Abstract
Intraneuronal filamentous tau inclusions such as neurofibrillary tangles (NFTs) are neuropathological hallmarks of Alzheimer’s disease (AD) and related sporadic and familial tauopathies. NFTs identical to those found in AD brains have also been detected in the hippocampus and entorhinal cortex of cognitively normal individuals as they age. To recapitulate age-induced NFT formation in a mouse model, we examined 12- to 24-month-old transgenic (Tg) mice overexpressing the smallest human brain tau isoform. These Tg mice develop congophilic tau inclusions in several brain regions including the hippocampus, amygdala, and entorhinal cortex. NFT-like inclusions were first detected in Tg mice at 18 to 20 months of age and they were detected by histochemical dyes that bind specifically to crossed β-pleated sheet structures (eg, Congo red, Thioflavin S). Moreover, ultrastructurally these lesions contained straight tau filaments comprised of both mouse and human tau proteins but not other cytoskeletal proteins (eg, neurofilaments, microtubules). Isolated tau filaments were also recovered from detergent-insoluble tau fractions and insoluble tau proteins accumulated in brain in an age-dependent manner. Thus, overexpression of the smallest human brain tau isoform resulted in late onset and age-dependent formation of congophilic tau inclusions with properties similar to those in the tangles of human tauopathies, thereby implicating aging in the pathogenesis of fibrous tau inclusions.
Abnormal tau proteins are implicated in mechanisms of brain degeneration in Alzheimer’s disease (AD), frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17), Pick’s disease, and related neurodegenerative tauopathies. Indeed, the neuropathological hallmarks of tauopathies are abundant aggregates of paired helical filaments (PHFs) and/or straight filaments composed of aberrantly phosphorylated tau proteins in central nervous system (CNS) neurons and/or glia. 1,2 In addition to neurodegenerative tauopathies, cognitively normal individuals develop a small number of neurofibrillary tangles (NFTs) in hippocampus and limbic structures as they age. 3 Thus, aging is a risk factor for the formation of NFTs in specific brain regions. Six highly soluble tau isoforms are generated from a single gene by alternative splicing and expressed predominantly in axons of the adult human brain. 4-6 The functions of these proteins include binding to and stabilizing microtubules (MTs) in the polymerized state. 7,8 However, it has been shown that abnormally phosphorylated filamentous tau proteins cannot perform these important functions and aggregate in neurons to form insoluble NFTs in AD 9-11 or similar fibrillary neuronal and/or glial inclusions in other tauopathies. 1,2
To study the pathogenesis of neurodegenerative tauopathies, we previously generated transgenic (Tg) mice that overexpressed the shortest human brain tau isoform in CNS neurons, and showed that 3- to 12-month-old Tg mice accumulate insoluble intraneuronal filamentous hyperphosphorylated tau inclusions accompanied by neurodegeneration in the spinal cord. 12 Thus, these tau Tg mice partially recapitulated the neuropathology of authentic tauopathies within 12 months of age. However, in humans, tauopathies rarely develop before the fifth decade of life, and disease onset is most common after age 60. 1,2
Although these Tg mice are relevant animal models of human tauopathies, 12,13 the inclusions in <12-month-old tau Tg mice differed from authentic NFTs in AD and other human tauopathies. For example, they were not detected by specific histochemical stains (eg, Thioflavin S, Congo red) that bind crossed β-pleated sheet structures and they contained a mixture of tau-, neurofilament (NF)-, and tubulin-immunoreactive straight filaments 10 to 20 nm in diameter. These aggregated filaments were found mostly in the proximal axons of spinal cord neurons, but they were also detected in cell bodies and dendrites of neurons in brain stem and telencephalon. Although the tau inclusions in young Tg mice, like human NFTs, were intensely stained by the Bodian silver method, they were not silver-impregnated by the Gallyas method, which detects most tangles in human tauopathies. The reasons for these differences are unclear, but they may reflect selective responses to the overexpression of human tau proteins in specific subtypes of mouse CNS neurons (eg, motor neurons) that contain high levels of NFs, species differences, or other factors. However, because the risk for developing NFTs in humans increases with advancing age, Tg mice that overexpress tau proteins provide novel opportunities to follow the natural history of tau pathologies during the lifespan of the animals and to investigate the effects of aging on the formation of filamentous NFT-like brain tau lesions. Indeed, we exploited this opportunity here and show that beyond 18 months, advancing age resulted in the formation of congophilic NFT-like tau inclusions in a subset of neurons of tau Tg mice that recapitulate the properties of their human counterparts.
