Abstract
A human midgut carcinoid tumor was successfully transplanted into nude mice and propagated for five consecutive generations (30 months) with well-preserved phenotype. Tumor cells in nude mice expressed identical neuroendocrine markers as the original tumor, including somatostatin receptors (somatostatin receptors 1 to 5) and vesicular monoamine transporters (VMAT1 and VMAT2). Because of the expression of somatostatin receptors and VMAT1 and VMAT2 the grafted tumors could be visualized scintigraphically using the somatostatin analogue 111In-octreotide and the catecholamine analogue 123I-metaiodobenzylguanidine. The biokinetics of the somatostatin analogue 111In-octreotide in the tumors was studied and showed a high retention 7 days after administration. Cell cultures were re-established from transplanted tumors. Immunocytochemical and ultrastructural studies confirmed the neuroendocrine differentiation. The human origin of transplanted tumor cells was confirmed by cytogenetic and fluorescence it situ hybridization analyses. Spontaneous secretion of serotonin and its metabolite, 5-hydroxyindole acetic acid, from tumor cells was demonstrated. The tumor cells increased their [Ca2+]i in response to β-adrenoceptor stimulation (isoproterenol) and K+-depolarization. All somatostatin receptor subtypes could be demonstrated in cultured cells. This human transplantable carcinoid tumor, designated GOT1, grafted to nude mice, will give unique possibilities for studies of somatostatin receptor- and VMAT-mediated radionuclide uptake as well as for studies of secretory mechanisms.
Midgut carcinoid tumors are derived from the enterochromaffin cells in the small intestine. In metastatic disease these tumors can give rise to severe hormonal symptoms because of excessive production of serotonin (5-HT) and tachykinins (substance P and neurokinins). Carcinoid tumors express numerous somatostatin receptors that can be used to alleviate hormonal symptoms by treatment with the long-acting somatostatin analogue octreotide. These receptors have also been used for visualization of tumors by scintigraphy and in receptor-guided surgery and may serve as targets for radionuclide therapy. 1 Studies of receptor mechanisms and secretory processes in these tumors have been hampered by the lack of suitable experimental models. Heterografting of human carcinoid tumors into privileged sites, eg, the anterior eye-chamber of immunosuppressed rats, has been tried but is restricted to the study of very small tissue pieces. 2 Midgut carcinoids can also be studied in primary cell cultures, but tumor cells proliferate slowly and survive only for limited periods of time. 3-6 Cell lines are ideal for studies of receptor mechanisms and secretory mechanisms, however, few carcinoid tumor cell lines are available today (ie, KRJ-I and BON). 7,8 The most widely used cell line, BON, was derived from a lymph node metastasis of a highly malignant pancreatic carcinoid. BON cells have been used to investigate responses to secretagogues and cytotoxic agents and express both epithelial markers (cytokeratin) and multiple amines and peptides [5-HT, chromogranin A (CgA), neurotensin, and pancreastatin]. In this report we present a transplantable human midgut carcinoid tumor that can be propagated in nude mice for extended periods of time with well-preserved neuroendocrine phenotype, including expression of somatostatin receptors and VMAT1 and VMAT2.
Materials and Methods
Case Report
A 55-year old woman presented with a severe midgut carcinoid syndrome associated with elevated urinary levels of 5-HIAA (630 μmol/24 hours; reference <50). Computed tomography, octreotide scintigraphy, and bone scintigraphy demonstrated metastatic spread to the liver, paraaortic and mediastinal lymph nodes, and to the skeleton. At surgery, tumor biopsies were obtained. Because the tumor burden was large, hepatic embolization therapy was considered to be a high-risk procedure so this patient was given systemic radionuclide therapy with 111In-labeled octreotide. 9
Tumor Cell Culture
Tumor material from one liver metastasis was obtained at surgery. Primary cell cultures were prepared as previously described. 10 In one of these primary cultures a markedly increased proliferation rate was noted after 3 months. These cells (referred to as GOT1 cells) were subsequently cultured in quantities to enable heterotransplantation to nude mice. The studies of secretory mechanisms and Ca2+ channel signaling were performed in cell cultures identically prepared from a tumor of the second generation from nude mice.
Transplantation of Tumor Cells to Nude Mice
For transplantation of tumor cells we used male BALB/cABom-nu mice, 3 to 4 weeks of age (Bomholtgaard, Ry, Denmark). The animal experiments were approved by the Ethical Committee for Animal Experiments, University of Göteborg.
Tumor Generation I
Tumor cells from cultures were harvested and grafted to 10 nude mice. Approximately 20 million tumor cells were injected subcutaneously into the back of the neck of each animal. When tumors became visible their size was estimated once a week after measurement with a caliper. Eight animals could be followed 6 to 12 months. At sacrifice, blood was collected and the tumor tissues were harvested.
Tumor Generation II to V
From the first generation of tumors new tumors were propagated by subcutaneous transplantation of two pieces of tumor tissue (1 × 1 × 2 mm each) per animal into 20 to 100 animals per generation. To date, five consecutive generations of tumors have been generated. In the fifth generation, the tumor growth rate in vivo was again estimated during a period of 3.5 months.
Cytogenetic and Fluorescence in Situ Hybridization (FISH) Analyses
Chromosome preparations were made from tumor cells obtained from a tumor of generation V. Cells were harvested after overnight exposure to colcemid followed by hypotonic treatment and fixation in methanol:acetic acid. 11 Slides were subsequently G-banded and analyzed according to the guidelines of the International System for Human Cytogenetic Nomenclature. 12
FISH analysis was performed on touch preparations prepared from a tumor of generation V as well as on unbanded metaphase chromosomes using whole chromosome painting probes for the human X chromosome and chromosome 1 (wcp X and 1; Vysis, Downers Grove, IL). The probes were labeled with Spectrum Green (X chromosome) and Spectrum Orange (chromosome 1) fluorophores. The conditions for hybridization and posthybridization washes were as recommended by the manufacturer. Nuclei and chromosomes were counterstained in blue with 4,6-diamino-2-phenylindole. Slides were examined in a Zeiss Axiophot epifluorescence microscope equipped with the appropriate filter sets. Fluorescence signals were digitalized, processed, and analyzed using the ProbeMaster FISH image analysis system (Perceptive Scientific International, Chester, England).
Binding and Scintigraphy of Radiolabeled Octreotide and 123I-Metaiodobenzylguanidine (MIBG)
The binding of the radiolabeled somatostatin analogue octreotide was studied on nude mice transplanted with tumors of generation I, II, and IV. d-Phe1-octreotide was labeled with 111In mainly according to the manufacturer (Mallinckrodt Medical B.V., Petten, The Netherlands). The fraction of peptide-bound 111In exceeded 99%. Each animal was injected intravenously with 0.25 to 4 MBq (0.1 μg) of 111In-octreotide 4 hours before killing. Tumors and blood samples were collected and weighed, and the 111In activity was measured in a γ counter. The activity concentration of the radionuclide was expressed as the fraction of injected activity per unit mass of the tissue (%IA/g) and the tumor-to-blood activity concentration ratio, T/B, was determined. 13 Before killing scintigraphy was performed with a γ camera.
