biopsies were stained for baboon IgM. Deposits of IgM are observed in the heart, but not in the lung. Original magnification, ×400
Evidence of Antigen Shedding from Pulmonary Xenotransplants
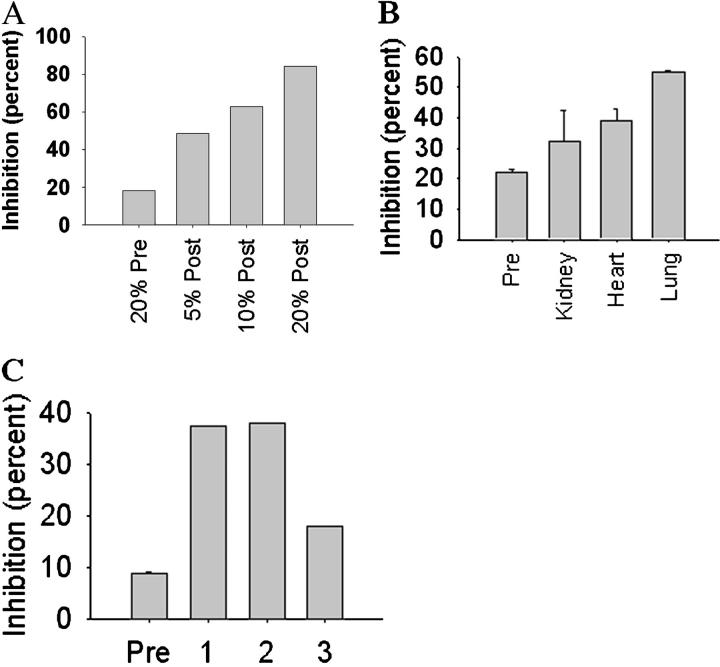
Electron micrographs of porcine lung xenografts reveal shedding of membrane fragments from vascular surfaces into the circulation, as compared to normal porcine lungs (Figure 4) ▶ . To test whether the material shed from the lung during perfusion with baboon blood contains antigens recognized by human xenoreactive antibodies, we collected baboon blood after perfusion through porcine lungs and confirmed that the blood lacked detectable levels of xenoreactive antibodies (data not shown). Serum from the perfused blood was then added to human serum known to contain xenoreactive natural antibodies, to test whether the binding of human IgM to porcine pulmonary microvascular endothelial cells might be blocked, as evidence of the presence of shed antigen. Figure 5A ▶ shows that the serum from baboon blood, which passed through porcine lungs, inhibited binding of human IgM by 84% in repeated experiments from two experiments. Blocking required the treatment of the baboon serum with dithiothreitol to release IgM that had complexed with the porcine antigen. This result suggests that porcine antigen was indeed shed into the blood and was then saturated with xenoreactive antibodies. To compare relative amounts of antigen shed from different organs, baboon blood was collected before and after perfusion through a porcine kidney, a porcine heart, or a porcine lung and then tested for the presence of antigen. Figure 5B ▶ shows that blood that had been perfused through porcine kidneys and hearts contains antigen capable of blocking the binding of human IgM to porcine aortic endothelial cells. However, the amounts of porcine antigen in blood perfused through porcine kidneys or hearts is notably less than in blood that had been perfused through porcine lungs. We next tested blood from recipients of porcine lung xenografts to determine whether antigen was shed in vivo. Serum prepared from baboon blood taken 15 to 20 minutes after porcine lung xenotransplantation inhibited human IgM binding to porcine pulmonary microvascular endothelial cells by 20 to 38% (Figure 5C) ▶ , compared to pretransplant baboon serum, indicating that antigen was shed from the transplanted organ. The limited ability of serum from pulmonary transplant recipients to inhibit IgM binding to porcine cells probably reflects the clearance of antigen associated with immune complexes from the blood in vivo, as described below.
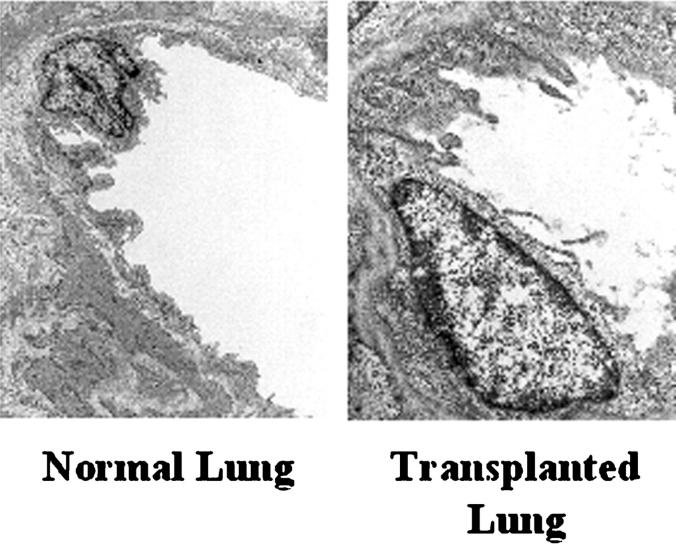
Figure 4.
Shedding of endothelial membrane from pulmonary xenografts. Tissues from porcine pulmonary xenografts in baboons and from normal pigs were fixed in 1% glutaraldehyde and 4% formaldehyde embedded in Spurr’s resin and stained with lead citrate. Ultrathin sections were studied by transmission electron microscopy. Shedding of material from the endothelium can be seen in transplanted lungs but not in normal lungs. Original magnification, ×4,000.