Materials and Methods
Tau Tg Mice
A transgene including a cDNA of the shortest human tau isoform (T44) driven by the mouse PrP promoter and 3′ untranslated sequences was used to create tau Tg mice on a B6D2/F1 background, and studies characterizing the three lines of tau Tg mice generated with this transgene were described earlier. 12 The heterozygous Tg mouse lines 7, 43, and 27 overexpressed human tau proteins at levels approximately 5-, 10- and 15-fold higher than endogenous mouse tau, respectively. The heterozygous line 27 with the highest levels of Tg tau was not viable beyond 3 months, and none of the homozygous mice generated from any of these lines survived beyond 3 months. Therefore, we conducted the studies described here on 3- to 24-month-old heterozygous line 7 tau Tg mice and wild-type (WT) littermate control mice.
Tissue Processing, Histology, and Immunohistochemistry
Tg and WT mice from 3 to 24 months of age were anesthetized and perfused transcardially with fixative containing 70% ethanol and 150 mmol/L NaCl or 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4. A representative series of 6-μm-thick paraffin sections of Tg and WT mouse brain and spinal cord as well as similar positive control sections of cortex from autopsy confirmed AD brain with abundant amyloid plaques and NFTs were examined with Gallyas silver, Thioflavin S, and Congo red histochemical stains to detect tau pathology as described. 12,14-16 A series of adjacent sections were also probed by immunohistochemistry using well characterized antibodies to abnormal tau and other proteins that form AD or other neurodegenerative brain lesions, 16,17 including rabbit polyclonal antibodies to human recombinant tau (17026,1:2000), mouse monoclonal antibodies (mAbs) to phosphorylation-independent tau epitopes (T14, 1:500; T46, 1:500), mouse mAbs to phosphorylated human tau (PHF1, 1:250), rabbit polyclonal antibodies to tau exon 2 (1:1000) and exon 3 (1:1000), rabbit polyclonal antibodies to the low-molecular-weight NF triplet (NFL) protein (anti-NFL, 1:2000), mouse mAbs to the middle-molecular-weight NF (NFM) subunit (RMO189, 1:1), mouse mAbs to the high-molecular-weight NF (NFH) subunit (RMdO9, 1:1; RMO24, 1:1), mouse mAbs to α- (1:500) and β-tubulin (1:500), and mouse mAb 1510 to ubiquitin (1:1000). Also, selected sections were double-labeled with Thioflavin S histochemistry and by immunofluorescence using the 17026 polyclonal anti-tau antibody (1:2000) to determine the extent to which tau immunoreactivity and Thioflavin S staining colocalized in these lesions.
Immuno-Electron Microscopy (EM) of CNS Tau Inclusions in Tg Mice
Pre-embedding immuno-EM was performed on 24-month-old Tg and WT brains from lethally anesthetized mice after perfusion fixation as described above using 10 ml of 0.05% glutaraldehyde and 0.5% paraformaldehyde in 0.1 mol/L cacodylate buffer, pH 7.4, followed by 50 ml of 0.2% glutaraldehyde and 2% paraformaldehyde in 0.1 mol/L cacodylate buffer. The L5 segments of the spinal cord were removed and postfixed in 4% paraformaldehyde, 0.2% glutaraldehyde, and 0.2 picric acid in 0.1 mol/L cacodylate buffer overnight. Vibratome (50 μm) brain sections were quenched in 0.1% sodium borohydride in Tris buffered saline (TBS) for 10 minutes, treated for another 10 minutes with 20% ethanol, blocked in 5% donor horse serum in PBS with 0.1% cold water fish skin gelatin and 1% chicken egg albumin for 60 minutes, and then incubated with 17026 (1:500) and PHF1 (1:25) in 0.1% bovine serum albumin (BSA) and PBS overnight at room temperature. Immuno-EM was performed using the diaminobenzidine (DAB) plus silver-gold enhancement method with biotinylated goat anti-rabbit IgG secondary antibodies (for 17026) or gold anti-mouse IgG antibodies for PHF1 (Vector, Houston, TX; 1:100). The secondary antibodies were applied for 2 hours at room temperature for each series of sections. After visualizing DAB-labeled profiles by routine immuno-EM methods, sections of interest were subjected to silver-gold intensification by incubation in silver methenamine developer containing 3% methenamine, 5% silver nitrate, and 1% sodium tetraborate at 60°C for 10 minutes using procedures similar to those described elsewhere. 17 The reaction was stopped with 2% sodium acetate and then stabilized in 3% sodium thiosulphate for 5 minutes. Sections were subjected to gold toning by incubating them in 0.1% gold chloride for 5 minutes, stabilizing them with 3% sodium thiosulfate for 5 minutes, followed by fixation with 2% glutaraldehyde in phosphate buffer overnight for subsequent EM analysis as described. 12,17