The binding and scintigraphy of MIBG (10 to 12 MBq) was studied in eight nude mice transplanted with tumors of generation IV at 4 hours (n = 2), 24 hours (n = 3), and 48 hours (n = 3) after injection.
Biokinetics of Radiolabeled Octreotide
Each animal was injected into a tail vein with 2 MBq (0.1 μg) of 111In-octreotide. The 111In activity in the syringe was measured before and after injection of the radiopharmaceutical in a well-type ionization chamber to obtain the injected activity for each animal. These animals were killed 0.5 hours (n = 5), 24 hours (n = 5), 3 days (n = 5), and 7 days (n = 5) after injection. The 111In activity in tumor, blood, and whole body was measured using a Wallace 1480 γ counter (WIZARD 3”; Wallace, Oy, Finland). The 111In-activity concentration in the tissue was expressed as the fraction of the injected activity per unit mass of the tissue (%IA/g).
Assays of 5-HTP, 5-HT, and 5-HIAA
The 5-HT precursor 5-hydroxytryptophan (5-HTP), serotonin (5-HT), and the main metabolite 5-hydroxyindole acetic acid (5-HIAA) in conditioned culture media were determined by reverse-phase HPLC with electrochemical detection. 6 For measurement of 5-HTP, 5-HT, and 5-HIAA in nude mice, whole blood was lysated in sterile water. Proteins were denatured with ZnSO4 and NaOH. The blood samples were thereafter centrifuged at 3,500 × g for 30 minutes and aliquots (20 μl) of the supernatant were injected onto the column.
Immunocytochemistry
Tumor tissues (primary tumor and tumors from nude mice) were fixed in buffered formalin for 4 to 24 hours and embedded in paraffin wax. Sections were incubated with primary antibodies overnight (Table 1) ▶ . Bound antibodies were visualized by an indirect immunoperoxidase technique. Cultures were fixed in 4% paraformaldehyde in phosphate-buffered saline at pH 7.4 for 4 hours and incubated for indirect immunofluorescence using primary antibodies listed in Table 1 ▶ . Control cultures were incubated identically, except for the primary antibodies, which were replaced by normal rabbit serum/mouse IgG. Cultures were examined with confocal laser scanning microscopy using a Zeiss Laser Scanning Microscope (LSM 410). 5
Table 1.
Primary Antibodies Used for Immunocytochemistry
| Antibody | Dilution (immuno- fluorescence) | Dilution (immuno- peroxidase, primary) | Dilution (immuno- peroxidase, nude mice) | Clone | Catalog no. | Company |
|---|---|---|---|---|---|---|
| Anti-human cytokeratin (mc) | 1:50 | 1:50 | 1:50 | MNF 116 | M 821 | DAKO, Glostrup, Denmark |
| Anti-E-Cadherin (mc) | 1:20 | 1:20 | — | 36 | C20820 | Transduction Laboratories, Lexington, KY |
| Anti-E-Cadherin (human) (mc) | 1:200 | — | 1:1000 | HECD-1 | 13-1700 | Zymed Laboratories, Inc., San Francisco, CA |
| NCAM (mc) | 1:50 | 1:50 | 1:20 | ERIC1 | sc-106 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA |
| VMAT1 (pc) | — | 1:500 | sc-7718 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA | ||
| VMAT1 (pc) | 1:100 | 1:10000 | Gift from Jefferey D. Erickson, Ph.D., NIH, Maryland | |||
| VMAT2 (pc) | 1:100 | 1:10000 | — | Jefferey D. Erickson | ||
| VMAT2 (pc) | — | 1:500 | AB1767 | Chemicon, Temecula, CA | ||
| CgA (mc) | 1:1000 | — | 1:1000 | LK2H10 | 1199021 | Boehringer Mannheim, Mannheim, Germany |
| Synaptophysin (mc) | 1:10 | 1:10 | 1:10 | SY38 | M0776 | DAKO, Glostrup, Denmark |
| Vimentin (mc) | 1:10 | 1:40 | 1:400 | V9 | M0725 | DAKO, Glostrup, Denmark |
| Serotonin (mc) | 1:20 | — | 1:20 | H209 | M758 | DAKO, Glostrup, Denmark |
| SV2 (mc) | 1:250 | 1:250 | 1:500 | Gift from Reinhardt Jahn, Buckley Kelly | ||
| Substance P (pc) | 1:200 | 1:200 | — | RPN.1572 | Amersham, Buckinghamshire, UK | |
| Substance P (pc) | — | — | 1:400 | MAB356 | Chemicon, Temecula, CA | |
| Neurofilament | — | — | 1:100 | 2F11 | M0762 | DAKO, Glostrup, Denmark |
mc, monoclonal; pc, polyclonal; —, not incubated.
Electron Microscopy
Tumor tissue was fixed in 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer (pH 7.2) for 24 to 48 hours and postfixed for 1 hour in 1% OsO4 in the same buffer. After dehydration the specimens were embedded in epoxy resin (TAAB 812). Ultrathin sections were contrasted with uranyl acetate and lead citrate and examined in a Philips CM 12 electron microscope.
Northern Analysis
Cells from primary cultures and tumor tissues from nude mice were harvested and total RNA was prepared by acid guanidinum thiocyanate-phenol-chloroform extraction. 14 Electrophoresis and hybridization with 32P-labeled antisense RNA probes for the five somatostatin receptor subtypes was performed as previously described. 15 As performed this analysis gave quantitative information of the expression of somatostatin receptor subtypes.
Intracellular Calcium Determinations
Cells attached to coverslips were washed and loaded for 30 minutes with FURA-2Am (1 μmol/L; Sigma) in the presence of 0.01% pluronic F-127 (Sigma) in a modified HEPES medium supplemented with 11.1 mmol/L glucose as previously described. 16 After loading, the cells were washed and the coverslips mounted in a temperature-controlled (37°C) chamber (volume, 110 μl) placed over a 100 X Fluor objective (Nikon, Tokyo, Japan) on the stage of an inverted Nikon microscope (DIAPHOT-TMD); 75 W xenon lamp. The cells were superinfused (flow rate of 1 ml/min) in the HEPES medium supplemented with 11.1 mol/L glucose and 0.5% bovine serum albumin to which was added isoproterenol, carbachol, or KCl (all from Sigma) according to the experimental protocol. The fluorescence of FURA-2 was recorded with dual wavelength excitation spectrophotofluorometry (emission wavelength, 510 nm; excitation wavelengths, 350 and 380 nm) and the [Ca2+]i was calculated according to Grynkiewicz and colleagues. 17
Statistical Analysis
The tumor size doubling time, 5-HT concentration in peripheral blood of nude mice, 111In-octreotide and 123I-MIBG concentration in tumor tissues, and [Ca2+]i are given as mean ± SEM. For statistical analysis the Students t-test was used.
