Abstract
Decorin is a small extracellular chondroitin/dermatan sulfate proteoglycan that has previously been shown to be involved in the angiogenesis-like behavior of endothelial cells (ECs) in vitro. There is also evidence that decorin plays a role in angiogenesis in vivo. In this study we sought to further explore the involvement of decorin in angiogenesis in vivo, especially in that associated with inflammation. We found by CD31 immunostaining of ECs that in giant cell arteritis there are capillary blood vessels not only in the adventitia as in uninvolved temporal artery wall, but also in the media and the external zone of the thickened intima. Localization of decorin by antiserum LF-30 in adjacent sections showed that in normal temporal artery wall decorin resides mainly in the media and the adventitia, whereas in inflamed temporal artery wall decorin is distributed throughout the vessel wall including the intima. Furthermore, the most intense reaction for decorin was evident in ECs of capillary neovessels within the media and the thickened intima of inflamed temporal artery wall. Decorin was also found in capillary ECs in certain pathological and physiological conditions in which the pivotal role of angiogenesis is more generally accepted. Pyogenic granulomas, granulation tissue of healing dermal wounds, and ovaries at different phases of follicle and corpus luteum formation all contained widely distributed CD31-positive capillaries. Decorin, on the other hand, was found in capillary ECs in pyogenic granulomas and granulation tissue, but not in those in the ovaries. The assessment of the degree of inflammation in the specimens with the presence of CD68-positive macrophages showed that the pyogenic granuloma, granulation tissue, and giant cell arteritis specimens were rich in macrophages around the decorin-positive capillaries. In contrast, the ovarian specimens were populated with fewer macrophages and even they were not located in close vicinity of capillaries negative for decorin. Our results confirm that decorin is involved in angiogenesis in vivo and, particularly, in conditions in which the inflammatory component is dominant.
Angiogenesis, the formation of new capillaries from pre-existing vessels through sprouting or intussusception, is a critical event in a variety of physiological processes (eg, embryonic development, ovarian follicle maturation, and tissue repair), as well as in pathological processes (eg, development of diabetic retinopathy and tumor growth and metastasis). 1,2 Angiogenesis also occurs during chronic inflammatory diseases including various forms of inflammatory vasculopathies such as giant cell arteritis (GCA) 3 and atherosclerosis. 4,5 At present, a redundancy of molecules are known to be variously involved in the regulation of the angiogenic process. 6,7 These molecules that either promote or inhibit angiogenesis are not only growth factors or cytokines, but also include a number of extracellular matrix components. 6,8-10 An extracellular matrix molecule that has been linked with angiogenesis is a small extracellular leucine-rich chondroitin/dermatan sulfate proteoglycan decorin. 11 Originally, it was demonstrated that this small proteoglycan is not expressed in detectable amounts by macrovascular endothelial cells (ECs) when they form a cobblestone-like monolayer in culture. 12 A similar observation was made in vivo in human fetal tissues. 13 However, later on it was found that when cultured macrovascular ECs spontaneously change their morphology from a polygonal shape to a sprouting phenotype they concomitantly initiate decorin synthesis and deposition indicating that decorin is associated with in vitro angiogenesis. 14 Recently, evidence for a causal role of decorin in the formation of capillary blood vessels in vitro was demonstrated when it was shown that macrovascular ECs that are transduced to overexpress decorin form tubes in collagen lattices, whereas control cells lacking the decorin construct fail to form the same structures. 15 Currently, there is also some evidence that decorin plays a role in angiogenesis in vivo as suggested by the presence of decorin in microvessels in human atherosclerotic plaques 16 and in ECs in human granulomatous tissue. 15 However, decorin is absent in the endothelium of resting capillaries. 17 In the present study, we sought to further confirm whether decorin is involved in angiogenesis in vivo and, in particular, whether inflammation might be a key element in inducing decorin expression by capillary ECs as indirectly suggested by the data of the mentioned studies. 15-17 Using temporal artery specimens obtained from patients with GCA and uninvolved control patients we first demonstrate, in agreement with previous studies, 3 that intimal thickening and angiogenesis are coupled in GCA. Next, we show that the capillary neovessels within the inflamed temporal artery wall contain decorin. We also demonstrate that decorin is present in ECs of capillary neovessels in pyogenic granulomas and in granulation tissue of healing dermal wounds. In contrast, ECs of capillary blood vessels in ovarian specimens representing different phases of follicle and corpus luteum formation are negative for decorin. Therefore, because the ovarian specimens do not exhibit substantial inflammation as assessed by the presence of macrophages, while the other specimens of this study are rich in pericapillary macrophages, we suggest that decorin is produced by capillary ECs in angiogenesis in conditions in which the inflammatory component is dominant.