Western Blot Analysis of Soluble and Insoluble Tau Proteins in Tg and WT Mice
Brain and spinal cord of 3-, 12-, and 24-month-old WT mice and 3-, 12-, 18-, 21-, and 24-month-old Tg mice were dissected from lethally anesthetized mice, and methods similar to those described recently 12,15,18,19 were used in the isolation procedures here. Briefly, brain tissues were sequentially extracted with ice-cold high salt RAB buffer [0.1 mol/L MES, 1 mmol/L EGTA, 0.5 mmol/L MgSO4, 0.75 mol/L NaCl, 0.02 mol/L NaF, 1 mmol/L phenylmethylsulfonyl fluoride, 0.1% protease inhibitor cocktail (100 μg/ml each of pepstatin A, leupeptin, TPCK, TLCK, and soybean trypsin inhibitor, and 100 mmol/L EDTA), pH 7.0],) follow by RIPA buffer (50 mmol/L Tris, 150 mmol/L NaCl, 1% NP40, 5 mmol/L EDTA, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, pH 8.0), and finally with 70% formic acid (FA). Equal amounts of samples were subsequently resolved on 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred onto nitrocellulose membranes. Western blot analysis was performed by previously reported procedures 12,15,18,19 using the 17026 rabbit polyclonal anti-tau antibody and the T49 mAb specific for endogenous mouse tau as the primary antibodies, and 125I-labeled Protein A or 125I-labeled goat anti-mouse IgG (IgG; New England Nuclear, Boston, MA) as secondary antibodies. The radiolabeled blots were exposed to PhosphorImager plates for various time periods, and the protein bands were visualized and quantified with ImageQuant software (Molecular Dynamics Inc., Sunnyvale, CA).
Isolation and EM of Insoluble Tau from Tg Mouse CNS
To monitor the tau aggregates in Tg mouse spinal cord and brain, transmission EM and immuno-EM were performed on insoluble tau isolated from the CNS of Tg mice. Briefly, spinal cord and brain from 12 Tg and 2 WT 1-year-old mice were dissected from lethally anesthetized animals, and enriched tau filament preparations were prepared as described previously using a sucrose gradient. 11,19 The different sucrose fractions were stored in 50 mmol/L ammonium acetate until analyzed by transmission EM and immuno-EM. To do this, 3 μl of 2.5–2.25 mol/L sucrose fractions from Tg and WT mice were placed on Formvar-coated nickel grids, and negative staining was performed as described and examined with a JEM1010 electron microscope (Peabody, MA) at 80 KV. 11,19
Results
Fibrillary Tau Lesions in Cortical Neurons of 18- to 24-Month-Old Tg Mice Exhibit the Morphology, Immunohistochemical Properties, and Histochemical Profile of AD NFTs
Fibrillary tau inclusions and the occasional associated neuropil thread-like lesions in aged tau Tg mice were immunostained with antibodies to phosphorylation-independent and phosphorylation-dependent tau epitopes in a similar manner to the tau inclusions in <12-month-old line 7 tau Tg mice as well as authentic human tangles (Figure 1, A and B) ▶ . 9,11,12 However, in contrast to tau Tg mice <12 months old, 12 Congo red and the Gallyas silver method stained filamentous lesions in perikarya of cortical neurons in brains of 18-month-old tau Tg mice, and these Congo red and Gallyas stained inclusions exhibited morphological and staining properties similar to AD NFTs (Figure 1, C and D) ▶ . Moreover, these NFT-like lesions showed intense tau immunoreactivity (Figure 1E) ▶ that colocalized with Thioflavin S labeling (Figure 1F) ▶ . Thus, these results indicate that the filamentous tau lesions in the older Tg mice contain structural elements with crossed β-pleated sheet conformations similar to those found in all amyloids and AD NFTs. 1,2 Filamentous tau inclusions with the properties described above were observed primarily in neuronal perikarya of the hippocampus, entorhinal cortex and amygdala, but less frequently in neocortex, and not in the spinal cord of 18- to 24-month-old Tg mice. Semiquantitative analyses showed that the frequency of occurrence of mouse tangle-like structures is about one to two per mouse brain section at 24 months.
Figure 1.