Figure 5.
Presence of immune complexes in baboon blood exposed to porcine lungs. Baboon blood that had been passed through porcine lungs, kidneys, or hearts (A and B) or that had been collected from recipients of pulmonary xenotransplants (C) was tested for the presence of porcine antigens by analysis of blocking of human xenoreactive antibodies. A: Blocking of human xenoreactive IgM by porcine antigen in baboon blood that had been passed through porcine lungs. Serum prepared from baboon blood before (pre) and after (post) perfusion through a porcine lung was diluted with PBS and treated with 10 mmol/L dl-dithiothreitol for 15 minutes at 37°C to depolymerize IgM and release bound antigen. The reduced serum was dialyzed against PBS in 10,000 MWCO cassettes to remove the dithiothreitol, and incubated with 5% human serum. The binding of human xenoreactive IgM to cultured porcine endothelial cells was tested by ELISA. Percent inhibition ±SE of triplicate determinations was based on the binding of 5% human serum IgM as a standard (standard errors are too small to be seen). The blocking of human xenoreactive antibodies indicated that the baboon blood contains porcine antigen that had been complexed with baboon antibodies. B: Porcine antigen in baboon blood perfused through various porcine organs. Porcine antigen was quantitated by measuring blocking of human xenoreactive IgM to porcine endothelial cells. Serum prepared from baboon blood before (pre) and after perfusion through a porcine lung, kidney, or heart was treated with dithiothreitol and dialyzed as described in A and incubated with 5% human serum. The binding of human xenoreactive IgM to cultured porcine endothelial cells was tested by ELISA. Percent inhibition ±SE of duplicate determinations was based on the binding of 5% human serum IgM as a standard. C: Blocking of human xenoreactive IgM by porcine antigen in the blood of baboons that received porcine pulmonary xenografts. Serum prepared from the blood of three baboons before (pre) and after (baboon 1, 2, and 3) pulmonary xenotransplantation was tested as described in A for the presence of porcine antigen. The “pre” result shown is the mean ±SE from the three baboons. Standard errors are too small to be seen. After pulmonary xenotransplantation, baboon blood contains antigen that is capable of blocking human xenoreactive natural antibodies.
Identification of Antigen Shed from Porcine Pulmonary Xenografts
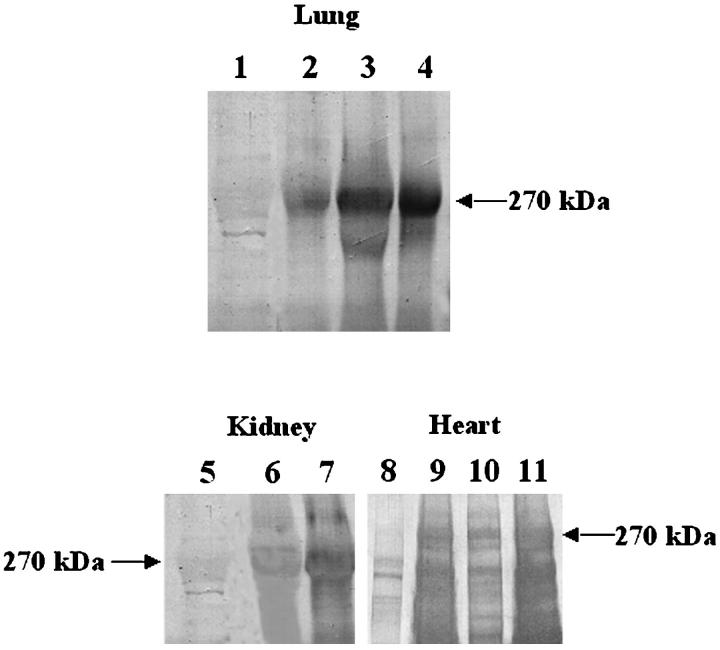
Porcine proteins shed into the circulation of baboons, after pulmonary, renal, or cardiac xenotransplantation, were characterized as follows. Serum from blood, collected before and after transplantation, was treated with PEG to precipitate immune complexes and the precipitates were separated by SDS-PAGE, transferred to PVDF, and tested for reactivity with human IgM. Figure 6 ▶ shows that posttransplant, but not pretransplant, serum from baboons contains complexes that yield a major band at ∼270 kd reactive with IgM in human serum. A visible but nonreactive band at ∼270 kd in the pretransplant serum is identified as baboon vWF below.
Figure 6.