Materials and Methods
Tissue Specimens
Temporal artery biopsy specimens were obtained from seven adult patients with GCA (five women and two men) and from seven uninvolved adult control patients (four women and three men). The diagnosis of GCA was based on classical clinical and histological criteria. 18 Pyogenic granuloma specimens were derived from the skin of five adult patients (two women and three men). Granulation tissue specimens of healing dermal wounds were collected from five adult patients (two women and three men). Ovarian specimens were obtained from four premenopausal healthy women (mean age, 48 years; range, 39 to 55 years) undergoing elective hysterectomy. Two of the specimens were in the follicular phase and two in the luteal phase. All tissue specimens were fixed in 10% neutral-buffered formalin, and after paraffin-embedding 5-μm transverse sections were cut and used for immunocytochemistry.
Antibodies
Macrophages, smooth muscle cells (SMCs), and ECs were identified by immunocytochemistry in the tissue specimens using the following antibodies: macrophages by a monoclonal mouse antibody to CD68 (dilution 1:100; DAKO, Glostrup, Denmark), SMCs by a monoclonal mouse α-smooth muscle actin (dilution 1:400; Sigma, St. Louis, MO), and ECs by a monoclonal mouse antibody to CD31 (dilution 1:1; BioGenex, San Ramon, CA). The distribution of decorin in the specimens was examined using a polyclonal rabbit antiserum LF-30 (dilution 1:100 13 ), kindly provided by Dr. Larry Fisher, National Institute of Dental Research, Bethesda, MD. Previously it has been well established that this decorin antiserum LF-30 is monospecific. 12,13
Immunocytochemistry
Formalin-fixed, paraffin-embedded histological sections of temporal artery specimens were stained with hematoxylin and eosin (H&E), and processed for immunocytochemistry. 19 Transverse sections of 5 μm were deparaffinized and rehydrated in a descending ethanol series. Immunoreactions were performed using the avidin-biotin complex method (Histostain-Plus Kit; Zymed, San Francisco, CA), with appropriate dilutions of the specific antisera (see above). Antibodies were applied in phosphate-buffered saline (PBS) containing 1% bovine serum albumin, and the sections were incubated overnight at 4°C. Before reacting with the decorin-specific antiserum LF-30, the sections were treated with 0.05 U/ml chondroitin ABC lyase (Seikagaku Co., Tokyo, Japan) in enriched Tris buffer for 3 hours at 37°C. 20 After rinses with PBS, a biotin-conjugated secondary antibody was applied and incubated for 10 minutes at room temperature. The slides were washed twice with PBS, and thereafter incubated with streptavidin-conjugated horseradish peroxidase for 10 minutes. The staining was visualized with diaminobenzidine, and the sections were counterstained with H&E. Control staining of the specimens was performed as described above, but no secondary antibody was used.
Results
Capillary Neovessel Formation within Temporal Artery Wall Affected by GCA
In GCA, the inflamed vessel wall characteristically contained macrophage-derived giant cells as well as individual macrophages (Figure 1A) ▶ that were not found in the uninvolved temporal artery wall (Figure 1D) ▶ . In addition, the intima of the inflamed temporal artery wall was markedly thickened and a substantial increase in the number of SMCs was observed (Figure 1B) ▶ when compared with the intima of the uninvolved temporal artery wall that was thin and contained only occasional SMCs beneath the ECs lining the lumen (Figure 1E) ▶ . Finally, although in the uninvolved temporal artery wall there were capillary blood vessels only in the adventitia (Figure 1F) ▶ , in the inflamed temporal artery wall capillary blood vessels were also found in the media and the external zone of the thickened intima (Figure 1C) ▶ . Thus, in GCA intimal thickening of the arterial wall and capillary neovessel formation were coupled.
Figure 1.
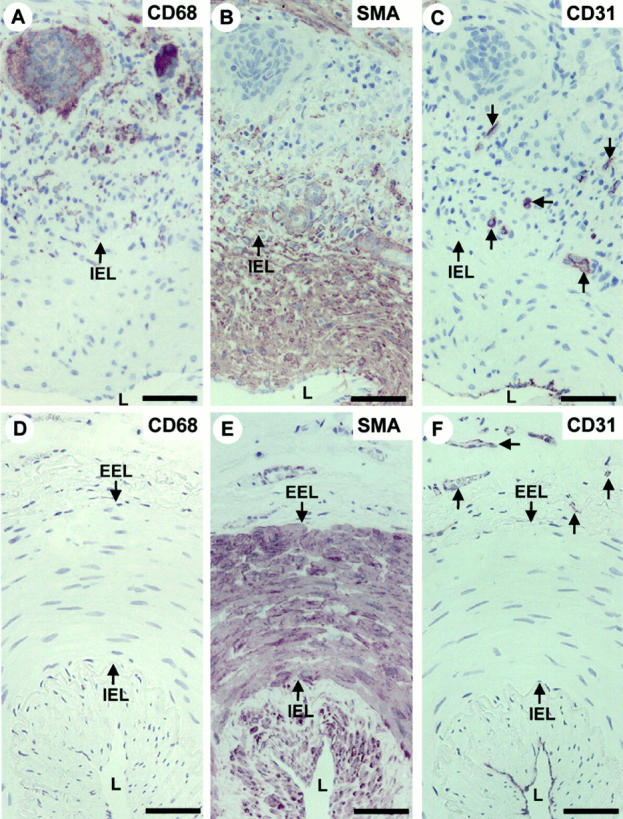
Capillary neovessel formation and intimal thickening are coupled in temporal artery wall affected by GCA. Immunoperoxidase staining of histological cross-sections of representative temporal artery specimens from a patient with GCA (A–C) and an uninvolved control patient (D–F). A and D: Macrophages identified with an antibody to CD68; B and E: SMCs identified with an antibody to α-smooth muscle actin (SMA). C and F: ECs identified with an antibody to CD31. Immunocytochemistry reactions are brown and counterstain for nuclei by hematoxylin is blue. The IEL and EEL arrows indicate the location of internal and external elastic laminae, respectively. The lumen of the vessels is indicated by L. Scale bar, 50 μm. Note that in the normal temporal artery wall capillary blood vessels are located only in the adventitia (F, arrows), whereas in the temporal artery wall affected by GCA there are capillary blood vessels also in the media and the external zone of the thickened intima (C, arrows).