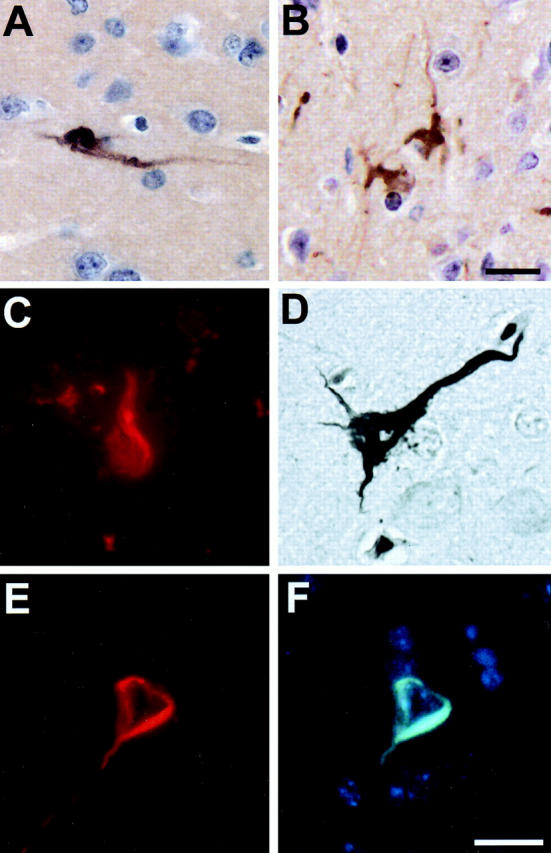
Filamentous tau lesions in the brains of 24-month-old Tg mice. A: NFT-like intraneuronal tau lesions in entorhinal cortex stained with anti-tau antibody 17026. B: NFT-like intraneuronal tau lesions associated with neuropil thread-like structures in the amygdala stained with the PHF1 mAb. C: Congo red detected flame-shaped intraneuronal tangles in the hippocampus. D: Silver-positive NFT-like intraneuronal inclusion in neocortex stained with the Gallyas method. E and F: Double-labeled NFT-like intraneuronal inclusion in the entorhinal cortex stained with the 17026 anti-tau antibody (E) and Thioflavin-S (F). All photomicrographs are at the same magnification. Scale bar, 10 μm.
Our previous studies have shown that spinal cord axonal tau inclusions in the younger Tg mice primarily contain aggregated human tau but they contain little or no endogenous mouse tau, since these inclusions were detected by an antibody to human tau (Figure 2A) ▶ but not by antibody specific to exon 2 that is present in mouse tau but not human fetal tau (Figure 2B) ▶ . By contrast, the Thioflavin S-positive inclusions in entorhinal cortex neurons of older Tg mice contained both the transgenic human tau protein as well as endogenous mouse tau since these brain inclusions were double-labeled with T14, a human specific anti-tau mAb (Figure 2C) ▶ and both the exon 2- and (Figure 2D) ▶ the exon 3-specific antibodies (data not shown). Although our previous study showed that the tau-positive inclusions detected in the spinal cord of young Tg mice were labeled by antibodies to NFL and β-tubulin, 12 those in the brain of old Tg mice were not immunostained by these antibodies (Figure 2, E–H) ▶ . Moreover, as the animals age, the spinal cord inclusions in 24-month-old mice continue to be immunostained with antibodies to neurofilaments and tubulin, whereas the brain inclusions in aged Tg mice were not labeled by these antibodies at any time (data not shown). 12 Finally, similar to authentic AD NFTs, we showed that the intraneuronal inclusions of the old Tg mice are ubiquitinated, since ubiquitin immunoreactivity colocalized with tau staining in double immunofluorescence studies (Figure 2, I and J) ▶ .
Figure 2.

Double immunofluorescence staining of filamentous tau lesions in CNS neurons of 24-month-old Tg mice. A–D: Double label immunofluorescence of a spinal cord spheroid (A, B) and NFT-like intraneuronal tau lesion in entorhinal cortex (C, D) of a 24-month-old Tg mouse using the T14 mAb (A, C) and the exon 2-specific antibody (B, D). Only the NFT-like lesions in entorhinal cortex show exon 2 immunoreactivity. E–H: Double immunofluo-rescence staining of NFT-like intraneuronal tau lesions using the T14 mAb (E, G), the anti-NFL antibody (F), and the anti-β-tubulin antibody (H) showing that the tau immunoreactivity does not colocalize with NFL or β-tubulin. (I, J) Double label immunofluorescence staining of a NFT-like tau lesion with the 17026 anti-tau antibody (I) and the anti-ubiquitin mAb (J) showing colocalization of tau and ubiquitin. All photomicrographs are at the same magnification. Scale bar, 10 μm.
Inclusions in Older Tg Mice Contain Exclusively Straight Tau Filaments
To determine whether or not the tau inclusions in the old Tg mice are comprised of PHFs or straight filaments, in situ immuno-EM was performed on brain samples of 24-month-old Tg and WT mice. No immunoreactive tau inclusions were detected in the brains of WT mice. The filamentous inclusions in Tg mouse hippocampal neurons were immunolabeled with anti-tau antibody 17026 (Figure 3 ▶ A, C, and E) as well as with the mAb PHF1 specific for a phosphorylation-dependent epitope on tau (Figure 3, B, D, and F) ▶ . The straight and smooth ultrastructure of the filaments was apparent in preparations decorated with the 17026 anti-tau antibody at high magnification (Figure 3, C and E) ▶ . The diameter of these filaments was 10 to 20 nm. Irregularly arranged PHF1-labeled tau filaments were also found in the neuronal perikarya and neurites (Figure 3F) ▶ . Finally, these straight filaments are not immunolabeled by antibodies to NF subunits or to tubulin (data not shown).
