Abstract
This study shows a strong correlation between the metastatic potentials of breast carcinoma cell lines and their ectopic expression of interleukin-8 (IL-8). Correlations exist for both constitutive and induced levels of IL-8 released. A correlation was also observed between cell morphology, metastatic potential, and IL-8 profile. Metastatic lines are fusiform in appearance, whereas, nonmetastatic lines are epithelioid. The metastatic potential of two breast carcinoma lines was examined using an orthotopic model of spontaneous metastasis. Metastatic cells formed rapidly growing, poorly differentiated primary tumors that metastasized. Nonmetastatic cells formed rapidly growing differentiated primary tumors that did not produce detectable metastases. Comparison of IL-8 expression by the parental cells and cell cultures developed from primary and metastatic tumors, demonstrates that IL-8 released by cultured cells from the primary tumor is higher than that of the parental cells, and IL-8 released by cultured cells derived from the metastatic lung tumors is greater than that released by cultured cells derived from the primary tumor. These data demonstrate a strong correlation between the metastatic phenotype of a cell and its IL-8 expression, suggesting a role for IL-8 in promoting the metastatic potential of breast tumor cells.
Metastasis is the process by which tumor cells spread from their site of origin to remote sites after gaining access to the circulatory system, culminating in the establishment of tumors at distant sites. 1 It has been established that a major, if not essential, requirement for metastasis, is angiogenesis, the recruitment of new blood vessels. 2 Newly acquired vessels supply tumor cells with oxygen and nutrients required for sustained growth. Mediators of angiogenesis responsible for tumor neovascularization may be derived directly from the tumor cells, tumor-associated inflammatory cells, or adjacent mesenchymal cells. 3 Understanding the mechanisms contributing to the metastatic potential of tumor cells will enhance the prospect of developing a means to impede the process of metastasis.
For the past 2 decades, members of the α-chemokine family have been observed as tumor cell products thought to contribute to growth and progression of tumor cells. 3 Interleukin-8 (IL-8), a member of this family of small basic peptides, was first purified on the basis of its neutrophil chemoattractant activity. 4 A clinical study examined 56 normal and 73 neoplastic breast tissues for mRNA levels of 13 cytokines. The only correlation found in this study was a higher IL-8 level in the neoplastic breast tissues than in the normal tissue (P = 0.0017). 5 The release of IL-8 by melanoma cells has been reported to be a contributing factor to both their growth and metastatic potential in vivo. Preliminary screening of human melanoma cell lines demonstrated that six of eight cell lines produce significant levels of IL-8. 6 In an experimental metastasis model, it was established that the metastatic potential of several clones derived from human melanoma cell lines correlated positively with the levels of IL-8 produced in vitro. 7 Another series of experiments demonstrated, in a non-small cell lung carcinoma tumor model, the in vivo growth rate and number of spontaneous lung metastases formed, correlated with the level of IL-8 secreted. In other supporting experiments, it was shown that passive immunization of severe combined immunodeficiency (SCID) mice with a neutralizing anti-IL-8 monoclonal antibody depressed the rate of tumor growth by >40% and was associated with an accompanying decline in lung metastases. This anti-IL-8 antibody, however, did not inhibit the in vitro growth of these non-small cell lung carcinoma cells, demonstrating IL-8 can enhance tumor growth and metastasis in vivo; the latter suggests the in vivo effects occur independently of an autocrine role. 8 A further assessment of the role of IL-8 expression in tumor metastasis was accomplished by transfecting an IL-8 expression system into a nonmetastatic human melanoma line that did not produce IL-8. The expression of IL-8 induced a metastatic phenotype in these cells. 9 Collectively, these data indicate that ectopic expression of IL-8 can contribute to an increased metastatic potential and that down-regulation of IL-8 in tumors should have a salutary effect.
Recently IL-8 was also found to be an endothelial cell chemoattractant in vitro and an angiogenic factor in vivo. 10-12 The angiogenic properties of IL-8 may explain the correlation between the growth and metastatic potential of a tumor cell line and its ectopic release of IL-8 that could enhance tumor neovascularization. It is commonly thought by many in the field that newly acquired blood vessels will contribute to the growth of tumors by increasing the rate at which oxygen and nutrients are supplied to the tumor cells and facilitating the removal of byproducts of cellular metabolism. The newly acquired blood vessels are less mature, having little or no basement membrane, and when juxtaposed to the tumor can facilitate metastasis by providing a ready means by which tumor cells can enter the circulation. Once in the circulation, tumor cells can be carried to distant sites where they can extravasate through postcapillary venules and establish metastatic centers. Cells expressing high levels of IL-8 would be at a selective advantage because they would not only acquire their own blood supply at their new site, but would also attract neutrophils. Release of enzymes by these inflammatory cells could facilitate tumor establishment via tissue remodeling.
