Abstract
A strategy for hepatocyte transplantation was recently developed whereby massive replacement of the recipient liver is achieved after a combined treatment with retrorsine, a pyrrolizidine alkaloid, and partial hepatectomy. We now investigated whether liver repopulation could occur in this animal model in the absence of any exogenous growth stimuli (eg, partial hepatectomy) for the transplanted cells. Dipeptidyl-peptidase type IV-deficient (DPPIV−) rats were used as recipients. Rats were given two injections of retrorsine (30 mg/kg each, 2 weeks apart), followed by transplantation of 2 × 106 hepatocytes isolated from a normal, syngeneic, DPPIV+ donor. At 2 weeks after transplantation, clusters of DPPIV+ hepatocytes occupied 3.3 ± 0.9% of host liver, increasing to 38.2 ± 6.3% at 2 months, and to 65.9 ± 8.8% at 5 months. By 1 year, >95% of the original hepatocytes were replaced by donor-derived cells. Serum parameters related both to hepatocyte function and integrity (including glucose, bilirubin, total proteins, cholinesterase, alanine aminotransferase, and alkaline phosphatase) were in the normal range in retrorsine-treated and repopulated animals. These results provide further insights toward developing strategies for effective liver repopulation by transplanted hepatocytes with reduced toxicity for the host and potential clinical applicability.
Transplantation of isolated hepatocytes is increasingly being considered as a possible alternative to whole organ replacement, which is currently the only available therapy to treat both inherited and acquired end-stage liver disease. 1-4 In fact, in many chronic liver disorders, particularly of inherited origin, the primary defect resides in the parenchymal cell, ie, the hepatocyte, and it would seem, therefore, unnecessary to replace the entire organ. 4,5
The liver has been among the first targets for strategies based on transplantation of isolated cells. 5-7 In recent years, this approach has been extended from experimental animals to humans, with the first reported cases of hepatocyte transplantation to treat familial hypercholesterolemia 8 and hyperbilirubinemia associated to Crigler-Najjar syndrome. 9 The procedure was shown to be technically feasible; however, studies to date have reported only modest reductions in serum cholesterol and bilirubin, respectively. A major obstacle toward achieving better clinical efficacy is the limited extent to which transplanted cells proliferate in the recipient liver. 8,9 This comes as no surprise, given the very low level of cell turnover present in this organ under normal conditions. 10
In the past few years, significant progress in this field has been made by the development of several experimental models for extensive liver repopulation via transplantation of isolated hepatocytes. 11-14 The uPA transgenic mouse model was the first to demonstrate the biological possibility of massive replacement of a diseased liver via exogenously provided normal cells. 11 In this system, endogenous hepatocytes expressing the targeted uPA transgene are selectively deleted and replaced by normal cells. A similar principle of selective survival advantage seems to form the basis for the massive proliferation of transplanted cells in the liver of the FAH-null mouse, a model for human hypertyrosinemia type I. 12 In both cases, the essential feature of the model resides in the inherent genetic defect of resident cells which causes toxicity and leads to their selective death and replacement.
Using a novel approach, we have recently reported near-total liver repopulation by transplanted normal hepatocytes in rats treated with retrorsine (RS). 13 In this system, rats with a normal genetic background are exposed to RS, a naturally occurring pyrrolizidine alkaloid which causes a long lasting block of hepatocyte cell cycle. 15-17 Two-thirds partial hepatectomy (PH) is then performed and is followed immediately by transplantation of normal hepatocytes isolated from a syngeneic donor. Under these conditions, >95% liver replacement by donor-derived cells is observed within 2 to 4 months after the operation. 13,18 A key component in this model is the persistent block imposed by RS on endogenous hepatocyte cell division, which allows for the selective expansion of transplanted cells. 13,19 Consistent with this interpretation, PH was found to be essential to achieve massive liver replacement within a few months after transplantation. However, it was repeatedly observed 13,20 that a low, but significant extent of repopulation (up to ∼15% within 6 weeks) 20 was also present in animals not receiving PH. Given the potential relevance of these findings, we decided to conduct a long-term study to follow the fate of transplanted hepatocytes in rats previously given RS and in the absence of any exogenously elicited growth stimulus, such as PH. The dipeptidyl-peptidase type IV-deficient (DPPIV−) F344 rat model for hepatocyte transplantation was used, as in previous reports. 13,20 Results indicated extensive (70 to 90%) replacement of the recipient liver by donor-derived cells at 6 to 9 months after transplantation, in both male and female rats. Moreover, serum analysis, performed up to 1 year after hepatocyte infusion, revealed normal values for several parameters related to liver function in the transplanted, repopulated animals.
Materials and Methods
A colony of DPPIV− F344 rats has been established in our laboratory, at the Department of Medical Sciences and Biotechnology, University of Cagliari (with animals supplied by the Marion Bessin Liver Research Center, Albert Einstein College of Medicine, New York, NY). Donor DPPIV+ F344 rats were purchased from Charles River, Milan, Italy. All animals were maintained on alternating 12 hour light/dark daily cycles, with food and water available ad libitum. They were fed Purina Rodent Lab Chow diet (Ditta Piccioni, Italy) throughout the experiments. Both male and female F344 DPPIV− rats were used. All animals received humane care according to the criteria outlined in National Institutes of Health Publication no. 86-23, revised in 1985.