Isolation of immune complexes from baboon blood after pulmonary, renal, or cardiac xenotransplantation. Serum isolated from baboon blood after pulmonary, renal, or cardiac xenotransplantation was diluted 5:1 with 12% PEG 8,000 and 60 mmol/L EDTA in VBS for 16 hours at 4°C to precipitate immune complexes. The solution was centrifuged at 2,000 × g for 20 minutes at 4°C to pellet immune complexes. Precipitates were resuspended in PBS, reduced, separated by SDS-PAGE (7.5% PA) and transferred to PVDF and stained as follows. Lane 1, pretransplant baboon serum reacted with human serum and then with anti-human IgM. Lane 2, baboon serum, taken 1 hour after xenotransplantation of a porcine lung, reacted with human serum and then with anti-human IgM. Lane 3, baboon serum, taken 2 hours after xenotransplantation of a porcine lung, reacted with human serum and then with anti-human IgM. Lane 4, baboon serum, taken 4 hours after xenotransplantation of a porcine lung, reacted with human serum and then with anti-human IgM. Lane 5, pretransplant baboon serum reacted with human serum and then with anti-human IgM. Lane 6, baboon serum taken 30 minutes after xenotransplantation of a porcine kidney, reacted with human serum and then with anti-human IgM. Lane 7, baboon serum taken 60 minutes after xenotransplantation of a porcine kidney, reacted with human serum and then with anti-human IgM. Lane 8, pretransplant baboon serum reacted with human serum and then with anti-human IgM. Lane 9, baboon serum taken 15 minutes after xenotransplantation of a porcine heart, reacted with human serum and then with anti-human IgM. Lane 10, baboon serum taken 30 minutes after xenotransplantation of a porcine heart, reacted with human serum and then with anti-human IgM. Lane 11, baboon serum taken 60 minutes after xenotransplantation of a porcine heart, reacted with human serum and then with anti-human IgM. A protein in the blood of baboons after pulmonary, renal, and cardiac xenotransplantation with an apparent molecular weight of 270 kd is reactive with human xenoreactive IgM.
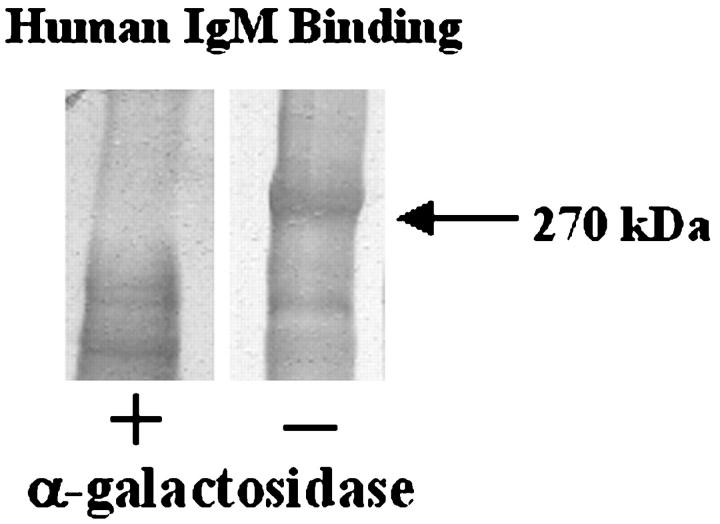
To test whether human IgM bound via Galα1-3Gal, as is described for other endothelial cell antigens, 17,27-29 PEG-precipitated serum components from baboons with porcine pulmonary xenotransplants were digested with α-galactosidase, an enzyme that cleaves terminal α-galactose residues, and then tested for reactivity with IgM. Figure 7 ▶ shows reactivity of human IgM with the 270-kd protein, abrogated by α-galactosidase digestion, indicating that the major porcine antigen contained Galα1-3Gal modification.
Figure 7.
Presence of Galα1-3Gal in immune complexes in the blood of baboons, after pulmonary xenotransplantation. Immune complexes in the serum taken from baboon blood after a pulmonary xenotransplantation were diluted 5:1 with 12% PEG 8000 and 60 mmol/L EDTA in VBS for 16 hours at 4°C to precipitate immune complexes. The solution was centrifuged at 2,000 × g for 20 minutes at 4°C to pellet immune complexes. Precipitates were resuspended in digestion buffer (100 mmol/L NaCl and 50 mmol/L sodium acetate). Samples were incubated with (+) or without (−) α-galactosidase (1 U/ml) for 5 hours at 37°C. Proteins were precipitated with ethanol, reduced, separated by SDS-PAGE (7.5% PA), and transferred to PVDF. The blots were reacted with human serum as a source of xenoreactive IgM and then with anti-human IgM. Lane 1, α-galactosidase digested proteins targeted by human IgM, Lane 2, control proteins targeted by human IgM. Digestion of samples with α-galactosidase abrogates the binding of human xenoreactive IgM.
