Abstract
The important issue addressed by the studies presented here is the mechanism of neutrophil-mediated damage to endothelial and epithelial cells during inflammation. Binding of neutrophil-released granule proteins to endothelial cells may be involved in vascular damage in patients with inflammatory vascular diseases. We have determined whether granule proteins proteinase 3(PR3) and/or myeloperoxidase (MPO) are internalized into endothelial cells, as examined by UV light, confocal, and electron microscopy. Coincident induction of apoptosis and/or the generation of intracellular oxidants were monitored. The results indicate that human endothelial cells (human umbilical vein endothelial cells, human umbilical arterial endothelial cells, human lung microvascular endothelial cells) internalize both PR3 and MPO, which are detected on the cell surface, in the cytoplasm, and possibly nuclear. Epithelial cells (small airway epithelial cells) internalized MPO but not PR3, implying that the mechanism of PR3 internalization may be cell-type specific and different from that of MPO. Internalization of PR3, but not MPO, correlated with activation of apoptosis. Internalization of MPO correlated with an increase in intracellular oxidant radicals. The requirement for the proteolytic activity of PR3 for the induction of apoptosis was examined by generating PR3-truncated fragments that did not contain the components of the catalytic triad. An apoptotic function was localized to the C-terminal portion of PR3. These studies reveal novel mechanisms by which the neutrophil granule proteins PR3 and MPO contribute to tissue injury at sites of inflammation.
Neutrophilic inflammatory infiltrate is a common pathological feature in acute inflammatory disease. 1-3 The primary function of neutrophils is the phagocytosis and destruction of microorganisms, which may result in acute inflammation in the surrounding tissue. On sensitization by inflammatory signals, neutrophils adhere to the endothelium adjacent to the infected tissue. After attachment, the neutrophils migrate to the infected site where they release oxygen radicals and their internal constituents, including azurophilic granule proteins. 1-5 Of the components present in these granules, special attention has been paid to PR3 and MPO, because the discovery that patients with Wegener’s granulomatosis, microscopic polyangiitis, and Churg-Strauss syndrome have circulating anti-PR3 or anti-MPO autoantibodies. 6-9 In recent years, a number of potential pathogenic mechanisms by which PR3 and MPO have detrimental effects on tissues and cells have been evaluated in multiple experimental models. 10-14
Structurally, PR3 belongs to the serprocidin subgroup of the chymotrypsin-like protease superfamily, comprised of neutrophil elastase, cathepsin G, and azurocidin. 15-17 With the exception of azurocidin, which is a pseudoprotease, these proteases degrade extracellular matrix macromolecules, such as elastin, fibronectin, laminin, vitronectin, and type IV collagen. 4,5,18,19 Sequence analysis of PR3 showed it to be identical to myeloblastin, a gene cloned as a differentiation marker from promyelocytic leukemia HL60 cells. 20 PR3 has been shown to be involved in retinoic acid-induced differentiation of HL60 cells. 20 Apparently, retinoic acid indirectly activates PR3, which then hydrolyzes hsp27/28, a component of mitogenic signal transduction pathways. 21,22 Other known substrates of PR3 include transcription factors (nuclear factor-κB and SP1) and cytokines (tumor necrosis factor-α, transforming growth factor-β1, and interleukin-1β). 23-26 Although proteolytic activity of PR3 is involved in processing these molecules, there are accumulating data that indicate that PR3 can have direct effects on intracellular processes in the absence of proteolytic activity. For example, a secreted, inactive proform of PR3 is shown to down-modulate DNA synthesis in normal hematopoietic progenitor cells. The inhibitory effect of secreted PR3 is reversible by granulocyte-macrophage colony-stimulating factor, implying that PR3 can function as a counterbalance to regulators of proliferation. 27 Enzymatically inactive PR3 has been shown to induce interleukin-8 production, whereas enzymatic activity is essential for elastase to stimulate this effect. 28 We have reported that inactive PR3 induced apoptosis of bovine pulmonary artery endothelial cells 29 and subsequent studies confirmed these findings. 30 Hence, PR3 seems to be a multifunctional protein endowed with the capacity to influence cell cycle, differentiation, and cell death.
It has been suggested that pulmonary injury and renal glomerular damage may be caused by the MPO system. MPO degrades H2O2 in the presence of chloride to produce hypochlorite (OCl−). The products of the MPO-H2O2-chloride system are powerful oxidants that can have profound biological effects. When MPO and H2O2 are released to the outside of the cell, a reaction with chloride can induce damage to adjacent tissue and thus contribute to the pathogenesis of disease. 13,14
The important issue addressed by the study presented here is the mechanism of neutrophil-mediated damage to endothelial and epithelial cells during inflammation. We investigate whether or not granule proteins, PR3 and MPO, are internalized into endothelial and epithelial cells and whether internalization has an effect on these cells. Precedence for nuclear import of granule proteases is provided by reports showing that granzyme B, a protease from granules of T lymphocytes and natural killer cells and a homologue of PR3, can be transported to the nucleus in treated Cos cells and the cells die by apoptosis. 31 Signal transduction events, associated with granzyme B-induced apoptosis, included activation of the cyclin-dependent kinase cdc-2. 32 Further, it was shown that specific inhibitors of the proteolytic activity of granzyme B had no effect on the nuclear import, implying that proteolytic activity was not essential for nuclear targeting.
We report that human endothelial cells bind, sequester, and route neutrophil proteins, PR3, and MPO, from the cell surface to the nucleus where they co-localize with chromatin. PR3 internalization is concomitant with apoptosis in contrast to MPO. Epithelial cells do not have the capacity to internalize PR3 and do not die by apoptosis. The apoptotic function of PR3 was mapped to a 100-amino acid fragment of the molecule, which contains no component of the catalytic triad. MPO internalization is not restricted by cell type, implying that the mechanism is not the same as that for PR3 internalization. Internalized MPO confers increased intracellular oxidant production.
Materials and Methods
Preparation of Granule Proteins
Granule proteins were isolated from leukocytes of human donors with leukemia. 29 PR3 was purified by a monoclonal anti-PR3 affinity column (monoclonal anti-PR3 IgG, 12-1D6-1D4, developed in our laboratory) and a Bio-Rex 70 column (Bio-Rad Laboratories, Richmond, CA) and MPO by a Resource S cation exchange column (Pharmacia, Uppsala, Sweden) and a Superdex 200 gel filtration column (Pharmacia). 29 The purity of PR3 and MPO was determined by amino acid sequencing, enzyme-linked immunosorbent assay, Western blot analysis, and enzymatic activity. Enzymatic activity of MPO was 172 U/mg protein using 4-aminoanipyrine as substrate. 33 PR3 proteolytic activity was very low, using Boc-Ala-Onp as substrate. 29 Protein preparations were determined to be endotoxin-free. Proteolytically active PR3 (109 U/mg protein) was a generous gift from Dr. J. Wieslander (Wieslab AB, Lund, Sweden). Triton X-100 was removed from active PR3 using Extracti-Gel D AffinityPak detergent-removing column (Pierce, Rockford, IL).