Results
Properties of the Primary Ileal Carcinoid
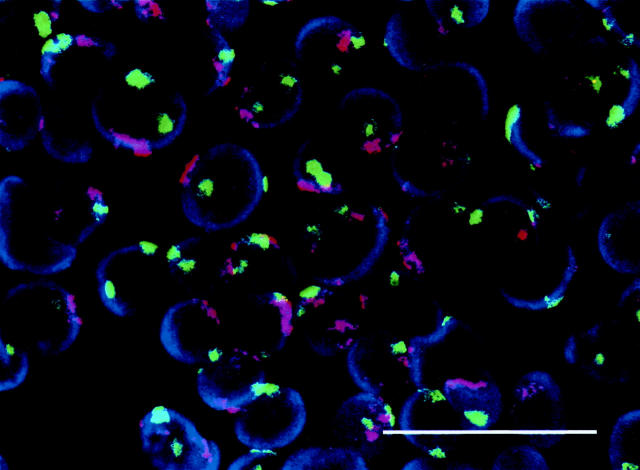
The primary tumor was a single lesion in the distal ileum measuring 2.5 cm in diameter. Light microscopic appearance was that of a typical ileal carcinoid with predominantly insular growth pattern. Immunocytochemical analysis demonstrated 5-HT- and substance P-production by tumor cells. The majority of tumor cells were positive for VMAT1 and VMAT2. General markers for neuroendocrine differentiation were also strongly positive (CgA, SV2, and NCAM) (Figure 1 ▶ and Table 2 ▶ ).
Figure 1.
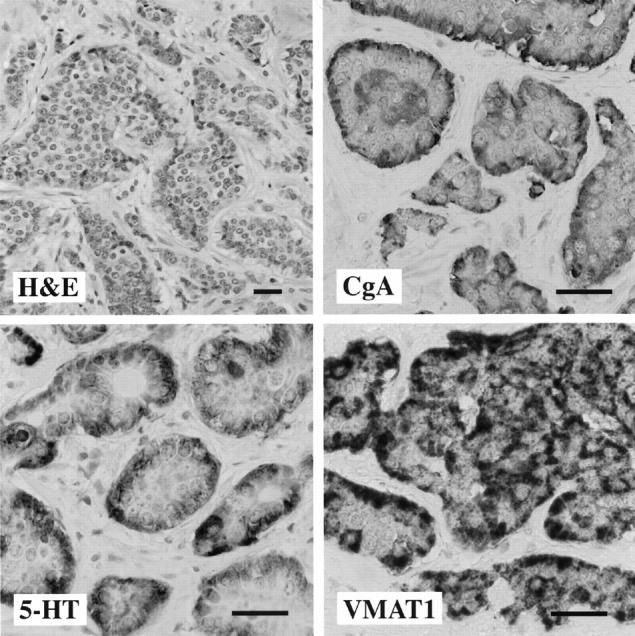
Morphology of the primary ileal carcinoid. Tumor cells grow in an insular pattern with abundant eosinophilic cytoplasm. A positive immunocytochemical reaction for CgA, serotonin (5-HT), and VMAT1 is observed in a majority of tumor cells. Scale bars, 30 μm.
Table 2.
Immunocytochemical Characterisation of the Transplantable Midgut Carcinoid
| Antigen | Ileal primary | Primary cell culture* | Tumors transplanted to nude mice | |||
|---|---|---|---|---|---|---|
| Tumor generation I | Tumor generation II | Tumor generation III | Tumor generation IV | |||
| Serotonin | +++ | + | + | + | ++ | + |
| Substance P | +++ | + | + | − | ++ | + |
| Chromogranin A | +++ | + | +++ | +++ | +++ | +++ |
| Synaptophysin | +++ | + | +++ | +++ | ++ | ++ |
| SV2 | +++ | + | +++ | +++ | +++ | +++ |
| VMAT1 | +++ | + | +++ | ++ | ++ | +++ |
| VMAT2 | ++ | + | +++ | +++ | +++ | +++ |
| NCAM(ERIC-1) | ++ | + | +++ | +++ | +++ | +++ |
| ECAD | +++ | + | +++ | +++ | +++ | +++ |
| Cytokeratin (MNF116) | +++ | + | +++ | +++ | +++ | +++ |
| Vimentin | − | − | − | − | − | − |
| Neurofilament | − | − | − | − | − | − |
Findings on the original ileal carcinoid and primary cell culture, before transplantation, is given for comparison.
+, 1–25% of tumor cells positive; ++, 25–75% of tumor cells positive; +++, 75–100% of tumor cells positive.
*, Only +/− scale was applied.
Properties of Tumor Cells before Transplantation
The epithelial phenotype was confirmed by positive immunocytochemical reactions for cytokeratin and E-cadherin, and negative reactions for vimentin and neurofilament. Immunocytochemical analysis demonstrated a strong expression of several neuroendocrine markers. A strong positive reaction was obtained for 5-HT, substance P, and for VMAT1 and VMAT2. In the cytoplasm of tumor cells SV2 immunoreactivity was abundant, whereas NCAM was located on the plasma membrane (Table 2) ▶ . Electron microscopy showed numerous secretory granules in the cytoplasm of tumor cells. These granules had the appearance typical for midgut carcinoids, ie, pleiomorphic, dense-core granules, measuring 100 to 400 nm in diameter.
Growth of Tumor Cells in Nude Mice
Tumor Generation I
Out of the 10 injected animals, eight were followed for 6 to 12 months after grafting of tumor cells. One animal was lost at transplantation and one was sacrificed before development of tumors, because of infectious complications. Gross tumors developed in 6 of 8 animals (take rate = 75%). The first tumors were observed 6 months after tumor cell injection. The doubling time for these tumors was estimated to 17.5 ± 2.2 days (n = 6; range, 8.9 to 22.7 days) (Figure 2) ▶ .
Figure 2.