Hepatocyte transplantation was performed with a modification of our recently published protocol. 13 Briefly, F344 DPPIV− rats, both male and female, weighing 80 to 100 g, were given two intraperitoneal injections of RS (Sigma Chemical Co., St. Louis, MO), 30 mg/kg each, 2 weeks apart. Two weeks after the last injection of RS, each animal received 2 × 10 6 freshly isolated hepatocytes via portal vein infusion. Hepatocytes were isolated from normal young adult DPPIV+ F344 donor rats according to a standard two step collagenase perfusion technique. 21 The isolated cell fraction used for transplantation studies was judged to be ∼95% hepatocytes by morphological analysis and cell viability was consistently between 85 to 95%, as determined by trypan blue dye exclusion. Control groups received either RS alone or no treatment. Four to six animals were killed at various intervals during the experiment, as indicated in the Results section. Where indicated, a single injection of 5′-bromodeoxyuridine (BrdU) (50 mg/kg i.p.; Sigma Chemical Co.) was given to the animals 2 hours before sacrifice. Liver samples were fixed in 10% buffered formaldehyde or snap-frozen. Histochemical determination of DPPIV enzyme activity and quantitation of DPPIV-positive areas in the liver were performed as described. 13,20 Three random sections were cut from each liver lobe of each animal and stained for DPPIV enzyme activity. Sections were then projected into a magnetic graphics tablet (NewSketch 1212 HR; Genius, KEY System Corp., Langenfeld, Germany), and quantitated with the help of a computer-assisted image analyzer. Liver DNA content was estimated according to published techniques, 20 whereas serum analysis was performed using automated Synchron Clinical System CX7 Delta (Beckman Coulter). Glutathione-S-transferase 7-7 (GST 7hyphen]7) and BrdU-labeling were detected by immunohistochemical methods. 22 Statistical analysis was performed using the Student’s t-test.
Results
Transplanted Hepatocytes Repopulate Extensively the Liver of RS-Treated Male and Female Rats in the Absence of Exogenous Growth Stimuli
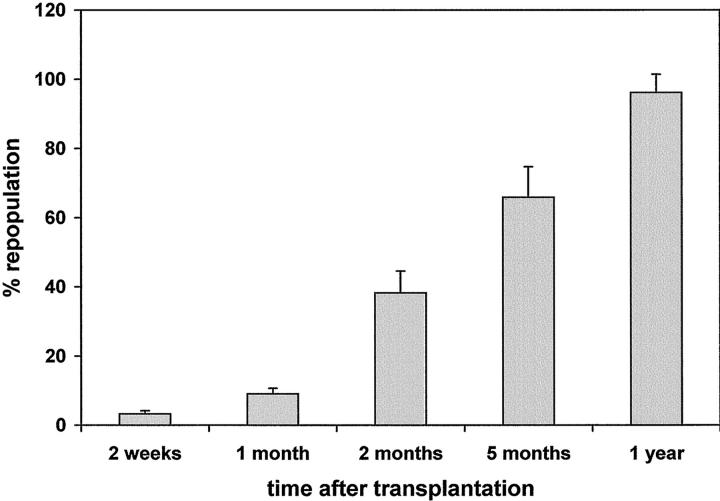
Animals were treated according to the standard protocol for hepatocyte transplantation with RS; 13 however, a major modification was introduced, in that no PH was performed at the time of cell infusion; moreover, in the absence of PH, (which cannot be performed before 4 weeks after treatment with RS, because it causes significant animal loss), we decided to perform cell transplantation at 2 weeks after the last dose of RS, reasoning that it was no longer necessary to maintain a 4-week gap. All groups tolerated well the experimental procedure. At 2 weeks after cell transplantation, numerous clusters (30 to 40/cm2) of DPPIV+ donor hepatocytes were present throughout the DPPIV− recipient liver; they comprised 5 to 10 cells/cluster/cross section, occupying 3.3 ± 0.9% of the total liver area (Figure 1 ▶ and Figure 2A ▶ ). Clusters were composed of approximately normal-sized hepatocytes, whereas the recipient liver was extensively megalocytic. 13,17 At 1 month, DPPIV+ clusters had enlarged in size to 10 to 40 cells/cross section and comprised 9.1 ± 1.6% of the total area (Figure 1 ▶ and Figure 2B ▶ ). They were mostly round or oval in shape; however, some appeared elongated along the hepatic plates. No signs of compression were evident in the surrounding parenchyma. Surrounding (DPPIV−) parenchymal cells were still megalocytic; however, collections of small DPPIV− hepatocytes (20 to 30 cells/focus) were evident, with a frequency of approximately one in 10 compared to DPPIV+ clusters. These have been referred to as “regenerative nodules” 13,17,23 and seem to be similar to those recently described by Gordon and colleagues 24 during liver regeneration in rats given RS.
Figure 1.