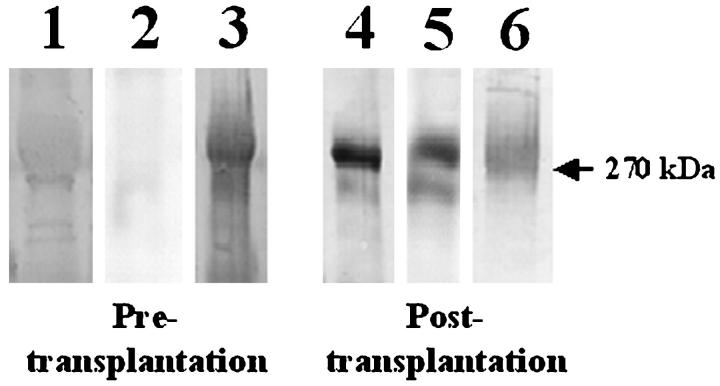
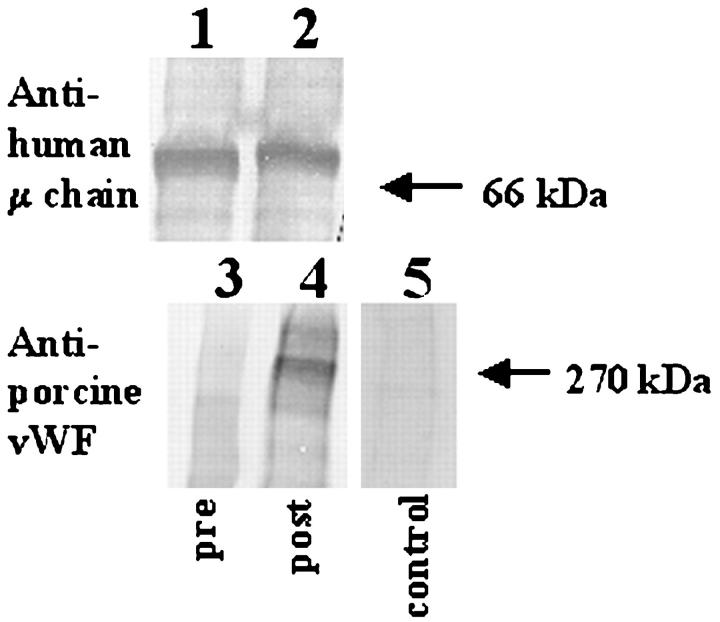
Figure 8 ▶ shows that the 270-kd protein in the posttransplant serum of baboons with pulmonary xenografts, which is reactive with human IgM, also reacts with mouse anti-porcine vWF antibodies. This finding is consistent with previous observations that porcine vWF from aortic endothelial and pulmonary microvascular endothelial cells is recognized by human xenoreactive IgM through Galα1-3Gal epitopes. 15,22 Serum from baboons taken before and after pulmonary xenotransplantation also contains baboon vWF (lanes 3 and 6), which migrates to 270 kd on Western blots but is not recognized by human xenoreactive natural antibodies (lane 1) (Figure 8) ▶ .
Figure 8.
Identification of 270-kd antigen recognized by xenoreactive human IgM. Serum obtained from baboon blood before (pre) or 3 hours after porcine pulmonary xenotransplantation (post) was diluted 5:1 with 12% PEG 8000 and 60 mmol/L EDTA in VBS for 16 hours at 4°C and then centrifuged at 2,000 × g for 20 minutes at 4°C to pellet immune complexes. Precipitates were resuspended in PBS, reduced, separated by SDS-PAGE (7.5% PA), and transferred to PVDF and stained as follows. Lane 1, pretransplant serum reacted with human serum and then anti-human IgM. Lane 2, pretransplant serum reacted with mouse anti-porcine vWF and then goat anti-mouse IgG. Lane 3, pretransplant serum reacted with rabbit anti-human vWF and then mouse anti-rabbit IgG. Lane 4, posttransplant serum reacted with human serum and then anti-human IgM. Lane 5, posttransplant serum reacted with mouse anti-porcine vWF and then goat anti-mouse IgG. Lane 6, posttransplant serum reacted with rabbit anti-human vWF and then mouse anti-rabbit IgG. This suggests that porcine vWF in baboon blood after pulmonary xenotransplantation reacts with human xenoreactive IgM.
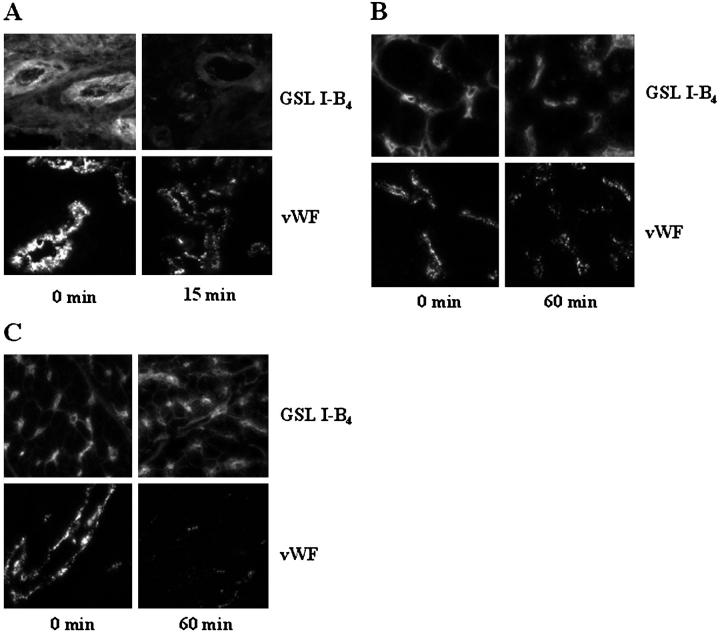
To determine whether vWF or other Galα1-3Gal-containing proteins are, in fact, lost from the endothelium of transplanted organs, tissues from porcine lungs and kidneys transplanted into baboons were assayed for reactivity with anti-porcine vWF and with GSL I-B4, a lectin specific for α-galactose residues, shortly after xenotransplantation. Although porcine lungs exhibit reactivity with both GSL I-B4 lectin and anti-porcine vWF before transplantation into baboons, there is a marked decrease in vWF and GSL I-B4 reactivity in the microvasculature 15 minutes after transplantation into baboons (Figure 9A) ▶ , suggesting that vWF, and perhaps other Galα1-3Gal-containing proteins, are lost rapidly on xenotransplantation. Porcine kidneys (Figure 9B) ▶ and porcine hearts (Figure 9C) ▶ also exhibit a loss of vWF from the microvasculature, suggesting that vWF may be released from the endothelium after transplantation of organs other than the lung within the first hour of reperfusion. In contrast, much of the Galα1-3Gal remains in the microvasculature of the porcine kidney and heart after transplantation compared to the lung, as measured by GSL I-B4. This finding is consistent with studies done on cardiac xenografts several days after transplantation that reveal little or no depletion of Galα1-3Gal measured by GSL I-B4. 30,31
Figure 9.