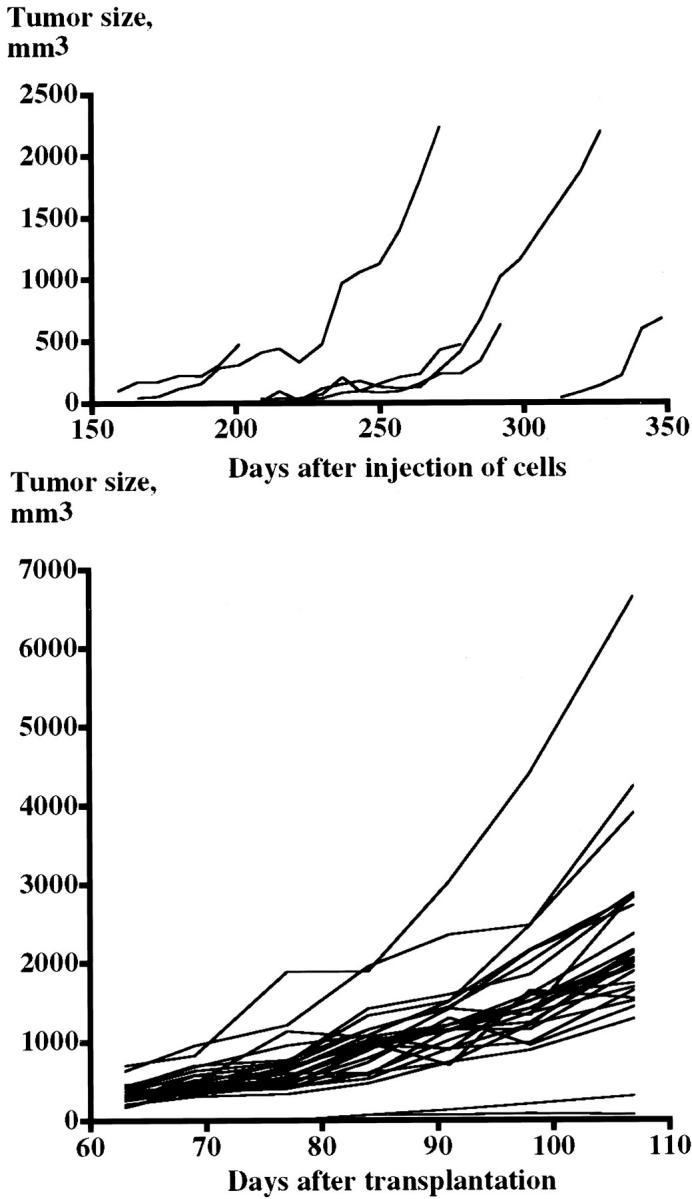
Diagrams showing the increase in tumor size throughout time in tumor generation I (top, n = 6) and tumor generation V (bottom, n = 27). The doubling times were 17.5 ± 2.2 days (range, 8.9 to 22.7 days) in tumor generation I and 15.6 ± 0.6 days (range, 5.4 to 21.5 days) in tumor generation V.
Tumor Generations II to V
In these generations the first tumors were observed already after 4 to 6 weeks. The take rates were 67% in generation II, 89% in generation III, 98% in generation IV, and 100% in generation V. The estimated doubling time in generation V was 15.6 ± 0.6 days (n = 31; range, 5.4 to 21.5) (Figure 2) ▶ . The tumors have now been continuously propagated for 30 months in nude mice.
The Transplanted GOT1 Tumor Cells Are of Human Origin
FISH analysis of the touch preparation revealed that >95% of the cells analyzed were of human origin. Hybridization with human painting probes showed that the majority of nuclei contained two X chromosome-specific and two chromosome 1-specific signals (Figure 3) ▶ . The few cells that were negative for the human wcp probes had generally larger nuclei than did those that were positive for the probes.
Figure 3.
FISH analysis of a touch preparation made from a transplanted tumor of generation V. Hybridization with wcp probes specific for the X chromosome (green signal) and chromosome 1 (red signal) reveals human chromosome-specific signals in the vast majority of cell nuclei (blue staining). Scale bar, 30 μm.
Cytogenetic analysis of a 17-hour culture confirmed that >95% of the metaphases had a human chromosome complement. The chromosome counts of all but one of the 46 metaphases analyzed were in the hypodiploid region with a modal number of 44 chromosomes (range, 38 to 45 chromosomes). All metaphases had clonal structural rearrangements including several marker chromosomes. No cell with a normal human karyotype was observed. Only a small percentage (<5%) of cells had mouse karyotype.
Immunocytochemical Properties and Amine Production of Transplanted Tumors
Microscopic examination of tumors demonstrated a characteristic pattern of insular growth with tumor cells positive for CgA, 5-HT, VMAT1, and VMAT2 (Figure 4) ▶ . One tumor from each of the first four generations was examined. The neuroendocrine differentiation was well maintained over the four generations (Table 2) ▶ . Electron microscopy confirmed presence of numerous electron-dense granules in the cytoplasm of the tumor cells.
Figure 4.
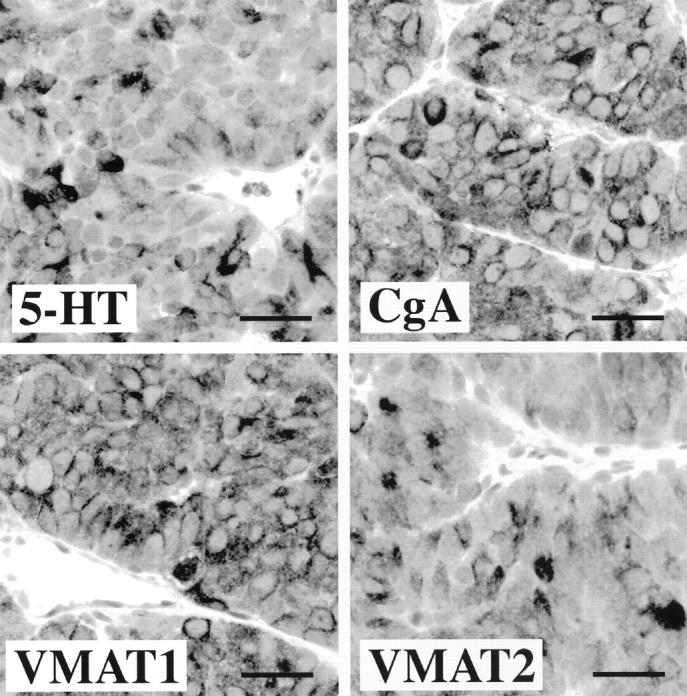
Immunocytochemical characterization of carcinoid tumors grown in nude mice. Tumor cells are positive for 5-HT, CgA, and VMAT1 and VMAT2. The immunohistochemical profile in grafted tumor cells was the same as in the primary midgut carcinoid tumor. Scale bars, 30 μm.
The average 5-HT concentration in peripheral blood of nude mice with generation II tumors was 5,103 ± 795 μmol/L (n = 7) versus 1,784 ± 225 μmol/L (n = 8) in animals without tumor (P < 0.001).
Somatostatin Receptors in Transplanted Tumors
Northern analysis revealed expression of all five somatostatin receptor subtypes in tumor cells from the primary cell culture. The somatostatin receptor expression was also investigated in tumors from nude mice and all five somatostatin receptor subtypes were expressed in all tumor generations investigated (generations I, II, and III). The only exception was somatostatin receptor 2, which was not detectable in generation III. The transcript sizes were as expected (Figure 5) ▶ .
Figure 5.