In view of the potential role of IL-8 in metastasis, the expression of IL-8 by four human breast carcinoma cell lines was examined to ascertain if the levels of IL-8 released correlate with their reported metastatic potential. The metastatic potentials of these cell lines were assigned based on their ability to spontaneously metastasize from xenographs in immunosuppressed mice. Our findings indicate the metastatic cells express a higher basal level of IL-8 than the nonmetastatic cells and that IL-8 induction by the inflammatory mediators, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), is ∼200-fold greater in the metastatic cells than in the nonmetastatic cells. A correlation between cellular morphology and metastatic potential was also observed.
Materials and Methods
Cell Lines and Culture Conditions
The MDA-MB-231 (referred to as MDA-231) and MCF-7 cell lines (American Type Culture Collection, Rockville, MD) were cultured with antibiotic-free Dulbecco’s modified Eagle’s medium (DMEM) (no. 10-017-CV; Mediatech, Herndon, VA) plus 10% fetal bovine serum (FBS) in sterile tissue-culture flasks and incubated at 37°C/6%CO2. The MDA-MB-435 (referred to as MDA-435) and T47D cell lines (ATCC) were cultured with antibiotic-free RPMI 1640 (no. 10-040-CV; Mediatech) plus 10% FBS in sterile tissue culture flasks (Falcon, Oxnard, CA no. 353111) and incubated as stated above. The cell lines were certified to be mycoplasm-free. Cells were subcultured by trypsinizing in 5 mg/ml of trypsin (no. T-4799; Sigma, St. Louis, MO) and 0.5 mmol/L of ethylenediaminetetraacetic acid in Hanks’ balanced salt solution without Ca++ or Mg++ in a laminar flow hood during their logarithmic phase of growth.
Enzyme-Linked Immunosorbent Assay (ELISA) for Human IL-8
The breast carcinoma cell lines were seeded in six-well plates containing 2 ml of their respective complete media. At 80% confluency, the media was aspirated and 2 ml of fresh complete media was introduced to each well. The six-well plates were then treated with IL-1β (a gift from Jim Cone of Otsuka Pharmaceutical Company, Rockville, MD) or TNF-α (Preprotech Inc., Rocky Hill, NJ) in the concentrations shown in Figure 1 ▶ . After a 24-hour treatment, 1.5 ml of media was collected from each well, clarified of cells and cellular organelles, stored at −20°C, and the number of cells per well determined. The IL-8 ELISA was performed according to the manufacturer’s instructions (OptEIA human IL-8 kit; PharMingen, San Diego, CA). This kit is specific for human IL-8, neither NIH 3T3 cells nor tissue culture strains developed from athymic mice tested with the IL-8 kit released IL-8 cross-reacting products constitutively or when induced with IL-1β or TNF-α.
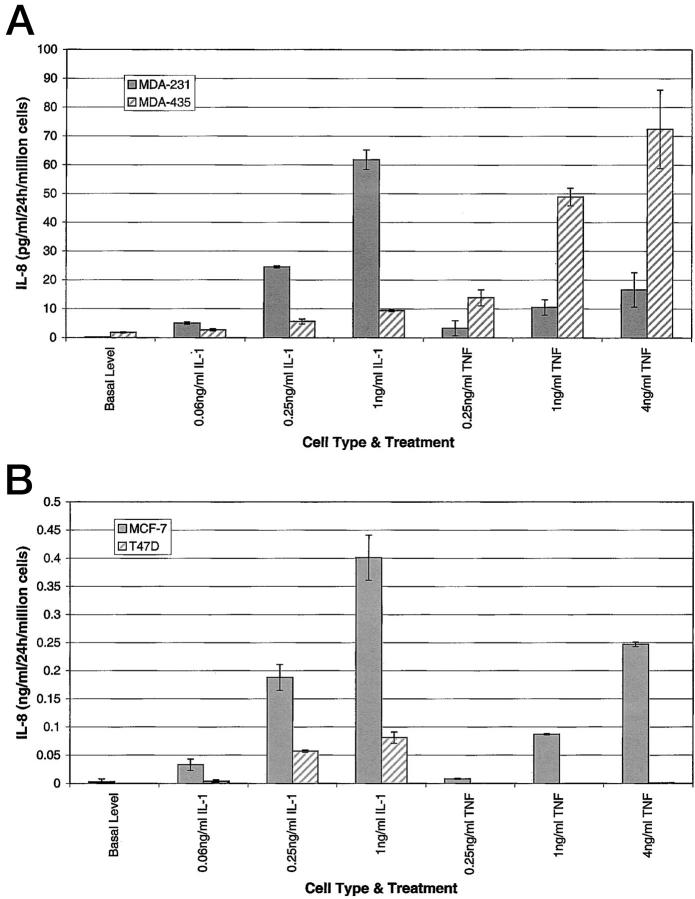
Figure 1.