Cell Culture Conditions
Pooled human umbilical vein endothelial cells (HUVECs), single-donor human umbilical arterial endothelial cells (HUAECs), single-donor human lung microvascular endothelial cells (HMVEC-Ls), and single-donor human small airway epithelial cells (SAECs) were obtained from Clonetics (San Diego, California, CA). HUVECs and HUAECs were cultured in EGM BulletKit, HMVEC-Ls in EGM-2 MV BulletKit, and SAECs in SAGM BulletKit media (Clonetics). Media contained growth factors, 2% fetal calf serum (FCS), antibiotics, and other supplements, but there was no FCS in the SAGM BulletKit medium. HUVECs and SAECs, passage 3 or 4, and HUAECs and HMVEC-Ls, passage 5 or 6, were used for experiments. Monkey epithelial cells (Cos-7) were maintained in Dulbecco’s modified Eagle’s medium-H, 10% fetal bovine serum, and antibiotics. EA.hy926 cells (HUVECs fused to A549 human lung epithelial carcinoma) were cultured in the same medium plus hyproxanthine aminopterin thymidine (HAT) supplement.
Translocation of Granule Proteins into Cells
Cells on microscope cover glasses were incubated with 10 μg/ml of PR3 or MPO for 5 minutes to 16 hours at 37°C in media without growth factors or FCS. For immunofluorescent staining, cells were fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS), permeabilized with acetone, and blocked with 0.2 mol/L glycine and 5% donkey serum in PBS. Primary antibodies were rabbit anti-PR3 (Wieslab AB) or rabbit anti-MPO antibody (DAKO, Carpinteria, CA) and secondary antibody, fluorescein isothiocyanate-conjugated affinipure F(ab′) 2 fragment donkey anti-rabbit IgG (H+L) (Jackson, West Grove, PA), was used. Cells were mounted and viewed by a Nikon FXA research light microscope (Nikon, Garden City, NY) for standard immunofluorescence staining. A Zeiss confocal laser-scanning microscope LSM 110 (Carl Zeiss, Thornwood, NY) for confocal immunofluorescence staining, with an optical section thickness is 0.5 μm using the 63× plan apo 1.4 NA objective. The cells were analyzed from top to bottom in 15 different planes. The appropriate negative controls were performed concurrently, consisting of untreated cells stained with primary antibodies and/or secondary antibody, PR3-treated cells with anti-MPO antibody as primary antibody and MPO-treated cells with anti-PR3 antibody as primary antibody, and treated cells with primary antibodies or secondary antibody alone.
For immunogold labeling, cells were processed according to the methods described by Madden. 34 Samples were blocked in 5% goat serum, incubated with primary antibodies, monoclonal anti-PR3 IgG (4A3) (Wieslab AB) or polyclonal anti-MPO IgG (DAKO), for 2 hours, and subsequently incubated with secondary antibodies, goat anti-mouse 10-nm colloidal gold (Amersham Life Science, Arlington Heights, IL) for monoclonal primary antibody or protein A 10 nm colloidal gold (Ted Pella, Redding, CA) for polyclonal primary antibody, for 1 hour at room temperature. The grids were observed and photographed using a LEO EM-910 transmission electron microscopy (LEO Electron Microscopy, Thornwood, NY). The appropriate negative controls were performed concurrently, consisting of untreated cells stained with anti-PR3 IgG or anti-MPO IgG and treated cells stained with normal mouse or rabbit IgG (DAKO).
Assessment of Apoptosis
Cells in flasks were treated with 1 to 10 μg/ml of isolated and enzymatically inactive PR3 or 1 to 100 μg/ml MPO for 6 to 24 hours in medium containing 2% FCS, but without growth factors. For all assays, both detached and adhered cells were harvested and combined. Apoptosis was assessed by characteristic morphological changes using an UV light microscope (Nikon), and by DNA content using flow cytometry. For UV light microscopy, cells were stained with DNA dyes, either 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, St. Louis, MO) or 20 μg/ml propidium iodide (Sigma). Apoptotic cells were identified by chromatin condensation and fragmentation. 35 Data reported was from six samples of at least three independent experiments, counting 500 cells from randomly selected fields. For flow cytometry, cells were fixed in 70% ethanol/PBS, incubated at 4°C overnight, and resuspended in propidium iodide staining mixture, as described previously. 29 DNA content was determined with a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) linked to a Cicero/Cyclops system (Cytomation, Fort Collins, CO) for data acquisition and analysis. Apoptotic cells were identified by a decrease in DNA content resulting in an apoptotic subG0/G1 population peak. At least 20,000 cells were analyzed in each sample and data were reported from at least three independent experiments.
Subcloning of PR3-N, PR3-M, and PR3-C cDNA Sequences
PR3cDNA/pAcC4 plasmid was a generous gift from Dr. Joelle E. Gabay, Cornell University Medical College. Nucleotides 1–313 (PR3-N), 314–604 (PR3-M), and 620–741 (PR3-C) were amplified by polymerase chain reaction (PCR). Primers were designed to generate restriction sites at each end and a kozak sequence at the 5′ end. Parameters were 30 cycles of 94°C for 30 seconds, 62°C for 30 seconds, and 72°C for 2 minutes. The primers for PR3-N cDNA were 5′-GATATCGCCACCATGGGCGCTCACCGGCCCC-3′ (forward) and 5′-TGGAATTCTCTGAGCCACCGAGAAGTG-3′ (reverse); PR3-M cDNA were 5′-GATATCGCCACCATGGGCGTGTTTCTGAACAACTACGAC-3′ (forward) and 5′-TGGAATTCTTCCGAAGCAGATGCCGGCCTT-3′ (reverse), and PR3-C cDNA primers were 5′-GATATCGCCACCATGGGCCTGATCTGTGATGGCATCATC-3′ (forward) and 5′-TGGAATTCTCAGCGTGGAACGGATCCAGT-3′ (reverse). Amplification products were subcloned into pCR-Blunt (Zero Blunt PCR Cloning Kit; Invitrogen, Carlsbad, CA) and sequenced using an M13 forward primer (5′-GTAAAACGACGGCCAG-3′) (UNC-CH Automated DNA Sequencing Facility, Chapel Hill, NC). Insert was retrieved from pCR-blunt and subcloned into pcDNA3.1(−)/Myc-HisA (Invitrogen)
Analysis of Apoptosis Induced by PR3 Fragments
Cos-7 cells were electroporated 24 hours after trypsinization with 30 μg of plasmid DNA in serum-free RPMI at 300 V and 960 μF using the Gene Pulser II Electroporation System (Biorad, Hercules, CA). Cells were cultured for 48 hours and conditioned medium was collected, centrifuged, and added to EA.hy926 cells. Apoptosis was quantitated by FACScan analysis of terminal dUTP nick-end labeling (TUNEL) cells using fluorescein isothiocyanate-dUTP and terminal transferase (TdT) (Boehringer Mannheim, Mannheim, Germany). Briefly, the cells were fixed with 1% paraformaldehyde in PBS and permeabilized with 70% ethanol and labeling reaction was performed. One half of each cell sample was used to determine background fluorescence by omitting TdT from the reaction mixture. The TdT was added to the second half and increases in fluorescence (FL1) were evaluated. Clumps and doublets were excluded by using forward scatter versus propidium iodide fluorescence (FL2-W).