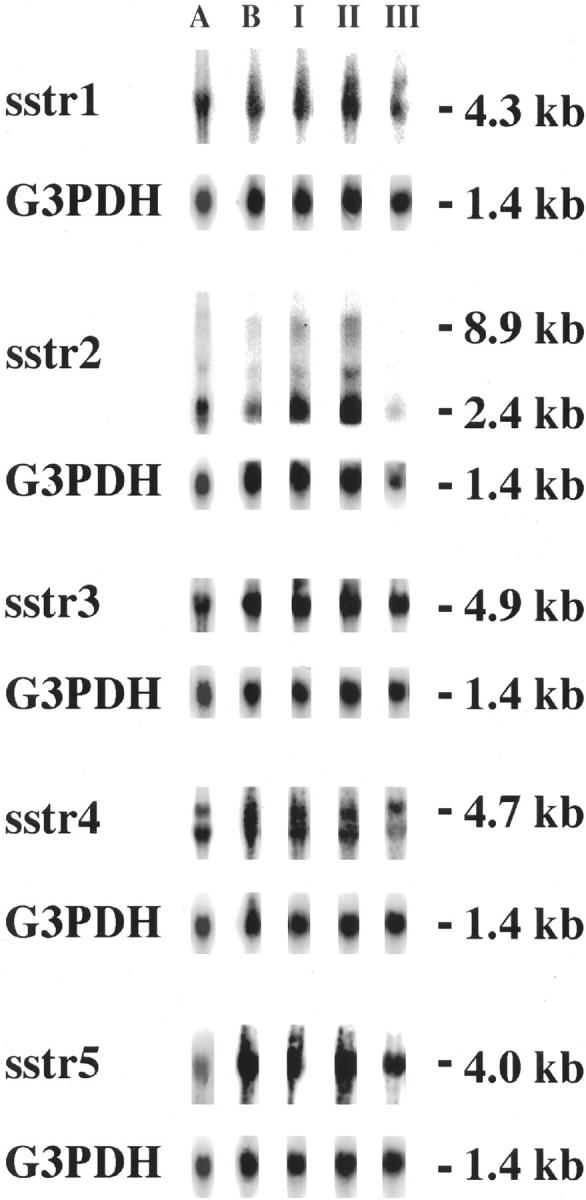
Quantitative analysis of somatostatin receptor expression. Northern analysis was performed on carcinoid tumor cells in primary culture before transplantation (A), tumor cells in cell culture derived from a transplanted tumor from nude mice (B) and tumors from generation I to III (I, II, and III). All five somatostatin receptor subtypes were expressed by the tumor cells in culture as well as tumors in nude mice. The expression of somatostatin receptors in the three generations was identical except for somatostatin receptor 2 in generation III, which was not detectable. G3PDH was used as housekeeping gene to confirm the integrity of the blotted mRNA.
Binding and Scintigraphy of Radiolabeled Octreotide and MIBG
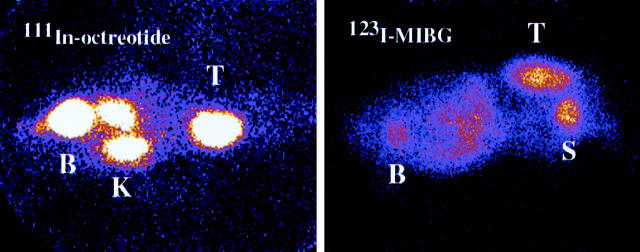
The concentration of 111In-octreotide in tumor at 4 hours after injection was very high for tumor generation I with 13% IA/g (n = 1), and lower values for generation II with 0.87 ± 0.32% IA/g (n = 3) and generation IV with 0.40 ± 0.08% IA/g (n = 10). The corresponding T/B values were 250, 63 ± 39, and 20 ± 4. Scintigraphy of the nude mice with tumor generation I showed high uptake in the tumor (Figure 6) ▶ , but also uptake/distribution of the radionuclide in the kidneys and urinary bladder.
Figure 6.
Octreotide scintigraphy (left) of a nude mouse with a carcinoid tumor located in the back of the neck (anteroposterior view). The mouse was examined in a γ camera 10 minutes after injection of 111In-octreotide. Uptake in the tumor (T) is indicated as well as uptake in the kidneys (K) and the urinary bladder (B). MIBG scintigraphy (right) of a nude mouse with a carcinoid tumor growing in the back of the neck (lateral view). The mouse was examined in a γ camera for 30 minutes 24 hours after the injection of 123I-MIBG. Uptake in the tumor (T) is indicated as well as uptake in the kidneys (K) and the urinary bladder (B).
The concentration of 123I-MIBG in tumor tissue of generation IV was 0.93 ± 0.06% IA/g (n = 2) at 4 hours, 1.1 ± 0.1% IA/g (n = 3) at 24 hours, and 0.54 ± 0.08% IA/g (n = 3) at 48 hours after injection. The corresponding T/B values were 12 ± 2, 28 ± 5, and 83 ± 13. Scintigraphy at 24 hours showed high uptake in the tumor, but also some uptake/distribution of the radionuclide in the salivary glands and urinary bladder (Figure 6) ▶ .
Biokinetics of Radiolabeled Octreotide
The tumors accumulated 111In after intravenous injection of 111In-octreotide. After a rapid release during the first 24 hours the decline in 111In-activity concentration was very slow for the tumor whereas it was considerably faster for blood and whole body. Seven days after the injection 17% of the initial 111In-activity concentration still remained in the tumors versus 0.05% and 3.3% in blood and whole body, respectively (Figure 7) ▶ . The concentration of 111In-activity in tumor tissue was 1.25 ± 0.14% IA/g (n = 5) at 0.5 hours, 0.33 ± 0.09% IA/g (n = 5) at 24 hours, 0.29 ± 0.02% IA/g (n = 5) at 3 days, and 0.22 ± 0.06 (n = 5) at 7 days after injection.
Figure 7.
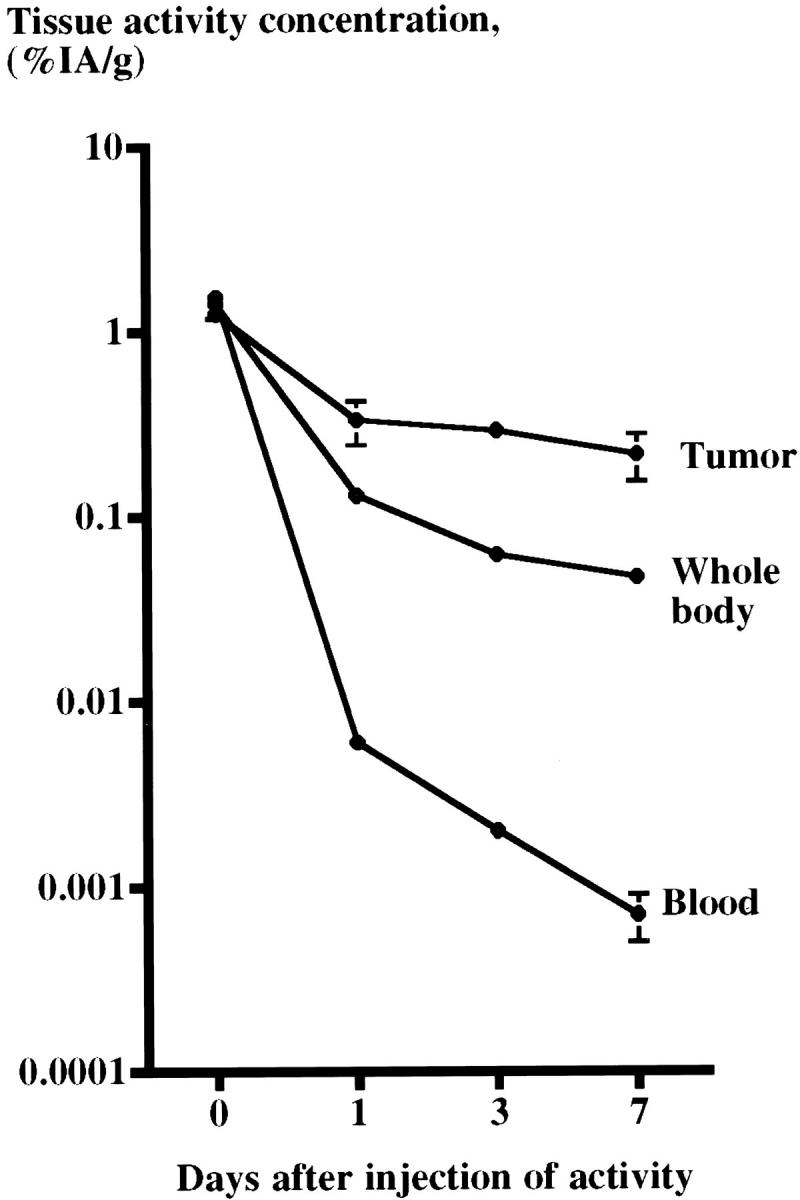
111In-activity concentration (%IA/g) in tumors transplanted to nude mice, blood, and whole body. After a rapid decline during the first 24 hours the decline in 111In-activity concentration was very slow for the tumor. Still 7 days after the injection 17% of the 111In-activity concentration at day 0 remained in the tumors whereas only 0.05% and 3.3% remained in the blood and whole body, respectively.