IL-8 expression by metastatic and nonmetastatic breast cancer cells. Cells were seeded in six-well plates in DMEM containing 10% FBS or RPMI 1640 containing 10% FBS. At 80% confluency, various concentrations of IL-1β or TNF-α were added to each well. At 24 hours, 1.5 ml of media was collected from each well, clarified of cells and organelles, and an IL-8 ELISA was performed. The values are calculated for 1 million cells. Table 1 ▶ is a numerical representation of the data.
cDNA Synthesis
To determine IL-8 mRNA production, MDA-231, MDA-435, MCF-7, and T47D cell lines were either grown in complete media and treated with 1 ng/ml IL-1β, or 4 ng/ml TNF-α, or grown in complete media alone. After a 3-hour treatment, the cellular RNA was harvested with Trizol reagent according to the manufacturer’s protocol (Life Technologies, Inc., Grand Island, NY). For cDNA, 1 μg of RNA, was reverse-transcribed with MLV reverse transcriptase according to the Superscript II reverse transcriptase manufacturer’s protocol (Life Technologies, Inc.).
Oligonucleotides
Primers were designed using a human IL-8 published sequence. 13 The 283-bp human IL-8 product was amplified using the following sequences: 5′-ATG ACT TCC AAG CTG GCC GT-3′ and 5′-CCT CTT CAA AAA CTT CTC CAC ACC-3′. β-actin primers producing a 643-bp product were also designed using the following sequences: 5′-CAT GGA TGA TGA TAT CGC CG-3′ and 5′-TCT CCT TAA TGT CAC GCA CGA-3′. Both sets of primers were constructed at the University of Minnesota Microchemical Facility.
Determination of IL-8 mRNA Production in Metastatic and Nonmetastatic Cells
A polymerase chain reaction (PCR) master mix containing PCR buffer, 25 mmol/L MgCl2 (FisherBiotech, Itasca, IL), 10 mmol/L dNTPs, 10 μmol/L of each IL-8 primer, 1.25 U Taq polymerase (FisherBiotech), and sterile water to 49 μl was added to 1 μl of cDNA. Amplification was performed using a PTC-100 programmable thermal controller (MJ Research, Inc., Watertown, MA) with the following cycle: 95°C for 5 minutes (1 cycle), followed by 35 cycles of 94°C for 1 minute, annealing at 65°C for 1 minute, extension at 72°C for 30 seconds, and concluding with a 10-minute extension at 72°C. The PCR products were analyzed on a 1% agarose gel stained with ethidium bromide.
Animal Studies
Female, athymic nu/nu mice (4 to 6 weeks old) were purchased from the National Cancer Institute (Frederick, MD) to be used in an orthotopic model of spontaneous metastasis. One week after arrival of the mice, human breast carcinoma cells (2.5 × 10 5 cells suspended in DMEM at 1 × 10 7 cells/ml) were injected directly into the second left mammary fat pad of each mouse through an incision just below the second nipple. The cells were injected in a volume of 25 μl using a 0.3-ml syringe with a 29-gauge needle (Monoject, St. Louis, MO, 006-0291). The mice receiving the estrogen-dependent MCF-7 cells were also implanted with 90-day release 17-β-estradiol pellets, which contain 0.72 mg of estrogen per pellet (Innovative Research of America, Sarasota, FL). Tissue from the primary tumor and lungs were saved for cell culture and histological analysis.