Figure 3.

Intraneuronal fibrillary tau lesions contain tau immunoreactive straight filaments. Pre-embedding immuno-EM shows tau immunoreactive straight filaments using the 17026 (A, C, and E) and PHF1 (B, D, and F) antibodies. The tau immunoreactive filaments labeled with 17206 anti-tau antibody in A are shown at higher magnification (see arrows in C) (bottom box) and E (top box) from areas marked by the squares in A. Arrows and arrowheads in B point to PHF1-labeled filaments around the nucleus and in neurite, respectively. Irregularly arranged PHF1-labeled straight filaments in neuronal perikarya and processes (see arrows in F) from the area in the square in D. Scale bars, 2 μm (A, B, and D) and 500 nm (C, E, and F).
Insoluble Tau Progressively Accumulates with Age in the CNS of Tau Tg Mice
Since aggregated tau inclusions are highly insoluble, we analyzed the solubility of tau in 3- to 24-month Tg and WT mouse by sequential extraction from brain and spinal cord samples using three different buffers with increasing extraction strengths (Figure 4) ▶ . The spinal cord and brain samples were sequentially extracted with aqueous high salt buffer, sodium dodecyl sulfate containing RIPA buffer, and 70% FA. The tau proteins in each of the three fractions were then analyzed by quantitative Western blotting with the anti-tau mAb T49 (which is specific for mouse tau) and 17026 (which binds to both human and mouse tau). As shown in Figure 4A ▶ , about 90% of endogenous mouse tau in the WT mouse brain and the spinal cord was soluble in high salt buffer, and no tau immunoreactivity was detected in the FA-soluble fraction. By contrast, the overexpression of human tau in the Tg mice caused a redistribution of the endogenous mouse tau from an aqueous soluble pool to an insoluble pool in brain but not in spinal cord in an age dependent manner. Indeed, about 40% of the endogenous mouse tau proteins were recovered in the RIPA fraction and >1% from the FA fraction from of old Tg mice. The presence of more RIPA-soluble endogenous mouse tau in brain compare to spinal cord supports our immunohistochemical data showing that mouse tau proteins are present in the fibrillary inclusions in brain but not spinal cord of old Tg mice.
Figure 4.
Accumulation of insoluble tau proteins in the CNS of old tau Tg mice. Brain cortex and spinal cord of 3-, 12-, and 24-month WT and 3-, 12-, 18-, 21-, and 24-month Tg mice were sequentially extracted with RAB high salt buffer, RIPA buffer, and 70% FA, and immunoblotted with antibodies T49 (A) or 17026 (B) and quantified using the appropriate 125I-labeled secondary antibodies. In the Tg mice, high salt insoluble tau recovered from RIPA and FA fractions progressively accumulated in brain and spinal cord. The accumulated tau in RIPA and FA extracted fractions was mainly Tg tau and there was no obvious difference between the levels of endogenous mouse tau in RIPA fractions in WT and Tg mice spinal cord, whereas in the cortex, the levels of mouse tau in RIPA fractions of Tg mice were significantly higher than those of WT mice.
We next compared the solubility of human tau with that of endogenous mouse tau in progressively aging Tg and WT mice (Figure 4B) ▶ . Because the expression of human tau was about 5.7-fold higher than endogenous mouse tau, the immunoreactive tau protein bands detected by antibody 17026 in the Tg mice primarily represent the human tau proteins. We found that the high salt extractable tau remained relatively constant at around 75 to 80% in the aging Tg mouse CNS samples compared to control samples, although high salt insoluble tau recovered in the RIPA- and FA-soluble fractions accumulated progressively in both brain and spinal cord of the Tg mice with advancing age. Significantly the increase in FA-extractable tau in 18- to 24-month-old Tg mouse brains parallels our ability to detect congophilic tau tangles in the brains of these mice.
Insoluble Tau Isolated from Tg Mouse CNS Contains Straight Filaments
To determine whether or not tau filaments can be recovered from detergent-insoluble tau fractions of Tg mouse CNS, sucrose gradient fractionation was performed as previously described. 11 Examination of negatively stained preparations of sucrose gradient fractions by EM demonstrated that insoluble CNS tau-rich fractions contained straight filaments with a diameter of 10 to 20 nm (Figure 5A) ▶ . Immuno-EM on these samples showed that the filaments are comprised of assembled tau proteins, since they are decorated only by the 17026 anti-tau antibody (Figure 5, B–D) ▶ but not by antibodies to NFL (Figure 5E) ▶ , peripherin (Figure 5F) ▶ , NFM, NFH, or α- or β- tubulin (data not shown).