Cell Cultures Generated from Transplanted Tumor (Generation II)
Spontaneous Secretion of 5-HTP, 5HT, and 5-HIAA by Tumor Cells
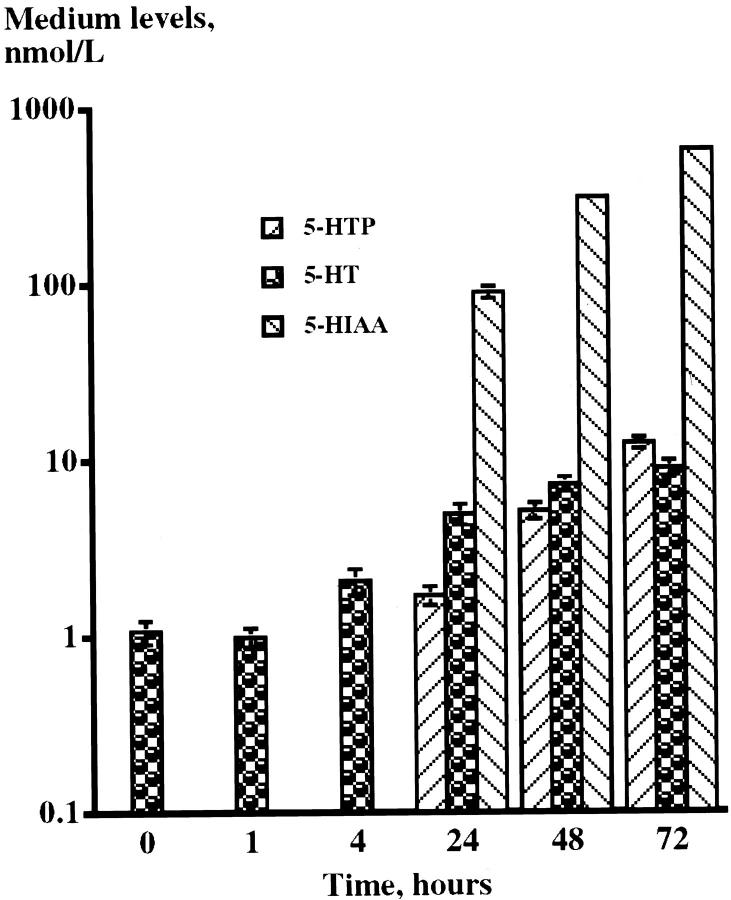
The spontaneous secretion of 5-HTP, 5HT, and 5-HIAA into culture medium was followed up to 72 hours after change of culture medium. 5-HT levels started to rise 4 hours after the change of medium and was elevated eightfold at 72 hours. The medium levels of 5-HTP and 5-HIAA started to rise at 24 hours after change of medium with very high levels of 5-HIAA (Figure 8) ▶ .
Figure 8.
Spontaneous secretion of 5-HTP, 5HT, and 5-HIAA into medium of cell cultures established from a transplanted tumor of generation II. The medium levels of 5-HTP and 5-HIAA started to rise 24 hours after change of medium with very high levels of 5-HIAA indicating a rapid turnover of 5-HT by the tumor cells.
Intracellular Calcium Determinations
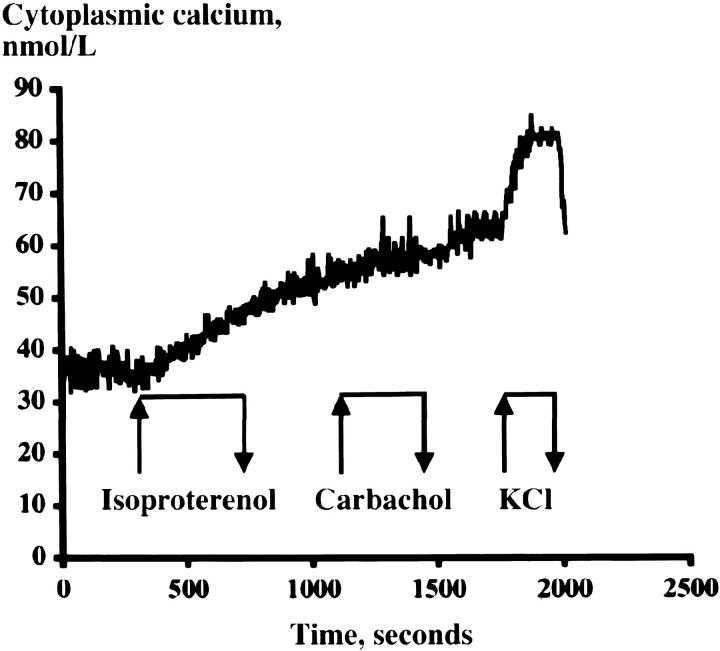
The baseline [Ca2+]i was 37 ± 4 μmol/L (n = 4). Isoproterenol (10 μmol/L) slightly increased [Ca2+]i, whereas carbachol (100 μmol/L) had no additional effect. Depolarization by KCl (20 mmol/L) rapidly increased [Ca2+]i in a reversible manner (Figure 9) ▶ .
Figure 9.
Effect of isoproterenol (10 μmol/L), carbachol (100 μmol/L), and depolarization by KCl (20 mmol/L), respectively, on [Ca2+]i in single superinfused tumor cells prepared from heterografted tumors of generation II. The trace is representative of three different experiments.