Results
IL-8 Produced in Vitro
Four tumorigenic breast carcinoma lines were examined for IL-8 release to determine whether a correlation exists between the release of IL-8 by a tumor cell line and its potential to metastasize. The metastatic cell lines, MDA-231 and MDA-435, are estrogen receptor-negative. 14 The cells used to establish the MDA-231 line were isolated from a pleural effusion taken from a patient with a breast adenocarcinoma, and the MDA-435 line was derived from a pleural effusion obtained from a patient with metastatic breast ductal carcinoma. The nonmetastatic, tumorigenic lines, MCF-7 and T47D, are estrogen receptor-positive cells. 14 The MCF-7 tumorigenic breast carcinoma line was initiated using cells from a pleural effusion that originated as a breast adenocarcinoma. A pleural effusion from a patient with a breast ductal carcinoma gave rise to the T47D breast carcinoma line. The assignment of the metastatic potential of these cell lines was dependent on their ability to metastasize in immunosuppressed mice. Conditioned media from the four tumor lines were examined for the presence of IL-8. The conditioned media from both metastatic cell lines contained substantial quantities of IL-8, ranging between 0.2 and 5.7 ng/ml/24 hours/10 6 cells (Figure 1A) ▶ . The basal level of IL-8 released by the nonmetastatic tumor cell lines was very low or undetectable by ELISA, ie, in our assay <0.005 ng/ml/24 hour/10 6 cells (Figure 1B) ▶ .
Tumors often contain infiltrating monocytes and macrophages that not only play a possible role in the dislodging of tumor cells, causing local tissue remodeling, but may also serve as a source of inflammatory mediators, including IL-1β and TNF-α. 3 Because these mediators are known inducers of IL-8, 13,15 their effects on the IL-8 expression of these four tumor cell lines were examined. Both metastatic cell lines responded to IL-1β and TNF-α induction. The MDA-231 cells responded better to IL-1β than to TNF-α. TNF-α induced a much higher level of IL-8 release by MDA-435 cells than did IL-1β, in addition also inducing the highest levels of IL-8 expression observed under the conditions tested (Figure 1A) ▶ . The IL-8 response of the nonmetastatic cell lines, MCF-7 and T47D, to these mediators was minimal (Figure 1B) ▶ . The data in Figure 1, A and B ▶ , is compiled in Table 1 ▶ to provide a numerical comparison. There is a 180-fold difference between the maximum levels of IL-8 in the metastatic and nonmetastatic cell lines. Similarly, the constitutive expression of IL-8 by the nonmetastatic cells, MCF-7 and T47D, was very low. The MCF-7 cells produced low levels of IL-8 in response to IL-1β and TNF-α; the T47D cells produced low levels of IL-8 in response to IL-1β, but did not respond to TNF-α. Perhaps most important of all, we observed the basal levels of IL-8 released by the metastatic cell lines were comparable to or higher than the highest induced levels of IL-8 released by the nonmetastatic cell lines.
Table 1.
IL-8 Release by Nonmetastatic and Metastatic Breast Carcinoma Cell Lines (ng/ml/24 hr/Million Cells)
| Nonmetastatic cell lines | Metastatic cell lines | |||
|---|---|---|---|---|
| MCF-7 | T47D | MDA-231 | MDA-435 | |
| Basal level | 0.003 ± 0.005 | 0.000 ± 0 | 0.222 ± 0.052 | 1.752 ± 0.208 |
| 0.06 ng/ml IL-1β | 0.033 ± 0.01 | 0.004 ± 0.002 | 5.018 ± 0.388 | 2.659 ± 0.358 |