ECs of Capillary Neovessels within the Inflamed Temporal Artery Wall Produce Decorin
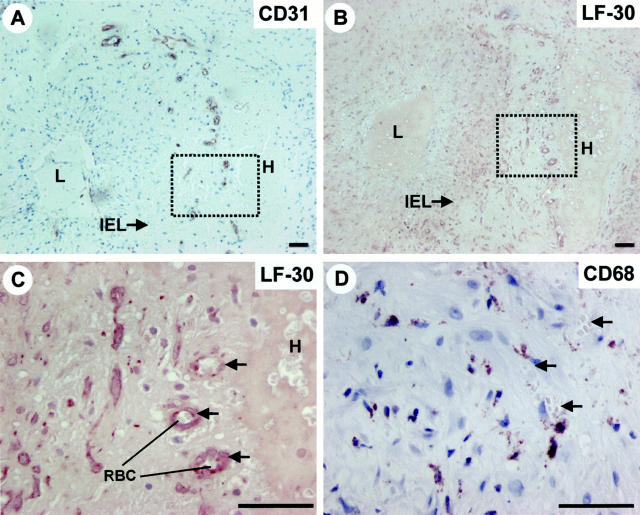
In the uninvolved temporal artery wall decorin was mainly localized in the media and the adventitia, whereas the intima exhibited only spotted reactions for decorin because of the presence of individual SMCs (Figure 2A ▶ ; see also Figure 1E ▶ for the location of SMCs). In contrast, in the temporal artery wall affected by GCA decorin was distributed throughout the vessel wall including the intima (Figure 2C) ▶ . In addition, ECs of the capillary neovessels within the media and the external zone of the thickened intima (Figure 2, E and F) ▶ showed the most intense reaction for decorin (Figure 2G) ▶ . These decorin-positive capillary neovessels were surrounded by macrophages (Figure 2H) ▶ . In the present study we also analyzed a temporal artery specimen accompanied with an intramedial hematoma because of the dissection of the vessel wall. This dissecting area with a hematoma was surrounded by several capillary neovessels (Figure 3A) ▶ that reacted intensively for decorin (Figure 3, B and C) ▶ . Furthermore, this capillary-rich area was populated with macrophages (Figure 3D) ▶ . Thus, the above results strongly supported the idea that decorin is produced by capillary ECs during angiogenesis associated with inflammation.
Figure 2.
Decorin is produced by ECs of capillary neovessels within the media and the thickened intima of temporal artery wall affected by GCA. Immunoperoxidase staining of histological cross-sections of representative temporal artery specimens from an uninvolved control patient (A and B), and from a patient with GCA (C–H). Immunocytochemistry reactions are brown and counterstain for nuclei with hematoxylin is blue. LF-30 indicates immunostaining for decorin (A, C, and G), and CD31 (E and F) and CD68 (H) indicate the location of ECs and macrophages in the specimens, respectively. The dot-lined rectangular regions shown in C and E correspond to each other in adjacent sections. G represents a magnified illustration of the dot-lined rectangular region in C, whereas F represents a magnified illustration of the dot-lined rectangular region shown in E. H represents the same region as that shown in F and G, but in an adjacent section. The arrows in F, G, and H indicate corresponding capillary neovessels in adjacent sections. B and D (marked by Contr) represent the negative control staining for the decorin staining shown in A and C, respectively. EEL, external elastic lamina; IEL, internal elastic lamina; L, lumen; RBC, red blood cell. Scale bar, 50 μm.
Figure 3.