Detection of Intracellular Oxidants Generated by MPO
Intracellular oxidants generation was detected by oxidation of nonfluorescent dihydrorhodamine (DHR) (New Concept Scientific. Burlington, Ontario, Canada) to fluorescent rhodamine. Cells were incubated with 10 μmol/L DHR for 1 hour in medium without FCS or growth factors, with the subsequent addition of MPO (1 to 50 μg/ml) or PR3 (1 to 10 μg/ml) at 37°C for 4 to 24 hours. Intracellular oxidant generation was determined by three methods. Cell fluorescence was observed by UV light microscopy. By flow cytometry, cells were harvested with trypsin/ethylenediaminetetraacetic acid and cell fluorescence were examined in FL1 green channel in which at least 20,000 cells were analyzed. For fluorometry, treated cells were washed, harvested, and lysed by sonication in 20 mmol/L Tris-HCl, pH 7.4, buffer. The fluorescence of cell lysates was measured by a fluorometer FLUOstar 403 (BMG Lab Technologies, Offenburg, Germany), 5 time flashes per sample (excitation wavelength of 485 nm, emission wavelength of 538 nm). 36 For blocking studies, MPO was pre-incubated with a 10-fold dose of catalase (Calbiochem, La Jolla, CA) for 30 minutes before the addition to cells. The appropriate negative controls were performed concurrently, consisting of cells incubated with medium or DHR alone, and no cell plate incubated with DHR and granule proteins.
Statistical Analysis
Analysis of variance was used to determine whether any differences between group means were seen at specific dose and time points within each experiment. When overall differences were found within the experiment, Dunnett’s t-test was used to further compare means of each treatment and its associated dose to the mean of the control group specific to the experiment. 37 This test evaluates the minimum significant difference between each group and control as compared to the critical value of Dunnett’s T at the P value of 0.05, whereas controlling for multiple testing. PROC ANOVA and PROC GLM in SAS were used for calculating the statistical analyses (SAS/STAS User’s Guide, SAS Institute, Cary, NC, 1989).
Results
Internalization
Translocation of PR3 into Endothelial Cells
To examine potential mechanisms of PR3-induced apoptosis, we determined whether endothelial cells had the capacity to internalize PR3. PR3-treated cells were examined by three standard techniques: immunofluorescence microscopy, immunofluorescence confocal laser scanning microscopy, and electron microscopy. By immunofluorescence microscopy, HUVECs treated with inactive PR3 showed surface and intracellular staining by 10 minutes (Figure 1B) ▶ . HUVECs that had not been exposed to PR3 did not show staining (Figure 1A ▶ and Figure 2A ▶ ). After 2 hours, the intensity of the fluorescence increased, indicating that the level of PR3 in the cytoplasm of the cell had increased (Figure 1C) ▶ . To confirm these data and to address the issue of nuclear localization, the PR3-treated cells were analyzed using confocal laser scanning microscopy. Selected micrographs are shown in Figure 2 ▶ . PR3 staining was detected on the cell surface, with diffuse staining in both the cytoplasm and the nucleus by 10 minutes (Figure 2B) ▶ . By 2 hours, the level of PR3 staining increased, indicating that PR3 uptake continued throughout the 2 hours of incubation (Figure 2C) ▶ .
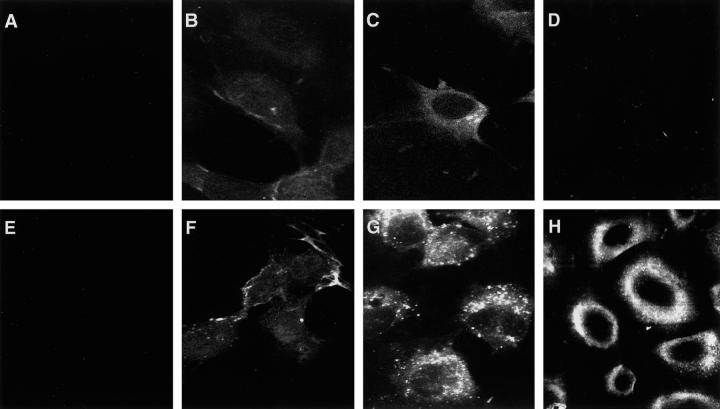
Figure 1.
Assessment of internalization by immunofluorescence microscopy. Photomicrographs of HUVECs (A–C and E–G) and SAECs (D and H). Untreated HUVECs did not show staining with either anti-PR3 antibody (A) or anti-MPO antibody (E). HUVECs incubated with PR3 (B) (10 μg/ml) or MPO (F) (10 μg/ml) displayed weak intracellular staining by 10 minutes. HUVECs treated with PR3 (C) or MPO (G) for 2 hours exhibited prominent intracellular staining. SAECs treated with PR3 for 2 hours (D) were negative for intracellular staining; SAECs treated with MPO (H) showed prominent staining.
Figure 2.
Assessment of internalization by immunofluorescence confocal laser-scanning microscopy. Photomicrographs of HUVECs (A–C and E–G) and SAECs (D and H). HUVECs treated with medium alone did not show staining with either anti-PR3 antibody (A) or anti-MPO antibody (E). HUVECs treated with PR3 (10 μg/ml) (B) or MPO (10 μg/ml) (F) displayed surface staining and weak intracellular staining by 10 minutes. HUVECs treated with PR3 (C) or MPO (G) for 2 hours resulted in intracellular staining with prominent staining in the cytoplasm. SAECs treated with PR3 for 2 hours were negative for intracellular staining (D). SAECs treated with MPO for 2 hours showed strong cytoplasmic staining (H).
Electron microscopy was used to study the compartmentalization of inactive PR3, once inside the cell. Selected micrographs are shown in Figure 3 ▶ . After 10 minutes of treatment, PR3 was detected in cytoplasmic extrusions (Figure 3A) ▶ . We also observed PR3 on the surface membrane, at attachment plaques, in the nucleus and in the nucleoli (data not shown). By 2 hours, PR3 was localized in the nucleus and associated with nuclear heterochromatin (Figure 3C) ▶ , in nucleoli, in the cytoplasm, and in secondary lysosomes, but not on the cell surface or in attachment plaques (data not shown). Endothelial cells harvested from artery (HUAECs) and lung microvascular (HMVEC-Ls), when incubated with PR3, showed staining patterns similar to those described above for HUVECs (data not shown).
Figure 3.

Assessment of internalization by electron microscopy. Electron micrographs of HUVECs stained by immunogold labeling. PR3 (A) (gold particles, arrows) was present in cytoplasmic extensions by 10 minutes and in the nucleus associated with heterochromatin by 2 hours (C); MPO (B) (gold particles, arrows) was associated with nuclear heterochromatin by 10 minutes and by 2 hours was detected in the cytoplasm and in secondary lysosomes (D).
Translocation of MPO into Endothelial Cells
We determined if endothelial cells internalize MPO. Analysis of MPO-treated cells showed that MPO was translocated into HUVECs with a staining pattern similar to that of PR3. Examination of HUVECs treated with MPO for 10 minutes showed weak intracellular staining, as assessed by immunofluorescence microscopy (Figure 1F) ▶ . After 2 hours, strong cytoplasmic staining was observed displaying a punctate pattern (Figure 1G) ▶ . Analysis by confocal laser scanning microscopy showed prominent surface membrane staining of MPO by 10 minutes (Figure 2F) ▶ , with some staining in the cytoplasm and nucleus, and by 2 hours a strong cytoplasmic signal was detected with some nuclear staining (Figure 2G) ▶ .