Figure 5.
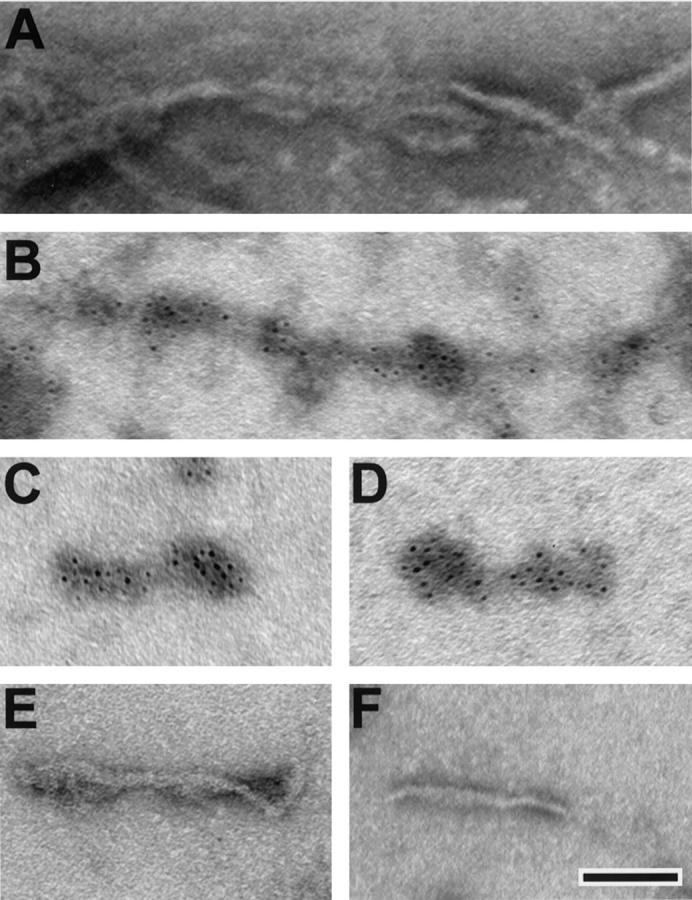
Immunogold labeling of tau filaments isolated from the old Tg mouse CNS. A: Negative staining alone shows a bundle of straight 10- to 20-nm diameter filaments in the insoluble fraction from the Tg mouse CNS. Using immuno-EM, intense immunogold labeling of these filaments is seen using the 17026 anti-tau antibody (B–D), but no immunogold labeling of the filaments was seen using the antibodies to NFL (E) and peripherin (F). All electron photomicrographs are at the same magnification. Scale bar, 200 nm.
Discussion
The experiments described in this study provide the first compelling evidence that congophilic tau tangles resembling human NFTs develop in the hippocampus and associated limbic areas of tau Tg mice in an age-dependent manner. Several criteria were used to show that the characteristics of mouse tau tangles and authentic human NFTs are similar. These include (i) the ability of histochemical dyes that bind crossed β-pleated sheet structures ie, Congo Red and Thioflavin S, to stain tangles in the brain of old Tg mice; (ii) the ability of Tg mouse tangles to be impregnated by the Gallyas silver method since Gallyas detects most tangles in human tauopathies; (iii) the formation of 10- to 20-nm-diameter straight filaments inside perikarya of neurons in the hippocampus and related limbic structures that are immunodecorated by anti-tau antibodies; (iv) the presence of tau protein but not NFs or tubulin in isolated filaments from detergent insoluble fractions of the Tg mouse CNS; and (v) the detection of ubiquitin immunoreactivity in the Tg mouse tangles. Thus, we suggest that the cortical tangles in older Tg mice resemble NFTs in the hippocampus of cognitively normal elderly individuals or the NFTs that develop early in the course of AD. However, the major difference between the mouse tangles described here and authentic human NFTs is that straight tau filaments, rather than PHFs, are the building blocks of the Tg mouse tangles. This may be due in part to altered tau isoform ratios expressed in our Tg mice, since AD NFTs contain all six tau isoforms, whereas the tau Tg mice express only the three endogenous mouse tau isoforms (4R-tau) with 4 MT binding repeat and the smallest human tau isoform with 3 MT binding repeats (3R-tau).