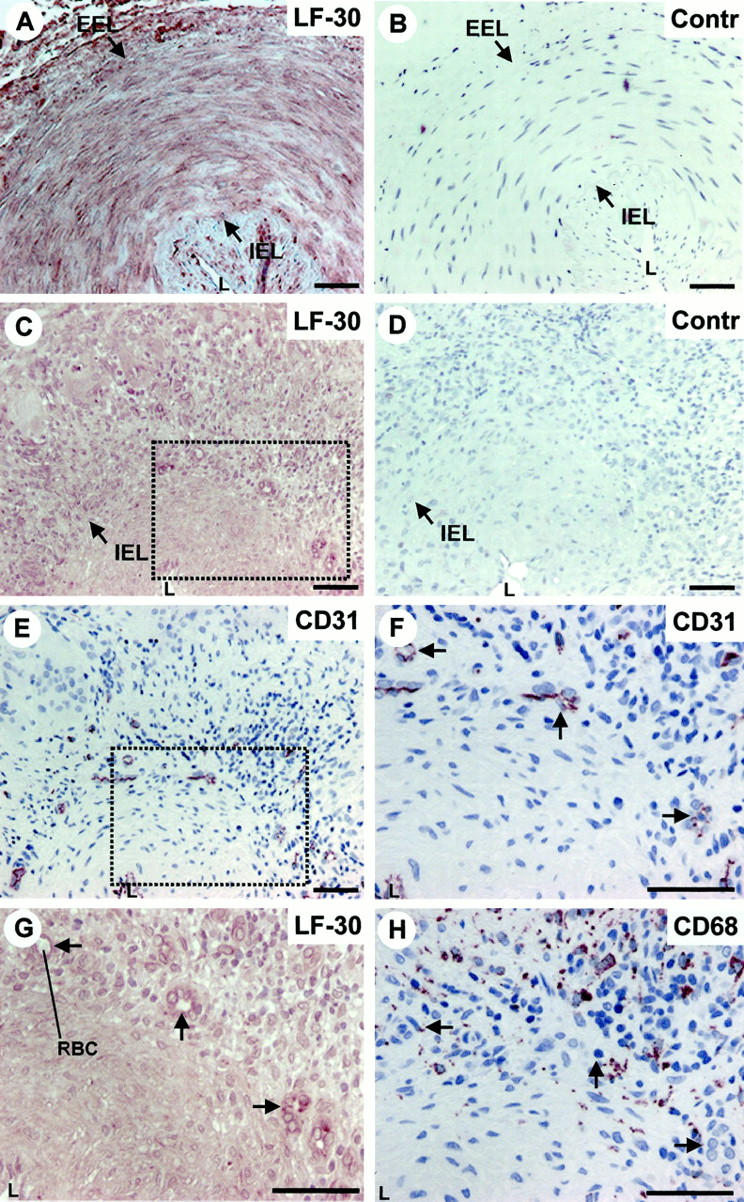
Capillary neovessels surrounding a hematoma within the media of an inflamed temporal artery wall are positive for decorin. Immunoperoxidase staining of adjacent histological cross-sections of GCA affected temporal artery specimen exhibiting an intramedial dissection with a hematoma (A–D). Immunocytochemistry reactions are brown and counterstain for nuclei by hematoxylin is blue. CD31, LF-30, and CD68 indicate immunostainings for ECs, decorin, and macrophages, respectively. The dot-lined rectangular regions shown in A and B correspond to each other in adjacent sections. C represents a magnified illustration of the dot-lined rectangular region shown in B. D represents the same region as that shown in C, but in an adjacent section. The arrows in C and D indicate corresponding capillary neovessels in adjacent sections. Red blood cells (RBC) in the lumen of two capillary neovessels are indicated to further confirm that the strongly decorin-positive structures represent capillary blood vessels. IEL, internal elastic lamina; L, lumen; H, intramedial hematoma. Scale bar, 50 μm.
Decorin Is Produced by Capillary ECs during Angiogenesis Associated with a Profound Inflammation
To further explore whether inflammation is critical in inducing decorin production by ECs during angiogenesis, we next stained tissue specimens representing pathological and physiological conditions, in which angiogenesis and inflammatory component are more generally known to be coupled. These conditions included pyogenic granuloma and granulation tissue of healing dermal wounds. We also stained ovarian specimens obtained from premenopausal women during hysterectomy, as it is known that the human reproductive system (eg, ovarian follicle and corpus luteum formation) is one of the few examples, or possibly the only one, in which angiogenesis occurs physiologically without any substantial inflammation. 21,22 Pyogenic granulomas, granulation tissue of healing dermal wounds, and ovarian specimens all contained widely distributed capillary blood vessels identified by CD31 staining (Figure 4, A and D ▶ , and Figure 5 ▶ ; A, D, and G). Decorin was detected in capillary ECs of the pyogenic granulomas (Figure 4B) ▶ and granulation tissue of healing dermal wounds (Figure 4E) ▶ , but not in those of the ovaries (Figure 5 ▶ ; B, E, and H). Reactions for macrophages in the adjacent sections of the above specimens collectively demonstrated that positive decorin staining in capillary ECs is associated with the presence of pericapillary macrophages (Figure 4, C and F ▶ , and Figure 5 ▶ ; C, F, and I). These results were parallel to the findings of decorin in the inflamed temporal artery wall where decorin was also present in capillary ECs during angiogenesis associated with a profound inflammation.