By electron microscopy, MPO was localized with heterochromatin in the nucleus within 10 minutes (Figure 3B) ▶ . Additional staining was observed at the plasma membrane, in the cytoplasm, at attachment plaques, in the nucleus and in the nucleoli (data not shown). Within 2 hours, MPO could be localized to secondary lysosomes (Figure 3D) ▶ , in the cytoplasm, nucleus and nucleoli, but not on the cell surface and not in attachment plaques (data not shown). Endothelial cells harvested from artery (HUAECs) and lung microvascular (HMVEC-Ls), when incubated with MPO, showed staining patterns similar to those described above for HUVECs (data not shown).
Translocation of MPO, but Not PR3, into Epithelial Cells
To determine whether translocation of PR3 and MPO was a phenomenon specific to endothelial cells, or if other cell types could internalize these proteins, epithelial cells (SAECs) were treated and examined as described above. Internalization of PR3 could not be detected at any time point, with very weak surface staining at 2 hours (Figure 1D) ▶ , and similar results were obtained by confocal microscopy and electron microscopy (Figure 2D) ▶ . Surprisingly, SAECs, treated with PR3, began to float off of the plate at 2 hours (∼30%) to 4 hours (∼60%). We analyzed the cells at multiple time points before detachment (5 minutes, 10 minutes, 15 minutes, 30 minutes, 1 hour, and 2 hours), and after detachment (4 hours) for PR3 staining. Of cells that were still attached at 2 hours, no PR3 internalization was detected. We reasoned that, even if the SAECs had fewer receptors for PR3, some positivity should have been detected by this time.
In contrast, MPO was internalized by SAECs and by 2 hours prominent staining was localized around the nucleus (Figure 1H) ▶ . Confocal microscopy showed similar staining (Figure 2H) ▶ . Analyses of internalization by electron microscopy showed staining patterns similar to those described above for HUVECs, with the exception that MPO was not found in the nucleus in SAECs (data not shown). These data imply that MPO may enter SAECs through a mechanism different from that of PR3.
These studies indicate that neutrophil granule proteins, MPO and PR3, cross the endothelial cell membrane. The electron microscopy studies indicate that both proteins can localize to the nucleus. We report that this phenomenon is not unique to endothelial cells harvested from a specific site, but is a common feature observed in HUVECs, HUAECs, and HMVEC-Ls. Interestingly, lung small airway epithelial cells do not seem to have the capability to take up PR3, but are highly receptive to MPO internalization, strongly suggesting that the mechanisms of internalization are unique.
Association of Apoptosis with Internalization of PR3, but Not MPO
To determine whether there is an association between internalization of PR3 and PR3-induced apoptosis, endothelial cells were treated with inactive PR3 and apoptosis was quantified. Flow cytometry was used to detect cells with a less than G0/G1 DNA content, indicative of apoptosis, displayed as a subG0/G1 peak (Figure 4A) ▶ . A dose- and time-dependent increase in apoptosis was found in PR3-treated HUVECs, compared to controls: 19.9 ± 6.7% versus 7.6 ± 1.7% with 10 μg/ml for 12 hours; 16.0 ± 3.7% versus 8.0 ± 3.0% with 5 μg/ml for 24 hours; and 28.4 ± 1.1% versus 8.0 ± 3.0% with 10 μg/ml for 24 hours.
Figure 4.

PR3 internalization induces apoptosis. A: Flow cytometric analysis. HUVECs treated with PR3 showed a concentration-dependent increase in apoptotic cells (shown as a subG0/G1 peak). UV light microscopy of DAPI-stained cells showed uniform nuclei in the untreated group (B); PR3 treated cells (12 hours) showed an apoptotic morphology with nuclear fragmentation (C). Dose-response curve showing percentage of apoptotic HUVECs (24 hours), as assessed by UV light microscopy (D). Time-dependent response curve of cells that were treated with 10 μg/ml PR3 or 100 μg/ml MPO (E). *, Statistically different from control (P = 0.0001). Values are means ±SEM; n = 6. PR3, but not MPO, induces HUVEC apoptosis in a dose- and time-dependent manner.
Apoptotic cells exhibiting nuclear fragmentation were quantified using UV light microscopy. Untreated HUVECs revealed uniform nuclei and intact plasma membranes (Figure 4B) ▶ . HUVECs treated with PR3 demonstrated fragmented nuclei of different sizes (Figure 4C) ▶ . A statistically significant increase in apoptotic HUVECs was observed in a dose- and time-dependent manner with 11.7 ± 2.8% apoptotic cells in 10 μg/ml of PR3 for 24h (Figure 4, D and E) ▶ . To determine whether inactive PR3 would induce apoptosis in endothelial cells harvested from sites other than umbilical vein, cells from umbilical artery and lung microvasculature were analyzed for fragmented, condensed DNA. Arterial endothelial cells, HUAECs, treated with 10 μg/ml of PR3 for 24 hours showed 10.3 ± 3.1% apoptosis, whereas lung microvascular cells, HMVEC-Ls, showed only 3.0 ± 1.8%. Compared with control values of 1.5 ± 1.1% in HUVECs, 1.3 ± 0.8% in HUAECs and 1.0 ± 0.9% in HMVEC-Ls, the changes were statistically significant with PR3 treatment in HUVECs and HUAECs, but not in HMVEC-Ls.
These results provide evidence that PR3-induced apoptosis may be linked with internalization of the protein. If this hypothesis is correct, then SAECs, which did not internalize PR3, should not undergo apoptosis with PR3 treatment. Examination of PR3-treated SAECs showed no morphological changes, by UV light microscopy, indicative of apoptosis, nor were any apoptotic cells detected by flow cytometry (0.69% with 10 μg/ml of PR3 for 7 hours; controls 0.68%). Of note, SAECs treated with inactive PR3 began to detach from the dish by 2 hours and a majority of the cells were floating by 7 hours (∼90%). Both attached and detached cells were harvested analyzed and no apoptosis was detected.
We determined whether internalization of MPO by HUVECs had an apoptosis-inducing effect. Assessment of nuclear fragmentation by UV light microscopy showed that MPO did not trigger apoptosis, even at doses up to 100 μg/ml and for a time up to 24 hours (Figure 4, D and E) ▶ . Nor were any apoptotic cells (above background) detected by flow cytometry, including cells treated with MPO concentrations ranging from 1 to 100 μg/ml for 6 to 24 hours. Additionally, no apoptosis was detected in HUAECs, HMVEC-Ls and SAECs after MPO internalization (data not shown).
These studies strongly suggest that PR3-induced apoptosis requires internalization. The mere process of internalization of extracellular proteins alone is not sufficient to cause apoptosis, based on data showing that MPO internalization does not result in apoptosis.