Loss of Galα1-3Gal and vWF from porcine xenografts. Tissues from porcine pulmonary, renal, and cardiac xenografts were assayed for reactivity with GSL I-B4, a lectin specific for Galα1-3Gal, and mouse anti-porcine vWF. A: Porcine pulmonary tissues taken 15 minutes after transplantation into baboons have markedly less vWF and less reactivity with GSL I-B4 than porcine pulmonary tissues taken before transplantation (0 minutes). Original magnification, ×400. B: Porcine renal tissues taken 60 minutes after transplantation into baboons have less vWF but only focal or no decrease of Galα1-3Gal based on reactivity with GSL I-B4 than porcine renal tissues taken before transplantation (0 minutes). Original magnification, ×400. C: Porcine cardiac tissues taken 60 minutes after transplantation into baboons have less vWF but only focal or no decrease of Galα1-3Gal based on reactivity with GSL I-B4 than porcine cardiac tissues taken before transplantation (0 minutes). Original magnification, ×400.
Characterization of Immune Complexes in Pulmonary Xenograft Recipients
To determine whether porcine vWF shed from pulmonary xenografts is complexed with IgM of the recipient, plasma from pulmonary xenograft recipients was passed over affinity matrix bearing Galα1-3Gal to isolate anti-Galα1-3Gal antibodies and the retained fractions were tested for porcine vWF. Figure 10 ▶ shows that Galα1-3Gal affinity columns isolated baboon IgM from both pre- and postpulmonary transplant serum. Porcine vWF co-isolated with baboon IgM from postpulmonary transplant serum but not from pretransplant serum or from normal porcine serum, indicating that after xenotransplantation porcine vWF is complexed with baboon anti-Galα1-3Gal IgM.
Figure 10.
Identification of components in immune complexes in baboon serum after porcine pulmonary xenotransplantation. Sera obtained from baboon blood taken before and 15 minutes after pulmonary xenotransplantation were diluted 5:1 with 12% PEG 8000 and 60 mmol/L EDTA in VBS for 16 hours at 4°C and then centrifuged at 2,000 × g for 20 minutes at 4°C to pellet immune complexes. Precipitates were resuspended in PBS and incubated with Galα1-3Galβ (Bdi) -PAA immobilized on Sepharose CL6B. Pig serum was incubated with immobilized Galα1-3Galβ as a control. The beads were washed with PBS and then boiled in reducing sample buffer to release bound material. The released material was separated by SDS-PAGE (7.5% PA), transferred to PVDF, and stained as follows. Lane 1, released material from pretransplant serum reacted with goat anti-human IgM. Lane 2, released material from posttransplant serum reacted with goat anti-human IgM. Lane 3, released material from pretransplant serum reacted with mouse anti-porcine vWF and then with goat anti-mouse IgG. Lane 4, released material from posttransplant serum reacted with mouse anti-porcine vWF and then with goat anti-mouse IgG. Lane 5, released material from pig serum reacted with mouse anti-porcine vWF factor and then with goat anti-mouse IgG. Porcine vWF is associated with baboon IgM.
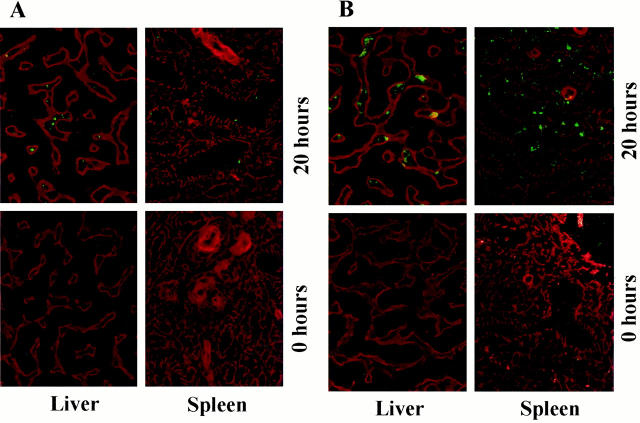
Immune complexes are removed from the circulation through the liver, spleen, and other organs. To test whether immune complexes containing porcine vWF were deposited in the organs of the recipient, liver and spleen from baboons that received porcine lung xenografts and contained circulating IgM/vWF immune complexes were tested by immunofluorescent microscopy for the presence of porcine vWF. Figure 11A ▶ shows that porcine vWF (fluorescent staining) was present in the livers and spleens of baboons with porcine lung xenografts, but not in the livers and spleens of baboons that had not received pulmonary transplants. The deposition of immune complexes can lead to the activation of complement at these sites. Liver and spleen from baboons containing porcine vWF also show evidence of C3 deposition (fluorescent staining) (Figure 11B) ▶ . Liver and spleen from baboons that did not receive pulmonary xenografts showed no evidence of C3 deposition.
Figure 11.
Deposition of immune complexes in pulmonary xenograft recipients. Liver and spleen taken from baboons before (0 hours) and after (20 hours) porcine pulmonary xenotransplantation were investigated by immunofluorescent microscopy for evidence of immune deposits. A: Fluorescent staining of anti-porcine vWF, with a rhodamine counterstain for basement membrane. Deposition of porcine vWF is evident in baboon liver and spleen after pulmonary xenotransplantation. Original magnification, ×400. B: Fluorescent staining of anti-human C3, with a rhodamine counterstain for basement membrane. Deposition of porcine C3 is evident in baboon liver and spleen after pulmonary xenotransplantation. Original magnification, ×400.