Liver replacement by DPPIV+-transplanted hepatocytes in DPPIV-deficient male rats treated with RS. Rats were injected with RS (2 doses, 30 mg/kg each, 2 weeks apart) followed 2 weeks later by transplantation of 2 × 10 6 DPPIV+ hepatocytes (via portal vein), isolated from a normal donor. Data represent mean ±SD of four to six animals per time point. Three random sections from each liver lobe were taken from each animal. Additional details are reported in Materials and Methods.
Figure 2.
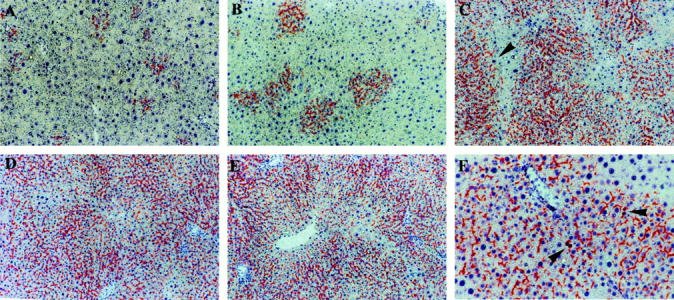
Liver replacement by DPPIV+-transplanted hepatocytes in DPPIV-deficient male and female rats treated with RS. A: Male recipient, 2 weeks after transplantation of 2 × 10 6 isolated hepatocytes. Clusters of DPPIV+ hepatocytes (five to 10 cells/cross section) were present throughout the liver, with a higher density around the portal triads. Surrounding parenchyma was diffusely megalocytic. B: Male recipient, 1 month after transplantation. DPPIV+ hepatocyte clusters had enlarged to 10 to 40 cells/cross section, occupying 9.1 ± 1.6% of the host liver. However, no signs of compression were evident in the tissue surrounding these clusters, where enlarged hepatocytes still represented the dominant cell type. C: Male recipient, 5 months after cell transplantation. Extensive replacement (65.9 ± 8.8%) of the host liver by DPPIV+-transplanted hepatocytes was present at this time, whereas residual megalocytes were discerned in DPPIV− areas of recipient origin (arrowhead). D: Male recipient, 1 year after transplantation. More than 95% of the liver was now occupied by DPPIV+ hepatocytes of donor origin, with only scattered DPPIV− megalocytes still present. E: Female DPPIV− recipient, 8 months after transplantation of 2 × 10 6 DPPIV+ hepatocytes. Results were similar to those seen in male animals (D), with 81.4 ± 7.6% of host liver replacement by transplanted cells. F: Female recipient, 8 months after transplantation. The animal was injected with BrdU 2 hours before killing and liver sample was processed with a dual histochemical-immunohistochemical technique for DPPIV enzyme activity (orange), localized to the hepatocytic membrane, and for BrdU incorporation into nuclei (dark red). BrdU-labeled nuclei of proliferating cells were still detected at this time point in the donor-derived hepatocyte population (arrowheads). Original magnifications: ×100 (A–E), ×200 (F).
By 2 months after cell transplantation, islands of DPPIV+ hepatocytes had expanded further, reaching 200 to 300 cells/cluster/cross section, with some clusters already becoming confluent. They represented 38.2 ± 6.3% of the liver mass. This proportion increased to 65.9 ± 8.8% at 5 months (Figure 1 ▶ and Figure 2C ▶ ) and was 96.1 ± 5.3% in animals treated with RS and killed 1 year after cell transplantation (Figure 1 ▶ and Figure 2D ▶ ). In the latter group, only residual scattered megalocytes were visible, along with rare (1.3 ± 0.9/cm2) islands of small DPPIV− hepatocytes comprising 100 to 300 cells and representing <3% of the total area. By contrast, no growth of DPPIV+ hepatocytes was seen at any time point (up to 1 year after cell transplantation) when cells were transplanted into normal untreated DPPIV− recipients (data not presented), in agreement with our previous findings. 13
In a parallel study, we investigated whether the liver of female rats treated with RS would also be replaced by normal transplanted hepatocytes when no exogenous proliferative stimulus was provided. This was a relevant issue to address, in light of a reported lower sensitivity of female versus male animals to the acute effects of RS. 25 DPPIV− female rats were injected with RS followed by DPPIV+ hepatocyte transplantation, as outlined above, and killed 2 months or 8 months thereafter. Surprisingly, results were very similar to those observed in male animals, with 33.9 ± 5.2% repopulation by DPPIV+ hepatocytes at 2 months and 81.4 ± 7.6% at 8 months (Figure 2E) ▶ . Moreover, as revealed by double labeling for DPPIV enzyme activity and BrdU immunohistochemical staining, proliferating DPPIV+ hepatocytes were still detected in the liver of female animals even 8 months after transplantation (Figure 2F) ▶ . This observation suggests that the process of liver repopulation was still active for many months after transplantation.
The extent of repopulation found in male and female animals in this study was higher than that described in our earlier reports in RS-treated animals not receiving PH. 13,26 However, more recently we observed as high as 15 to 20% repopulation in RS-treated female rats at 6 weeks after cell transplantation, in the absence of PH or any other exogenous stimulus. 20 Interestingly, both in the latter 20 and in the present study, cell transplantation was performed at 2 weeks after the second dose of RS, whereas in the first report, 13 this interval was 4 weeks. This suggests that interval between RS treatment and cell transplantation could be a determinant in the extent of repopulation in this model.