Identification of a Noncatalytic Domain of PR3 with Apoptotic Function
We have proposed that the apoptosis-inducing effects of PR3 described above were independent of proteolytic activity. The potential exists that the PR3 preparations used in those studies had very low levels of proteolytic activity. We hypothesized that if the proteolytic activity of PR3 is solely responsible for the apoptotic effects on HUVECs, then blocking this activity should block apoptosis. To test this, proteolytically active PR3 was used to induce apoptosis in HUVECs, plus and minus inhibitors. We found that addition of 2% fetal calf serum blocked only 35.7% apoptosis and addition of α-1-antitrypsin (α1-AT) blocked only 36.1%. Because not all apoptosis was blocked, the data support the hypothesis that PR3 can induce apoptosis through a mechanism (s) that is independent of its proteolytic function. However, there is the possibility the protease inactivators were only partially effective in blocking PR3 activity. Perhaps they are effective in vitro but once the proteinases enter the cell, inhibitor activity is lost. To address this issue, we developed a system to produce PR3 fragments that did not contain the full component of sequences required for the catalytic site. Based on crystal structure studies (Figure 5A) ▶ , only one of three components of the catalytic triad was in the first one-third of the molecule, the second component was in the middle portion and the third component was in the last third of the molecule. The catalytic triad forms the active site through protein folding. 38 Therefore, three unique fragments of PR3 were generated, each of which contained only one portion of the catalytic triad. These were subcloned into a mammalian expression vector that contains a myc-epitope tag and a histidine tail (pcDNA3.1 myc-his). Cos-7 cells were transiently transfected with vectors coding for the N-terminal peptide (amino acids 2–104), middle peptide (amino acids 105–204), or the C-terminal peptide (amino acids 205–247) and stained with a DNA dye to detect DNA fragmentation. Cos-7 cells transfected with the C-terminal peptide died by apoptosis 24 hours after transfection, showing fragmented nuclei (Figure 5B) ▶ . Cells transfected with PR3-N or PR3-M did not show this morphology (data not shown).
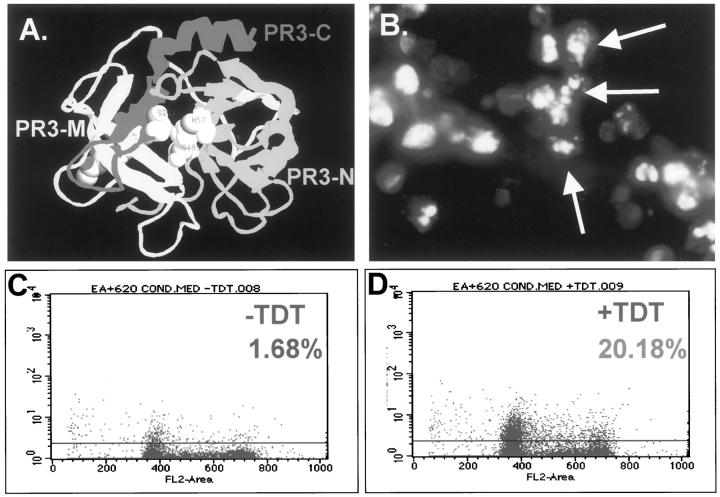
Figure 5.
Identification of a noncatalytic domain of PR3 with apoptotic function. Ribbon model of PR3 molecule (A): N-terminal fragment (PR3-N, light gray), middle fragment (PR3-M, white), and C-terminal fragment (dark gray, PR3-C). Cos-7 cells (B) transfected with PR3-C vector, stained with DAPI, show nuclear fragmentation. Flow cytometric analysis (C and D) of TUNEL-labeled EA. Hy926 cells treated with conditioned medium containing PR3-C. Limits were set using the minus TdT (C) to control for background fluorescence. Plus TdT (D) shows increased fluorescence indicative of apoptotic cells.
Having identified a fragment of PR3 with death-inducing function in Cos-7 cells, the critical question arises of whether this fragment will have a similar effect on endothelial cells. In this experiment the endothelial-like cell line, EA.hy.926, was used to test the effects of conditioned medium from culture plates of Cos-7 cells that had been transfected with PR3-N, PR3-M, or PR3-C 48 hours previously. The high level of expression of the transfected plasmid in Cos-7 cells generally results in the release of peptide into the medium. EA.hy.926 cells were used in this study because HUVECs require special medium; therefore, it was difficult to interchange the media with Cos-7 cells. However, we could interchange the media used for growing EA.hy.926 cells and Cos cells. Apoptosis was monitored by FACScan analysis of TUNEL-labeled cells, as shown in Figure 5 ▶ . The terminal transferase enzyme (TdT) required for end-labeling of the fragmented DNA was omitted to set parameters of background fluorescence (Figure 5C) ▶ . Increases because of addition of TdT indicate TUNEL-labeled apoptotic cells. EA.hy926 incubated with conditioned medium (24 hours) from PR3-C-transfected cells had 20 ± 3.0% TUNEL-positive cells (Figure 5D) ▶ . PR3-M-transfected cells had 4.75 ± 0.75% positive cells and PR3-N-transfected cells had 1.07 ± 2.6%. The data indicate that the C-terminal domain of PR3 can function as a pro-apoptotic molecule, at least in the cell types tested, whereas the N-terminal, and middle fragments cannot. These findings support the hypothesis that the mechanism of PR3-induced apoptosis does not necessarily require its proteolytic function.
Association of Increased Intracellular Oxidation with Internalization of MPO, but Not PR3
The data presented above indicate that internalization of PR3 can induce apoptosis in endothelial cells, but internalization of MPO does not, thus raising the question of whether MPO, once inside an endothelial cell, has any measurable function that could affect cellular integrity. To test this, intracellular oxidant generation was determined by monitoring the oxidation of nonfluorescent DHR to fluorescent rhodamine. By UV light microscopy, HUVECs incubated with MPO in the presence of DHR showed the generation of oxidants, displayed as green cytoplasmic fluorescence (Figure 6B) ▶ , whereas cells incubated with DHR alone did not (Figure 6A) ▶ . As analyzed by flow cytometry, MPO incubation in the presence of DHR for 6 to 24 hours induced an increase of fluorescence intensity in a dose- and time-dependent manner (Figure 6, E–G) ▶ . MPO increased oxidant levels by 60% with 1 μg/ml, by 99% with 5 μg/ml, and by 136% with 25 μg/ml treatment for 6 hours and by 349% with 25 μg/ml treatment for 24 hours (Figure 6, F and G) ▶ . Using fluorometric methods, lysates of HUVECs treated with 25 μg/mg MPO and DHR for 6 hours showed a 1.83-fold increase in oxidant production. Similar to HUVECs, SAECs incubated with MPO in the presence of DHR showed the generation of oxidants, displayed as green cytoplasmic fluorescence by UV light microscopy (Figure 6D) ▶ , whereas cells incubated with DHR alone did not (Figure 6C) ▶ . As measured by flow cytometry, oxidant levels increased by 208% with 25 μg/ml MPO treatment for 6 hours. Using fluorometric methods, lysates of SAECs treated with 25 μg/ml MPO for 6 hours showed a 1.78-fold increase. If MPO treatment results in increased levels of reactive oxygen species leading to increased levels of H2O2, then addition of catalase would result in reduced oxidant levels. Catalase directly catalyzes decomposition of H2O2 to ground-state O2. 39 Although catalase is not cell permeable, H2O2 can freely diffuse across cell membranes and therefore would be available for interaction with catalase in the tissue culture medium. 40 Addition of catalase significantly blocked MPO-induced increases in reactive oxygen species (Figure 6, F and G) ▶ , in contrast to 1 to 10 μg/ml of PR3 for 6 to 24 hours, which did not induce generation of intracellular oxidants in either HUVECs or SAECs (data not shown). The data indicate that MPO is functionally active once internalized into HUVECs or SAECs.