Discussion
For 3 decades, immune reactions have been classified into four categories: 32 type I, or anaphylactic, reactions reflect typical allergic responses. Type II, or cytotoxic, reactions are triggered by the binding of antibodies to antigens on the surface of target cells. Type III, or immune complex, reactions are caused by the formation of circulating antibody-antigen complexes and deposition in tissue spaces. Type IV, or delayed type hypersensitivity, reactions are mediated by T lymphocytes. The rejection of vascularized xenografts, triggered by binding of xenoreactive antibodies to antigens on the surface of donor endothelial cells and the subsequent activation of the complement cascade, 1-4,11 would seem to be a classic example of the type II reaction. Here, we report that in the xenotransplantation of the lung, a type III reaction caused by the association of xenoreactive antibodies with vWF shed from the endothelium of the newly transplanted organ, rather than a type II reaction, occurs.
The shedding of vWF factor from xenotransplants presumably reflects injury or inflammatory stimulation of the lung. vWF has been found to be secreted by endothelial cells exposed to hypoxia, 20 membrane attack complexes, 19 and mechanical injury. 33 All of these might be expected to occur in an organ xenotransplant. The shedding of porcine vWF from the xenotransplants presumably allows the formation of immune complexes with xenoreactive IgM. The identification of immune complex formation as a predominant type of immune reaction after xenotransplantation of the lung also helps to answer other more perplexing responses to lung xenotransplantation and identifies a new hurdle to clinical application of xenotransplantation.
The findings reported here account for the fate of xenoreactive antibody after pulmonary xenotransplantation, heretofore somewhat of a puzzle. Some years ago, we observed that although porcine cardiac and renal xenografts transplanted into primates have prominent deposits of recipient antibody in large and small blood vessels, pulmonary xenografts transplanted in primates have prominent deposits of recipient antibody on only pulmonary artery and not in small blood vessels. 8,10 Although depletion of xenoreactive antibodies was found to clearly prolong the survival of kidney and cardiac xenografts, 2,34 depletion of xenoreactive antibodies using anti-Galα1-3Gal, or anti-Ig columns, or by perfusion of blood through porcine kidneys does not prevent early injury to xenografts, whereas perfusion through porcine lungs does prevent early injury 9 (C. Lau, unpublished observations). The findings presented here suggest these differences between the xenotransplanted lung and the xenotransplanted kidney and heart might reflect the amount of antigen shed from pulmonary xenografts leading to formation of immune complexes in the circulation of the recipient. This observation has two implications for the pathophysiology and pathogenesis of lung injury. First, the diversion of antibodies and complement away from the surface of lung endothelium establishes a type of reaction that may differ in fundamental ways from hyperacute rejection suffered by other organs. Second, the assembly of antibody-antigen complexes after pulmonary xenotransplantation, as described here, is not necessarily detrimental for the graft, and it may, rather, provide some measure of protection from immune injury.
Our observations may also help explain why the administration of cobra venom factor can worsen the outcome of lung xenografts, whereas it dramatically improves the outcome of cardiac and renal xenografts. Cobra venom factor activates complement, generating anaphylatoxins to which the vasculature of the lung is profoundly sensitive. 9,35 Indeed, administration of cobra venom factor to some animals causes pulmonary shock and death. 36 The formation of immune complexes in pulmonary blood vessels undoubtedly causes the formation of some anaphylatoxins, and these may, in turn, cause the profound vasoconstriction that plagues lung xenografts. Whether and to which extent the shedding of antigen from the blood vessels of xenografts is beneficial may, thus, depend on the kinetics and quantity of antigen shedding and the rate of immune complex formation.
In addition to inciting the local formation of anaphylatoxins, immune complexes formed after xenotransplantation might influence the systemic response to xenotransplantation. The deposition of immune complexes in peripheral organs could trigger immune-complex disease. Although our experimental xenotransplants, studied throughout a period of hours, did not reveal pathological or clinical evidence of organ injury. Such injury might be anticipated in the days to weeks after pulmonary xenotransplantation. Furthermore, the delivery of immune complexes to lymphoid organs and local activation of complement could heighten the elicited immune response to the porcine organ. Although we have seen only limited immune responses after the transplantation of porcine hearts into baboons or exposure of human patients to porcine livers, 37,38 we might anticipate that the response to pulmonary transplantation might be more diverse and more intense. Among the immune responses of greatest concern would be responses to porcine histocompatibility antigens, which cross-react with HLA. Were they to occur, such cross-reactive responses could preclude subsequent allotransplantation.
Our observations should not be taken as evidence that antigenic material is shed only from pulmonary and not from other types of xenografts. We have found immune complexes, albeit in notably smaller amounts, in the blood of recipients of cardiac and renal xenografts. However, the amount of vWF shed from lung xenotransplants clearly exceeds the amount shed from kidney or heart xenotransplants; thus the increased amount of vWF probably reflects the greater mass of vasculature in the transplanted lung. We have also observed that the recipients of cardiac xenografts and patients whose blood has been perfused through porcine livers develop substantially higher levels of anti-Galα1-3Gal antibodies within days of exposure to porcine organs, suggesting some measure of immune stimulation had occurred. In light of these findings it is clear that type III immune reactions involving immune complex formation are evident in xenograft recipients and warrant further investigation into their role in graft injury and graft survival.