Repopulating Hepatocyte Clusters Do Not Express Increased Levels of GST 7-7
Hepatocytes transplanted into the liver of animals treated with RS undergo rapid proliferation to form discrete proliferative clusters, which then expand to eventually replace the bulk of the recipient liver. Given the long-standing concern regarding a possible association between repeated cell division cycles (clonal expansion) and increased risk for cell transformation, 27 we tested for increased expression of GST 7-7 in DPPIV+ hepatocyte clusters at various times during liver repopulation. Increased expression of GST 7-7 is in fact a common finding in hepatocyte foci and nodules developing during chemically induced liver carcinogenesis in rats, and is considered a marker to identify early focal lesions at risk for neoplastic transformation. 28 Serial sections were used for either histochemical staining of DPPIV enzyme activity or immunohistochemical detection of GST 7-7. Liver samples obtained at 2 weeks, and 1, 2, 5, and 8 months after cell transplantation were examined. Representative results are shown in Figure 3, A and B ▶ . No evidence was found for increased expression of GST 7-7 in DPPIV+ hepatocytes at any time point considered. Positive GST 7-7 staining was regularly present in bile ducts 17 and was found only in rare scattered hepatocytes of recipient origin. Histological findings on hematoxylin and eosin-stained liver sections were similar to those previously described. 13 Figure 3, C and D ▶ , reports liver histology of untreated (Figure 3C) ▶ or treated with RS and transplanted (Figure 3D) ▶ female rats, killed 9 months after starting the experiment. The liver architecture was primarily normal, except for some residual areas of megalocytosis. No proliferation of bile ductular structures was evident.
Figure 3.
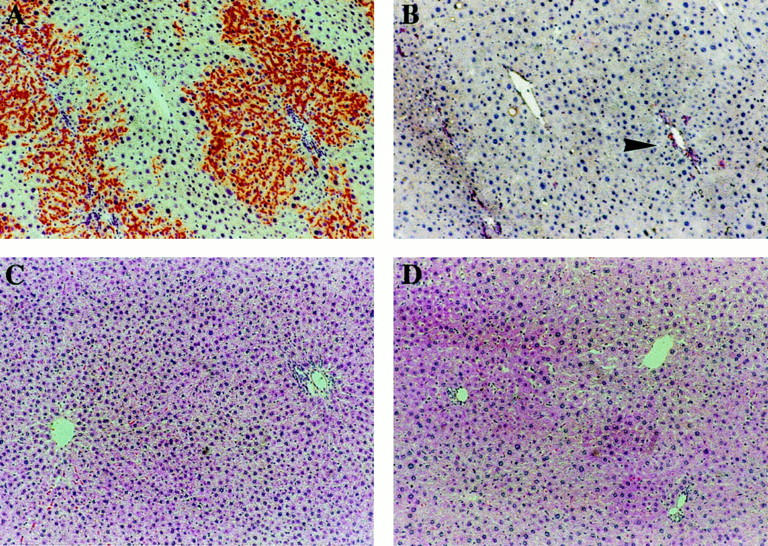
A and B: Serial sections of liver from a male recipient killed 5 months after transplantation. A: Histochemical staining for DPPIV activity shows large clusters of repopulating donor hepatocytes. B: Immunohistochemical analysis for the expression of GST 7-7 on serial section revealed no staining corresponding to DPPIV+ hepatocyte clusters present in A. The enzyme was regularly detected in bile ducts (arrowhead). C and D: Standard histology on formalin-fixed and H&E-stained liver samples obtained from female recipients 8 months after transplantation. C: Untreated control liver. D: RS-treated and transplanted liver. Liver architecture appeared essentially normal, except for the presence of scattered megalocytes most frequently located around the pericentral zone. Extent of liver replacement by transplanted cells was 81.4 ± 7.6% (see Figure 2E ▶ ).
Normal Liver Function after the RS Protocol with or without Hepatocyte Transplantation
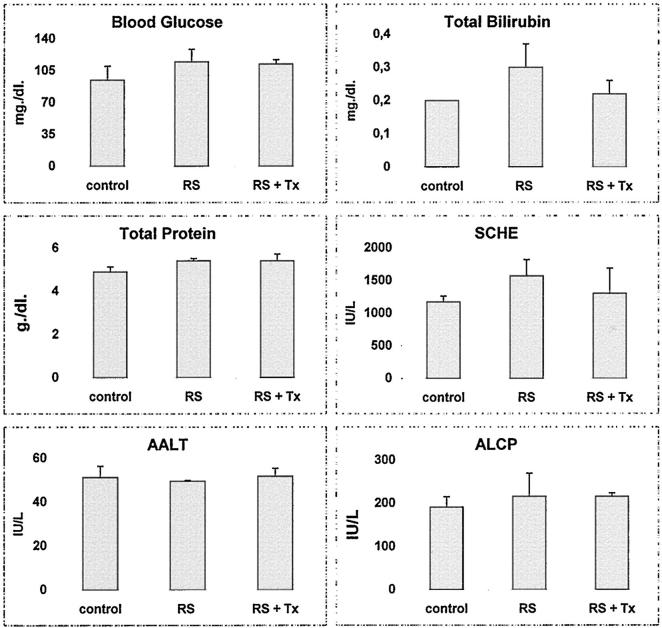
We have previously documented that transplanted hepatocytes repopulating the liver of RS-treated rats express a fully differentiated phenotype, according to a series of biochemical parameters detected by histochemical and immunohistochemical methods. 13 To further extend these observations at the whole animal level, several serum parameters related both the hepatocyte function and integrity (including glucose, bilirubin, total protein, cholinesterase, alanine aminotransferase, alkaline phosphatase), were monitored in rats presenting extensive liver repopulation. In addition, animals exposed to RS treatment with no transplantation were also examined and compared to normal untreated rats. Figure 4 ▶ presents results obtained in groups of female animals killed 9 months after starting the experiment. The extent of repopulation in the transplanted group was 81.4 ± 7.6% (data presented above). As reported in Figure 4 ▶ , no significant alterations in any of the considered parameters were detected in transplanted animals compared to normal reference levels. A similar pattern of results was also seen in male and female animals killed at various time points during the process of repopulation, up to 1 year after cell transplantation. Furthermore, exposure to RS alone, according to protocol developed for hepatocyte transplantation, 13 was also not associated with any significant alteration in any of the examined parameters, suggesting that the protocol per se does not compromise liver function.