| 0.25 ng/ml IL-1β | 0.188 ± 0.023 | 0.057 ± 0.002 | 24.510 ± 0.381 | 5.611 ± 0.867 |
| 1 ng/ml IL-1β | 0.401 ± 0.04 | 0.08 ± 0.01 | 61.818 ± 3.381 | 9.354 ± 0.325 |
| 0.25 ng/ml TNF-α | 0.008 ± 0.001 | 0.000 ± 0 | 3.315 ± 2.606 | 13.851 ± 2.778 |
| 1 ng/ml TNF-α | 0.087 ± 0.001 | 0.000 ± 0 | 10.530 ± 2.673 | 48.879 ± 3.076 |
| 4 ng/ml TNF-α | 0.247 ± 0.004 | 0.001 ± 0 | 16.616 ± 5.938 | 72.413 ± 13.63 |
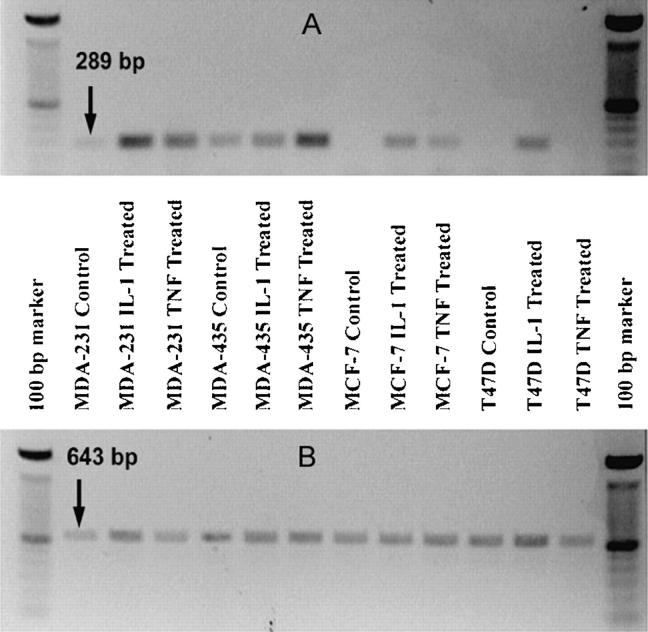
The IL-8 mRNA expressed in these cells under constitutive and induced conditions was examined using reverse transcriptase (RT)-PCR (Figure 2) ▶ . The mRNA of the two unstimulated metastatic cell lines gave rise to light bands of RT-PCR product. The bands produced from the mRNA of cells treated with either IL-1β or TNF-α were darker than the bands from the unstimulated cells. This is consistent with the ELISA data that shows the levels of IL-8 released by MDA-231 and MDA-435 cells on treatment with either IL-1β or TNF-α are dramatically increased above the constitutive levels. The mRNA from the unstimulated, nonmetastatic cell lines did not produce observable RT-PCR products. When induced with IL-1β, the mRNA from both the MCF-7 and T47D cells produced light bands of RT-PCR product. Treatment with TNF-α showed only the mRNA from the MCF-7 cells produced a visible RT-PCR product. Northern blot analysis of mRNA from MDA-435 and T47D cells also confirmed that the T47D cells produced little or no IL-8 mRNA and MDA-435 cells produced measurable quantities of IL-8 mRNA (data not shown).
Figure 2.
IL-8 mRNA production of stimulated and unstimulated metastatic and nonmetastatic cells. Total RNA was extracted from untreated (control) cells, and from cells that were stimulated for 3 hours with either 1 ng/ml IL-1β or 4 ng/ml TNF-α. First-strand cDNA was produced by amplification with 1 μg of RNA using oligo-dT primers. Thirty-five cycles of PCR were performed using primer sets amplifying a 289-bp segment of the human IL-8 gene (A). Twenty-five cycles of PCR were performed using a β-actin primer set amplifying a 643-bp segment of the human β-actin gene (B).
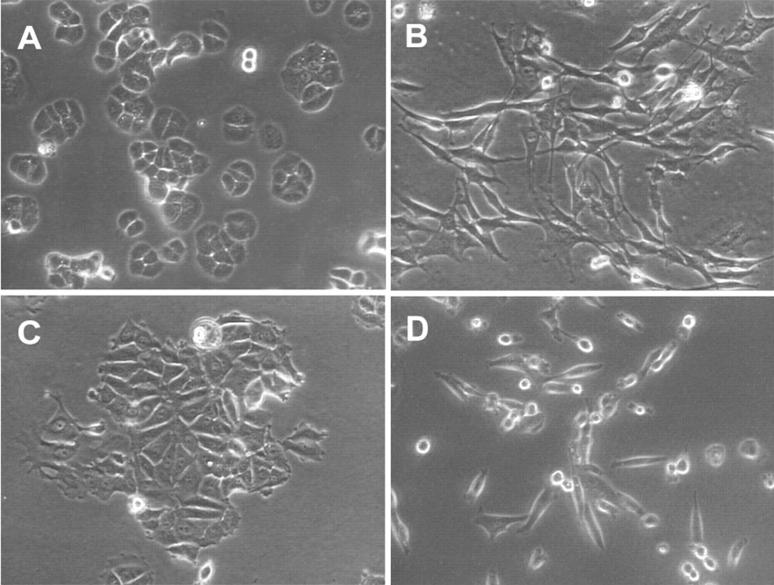
In addition to the differences in IL-8 release, there are obvious differences in the morphological appearance of metastatic and nonmetastatic cells in vitro. Metastatic cell lines are bipolar or fusiform in nature. They lack tight cell-cell junctions and do not form distinct colonies in monolayer cultures (Figure 3, B and D) ▶ . In contrast, morphologies exhibited by the nonmetastatic cell lines are more epithelioid (Figure 3, A and C) ▶ . Other investigators have shown that these nonmetastatic cells express E-cadherin at their cell-cell junctions. 14 In monolayer cultures these cells form distinct colonies or islands of nonmigratory cells. To determine whether the expressed cellular morphology may play a role in IL-8 expression and release, the conditioned media from a human dermal fibroblast (HDF) cell line, displaying a greater length-to-width ratio than either of the metastatic breast carcinoma cell lines, was examined. The level of IL-8 released constitutively by HDF cells, 0.89 ± 0.06 ng/ml/24 hours/10 6 cells, lies between the constitutive levels released by the MDA-231 and the MDA-435. When the HDF cells were stimulated with either 1 ng/ml IL-1β or 4 ng/ml TNF-α, they released 1541.24 ± 96.2 and 283.72 ± 11.93 of 0.06 ng/ml/24 hours/10 6 cells of IL-8, respectively.