Footnotes
Address reprint requests to Jeffrey L. Platt, M.D., Transplantation Biology, Medical Sciences Building, 2-66, Mayo Clinic, 200 First St., SW, Rochester, Minnesota 55905. E-mail: platt.jeffrey@mayo.edu.
Supported by grants from the National Institutes of Health (HL 46810 and HL52297) and from Ministerio de Educacion y Ciencia (Spain).
References
- 1.Perper RJ, Najarian JS: Experimental renal heterotransplantation. I. In widely divergent species. Transplantation 1966, 4:377-388 [DOI] [PubMed] [Google Scholar]
- 2.Cooper DKC, Human PA, Lexer G, Rose AG, Rees J, Keraan M, Du Toit E: Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transpl 1988, 7:238-246 [PubMed] [Google Scholar]
- 3.Platt JL, Fischel RJ, Matas AJ, Reif SA, Bolman RM, Bach FH: Immunopathology of hyperacute xenograft rejection in a swine-to-primate model. Transplantation 1991, 52:214-220 [DOI] [PubMed] [Google Scholar]
- 4.Dalmasso AP, Vercellotti GM, Fischel RJ, Bolman RM, Bach FH, Platt JL: Mechanism of complement activation in the hyperacute rejection of porcine organs transplanted into primate recipients. Am J Pathol 1992, 140:1157-1166 [PMC free article] [PubMed] [Google Scholar]
- 5.Brauer RB, Baldwin WM, III, Daha MR, Pruitt SK, Sanfilippo F: Use of C6-deficient rats to evaluate the mechanism of hyperacute rejection of discordant cardiac xenografts. J Immunol 1993, 151:7240-7248 [PubMed] [Google Scholar]
- 6.Platt JL, Vercellotti GM, Dalmasso AP, Matas AJ, Bolman RM, Najarian JS, Bach FH: Transplantation of discordant xenografts: a review of progress. Immunol Today 1990, 11:450-456 [DOI] [PubMed] [Google Scholar]
- 7.Bach FH, Winkler H, Ferran C, Hancock WW, Robson SC: Delayed xenograft rejection. Immunol Today 1996, 17:379-384 [DOI] [PubMed] [Google Scholar]
- 8.Kaplon RJ, Platt JL, Kwiatkowski PA, Edwards NM, Xu H, Shah AS, Michler RE: Absence of hyperacute rejection in pig-to-primate orthotopic pulmonary xenografts. Transplantation 1995, 59:410-416 [PubMed] [Google Scholar]
- 9.Pierson RNI, Kasper-Konig W, Tew DN, Young VK, Dunning JJ, Horsley J, Carey NRB, Wallwork J, White DJG: Hyperacute lung rejection in a pig-to-human transplant model. Transplantation 1997, 63:594-603 [DOI] [PubMed] [Google Scholar]
- 10.Daggett CW, Yeatman M, Lodge AJ, Chen EP, Gullotto C, Frank MM, Platt JL, Davis RD: Total respiratory support from swine lungs in primate recipients. J Thorac Cardiovasc Surg 1998, 115:19-27 [DOI] [PubMed] [Google Scholar]
- 11.Gewurz H, Clark DS, Cooper MD, Varco RL, Good RA: Effect of cobra venom-induced inhibition of complement activity on allograft and xenograft rejection reactions. Transplantation 1967, 5:1296-1303 [DOI] [PubMed] [Google Scholar]
- 12.Miyagawa S, Hirose H, Shirakura R, Naka Y, Nakata S, Kawashima Y, Seya T, Matsumoto M, Uenaka A, Kitamura H: The mechanism of discordant xenograft rejection. Transplantation 1988, 46:825-830 [DOI] [PubMed] [Google Scholar]
- 13.Leventhal JR, Dalmasso AP, Cromwell JW, Platt JL, Manivel CJ, Bolman RM, Matas AJ: Prolongation of cardiac xenograft survival by depletion of complement. Transplantation 1993, 55:857-866 [DOI] [PubMed] [Google Scholar]
- 14.Yeatman M, Daggett CW, Parker W, Byrne GW, Logan JS, Frank MM, Platt JL, Davis RD: Complement-mediated pulmonary xenograft injury: studies in swine-to-primate orthotopic single lung transplant models. Transplantation 1998, 65:1084-1093 [DOI] [PubMed] [Google Scholar]
- 15.Holzknecht ZE, Coombes S, Blocher BA, Lau CL, Davis RD, Platt JL: Identification of antigens on porcine pulmonary microvascular endothelial cells recognized by human xenoreactive natural antibodies. Lab Invest 1999, 79:763-773 [PubMed] [Google Scholar]
- 16.Cooper D, Good A, Koren E, Oriol R, Malcom A, Ippolito R, Neethling F, Ye Y, Romano E, Zuhdi N: Identification of α-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol 1993, 1:198-205 [DOI] [PubMed] [Google Scholar]
- 17.Sandrin MS, Vaughan HA, Dabkowski PL, McKenzie IFC: Anti-pig IgM antibodies in human serum react predominantly with Galα(1,3)Gal epitopes. Proc Natl Acad Sci USA 1993, 90:11391-11395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin SS, Holzknecht ZE, Parker W, Lindman BJ, Platt JL: Porcine endothelial cell membrane antigens recognized by human and baboon xenoreactive antibodies during organ perfusion. Transplant Proc 1996, 28:607. [PubMed] [Google Scholar]