Figure 4.
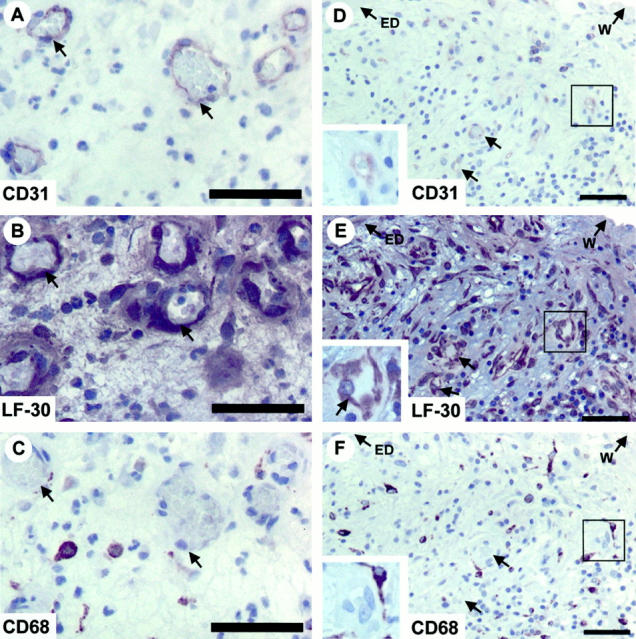
Decorin is produced by ECs of capillary neovessels associated with a profound inflammation. Immunoperoxidase staining of histological cross-sections of representative pyogenic granuloma (A–C) and granulation tissue of healing dermal wound (D–F) specimens. CD31, LF-30, and CD68 indicate immunostainings for ECs, decorin, and macrophages in adjacent sections, respectively. Immunocytochemistry reactions are brown and counterstain for nuclei by hematoxylin is blue. The arrows in A, B, and C indicate corresponding capillary neovessels in the adjacent sections of the pyogenic granuloma specimen. The lined rectangular regions presented in D, E, and F correspond to each other in adjacent sections of the granulation tissue of healing dermal wound specimen, and a magnified illustration of each region is shown in the inset of each panel, to visualize that CD31-positive capillary ECs (D) stain for decorin (E, arrow) and are also surrounded by CD68-positive macrophages (F). The two arrows shown in D, E, and F indicate corresponding capillary neovessels in adjacent sections. ED, epidermis; W, wound edge. Scale bar, 50 μm.
Figure 5.
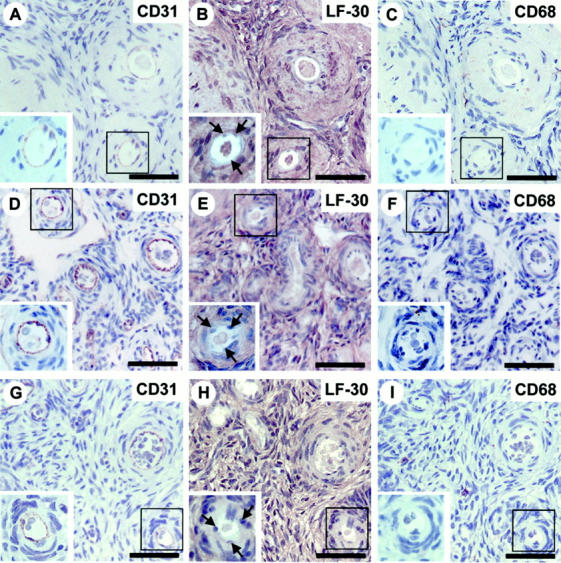
ECs of capillary neovessels in ovaries at different phases of follicle and corpus luteum formation are negative for decorin immunostaining. Immunoperoxidase staining of histological cross sections of ovary specimens at early follicular phase (A–C), late follicular phase (D–F), and early luteal phase (G–I). CD31, LF-30, and CD68 indicate immunostainings for ECs, decorin, and macrophages in adjacent sections, respectively. Immunocytochemistry reactions are brown and counterstain for nuclei by hematoxylin is blue. The lined rectangular region shown in each panel corresponds to each other within the same specimen. A magnified illustration of this region is presented in the inset of each panel to better visualize that the CD31-positive capillary ECs (A, D, and G) are negative for decorin staining (B, E, and H; arrows) and that the surrounding area of these capillary ECs is not populated by CD68-positive macrophages (C, F, and I). Scale bar, 50 μm.