Figure 4.
Serum analysis of samples obtained from female animals at 8 months after transplantation. Control: age-matched, untreated group; RS: RS-treated group; RS+TX; RS-treated and transplanted group. SCHE, serum cholinesterase; AALT, alanine aminotransferase; ALCP, alkaline phosphatase. Values are mean ± SD of four samples per group. No statistically significant differences were present among different groups in any of the considered parameters.
Reduced Liver Size in Rats Treated with RS
Pyrrolizidine alkaloids, including RS, are known for their ability to impose a persistent block on hepatocyte cell division. 17,29,30 This effect becomes particularly evident when the liver is challenged with a vigorous regenerative stimulus, such as that elicited by PH. 17 However, some reports have also pointed to a relative inability of the liver exposed to these alkaloids to maintain a balanced mass during normal organ turnover. 23 In support of these findings, we have recently observed a decrease in liver weight and liver DNA content in animals receiving two doses of RS (30 mg/kg each, as in the present study) and killed 2 weeks after the second injection. 17 This finding may become relevant in light of the results presented above (ie, extensive proliferation of transplanted hepatocytes in rats treated with RS, in the absence of exogenous growth stimuli). In fact, it is reasonable to conceive that the presence of a relatively small liver in animals treated with RS may set the stage for the selective proliferation of transplanted cells, which are not blocked by previous exposure to the alkaloid.
Based on the above considerations, we examined whether there was any alteration in the size of the liver and/or total liver DNA content in female animals treated with RS and killed several months later. Three groups of animals were included in this study: group I, untreated controls; group II, RS-treated only; and group III, RS plus cell transplantation (details are reported in the Materials and Methods). All groups were killed 9 months after the initial treatment. The results obtained confirmed and extended our previous observations. As reported in Figure 5A, a ▶ significant reduction in the relative weight of the liver was seen in the groups receiving RS (groups II and III) compared to controls (group I), with no intergroup differences between transplanted and nontransplanted animals (groups III and II, respectively). A similar pattern of results was seen when liver DNA content was considered. Both total DNA/liver (Figure 5B) ▶ and liver DNA/100 g body weight (Figure 5C) ▶ were significantly reduced (up to 35%) in RS-treated groups compared to untreated controls. Again, there was no statistically significant difference in either parameter between groups receiving RS or RS plus cell transplantation.
Figure 5.
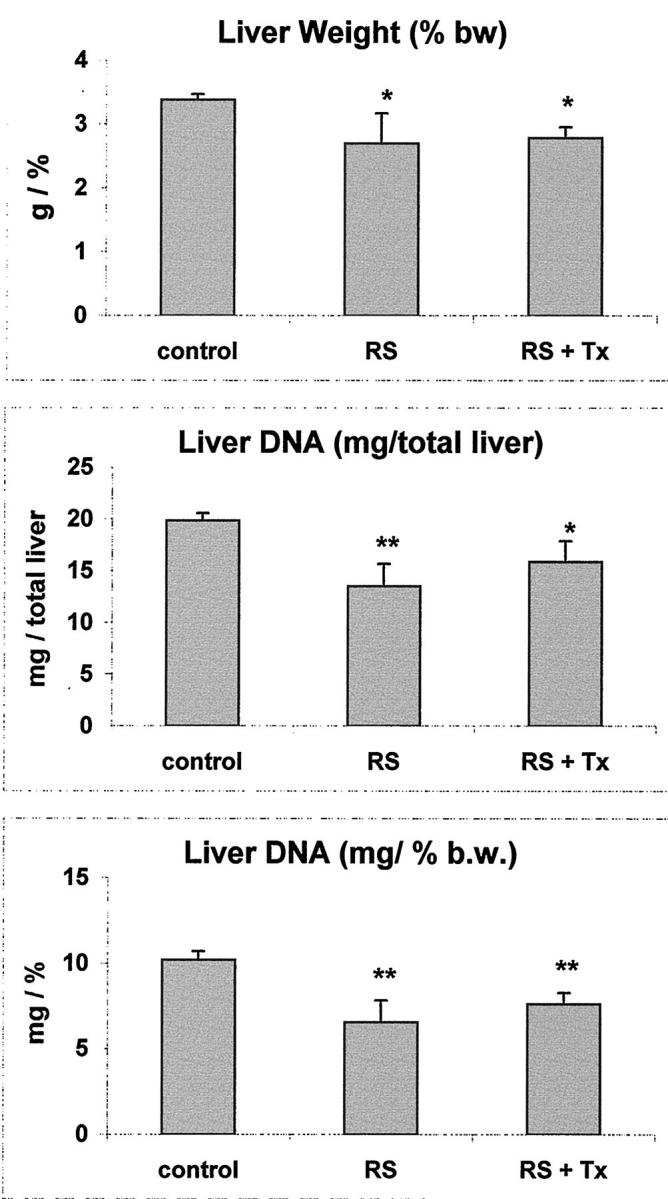
Liver size and liver DNA content in female animals at 8 months after transplantation. Control: age-matched, untreated group; RS: RS-treated group; RS+TX; RS-treated and transplanted group. Values are mean ±SD of four samples per group. Significantly different from control: *, P < 0.05; **, P < 0.01. No statistically significant differences were present between RS and RS+TX groups in any of the considered parameters.