Discussion
Increased understanding of the biology of carcinoid tumor cells and development of new approaches for diagnostic and therapeutic attempts require reliable human cell lines. In this study we present a human midgut carcinoid tumor that was transplanted to nude mice and successfully propagated for five consecutive generations with well-preserved neuroendocrine differentiation. The human origin of the cells was confirmed by cytogenetic and FISH analyses. Touch preparations from generation V showed that ≥95% of the cells were of human origin using painting probes for the human X chromosome and chromosome 1. Cytogenetic analysis revealed that the vast majority of metaphases had a human chromosome complement. The tumor had a hypodiploid stemline with several clonal structural and numerical abnormalities.
The neuroendocrine features of the transplanted tumors were those of a well-differentiated endocrine tumor, ie, strongly immunoreactive for general endocrine markers such as CgA and synaptophysin. Furthermore, the tumor cells expressed markers characteristic for midgut carcinoids, eg, 5-HT. Comparison of the immunohistochemical profiles and ultrastructural features confirmed a high degree of similarity between the original primary carcinoid and the transplanted tumors of all generations.111In-octreotide showed high uptake of the radionuclide and high T/B values in the tumors and Northern blot analysis showed expression of all five somatostatin receptor subtypes in the tumors. These tumors can therefore be used for diagnostic visualization by octreotide scintigraphy and also as a model for studies of somatostatin receptor-mediated radiation therapy. Quantitative analysis of the somatostatin receptor expression levels needs to be performed to give further information about the mechanism for 111In-octreotide uptake. To date biokinetic studies of radiolabeled somatostatin analogues in animal models have been restricted to somatostatin receptor-expressing exocrine pancreatic tumors, ie, CA20948 and AR4-2J. 18,19 The strong accumulation and slow release of 111In in the transplanted tumors of our model closely resembles the biokinetics of 111In of the original midgut carcinoid tumor, demonstrating the relevance of this new model. 20 For therapeutic purposes it may be of interest to modulate somatostatin receptor expression, eg, selective up-regulation of somatostatin receptor 2 for optimal binding to octreotide, which today is the analogue most widely used for targeted radionuclide therapy. Such receptor up-regulation can probably be induced by steroid hormones. 21
The uptake of radiolabeled MIBG has been shown to be high in pheochromocytomas, expressing both VMAT1 and VMAT2, but reduced to one third in midgut carcinoids, which often lack expression of VMAT2. 22 In vitro experiments have shown that MIBG may act as a substrate for chromaffin granules. 23 VMAT2 may thus be important for MIBG transport and tumor visualization. In this model we found that the transplanted tumors, cultured tumor cells and the original primary ileal carcinoid expressed both VMAT1 and VMAT2. 123I-MIBG scintigraphy visualized the tumors in nude mice and determination of the radionuclide concentrations in tumor tissue and blood showed very high T/B, which clearly indicates that radiolabeled MIBG may be used therapeutically in GOT1 tumors.
In cell cultures very high levels of the main 5-HT metabolite, 5-HIAA, were found. Because midgut carcinoid tumor cells contain both the deaminating enzymes monoamine oxidase A and B, the high 5-HIAA/5-HT ratio indicates a high turnover of 5-HT and complete intracellular metabolism with rapid release of the metabolite into the culture medium. 6
This study further shows that the tumor cells have mechanisms for homeostatic regulation of [Ca2+]i, which are similar to other neuroendocrine cells, eg, endocrine cells of the normal pancreatic islets 24 and chromaffin PC12 cells 25 in regard to baseline [Ca2+]i and the [Ca2+]i responses to isoproterenol, which is explained by opening of plasma membrane Ca2+ channels through activation of cyclic AMP and protein kinase A 25,26 and K+ depolarization. In contrast, the muscarinic agonist carbachol had no effect on [Ca2+]i, which is different from other cell systems 27 and suggests lack of functional muscarinic receptors in these cells or lack of a significant phospholipase C-dependent formation of inositol 1,4,5-trisphosphate. A previous study on human midgut carcinoid cells in a primary cell culture revealed the existence of voltage-gated Ca2+ currents through L-type Ca2+ channels studied by a patch-clamp technique. 28 Therefore, the GOT1 cells respond with changes in [Ca2+]i in response to formation of cyclic AMP and depolarization as other neuroendocrine cells.
This study presents evidence that this transplantable human GOT1 carcinoid expresses all recognized somatostatin receptors, the amine transporters VMAT1 and VMAT2 both in vitro and in vivo, bind and internalize radiolabeled somatostatin analogue and MIBG and exhibits neuroendocrine characteristics of handling [Ca2+]i. This cell line is therefore suited to understand biological aspects of human midgut carcinoids and to evaluate new modalities for therapy.
Acknowledgments
We thank Graeme I. Bell (University of Chicago, Chicago, IL), Friedrich Raulf (Preclinical Research, Novartis, Basle, Switzerland), and Susumo Seino (Chiba University School of Medicine, Japan) for generously supplying somatostatin receptor probes; and Siv Tuneberg, Pernilla Andersson, Annki Illerskog, Lena William-Olsson, Ellinor Andersson, Malin Bengtsson, Gülay Altiparmak, Kerstin Knutsson, and Ragnar Alm for expert technical assistance.
Footnotes
Address reprint requests to Lars Kölby, M.D., Institute of Surgical Sciences, Department of Surgery, Sahlgrenska University Hospital, S-413 45 Göteborg, Sweden. E-mail: lars.kolby@surgery.gu.se.
Supported by The Swedish MRC (5220 and 6834), The Swedish Cancer Society (2690, 3427 and 3911), I.B. and A. Lundberg Research Foundation, Assar Gabrielsson Foundation, The Swedish Society of Medicine, The Swedish Society for Medical Research, The Göteborg Medical Society, The King Gustav V Jubilee Clinic Cancer Fund, Göteborg, Sahlgrenska University Hospital Research Funds, Gunvor and Josef Anérs Stiftelse, Axel Linders Stiftelse, Gunvor, Arvid and Elisabet Nilssons Stiftelse, Serena Ehrenströms donationsfond, Wilhelm och Martina Lundgrens Vetenskapsfond, and Malmö University Hospital Foundation for the Prevention and Treatment of Cancer.