Discussion
The demonstration of this study that intimal thickening and the formation of new capillary blood vessels are coupled within the temporal artery wall affected by GCA is consistent with a recent study by Kaiser and colleagues. 3 The present findings that ECs of capillary neovessels in inflamed temporal artery wall, pyogenic granuloma, and granulation tissue of healing dermal wounds produce decorin and that these decorin-positive capillaries are surrounded by macrophages suggest the importance of decorin particularly in angiogenesis associated with a profound inflammation. This conclusion is also supported by the results of Schönherr and colleagues 15 that decorin is expressed by ECs in human granulomatous tissue. Moreover, Gutierrez and colleagues 16 have demonstrated that capillary blood vessels in the thrombi and advanced atherosclerotic plaques stain for decorin. As atherosclerosis is recently considered as an inflammatory disease, 5 the results of Gutierrez and colleagues 16 also provide indirect evidence that decorin plays a role in angiogenesis associated with an inflammatory reaction. In agreement with the above argument, ECs of capillary neovessels in ovaries representing different phases of follicle and corpus luteum formation were found to be negative for both decorin and a profound pericapillary inflammation assessed by the presence of macrophages. Furthermore, Bosse and colleagues 17 have previously shown that decorin is absent from the endothelium in resting capillaries. The result with decorin knock-out mice that the vasculature remains unaffected 23 does not necessarily mean that decorin is not involved in angiogenesis. It rather emphasizes that depending on the underlying cause of angiogenesis different molecules are involved in the process. 24
The functional role that decorin plays in inflammation-associated angiogenesis is not known at the moment. However, based on the current knowledge of decorin several potential mechanisms can be proposed. It is possible that by interacting with specific other angiogenesis-associated extracellular matrix molecules such as type I collagen 25,26 and fibronectin 27,28 and by influencing the organization of these molecules 23,29 decorin stabilizes the extracellular matrix assembly in a way that provides a template for ECs to form capillary tubes. 30,31 Decorin may also be involved in inflammatory angiogenesis through its effects on the activity of angiogenesis-regulating growth factors. Specifically, decorin has been shown to bind transforming growth factor-β (TGF-β), 32,33 which neutralizes TGF-β action 34 and thereby very likely also the frequently observed antiangiogenic effects of this cytokine. 35,36 Furthermore, there is indirect evidence that decorin promotes the activity of the fibroblast growth factor-2 (FGF-2). 37 The finding that decorin is a biological ligand for the epidermal growth factor receptor 38 provides an additional growth factor-dependent mechanism whereby decorin might contribute to angiogenesis. 39 Other potential mechanisms by which decorin could play a role in angiogenesis associated with inflammation include the apoptosis-preventing effect of decorin on ECs 15 as well as its stimulatory effect on collagenase gene expression 40 and phospholipase A2 activity 41 because each of these cellular and molecular events are of special importance in inflammatory angiogenesis. 42-44
Besides the functional role of decorin in inflammation-associated angiogenesis, the factors responsible for the induction of decorin production by ECs during the angiogenic process are not known. Kaiser and colleagues 3 have shown that the formation of new capillary blood vessels within the arterial wall affected by GCA is regulated by giant cells and CD68-positive macrophages, ie, by inflammatory cells. Furthermore, they have shown that this capillary neovessel formation correlates with the expression of interferon-γ and vascular endothelial growth factor. 3 However, in the recent studies by us and others it has been reported that these two cytokines are not capable of inducing decorin expression by ECs in vitro. 15,45 It has also been shown that decorin expression is not induced in ECs when exposed to several other angiogenic or inflammatory cytokines and growth factors such as tumor necrosis factor-α and interleukin-1β as well as FGF-2 and -7. 15,45 Furthermore, the phorbol ester 12-O-tetradecanoylphorbol-13-acetate has been shown to be ineffective in inducing decorin expression by ECs in culture. 15 Thus, it still remains to be clarified whether there is an individual cytokine or growth factor that by itself or in combination with other factors could turn on the decorin gene in ECs. Nevertheless, the results of the present study still emphasize the importance of macrophage-derived factors as inducers of decorin production by ECs during inflammation-associated angiogenesis.
In summary, the results of the present study have demonstrated that decorin is an integral component of new capillaries in angiogenesis in vivo, especially in that associated with a profound inflammation. The exact functional role of decorin in angiogenesis and the factors responsible for the induction of decorin production by ECs remain to be resolved.
Acknowledgments
We thank Ms. Päivi Auho and Sinikka Kollanus for excellent technical assistance; Sanna Oksjoki, M.B., for providing us with the ovarian specimens; and Dr. Larry Fisher, Ph.D., for supplying us with the antiserum LF-30 against decorin core protein.
Footnotes
Address reprint requests to Hannu Järveläinen, M.D., Ph.D., Department of Medical Biochemistry, University of Turku, Kiinamyllynkatu 10, FIN-20520 Turku, Finland. E-mail: hannu.jarvelainen@utu.fi.
Supported by The Turku University Foundation, The Medical Research Fund of Turku University Central Hospital, The Finnish Foundation for Cardiovascular Research, Aarne Koskelo Foundation, Paavo Nurmi Foundation, and The Orion-Farmos Research Foundation.