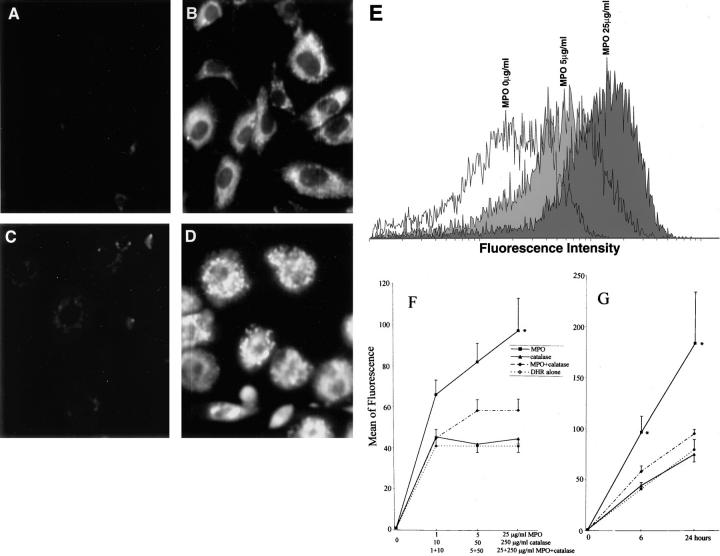
Figure 6.
Internalization of MPO results in generation of intracellular oxidants. Untreated HUVECs (A) and SAECs (C) incubated with DHR alone for 6 hours showed no cytoplasmic fluorescence. HUVECs (B) and SAECs (D) incubated with MPO (25 μg/ml) plus DHR for 6 hours showed increased intracellular oxidant generation. Flow cytometric analysis (E) showed an increase in mean of fluorescence intensity in HUVECs treated with 5 or 25 μg/ml MPO. Flow cytometric analysis (F and G) showing a dose- and time-dependent increase in intracellular oxidants generated by MPO treatment. Addition of catalase resulted in decreased intracellular oxidant generation. *, Statistically different from DHR alone (P < 0.05). Values are means ±SEM; n = 3. MPO causes the increase of intracellular oxidant generation in HUVECs in a dose- and time-dependent manner and the increase can be blocked by catalase.
Discussion
Our results provide new insights into potential mechanisms of neutrophil-associated tissue damage in inflammation. We report that endothelial cells bind, sequester, and route neutrophil PR3 and MPO from the cell surface through the cell. To our knowledge, after an exhaustive search of the database, this is the first report that neutrophil granule proteins, PR3 and MPO, are capable of entering cells and causing toxic events. Because PR3 and MPO are both released into the endothelial cell microenvironment during inflammation, our data suggest that PR3 and MPO induce changes in endothelial cells through a mechanism requiring internalization of the peptides. The significance of our data over what was previously known is that it adds a new dimension to mechanisms of PR3 and MPO effects, and future research will need to address potential interactions with cytoplasmic and/or nuclear proteins. Therefore, in addition to the conventional role of neutrophil proteins in mediating extracellular matrix degradation, we propose that granule proteins play a far more complex role in affecting endothelial cells than previously described.
Internalization of inactive PR3 seems to be linked with activation of apoptosis. The mechanism of PR3 internalization may be unique, compared to that of MPO; however, a systematic study will be required to confirm these initial observations.
We have demonstrated that the C-terminal domain of the PR3 molecule carries an apoptosis-inducing function, independent of its proteolytic function. The data imply that inactive PR3 may exert its action through penetration into the endothelial cell cytoplasm and/or nucleus and perhaps even the nucleolus. It now becomes obvious that understanding the mechanism of PR3-induced effects will be dependent on elucidation of natural PR3-interacting proteins. Some known substrates of PR3 are HSP28, nuclear factor-κB, and Sp1, proteins found in both the cytoplasm and the nucleus. 21-23 If inactive PR3 were to complex with these proteins, it is feasible that their normal functions could be altered, even in the absence of proteolytic cleavage. In support of this proposal some parallels can be drawn with the extensively studied protease, granzyme B, a cytotoxic T-cell granule protein with high homology to the neutrophil PR3. Characterization and identification of substrates demonstrated that granzyme B recognizes an 80-kd nuclear protein and cytoplasmic proteins of 50, 94, and 69 kd. 41 The 69-kd protein bound both the active and zymogen forms of granzyme B. The C-terminal fragment of PR3 has an interesting structure that may facilitate protein interactions and contribute to its pathogenic effects. The only α-helical domain in the PR3 molecule is one at the C-terminal end of the molecule. The helix is downstream of a hinge region, facilitating rotation and movement of the helix in an arm-like manner, which would allow this domain to interact with other proteins.
There is accumulating evidence that some neutrophil granule proteins have dual functions. Cathepsin G has a death function associated with the C-terminal portion of the protein, which has no proteolytic activity. 42 Interestingly, the amino acid sequence of this region of cathepsin G is highly homologous to PR3-C, the region of PR3 containing the apoptosis-inducing function. Elastase has a death function separable from and independent of its proteolytic activity. 43 It has recently been shown that azurocidin is internalized into endothelial cells and is targeted to mitochondrial compartments of the cell, but not in the nuclear compartment. 44 Interestingly, internalized azurocidin markedly reduces growth factor deprivation-induced apoptosis, and the authors have suggested that uptake of exogenous azurocidin contributes to the sustained viability of endothelial cells in the context of locally activated neutrophils. The question arises of whether azurocidin would block PR3-induced apoptosis, thus revealing complexities of the balance/counterbalance mechanisms of the neutrophilic system never before realized.
Endothelial cells have been shown to internalize two other neutrophil granule proteins, lactoferrin 45 and azurocidin. 46 Lactoferrin is thought to enter the endothelial cell once complexed to binding sites on the cell surface for cationic proteins, whereas azurocidin binds to endothelial cell surface proteoglycans. However, neither of these two proteins are proteases. Proteases that are internalized by endothelial cells include renin and tissue plasminogen activator, both of which are internalized through binding to the mannose receptor. 47,48 The mechanism of PR3 internalization has not been determined, however Taekema-Roelvink and co-workers 49 have shown that PR3 interacts with a 111-kd membrane molecule of HUVECs. Further identification of PR3 binding molecules is currently under investigation.
MPO internalization has important implications in pinpointing mechanisms involved in oxidative damage in vivo. MPO uses H2O2 to produce diffusible cytotoxic oxidants. 14 Internalization of MPO resulted in increased oxidant production through a catalase-inhibitable reaction. This means that MPO substrate H2O2 was present in these cells, in the absence of any other cytokine treatment. Vascular endothelial cells have been shown to produce basal levels of H2O2 at an intracellular site in the vicinity of peroxisomes and at a second site near the cell surface that is inaccessible to intracellular catalase. 50 It has been shown also that cultured cells have as much as threefold greater rate of release of H2O2, in certain culture mediums. 46 Therefore, it is highly possible that endothelial cells of vessels have low levels of intracellular H2O2. Titration of cellular H2O2 by MPO can result in decreased production of hydroxyl radical, thereby minimizing cell injury. 51 H2O2 can react with superoxide anion to form the highly reactive hydroxyl radical. Purified MPO has been shown to strongly inhibit hydroxyl radical production in a concentration-dependent manner. 52 Although MPO may have a protective effect in certain cases, MPO can react with H2O2 to form hypochlorous acid (HOCl), which is a more potent oxidant than H2O2, and excess hypochlorous acid formation can result in tissue damage. Active MPO has been shown to be a component of human atherosclerotic lesions, 53 and internalization of MPO could potentate this disease process.