The tangles that developed in the brains of aged tau Tg mice differ from the tau inclusions we described previously in the spinal cord and brain stem of younger (<12 months) tau Tg mice. For example, the tau inclusions in these younger tau Tg mice were not stained by the amyloid-specific dyes Thioflavin S or Congo red, which are diagnostic indicators of the presence of amyloid pathology by virtue of their specific binding to β-pleated sheet structures. Further, these tau inclusions contained straight filaments 10 to 20 nm in diameter that were admixed with NFs, 12 and, although human tangles may exhibit variable NF immunoreactivity, the PHFs in NFTs are composed of abnormally phosphorylated tau proteins. 1,2,12,13 Additionally, despite demonstrating that the tau inclusions in young Tg mice were intensely stained by the Bodian silver method, just like their counterparts in human tauopathies, they were not detected by the Gallyas method. Finally, most of the tau inclusions in the young Tg mice were in the proximal axons of neurons in the spinal cord.
The mechanism(s) whereby our tau Tg mice develop two different types of tau inclusions at different ages during the lifespan of the mouse and in different parts of the CNS is unknown. One possible explanation may be the differential responses of select subtypes of neurons to the overexpression of tau protein. Indeed, selective vulnerability of specific neuronal populations to neurodegeneration has been noted in previous studies of Tg mouse models. For example, the development of inclusions in spinal cord motor neurons has been observed in Tg mice overexpressing NF subunit proteins or mutant SOD1, and the formation of NF and/or SOD1 aggregates, respectively, in spinal cord motor neurons leads to motor neuron degeneration. 20-24 Moreover, it is likely that the overexpressed tau proteins in young Tg mice have a propensity to interact with NFs in motor neurons to form aggregates of tau and NFs such that they eventually cause neurodegeneration by blocking axonal transport. 12,13 In this regard, the young tau Tg mice recapitulate key aspects of human tauopathies including a progressive, age-dependent accumulation of argyrophilic, tau-immunoreactive inclusions in neurons of spinal cord, brainstem, and neocortex similar to amyotrophic lateral sclerosis/parkinsonism dementia complex (ALS/PDC) of Guam, although filamentous tau inclusions in ALS/PDC include all six human brain tau isoforms and similar inclusions in the Tg mice contain only the smallest human tau isoform. 12,13
By contrast, age-related NFTs are found in small numbers and primarily in the hippocampus and related limbic neurons as normal individuals reach an advanced age. Thus, we propose that the mouse tangles that form in Tg mice as they advance to the terminal phase of the murine lifespan recapitulate key features of age-related NFTs in humans, since the location of these Tg mouse tangles as well as the frequency of occurrence in aged Tg mice parallel those found in humans. Age-related NFTs have never been detected in rodents, perhaps due to their relative short life span. However, the overexpression of the human tau protein may change the dynamic equilibrium of tau in aging hippocampal neurons of the mouse such that the excess human tau protein can assemble with the endogenous mouse tau into structures very similar to authentic human tau tangles.
Although the mechanisms leading to the formation of different types of tau inclusions in young versus aged Tg mice remain to be clarified, these issues are amenable to experimental investigation now. For example, it is possible to apply powerful gene profiling methods for quantitative comparison of the relative expression levels of mRNAs in normal versus tangle-bearing neurons in human postmortem brains, 25 and these analyses now can be extended to Tg mouse models of human tauopathies. Thus, the induction of tau pathology in Tg mice that recapitulates the features of authentic human tau tangles as the mice progressively age to the limits of their lifespan provides model systems which will accelerate efforts to discover drugs to prevent tangle formation or disrupt and eliminate existing tangles in patients with a progressive tauopathy.
Acknowledgments
We thank our colleagues in the Center for Neurodegenerative Disease Research and the Biomedical Imaging Core of the University of Pennsylvania Medical School, and the Penn Alzheimer Center for their assistance. We also express our appreciation to the families of the patients studied here, who made this research possible.
Footnotes
Address reprint requests to Dr. Virginia M.-Y. Lee, Center for Neurodegenerative Disease Research, Maloney 3, HUP, 3600 Spruce Street, Philadelphia, PA 19104-4283. E-mail: vmylee@mail.med.upenn.edu.
Supported by grants from the National Institute on Aging of the National Institutes of Health and the Oxford Foundation.