References
- 1.Folkman J, Klagsbrun M: Angiogenic factors. Science 1987, 235:442-447 [DOI] [PubMed] [Google Scholar]
- 2.Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995, 1:27-31 [DOI] [PubMed] [Google Scholar]
- 3.Kaiser M, Younge B, Bjornsson J, Goronzy JJ, Weyand CM: Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol 1999, 155:765-774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien ER, Garvin MR, Dev R, Stewart DK, Hinoharam T, Simpson JB, Schwartz SM: Angiogenesis in human coronary atherosclerotic plaques. Am J Pathol 1994, 145:883-894 [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R: Atherosclerosis is an inflammatory disease. Am Heart J 1999, 138:S419-S420 [DOI] [PubMed] [Google Scholar]
- 6.Norrby K: Angiogenesis: new aspects relating to its initiation and control. APMIS 1997, 105:417-437 [DOI] [PubMed] [Google Scholar]
- 7.Risau W: Mechanisms of angiogenesis. Nature 1997, 386:671-674 [DOI] [PubMed] [Google Scholar]
- 8.Ingber DE, Folkman J: How does extracellular matrix control capillary morphogenesis? Cell 1989, 58:803-805 [DOI] [PubMed] [Google Scholar]
- 9.Klagsbrun M, Moses MA: Molecular angiogenesis. Chem Biol 1999, 6:R217-R224 [DOI] [PubMed] [Google Scholar]
- 10.St. Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW: Genes expressed in human tumor endothelium. Science 2000, 289:1197-1202 [DOI] [PubMed] [Google Scholar]
- 11.Iozzo RV: The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol 1997, 32:141-174 [DOI] [PubMed] [Google Scholar]
- 12.Järveläinen HT, Kinsella MG, Wight TN, Sandell LJ: Differential expression of small chondroitin/dermatan sulfate proteoglycans, PG-I/biglycan and PG-II/decorin, by vascular smooth muscle and endothelial cells in culture. J Biol Chem 1991, 266:23274-23281 [PubMed] [Google Scholar]
- 13.Bianco P, Fisher LW, Young MF, Termine JD, Robey PG: Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem 1990, 38:1549-1563 [DOI] [PubMed] [Google Scholar]
- 14.Järveläinen HT, Iruela-Arispe ML, Kinsella MG, Sandell LJ, Sage EH, Wight TN: Expression of decorin by sprouting bovine aortic endothelial cells exhibiting angiogenesis in vitro. Exp Cell Res 1992, 203:395-401 [DOI] [PubMed] [Google Scholar]
- 15.Schönherr E, O’Connell BC, Schittny J, Robenek H, Fastermann D, Fisher LW, Plenz G, Vischer P, Young MF, Kresse H: Paracrine or virus-mediated induction of decorin expression by endothelial cells contributes to tube formation and prevention of apoptosis in collagen lattices. Eur J Cell Biol 1999, 78:44-55 [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez P, O’Brien KD, Ferguson M, Nikkari ST, Alpers CE, Wight TN: Differences in the distribution of versican, decorin, and biglycan in atherosclerotic human coronary arteries. Cardiovasc Path 1997, 6:271-278 [DOI] [PubMed] [Google Scholar]
- 17.Bosse A, Schwarz K, Vollmer E, Kresse H: Divergent and co-localization of the two small proteoglycans decorin and proteoglycan-100 in human skeletal tissues and tumors. J Histochem Cytochem 1993, 41:13-19 [DOI] [PubMed] [Google Scholar]
- 18.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, Edworthy SM, Fausi AS, Leavitt RY, Lie JT, Lightfoot RW, Masi AT, McShane DJ, Mills JA, Wallace SL, Zvaifler NJ: The American College of Rheumatology Criteria for the classification of giant-cell arteritis. Arth Rheum 1990, 33:1122-1128 [DOI] [PubMed] [Google Scholar]
- 19.Elias JM, Margiotta M, Gaborc D: Sensitivity and detection efficiency of the peroxidase antiperoxidase (PAP), avidin-biotin peroxidase complex (ABC), and peroxidase-labeled avidin-biotin (LAB) methods. Am J Clin Pathol 1989, 92:62-67 [DOI] [PubMed] [Google Scholar]
- 20.Elenius K, Vainio S, Laato M, Salmivirta M, Thesleff I, Jalkanen M: Induced expression of syndecan in healing wounds. J Cell Biol 1991, 114:585-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modlich U, Kaup FJ, Augustin HG: Cyclic angiogenesis and blood vessel regression in the ovary: blood vessel regression during luteolysis involves endothelial cell detachment and vessel occlusion. Lab Invest 1996, 74:771-780 [PubMed] [Google Scholar]
- 22.Goede V, Schmidt T, Kimmina S, Kozian D, Augustin HG: Analysis of blood vessel maturation processes during cyclic ovarian angiogenesis. Lab Invest 1998, 78:1385-1394 [PubMed] [Google Scholar]
- 23.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV: Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 1997, 136:729-743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmeliet P: Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000, 6:389-395 [DOI] [PubMed] [Google Scholar]