Discussion
The first relevant conclusion from these studies is that massive liver replacement by transplanted normal hepatocytes can take place in rats treated with RS in the absence of any exogenous growth stimulus administered during the process of repopulation. As such, these results represent a significant extension of our previous findings in which near-total liver replacement was described in RS-treated rats when normal hepatocytes were transplanted in conjunction with PH. Although the kinetics of repopulation in the absence of PH was slower compared to that observed in the original study, 13 significant liver replacement was already present at 2 months after transplantation, (38.2 ± 6.3%), becoming extensive at 5 months (65.9 ± 8.8%) and further increasing to >90% between 8 months and 1 year. The significance of these findings is twofold: 1) they illustrate the possibility to achieve massive liver replacement by transplanted cells without the need for growth stimuli to be provided exogenously (eg, PH or other mitogenic agents); and 2) they suggest possible novel mechanisms sustaining the selective expansion of donor-derived normal cells.
For transplanted cells to proliferate, one needs to provide the necessary space in the recipient organ. 4 Thus, both in the uPA transgenic mouse 11 and in the FAH-null mouse, 12 the selective proliferation of transplanted normal cells is fostered by a process of chronic toxicity and cell death occurring in the resident hepatocyte population. Similarly, in the RS model of liver repopulation in the rat, as originally described, partial removal of liver tissue in a background of persistent mitoinhibition of resident hepatocytes was found to be essential for the rapid and massive expansion of transplanted cells. 13
However, our present data cannot be entirely explained within this conceptual framework. In fact, no exogenous growth stimulus (eg, PH) was introduced to drive proliferation of transplanted cells, nor there was any histological (Figure 3D) ▶ or serological evidence of chronic toxicity leading to necrosis of endogenous hepatocytes, either during the process of liver repopulation or in RS-treated animals not receiving cell transplantation (Figure 4, A through F) ▶ . It is still possible to consider that a mechanism of compensatory growth may contribute to the extensive proliferation of transplanted cells in the absence of exogenous stimuli. In fact, both liver weight and liver DNA content were reduced compared to appropriate controls (Figure 4) ▶ , in animals treated with RS and killed as long as 8 months later, in line with previous observations. 20 Such a relative deficit of liver mass persisting throughout a prolonged period may represent a sufficient growth compensatory stimulus for transplanted cells to expand, given the persistent cell cycle block exerted by RS on host hepatocytes. However, no significant recovery of liver mass and/or liver DNA content was seen in RS-treated and transplanted animals even in presence of levels of repopulation as high as 85% of the total liver. This finding suggests that the selective proliferation of transplanted normal cells is primarily sustained by additional mechanisms, different from simple compensatory growth.
It is clear from our data that donor-derived cells are not simply growing side by side with endogenous megalocytes blocked by RS, but they are actually replacing resident parenchymal cells. There is evidence indicating an increased susceptibility to the induction of apoptotic cell death in rat liver exposed to RS. 26 Most recently, apoptosis associated with enhanced levels of Bax protein was reported in the liver of rats treated with RS followed by PH. 31 The relevance of the latter findings to our present results needs to be carefully evaluated, because in this study liver replacement by transplanted cells occurred in the absence of PH. However, it is possible to hypothesize that a prolonged increase in the rate of apoptosis in RS-exposed endogenous hepatocytes may drive the growth of donor-derived cells; alternately, or in combination, the presence of normal transplanted cells may trigger selective deletion of RS-damaged resident hepatocytes, also possibly through apoptosis.