In summary, our data indicate the release of granule proteins by activated neutrophils and monocytes during acute inflammation may result in direct toxic effects by previously unrecognized mechanisms. The evidence indicates that PR3 enters endothelial cells and induces apoptosis through a function localized to the C-terminal death domain. We are very interested in understanding how C-PR3 is activating apoptosis. Exploration of the pathway(s) used by this fragment will require purified peptide. Efforts to generate this reagent have thus far been unsuccessful, because the C-PR3 fragment kills many of the expression systems that we have tried to use. In those systems that were not killed, we have yet to get purified protein. Studying the effects of internalization of neutrophil granule proteins or their fragments may well prove to be very important in understanding mechanism(s) of injury during acute inflammation.
Footnotes
Address reprint requests to Dr. Jia Jin Yang, School of Medicine, Division of Nephrology, The University of North Carolina at Chapel Hill, CB# 7155, 347 MacNider Building, Chapel Hill, NC 27599-7155. E-mail: jjyang@med.unc.edu.
Supported by National Institute of Diabetes and Digestive and Kidney Diseases grant DK 40208.
References
- 1.Dallegri F, Ottonello L: Tissue injury in neutrophilic inflammation. Inflamm Res 1997, 46:382-391 [DOI] [PubMed] [Google Scholar]
- 2.Varani J, Ginsburg L, Schuger L, Gibbs DF, Bromberg J, Johnson KJ, Ryan U, Ward PA: Endothelial cell killing by neutrophils. Synergistic interaction of oxygen products and proteases. Am J Pathol 1989, 135:435-438 [PMC free article] [PubMed] [Google Scholar]
- 3.Kunz M, Beutel S, Brocker EB: Leucocyte activation in erythema nodosum. Clin Exp Dermatol 1999, 24:396-401 [DOI] [PubMed] [Google Scholar]
- 4.Owen CA, Campbell EJ: The cell biology of leukocyte-mediated proteolysis. J Leukoc Biol 1999, 65:137-150 [DOI] [PubMed] [Google Scholar]
- 5.Borregaard N, Cowland JB: Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997, 89:3503-3521 [PubMed] [Google Scholar]
- 6.Goldschmeding R, van der Schoot CE, ten Bokkel Huinink D, Hack CE, van den Ende ME, Kallenberg CGM, von dem Borne AEGKR: Wegener’s granulomatosis autoantibodies identify a novel diisopropylfluorophosphate-binding protein in the lysosomes of normal human neutrophils. J Clin Invest 1989, 84:1577-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falk RJ, Jennette JC: Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 1988, 318:1651-1657 [DOI] [PubMed] [Google Scholar]
- 8.Kallenberg CGM, Brouwer E, Weening JJ, Cohen TJW: Anti-neutrophil cytoplasmic antibodies: current diagnostic and pathophysiological potential. Kidney Int 1994, 46:1-15 [DOI] [PubMed] [Google Scholar]
- 9.Jennette JC, Falk RJ: Anti-neutrophil cytoplasmic autoantibodies: discovery, specificity, disease associations and pathogenic potential. Adv Pathol Lab Med 1995, 8:363-378 [Google Scholar]
- 10.Kao RC, Wehner NG, Skubitz KM, Gray BH, Hoidal JR: Proteinase 3: a distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamsters. J Clin Invest 1988, 82:1963-1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballieux BEPB, Hiemstra PS, Mohamad NK, Hagen EC, van Es LA, van der Woude FJ, Daha MR: Detachment and cytolysis of human endothelial cells by proteinase 3. Eur J Immunol 1994, 24:3211-3215 [DOI] [PubMed] [Google Scholar]
- 12.Okrent DG, Lichtenstein AK, Ganz T: Direct cytotoxicity of polymorphonuclear leukocyte granule proteins to human lung-derived cells and endothelial cells. Am Rev Respir Dis 1990, 141:179-185 [DOI] [PubMed] [Google Scholar]
- 13.Saeki T, Kuroda T, Morita T, Suzuki K, Arakawa M, Kawasaki K: Significance of myeloperoxidase in rapidly progressive glomerulonephritis. Am J Kidney Dis 1995, 26:12-21 [DOI] [PubMed] [Google Scholar]
- 14.Johnson RJ, Couser WG, Chi EY, Adler S, Klebanoff SJ: New mechanism for glomerular injury. Myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest 1987, 79:1379-1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garwicz D, Lindmark A, Hellmark T, Gladh M, Jogi J, Gullberg U: Characterization of the processing and granular targeting of human proteinase 3 after transfection to the rat RBL or the murine 32D leukemic cell lines. J Leukoc Biol 1997, 61:113-123 [DOI] [PubMed] [Google Scholar]
- 16.Campanelli D, Detmers PA, Nathan CF, Gabay JE: Azurocidin and a homologous serine protease from neutrophils. Differential antimicrobial and proteolytic properties. J Clin Invest 1990, 85:904-915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenne DE: Structure of the azurocidin, proteinase 3, and neutrophil elastase genes. Implications for inflammation and vasculitis. Am J Respir Crit Care Med 1994, 150:S147-S154 [DOI] [PubMed] [Google Scholar]
- 18.Rao NV, Rao GV, Marshall BC, Hoidal JR: Biosynthesis and processing of proteinase 3 in U937 Cells. J Biol Chem 1996, 271:2972-2978 [DOI] [PubMed] [Google Scholar]
- 19.Rao NV, Wehner NG, Marshall BC, Gray WR, Gray BH, Hoidal JR: Characterization of proteinase-3 (PR-3), a neutrophil serine proteinase. Structural and functional properties. J Biol Chem 1991, 266:9540-9548 [PubMed] [Google Scholar]
- 20.Bories D, Raynal MC, Solomon DH, Darzynkiewicz Z, Cayre YE: Down-regulation of a serine protease, myeloblastin, causes growth arrest and differentiation of promyelocytic leukemia cells. Cell 1989, 59:959-968 [DOI] [PubMed] [Google Scholar]
- 21.Spector NL, Hardy L, Ryan C, Miller WH, Humes JL, Nadler LM, Luedke E: 28-kDA mammalian heat shock protein, a novel substrate of a growth regulatory protease involved in differentiation of human leukemia cells. J Biol Chem 1995, 270:1003-1006 [DOI] [PubMed] [Google Scholar]
- 22.Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA, Anderson CW: Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem 1992, 267:794-803 [PubMed] [Google Scholar]
- 23.Franzoso G, Biswas P, Poli G, Carlson LM, Brown KD, Tomita-Yamaguchi M, Fauci AS, Siebenlist UK: A family of serine proteases expressed exclusively in myelo-monocytic cells specifically processes the nuclear factor-kB subunit p65 in vitro and may impair human immunodeficiency virus replication in these cells. J Exp Med 1994, 180:1445-1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao J, Zhang F, Donnelly RJ, Spector NL, Studzinski GP: Truncation of Sp1 transcription factor by myeloblastin in undifferentiated HL60 cells. J Cell Physiol 1998, 175:121-128 [DOI] [PubMed] [Google Scholar]