References
- 1.Hong M, Trojanowski JQ, Lee VMY: Tau-based neurofibrillary lesions. Clark CM Trojanowski JQ eds. Neurodegenerative Dementias: Clinical Features and Pathological Mechanisms. 2000, :pp 161-176 McGraw Hill, New York [Google Scholar]
- 2.Lee VMY, Trojanowski JQ: Distinct tau gene mutations induce specific dysfunctions/toxic properties in tau proteins associated with specific FTDP-17 phenotypes. Lee VMY Trojanowski JQ Buee L Christen Y eds. Fatal Attractions: Protein Aggregates in Neurodegenerative Diseases. 2000, :pp 87-104 IPSEN Foundation, Paris [Google Scholar]
- 3.Hof PR, Bouras C, Morrison JH: Cortical Neuropathology In Aging And Dementing Disorders: Neuronal Typology, Connectivity And Selective Vulnerability. Peters A Morrison JH eds. Cerebral Cortex 14. 1999, :pp 175-276 Kluwer Academic/Plenum Publishers, New York [Google Scholar]
- 4.Andreadis A, Brown WM, Kosik KS: Structure and novel exons of the human tau gene. Biochemistry 1992, 31:10626-10633 [DOI] [PubMed] [Google Scholar]
- 5.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA: Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 3:519-526 [DOI] [PubMed] [Google Scholar]
- 6.Binder LI, Frankfurter A, Rebhun LI: The distribution of tau in the mammalian central nervous system. J Cell Biol 1985, 101:1371-1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW: A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA 1975, 72:1858-1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drechsel DN, Hyman AA, Cobb MH, Kirschner MW: Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell 1992, 3:1141-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VMY: Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 1993, 10:1089-1099 [DOI] [PubMed] [Google Scholar]
- 10.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA: Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 3:519-526 [DOI] [PubMed] [Google Scholar]
- 11.Lee VMY, Balin BJ, Otvos L, Trojanowski JQ: A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science 1991, 251:675-678 [DOI] [PubMed] [Google Scholar]
- 12.Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VMY: Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron 1999, 24:751-762 [DOI] [PubMed] [Google Scholar]
- 13.Lee VMY, Trojanowski JQ: Neurodegenerative tauopathies: human disease and transgenic mouse models. Neuron 1999, 24:507-510 [DOI] [PubMed] [Google Scholar]
- 14.Gallyas F: Silver staining of Alzheimer’s neurofibrillary changes by means of physical development. Acta Morphol Acad Sci Hung 1971, 19:1-8 [PubMed] [Google Scholar]
- 15.Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, Morris JC, Wilhelmsen KC, Schellenberg GD, Trojanowski JQ, Lee VMY: Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 1998, 282:1914-1917 [DOI] [PubMed] [Google Scholar]
- 16.Puchtler H, Sweat F, Levine M: On the binding of Congo red by amyloid. J Histochem Cytochem 1962, 10:355-364 [Google Scholar]
- 17.Galvin JE, Uryu K, Lee VMY, Trojanowski JQ: Axon pathology in Parkinson’s disease and Lewy body dementia hippocampus contains α-, β-, and γ-synuclein. Proc Natl Acad Sci USA 1999, 96:13450-13455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Souza I, Poorkaj P, Hong M, Nochlin D, Lee VMY, Bird TD, Schellenberg GD: Missense and silent mutations in tau cause FTDP-17 by altering alternative splicing. Proc Natl Acad Sci USA 1999, 96:5598-5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee VMY, Wang J, Trojanowski JQ: Purification of paired helical filament tau and normal tau from human brain tissue. Methods Enzymol 1999, 309:81-89 [DOI] [PubMed] [Google Scholar]
- 20.Côté F, Collard JF, Julien JP: Progressive neuropathy in transgenic mice expressing the human neurofilament heavy gene: a mouse model of amyotrophic lateral sclerosis. Cell 1993, 73:35-46 [DOI] [PubMed] [Google Scholar]
- 21.Lee MK, Marszalek JR, Cleveland DW: A mutant neurofilament subunit causes massive, selective motor neuron death: implications for the pathogenesis of human motor neuron disease. Neuron 1994, 13:975-988 [DOI] [PubMed] [Google Scholar]
- 22.Tu PH, Raju P, Robinson KA, Gurney ME, Trojanowski JQ, Lee VMY: Transgenic mice carrying a human mutant superoxide dismutase transgene develop neuronal cytoskeletal pathology resembling human amyotrophic lateral sclerosis lesions. Proc Natl Acad Sci USA 1996, 93:3155-3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Cork LC, Griffin JW, Cleveland DW: Increased expression of neurofilament subunit NF-L produces morphological alterations that resemble the pathology of human motor neuron disease. Cell 1993, 73:23-33 [DOI] [PubMed] [Google Scholar]
- 24.Zhang B, Tu PH, Abtahian F, Trojanowski JQ, Lee VMY: Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J Cell Biol 1997, 139:1307-1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ginsberg SD, Hemby SE, Lee VMY, Eberwine JH, Trojanowski JQ: Expression profile of transcripts in Alzheimer’s disease neurofibrillary tangle-bearing CA1 neurons identifies new potential mediators of neurodegeneration. Ann Neurol 2000, 48:77-87 [PubMed] [Google Scholar]