References
- 1.Wängberg B, Nilsson O, Johanson V, Kölby L, Forssell-Aronsson E, Andersson P, Fjälling M, Tisell L, Ahlman H: Somatostatin receptors in the diagnosis and therapy of neuroendocrine tumor. Oncologist 1997, 2:50-58 [PubMed] [Google Scholar]
- 2.Nilsson O, Wängberg B, Kölby L, Dahlström A, Ahlman H: Intraocular transplantation and primary cell cultures as experimental models for the study of human carcinoid disease. Ann NY Acad Sci 1994, 733:380-392 [DOI] [PubMed] [Google Scholar]
- 3.Ahlman H, Wigander A, Mölne J, Nilsson O, Karlsson JE, Theodorsson E, Dahlström A: Presence of nerve growth factor-like immunoreactivity in carcinoid tumour cells and induction of a neuronal phenotype in long-term culture. Int J Cancer 1989, 43:949-955 [DOI] [PubMed] [Google Scholar]
- 4.Debons-Guillemin MC, Launay JM, Roseto A, Peries J: Serotonin and histamine production by human carcinoid cells in culture. Cancer Res 1982, 42:1513-1516 [PubMed] [Google Scholar]
- 5.Nilsson O, Wängberg B, Theodorsson E, Skottner A, Ahlman H: Presence of IGF-I in human midgut carcinoid tumours—an autocrine regulator of carcinoid tumour growth? Int J Cancer 1992, 51:195-203 [DOI] [PubMed] [Google Scholar]
- 6.Westberg G, Ahlman H, Nilsson O, Illerskog A, Wängberg B: Secretory patterns of tryptophan metabolites in midgut carcinoid tumor cells. Neurochem Res 1997, 22:977-983 [DOI] [PubMed] [Google Scholar]
- 7.Pfragner R, Wirnsberger G, Niederle B, Behmel A, Rinner I, Mandl A, Wawrina F, Luo JS, Adamiker D, Hoeger H, Ingolic E, Schauenstein K: Establishment of a continuous cell line from a human carcinoid of the small intestine (KRJ-I): characterization and effects of 5-azacytidine on proliferation. Int J Oncol 1996, 8:513-520 [DOI] [PubMed] [Google Scholar]
- 8.Evers BM, Townsend CM, Jr, Upp JR, Allen E, Hurlbut SC, Kim SW, Rajaraman S, Singh P, Reubi JC, Thompson JC: Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology 1991, 101:303-311 [DOI] [PubMed] [Google Scholar]
- 9.Fjälling M, Andersson P, Forssell-Aronsson E, Gretarsdottir J, Johansson V, Tisell LE, Wängberg B, Nilsson O, Berg G, Michanek A, Lindstedt G, Ahlman H: Systemic radionuclide therapy using indium-111-DTPA-D-Phe1-octreotide in midgut carcinoid syndrome. J Nucl Med 1996, 37:1519-1521 [PubMed] [Google Scholar]
- 10.Wigander A, Lundmark K, McRae A, Mölne J, Nilsson O, Haglid K, Dahlström A, Ahlman H: Production of transferable neuronotrophic factor(s) by human midgut carcinoid tumour cells; studies using cultures of rat fetal cholinergic neurons. Acta Physiol Scand 1991, 141:107-117 [DOI] [PubMed] [Google Scholar]
- 11.Nordkvist A, Mark J, Gustafsson H, Bang G, Stenman G: Non-random chromosome rearrangements in adenoid cystic carcinoma of the salivary glands. Genes Chromosom Cancer 1994, 10:115-121 [DOI] [PubMed] [Google Scholar]
- 12.Mitelman F (ed): ISCN, An International System for Human Cytogenetic Nomenclature. Basel, S Karger, 1995
- 13.Forssell-Aronsson E, Fjälling M, Nilsson O, Tisell LE, Wängberg B, Ahlman H: Indium-111 activity concentration in tissue samples after intravenous injection of indium-111-DTPA-D-Phe-1-octreotide. J Nucl Med 1995, 36:7-12 [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N: Single-step method for RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 15.Nilsson O, Kolby L, Wangberg B, Wigander A, Billig H, William-Olsson L, Fjalling M, Forssell-Aronsson E, Ahlman H: Comparative studies on the expression of somatostatin receptor subtypes, outcome of octreotide scintigraphy and response to octreotide treatment in patients with carcinoid tumours. Br J Cancer 1998, 77:632-637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsson S, Ahren B: Cytosolic Ca2+ oscillations by gastrin releasing peptide in single HIT-T15 cells. Peptides 1999, 20:579-587 [DOI] [PubMed] [Google Scholar]
- 17.Grynkiewicz G, Poenie M, Tsien RY: A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985, 260:3440-3450 [PubMed] [Google Scholar]
- 18.Breeman WA, Hofland LJ, Bakker WH, van der Pluijm M, van Koetsveld PM, de Jong M, Setyono-Han B, Kwekkeboom DJ, Visser TJ, Lamberts SW, Krenning EP: Radioiodinated somatostatin analogue RC-160: preparation, biological activity, in vivo application in rats and comparison with [123I-Tyr3]octreotide. Eur J Nucl Med 1993, 20:1089-1094 [DOI] [PubMed] [Google Scholar]
- 19.Mäcke HR, Behe M, Froidevaux S, Heppeler A, Jermann E: DOTA-D-Phe(1)-Tyr(3)-octreotide (DOTATOC). A unique somatostatin receptor ligand for labeling with a variety of metallic radionuclides. J Nucl Med 1997, 38(Suppl):18P
- 20.Andersson P, Forssell-Aronsson E, Grétarsdóttir J, Johanson V, Wängberg B, Nilsson OMF, Ahlman H: Biokinetics and dosimetry after repeated injections of 111In-DTPA-D-Phe1-octreotide. Sixth International Radiopharmaceutical Dosimetry Symposium. 1996, Tennessee, Gatlinburg
- 21.Visser-Wisselaar HA, Hofland LJ, van Uffelen CJ, van Koetsveld PM, Lamberts SW: Somatostatin receptor manipulation. Digestion 1996, 57(Suppl 1):7-10 [DOI] [PubMed] [Google Scholar]
- 22.Kimmig BN: Radiotherapy for gastroenteropancreatic neuroendocrine tumors. Ann NY Acad Sci 1994, 733:488-495 [DOI] [PubMed] [Google Scholar]
- 23.Henry JP, Gasnier B, Desnos C, Scherman D, Krejci E, Massoulie J: The catecholamine transporter of adrenal medulla chromaffin granules. Ann NY Acad Sci 1994, 733:185-192 [DOI] [PubMed] [Google Scholar]
- 24.Hellman B, Gylfe E, Bergsten P, Grapengiesser E, Lund PE, Berts A, Dryselius S, Tengholm A, Liu YJ, Eberhardson M, Chow RH: The role of Ca2+ in the release of pancreatic islet hormones. Diabete Metab 1994, 20:123-131 [PubMed] [Google Scholar]
- 25.Ämmälä C, Ashcroft FM, Rorsman P: Calcium-independent potentiation of insulin release by cyclic AMP in single β-cells. Nature 1993, 363:356-358 [DOI] [PubMed] [Google Scholar]
- 26.Kaspar SP, Pelzer DJ: Modulation by stimulation rate of basal and cAMP-elevated Ca2+ channel current in guinea pig ventricular cardiomyocytes. J Gen Physiol 1995, 106:175-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilon P, Henquin JC: Influence of membrane potential changes on cytoplasmic Ca2+ concentration in an electrically excitable cell, the insulin-secreting pancreatic B-cell. J Biol Chem 1992, 267:20713-20720 [PubMed] [Google Scholar]
- 28.Glassmeier G, Strubing C, Riecken EO, Buhr H, Neuhaus P, Ahnert-Hilger G, Wiedenmann B, Scherubl H: Electrophysiological properties of human carcinoid cells of the gut. Gastroenterology 1997, 113:90-100 [DOI] [PubMed] [Google Scholar]