- 19.Hattori R, Hamilton KK, McEver RP, Sims PJ: Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem 1989, 264:9053-9060 [PubMed] [Google Scholar]
- 20.Pinsky DJ, Naka Y, Liao H, Oz MC, Wagner DD, Mayadas TN, Johnson RC, Hynes RO, Heath M, Lawson CA, Stern DM: Hypoxia-induced exocytosis of endothelial cell Weibel-Palade bodies. J Clin Invest 1996, 97:493-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daggett CW, Yeatman M, Chen EP, Lodge AJ, Srinivasan M, Byrne GW, Logan JS, Platt JL, Davis RD: Transgenic swine-to-primate pulmonary xenotransplantation. Surg Forum 1996, XLVII:413-415 [Google Scholar]
- 22.Holzknecht ZE, Platt JL: Identification of porcine endothelial cell membrane antigens recognized by human xenoreactive antibodies. J Immunol 1995, 154:4565-4575 [PubMed] [Google Scholar]
- 23.Yeatman M, Daggett C, Byrne G, Logan J, Platt J, Davis R: Human complement regulatory proteins in transgenic swine protect lungs from humoral injury during xenogeneic perfusion. Ann Thorac Surg 1999, 67:769-775 [DOI] [PubMed] [Google Scholar]
- 24.Platt JL, Turman MA, Noreen HJ, Fischel RJ, Bolman RM, Bach FH: An ELISA assay for xenoreactive natural antibodies. Transplantation 1990, 49:1000-1001 [DOI] [PubMed] [Google Scholar]
- 25.Benson RE, Catalfamo JL, Dodds WJ: A multispecies enzyme-linked immunosorbent assay for von Willebrand’s factor. J Lab Clin Med 1992, 119:420-428 [PubMed] [Google Scholar]
- 26.Platt JL, Holzknecht ZE: Porcine platelet antigens recognized by human xenoreactive natural antibodies. Transplantation 1994, 57:327-335 [DOI] [PubMed] [Google Scholar]
- 27.Good AH, Cooper DKC, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, Ye Y, Zuhdi N, Lamontagne LR: Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc 1992, 24:559-562 [PubMed] [Google Scholar]
- 28.Collins BH, Parker W, Platt JL: Characterization of porcine endothelial cell determinants recognized by human natural antibodies. Xenotransplantation 1994, 1:36-46 [Google Scholar]
- 29.Lin SS, Kooyman DL, Daniels LJ, Daggett CW, Parker W, Lawson JH, Hoopes CW, Gullotto C, Li L, Birch P, Davis RD, Diamond LE, Logan JS, Platt JL: The role of natural anti-Galα1-3Gal antibodies in hyperacute rejection of pig-to-baboon cardiac xenotransplants. Transpl Immunol 1997, 5:212-218 [DOI] [PubMed] [Google Scholar]
- 30.Alvarado CG, Cotterell AH, McCurry KR, Collins BH, Magee JC, Berthold J, Logan JS, Platt JL: Variation in the level of xenoantigen expression in porcine organs. Transplantation 1995, 59:1589-1596 [PubMed] [Google Scholar]
- 31.Parker W, Holzknecht ZE, Song A, Blocher BA, Bustos M, Reissner KJ, Everett ML, Platt JL: Fate of antigen in xenotransplantation: implications for acute vascular rejection and accommodation. Am J Pathol 1998, 152:829-839 [PMC free article] [PubMed] [Google Scholar]
- 32.Coombs RRA, Gell PGH: The classification of allergic reactions underlying disease. Clinical Aspects of Immunology. 1963, F. A. Davis Company, Edited by PGH Gell. Philadelphia
- 33.Reidy MA, Chopek M, Chao S, McDonald T, Schwartz SM: Injury induces increase of von Willebrand factor in rat endothelial cells. Am J Pathol 1989, 134:857-864 [PMC free article] [PubMed] [Google Scholar]
- 34.Alexandre GPJ, Gianello P, Latinne D, Carlier M, Dewaele A, Van Obbergh L, Moriau M, Marbaix E, Lambotte JL, Lambotte L, Squifflet JP: Plasmapheresis and splenectomy in experimental renal xenotransplantation. Hardy MA eds. Xenograft 25. 1989, :pp 259-266 Elsevier Science Publishers, New York [Google Scholar]
- 35.Yeatman M, Daggett CW, Parker W, Byrne GW, Logan JS, Platt JL, Davis RD: Complement-mediated pulmonary xenograft injury. Transplantation 1998, 65:1084-1099 [DOI] [PubMed] [Google Scholar]
- 36.Till GO, Johnson KJ, Kunkel R, Ward PA: Intravascular activation of complement and acute lung injury. J Clin Invest 1982, 69:1126-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCurry KR, Parker W, Cotterell AH, Weidner BC, Lin SS, Daniels LJ, Holzknecht ZE, Byrne GW, Diamond LE, Logan JS, Platt JL: Humoral responses in pig-to-baboon cardiac transplantation: implications for the pathogenesis and treatment of acute vascular rejection and for accommodation. Hum Immunol 1997, 58:91-105 [DOI] [PubMed] [Google Scholar]
- 38.Cotterell AH, Collins BH, Parker W, Harland RC, Platt JL: The humoral immune response in humans following ex vivo perfusion of porcine livers. Transplant Proc 1996, 28:546. [PubMed] [Google Scholar]