- 25.Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, Leimer AH, Cheronis J: Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci USA 1999, 96:6261-6266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csernok E, Szymkowiak CH, Mistry N, Daha MR, Gross WL, Kekow J: Transforming growth factor-beta (TGF-beta) expression and interaction with proteinase 3 (PR3) in anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Clin Exp Immunol 1996, 105:104-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skold S, Rosberg B, Gullberg U, Olofsson T: A secreted proform of neutrophil proteinase 3 regulates the proliferation of granulopoetic progenitor cells. Blood 1999, 93:849-856 [PubMed] [Google Scholar]
- 28.Berger SP, Seelen MAJ, Hiemstra PS, Gerritsma JSJ, Heemskerk E, van der Woude FJ, Daha MR: Proteinase 3, the major autoantigen of Wegener’s granulomatosis, enhances IL-8 production by endothelial cells in vitro. J Am Soc Nephrol 1996, 7:694-701 [DOI] [PubMed] [Google Scholar]
- 29.Yang JJ, Kettritz R, Falk RJ, Jennette JC, Gaido ML: Apoptosis of endothelial cells induced by the neutrophil serine proteases proteinase 3 and elastase. Am J Pathol 1996, 149:1617-1626 [PMC free article] [PubMed] [Google Scholar]
- 30.Taekema-Roelvink MEJ, van Kooten C, Janssens MC, Heemskerk E, Daha MR: Effect of anti-neutrophil cytoplasmic antibodies on proteinase 3-induced apoptosis of human endothelial cells. Scand J Immunol 1998, 48:37-43 [DOI] [PubMed] [Google Scholar]
- 31.Jans DA, Jans P, Briggs LJ, Sutton V, Trapani JA: Nuclear transport of granzyme B (fragmentin-2). J Biol Chem 1996, 271:30781-30789 [DOI] [PubMed] [Google Scholar]
- 32.Shi L, Chen G, He D, Bosc DG, Litchfield DW, Greenberg AH: Granzyme B induces apoptosis and cyclin A-associated cyclin-dependent kinase activity in all stages of the cell cycle. J Immunol 1996, 157:2381-2385 [PubMed] [Google Scholar]
- 33.Yang JJ, Jennette JC, Falk RJ: Immune complex glomerulonephritis is induced in rats immunized with heterologous myeloperoxidase. Clin Exp Immunol 1994, 97:466-473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madden V: Microwave processing of cell monolayers in situ for post-embedding immunocytochemistry with retention of ultrastructure and antigenicity. Microsc Microanal 1998, 4(Suppl 2):854-855 [Google Scholar]
- 35.Polunovsky VA, Wendt CH, Ingbar DH, Peterson MS, Bitterman PB: Induction of endothelial cell apoptosis by TNF alpha: modulation by inhibitors of protein synthesis. Exp Cell Res 1994, 214:584-594 [DOI] [PubMed] [Google Scholar]
- 36.Rochelle LG, Fischer BM, Adler KB: Concurrent production of reactive oxygen and nitrogen species by airway epithelial cells in vitro. Free Radic Biol Med 1998, 24:863-868 [DOI] [PubMed] [Google Scholar]
- 37.Dunnett CW: A multiple comparisons procedure for comparing several treatments with a control. J Am Stat Assoc 1955, 50:1096-1121 [Google Scholar]
- 38.Fujinaga M, Chernaia MM, Halenbeck R, Koths K, James MNG: The crystal structure of PR3, a neutrophil serine proteinase antigen of Wegener’s granulomatosis antibodies. J Mol Biol 1996, 261:267-278 [DOI] [PubMed] [Google Scholar]
- 39.Halliwell B: Free Radicals in Biology and Medicine. 1999:pp 134-140 Clarendon Press, New York
- 40.Bai J, Rodriguez AM, Melendez JA, Cederbaum AI: Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injury. J Biol Chem 1999, 274:26217-26224 [DOI] [PubMed] [Google Scholar]
- 41.Pinkoski MJ, Winkler U, Hudig D, Bleackley RC: Binding of granzyme B in the nucleus of target cells. J Biol Chem 1996, 271:10225-10229 [DOI] [PubMed] [Google Scholar]
- 42.Miyasaki KT, Qu XD, Harwig SS, Cho Y, Lehrer RI: Identification of CG-1, a natural peptide antibiotic derived from human neutrophil cathepsin G. Adv Dent Res 1995, 9:63-66 [DOI] [PubMed] [Google Scholar]
- 43.Garcia R, Gusmani L, Murgia R, Guarnaccia C, Cinco M, Rottini G: Elastase is the only human neutrophil granule protein that alone is responsible for in vitro killing of Borrelia burgdorferi. Infect Immun 1998, 66:1408-1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olofsson AM, Vestberg M, Herwald H, Rygaard J, David G, Arfors KE, Linde V, Flodgaard H, Dedio J, Muller-Esterl W, Lundgren-Akerlund E: Heparin-binding protein targeted to mitochondrial compartments protects endothelial cells from apoptosis. J Clin Invest 1999, 104:885-894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Courtoy PJ, Moguilevsky N, Retegui LA, Castracane CE, Masson PL: Uptake of lactoferrin by the liver. II. Endocytosis by sinusoidal cells. Lab Invest 1984, 50:329-334 [PubMed] [Google Scholar]
- 46.Paler-Martinez A, Panus PC, Chumley PH, Ryan U, Hardy MM, Freeman BA: Endogenous xanthine oxidase does not significantly contribute to vascular endothelial production of reactive oxygen species. Arch Biochem Biophys 1994, 311:79-85 [DOI] [PubMed] [Google Scholar]
- 47.Admiraal PJ, van Kesteren CA, Danser AH, Derkx FH, Sluiter W, Schalekamp MA: Uptake and proteolytic activation of prorenin by cultured human endothelial cells. J Hypertens 1999, 17:621-629 [DOI] [PubMed] [Google Scholar]
- 48.Biessen EA, van Teijlingen M, Vietsch H, Barrett-Bergshoeff MM, Bijsterbosch MK, Rijken DC, van Berkel TJ, Kuiper J: Antagonists of the mannose receptor and the LDL receptor-related protein dramatically delay the clearance of tissue plasminogen activator. Circulation 1997, 95:46-52 [DOI] [PubMed] [Google Scholar]
- 49.Taekema-Roelvink ME, Van Kooten C, Heemskerk E, Schroeijers W, Daha MR: Proteinase 3 interacts with a 111-kD membrane molecule of human umbilical vein endothelial cells. J Am Soc Nephrol 2000, 11:640-648 [DOI] [PubMed] [Google Scholar]
- 50.Kinnula VL, Mirza Z, Crapo JD, Whorton AR: Modulation of hydrogen peroxide release from vascular endothelial cells by oxygen. Am J Respir Cell Mol Biol 1993, 9:603-609 [DOI] [PubMed] [Google Scholar]
- 51.Varani J, Taylor CG, Riser B, Shumaker DK, Yeh KY, Dame M, Gibbs DF, Todd RF, III, Dumler F, Bromberg J, Killen PD: Mesangial cell killing by leukocytes: role of leukocyte oxidants and proteolytic enzymes. Kidney Int 1992, 42:1169-1177 [DOI] [PubMed] [Google Scholar]
- 52.Winterbourn CC: Myeloperoxidase as an effective inhibitor of hydroxyl radical production. Implications for the oxidative reactions of neutrophils. J Clin Invest 1986, 78:545-550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R: Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest 1996, 97:1535-1544 [DOI] [PMC free article] [PubMed] [Google Scholar]