- 25.Schönherr E, Witsch-Prehm P, Harrach B, Robenek H, Rauterberg J, Kresse H: Interaction of biglycan with type I collagen. J Biol Chem 1995, 270:2776-2783 [DOI] [PubMed] [Google Scholar]
- 26.Svensson L, Heinegard D, Oldberg A: Decorin-binding sites for collagen type I are mainly located in leucine-rich repeats 4–5. J Biol Chem 1995, 270:20712-20716 [DOI] [PubMed] [Google Scholar]
- 27.Lewandowska K, Choi HU, Rosenberg LC, Zardi L, Culp LA: Fibronectin-mediated adhesion of fibroblasts: inhibition by dermatan sulfate proteoglycan and evidence for a cryptic glycosaminoglycan-binding domain. J Cell Biol 1987, 105:1443-1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt G, Robenek H, Harrach B, Glossl J, Nolte V, Hormann H, Richter H, Kresse H: Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol 1987, 104:1683-1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinsella MG, Fischer JW, Mason DP, Wight TN: Retrovirally mediated expression of decorin by macrovascular endothelial cells. Effects on cellular migration and fibronectin fibrillogenesis in vitro. J Biol Chem 2000, 275:13924-13932 [DOI] [PubMed] [Google Scholar]
- 30.Jackson CJ, Jenkins KL: Type I collagen fibrils promote rapid vascular tube formation upon contact with the apical side of cultured endothelium. Exp Cell Res 1991, 192:319-323 [DOI] [PubMed] [Google Scholar]
- 31.Vernon RB, Lara SL, Drake CJ, Iruela-Arispe ML, Angello JC, Little CD, Wight TN, Sage EH: Organized type I collagen influences endothelial patterns during “spontaneous angiogenesis in vitro”: planar cultures as models of vascular development. In Vitro Cell Dev Biol Anim 1995, 31:120-131 [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi Y, Mann DM, Ruoslahti E: Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 1990, 346:281-284 [DOI] [PubMed] [Google Scholar]
- 33.Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E: Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 1994, 302:527-534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E: Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 1992, 360:361-364 [DOI] [PubMed] [Google Scholar]
- 35.Pepper MS: Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev 1997, 8:21-43 [DOI] [PubMed] [Google Scholar]
- 36.Vernon RB, Sage EH: A novel, quantitative model for study of endothelial cell migration and sprout formation within three-dimensional collagen matrices. Microvasc Res 1999, 57:118-133 [DOI] [PubMed] [Google Scholar]
- 37.Penc SF, Pomahac B, Winkler T, Dorschner RA, Eriksson E, Herndon M, Gallo RL: Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J Biol Chem 1998, 273:28116-28121 [DOI] [PubMed] [Google Scholar]
- 38.Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I: Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem 1999, 274:4489-4492 [DOI] [PubMed] [Google Scholar]
- 39.Pepper MS: Manipulating angiogenesis. From basic science to the bedside. Arterioscler Thromb Vasc Biol 1997, 17:605-619 [DOI] [PubMed] [Google Scholar]
- 40.Huttenlocher A, Werb Z, Tremble P, Huhtala P, Rosenberg L, Damsky CH: Decorin regulates collagenase gene expression in fibroblasts adhering to vitronectin. Matrix Biol 1996, 15:239-250 [DOI] [PubMed] [Google Scholar]
- 41.Sartipy P, Johansen B, Gasvik K, Hurt Camejo E: Molecular basis for the association of group IIA phospholipase A(2) and decorin in human atherosclerotic lesions. Circ Res 2000, 86:707-714 [DOI] [PubMed] [Google Scholar]
- 42.Jackson JR, Bolognese B, Mangar CA, Hubbard WC, Marshall LA, Winkler JD: The role of platelet activating factor and other lipid mediators in inflammatory angiogenesis. Biochim Biophys Acta 1998, 1392:145-152 [DOI] [PubMed] [Google Scholar]
- 43.Mach F, Schonbeck U, Fabunmi RP, Murphy C, Atkinson E, Bonnefoy JY, Graber P, Libby P: T lymphocytes induce endothelial cell matrix metalloproteinase expression by a CD40L-dependent mechanism: implications for tubule formation. Am J Pathol 1999, 154:229-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Connor DS, Schechner JS, Adida C, Mesri M, Rothermel AL, Li F, Nath AK, Pober JS, Altieri DC: Control of apoptosis during angiogenesis by survivin expression in endothelial cells. Am J Pathol 2000, 156:393-398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelimarkka L, Kainulainen V, Schönherr E, Moisander S, Jortikka M, Lammi M, Elenius K, Jalkanen M, Järveläinen H: Expression of small extracellular chondroitin/dermatan sulfate proteoglycans is differentially regulated in human endothelial cells. J Biol Chem 1997, 272:12730-12737 [DOI] [PubMed] [Google Scholar]