A second interesting issue raised by the present results pertains to the efficiency of the RS model of hepatocyte transplantation in male versus female rats. In fact, female rats were reported to be relatively resistant to the acute toxicity of RS, with an LD50 almost five times as high as that for male rats of the same age. 25 It is therefore surprising to find that, using the same standard dose of RS (30 mg/kg twice) in both male and female recipients, and in the absence of exogenous growth stimuli, female rats also undergo massive liver replacement by transplanted cells, with percentages of repopulation similar to those observed in males. These data are of particular significance and suggest that acute toxicity of RS, or at least part of it, can be avoided in a setting designed for liver repopulation through transplanted hepatocytes. Additional studies are in progress to explore this possibility.
We have previously documented that normal hepatocytes transplanted into RS-treated liver express a differentiated phenotype during the process of liver repopulation and perform normal biochemical functions. 13,18 Results presented in this report confirm and extend the above conclusion; in fact, both RS-treated and RS-treated and repopulated livers tested normal according to several serum parameters related to hepatocyte function and integrity (Figure 3) ▶ . It is noteworthy that RS treatment per se, when given according to the present protocol for hepatocyte transplantation, does not seem to cause significant impairment of liver function several months after exposure, despite the persistence of extensive areas of megalocytosis.
It is still debatable whether repeated cell proliferation per se represents a risk factor for the emergence of the neoplastic phenotype. 27,32 Under the experimental conditions described in the present studies, in which transplanted cells undergo a process of clonal expansion involving many cell divisions, no evidence for increased focal expression of GST 7-7 was found at any time point during the repopulation process (Figure 3, A and B) ▶ . To the extent that this enzyme is a reliable marker of neoplastic precursor lesions, 26 this result indicates that liver repopulation with transplanted normal hepatocytes in this model is not associated with the emergence of phenotypically altered cells on the pathway toward neoplasia. However, additional studies with longer periods of observation are warranted to settle this issue.
In conclusion, the present results provide important insights toward developing strategies for effective liver repopulation by transplanted hepatocytes with reduced toxicity for the host and potential clinical applicability. Extensive liver replacement could in fact be demonstrated in RS-treated animals in the absence any of exogenous growth stimuli, such as PH, thereby avoiding any additional treatment. Furthermore, acute toxicity of RS, including hepatocyte necrosis, seems unnecessary to set the stage for liver repopulation to occur in this model. A better understanding of the relevant mechanisms which are driving the growth of transplanted normal hepatocytes in RS-treated rat liver will help in designing better approaches toward the applicability of hepatocyte transplantation as an effective therapeutic strategy. 1-4
Acknowledgments
We thank Marinella Boi for her expert technical support and Tiziana Pusceddu for her excellent secretarial assistance.
Footnotes
Address reprint requests to Dr. Ezio Laconi, M.D., Ph.D., Sezione di Patologia Sperimentale, Ospedale Oncologico “A. Businco”, Via Jenner, 09125, Cagliari, Italy. E-mail: elaconi@unica.it.
Supported in part by Telethon, Italy (to P. P.), the Roche Organ Transplantation Research Foundation (to E. L.), and by grants RO1 DK17609 and P30 DK41296 from the National Cancer Institute, Bethesda, MD (to D. A. S.).
References
- 1.Lake JR: Hepatocyte transplantation. N Engl J Med 1998, 338:1464-1465 [DOI] [PubMed] [Google Scholar]
- 2.Strom SC, Chowdhury JR, Fox IJ: Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis 1999, 19:39-48 [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Gorla GR, Irani AN: Hepatocyte transplantation: emerging insights into mechanisms of liver repopulation and their relevance to potential therapies. J Hepatol 1999, 30:162-170 [DOI] [PubMed] [Google Scholar]
- 4.Grompe M, Laconi E, Shafritz DA: Principles of therapeutic liver repopulation. Semin Liver Dis 1999, 19:7-14 [DOI] [PubMed] [Google Scholar]
- 5.Kay MA, Woo SLC: Gene therapy for metabolic disorders. Trends Genet 1994, 10:253-257 [DOI] [PubMed] [Google Scholar]
- 6.Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Sowe A, Simmons RL, Najarian JS: Hepatocellular transplantation for metabolic deficiencies; decrease of plasma bilirubin in Gunn rats. Science 1976, 192:892-894 [DOI] [PubMed] [Google Scholar]
- 7.Groth CG, Arborgh B, Bjorken C, Sundberg B, Lundgren G: Correction of hyperbilirubinemia in the glucuronyl-transferase deficient rat by intraportal hepatocyte transplantation. Transplant Proc 1976, 9:313-316 [PubMed] [Google Scholar]
- 8.Grossman M, Rader DJ, Muller DWM, Kolansky DM, Kozarsky K, Clark DJ, III, Stein EA, Lupien PJ, Brewer HB, Jr, Raper SE, Wilson JM: A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med 1995, 1:1148-1154 [DOI] [PubMed] [Google Scholar]
- 9.Fox IJ, Roy-Chowdhury J, Kaufman SS, Goertzen TC, Roy-Chowdhury N, Warkentin TI, Dorko K, Sauter BV, Strom SC: Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med 1998, 338:1422-1426 [DOI] [PubMed] [Google Scholar]
- 10.Wright N, Alison MR: The liver. The Biology of Epithelial Cell Populations, 1984, vol. 2:pp 880-980 Clarendon Press, , ch. 25. Oxford [Google Scholar]
- 11.Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL: Replacement of diseased mouse liver by hepatic cell transplantation. Science 1994, 263:1149-1152 [DOI] [PubMed] [Google Scholar]
- 12.Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou C-N, Finegold M, Grompe M: Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tryosinaemia type I. Nat Genet 1996, 12:266-273 [DOI] [PubMed] [Google Scholar]
- 13.Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA: Long term, near total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol 1998, 158:319-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guha C, Sharma A, Gupta S, Alfieri A, Gorla GR, Gagandeep S, Rana S, Roy-Chowdhury N, Tanka KE, Vikram B, Roy-Chowdhury J: Amelioration of radiation-induced liver damage in partially hepatectomized rats by hepatocyte transplantation. Cancer Res 1999, 59:5871-5874 [PubMed] [Google Scholar]
- 15.McLean E: The toxic actions of pyrrolizidine (senecio) alkaloids. Pharmacol Rev 1970, 22:429-483 [PubMed] [Google Scholar]
- 16.Dabeva MD, Laconi E, Oren R, Petkov PM, Hurston E, Shafritz DA: Liver regeneration and α-fetoprotein mRNA expression in the retror-sine model for hepatocyte transplantation. Cancer Res 1998, 58:5825-5834 [PubMed] [Google Scholar]
- 17.Laconi S, Curreli F, Diana S, Pasciu D, De Filippo G, Sarma DSR, Pani P, Laconi E: Liver regeneration in response to partial hepatectomy in rats treated with retrorsine: a kinetic study. J Hepatol 1999, 31:1069-1074 [DOI] [PubMed] [Google Scholar]
- 18.Oren R, Dabeva MD, Petkov PM, Hurston E, Laconi E, Shafritz DA: Restoration of normal serum albumin levels in Nagase analbuminemic rats by hepatocyte transplantation. Hepatology 1999, 29:75-81 [DOI] [PubMed] [Google Scholar]
- 19.Laconi E: Differential growth: from carcinogenesis to liver repopulation. Am J Pathol 2000, 156:389-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pani P, Laconi S, Pillai S, Scintu F, Curreli F, Shafritz DA, Laconi E: Direct hyperplasia does not enhance kinetics of liver repopulation in a new model of hepatocyte transplantation in the rat. J Hepatol 1999, 31:354-359 [DOI] [PubMed] [Google Scholar]
- 21.Seglen PO: Preparation of isolated rat liver cells. Methods Cell Biol 1976, 13:29-83 [DOI] [PubMed] [Google Scholar]
- 22.Tomasi C, Laconi E, Laconi S, Greco M, Sarma DSR, Pani P: Effect of fasting/refeeding on the incidence of chemically-induced hepatocellular carcinoma in the rat. Carcinogenesis 1999, 20:1979-1983 [DOI] [PubMed] [Google Scholar]
- 23.Schoental RJ, Magee PN: Further observations on the subacute and chronic liver changes in rats after a single dose of various pyrrolizidine (senecio) alkaloids. J Pathol Bacteriol 1959, 78:471-481 [DOI] [PubMed] [Google Scholar]
- 24.Gordon GJ, Coleman WB, Hixson DC, Grisham JW: Liver regeneration in rats with retrorsine-induced hepatocellular injury proceeds through a novel cellular response. Am J Pathol 2000, 156:607-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattocks AR: Acute hepatotoxicity and pyrrolic metabolites in rats dosed with pyrrolizidine alkaloids. Chem-Biol Interact 1972, 5:227–242 [DOI] [PubMed]
- 26.Oren R, Dabeva MD, Karnezis AN, Petkov PM, Rosencrantz R, Sandhu JP, Moss SF, Wang S, Hurston E, Laconi E, Holt PR, Thung SN, Zhu L, Shafritz DA: Role of thyroid hormone in stimulating liver repopulation by transplanted hepatocytes. Hepatology 1999, 30:903-913 [DOI] [PubMed] [Google Scholar]
- 27.Ames BN, Gold LS: Too many rodent carcinogens: mitogenesis increases mutagenesis. Science 1990, 249:970-971 [DOI] [PubMed] [Google Scholar]
- 28.Satoh K, Kitahara A, Soma Y, Inaba Y, Hatayama I, Sato K: Purification, induction and distribution of placental glutathione transferase: a new marker enzyme for preneoplastic cells in the rat chemical hepatocarcinogenesis. Proc Natl Acad Sci USA 1985, 82:3964-3968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson JE: Effects of the pyrrolizidine alkaloid lasiocarpine-N-oxide on nuclear and cell division in the liver of rats. J Pathol Bacteriol 1965, 89:153-171 [DOI] [PubMed] [Google Scholar]
- 30.Jago MV: The development of the hepatic megalocytosis of chronic pyrrolizidine alkaloid poisoning. Am J Pathol 1969, 56:405-422 [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon GJ, Coleman WB, Grisham JW: Bax-mediated apoptosis in the livers of rats after partial hepatectomy in the retrorsine model of hepatocellular injury. Hepatology 2000, 32:312-320 [DOI] [PubMed] [Google Scholar]
- 32.Farber E: Cell proliferation as a major risk factor for cancer: a concept of doubtful validity. Cancer Res 1995, 55:3759-3762 [PubMed] [Google Scholar]