Figure 3.
Images of cell lines. Cells were plated, maintained for 72 hours in DMEM plus 10% FBS or RPMI 1640 plus 10% FBS, and then visualized by phase contrast microscopy (×200). A: T47D (nonmetastatic). B: MDA-435 (metastatic). C: MCF-7 (nonmetastatic). D: MDA-231 (metastatic).
Tumorigenic and Metastatic Potential of Breast Carcinoma Cell Lines
The tumorigenic and metastatic properties of two breast carcinoma cell lines, MDA-435 and MCF-7, were tested using an orthotopic model of tumor growth and spontaneous metastasis. The MCF-7 line is classified as nonmetastatic and the MDA-435 line as metastatic. After orthotopic injection, both breast carcinoma cell lines gave rise to primary tumors that were measurable within 2 weeks. Tumors growing out of the MDA-435 inoculations grew slightly faster than those growing out of the MCF-7 inoculations. By 10 weeks, the tumors produced by each of the cell lines had grown to ∼1 to 1.5 cm in diameter. The MDA-435 tumors began to penetrate the skin, at which time the animals were euthanized and tissue was collected. The MCF-7 tumors did not penetrate the skin on the chest and were allowed to grow for a week longer.
The histological appearance of the primary tumors that developed in response to the orthotopic injection of MCF-7 cells is distinctly different from those produced by MDA-435 cells. MCF-7 cells develop tumors with morphological features of a well-differentiated breast adenocarcinoma. The cells group together in glandular structures and display moderate pleomorphism, whereas primary tumors produced by MDA-435 cells are less well differentiated, and grow in solid homogeneous sheets of cells (Figure 4, A and B) ▶ . The histological appearance of the tumors formed by these carcinoma lines is analogous to their in vitro growth patterns, the MCF-7 cells grew as islands of tightly associated, E-cadherin-containing epithelioid cells 14 (Figure 3C) ▶ , whereas, the MDA-435 cells grew as individual fusiform cells with little or no cell-cell interactions (Figure 3B) ▶ . The MCF-7 cells, although tumorigenic (Figure 4A) ▶ , did not give rise to observable metastases in any animals (Figure 4C) ▶ . However, the primary tumors formed by the MDA-435 cells gave rise to large numbers (>50) of lung metastases in five of 10 animals. Metastatic tumors were not observed in the other five mice. A representative metastatic lung tumor is shown in Figure 4D ▶ .
Figure 4.
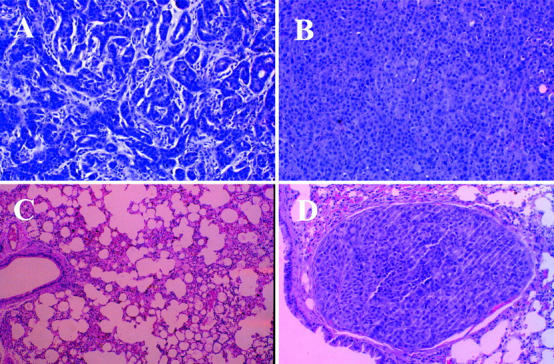
Histology of sections taken from mouse primary tumors and lung tissues. Sections taken from primary tumors and lungs of female athymic nu/nu mice orthotopically injected with either MCF-7 or MDA-435 cells were fixed in formaldehyde and embedded in paraffin. The slides were stained with H&E and visualized at ×40. A: MCF-7 adenocarcinoma, site of inoculation. B: MDA-435 adenocarcinoma, site of inoculation. C: MCF-7 lung, absence of metastases. D: MDA-435 lung, lumen of blood vessel is occluded by tumor embolus.
IL-8 Production by Primary and Metastatic Tumors
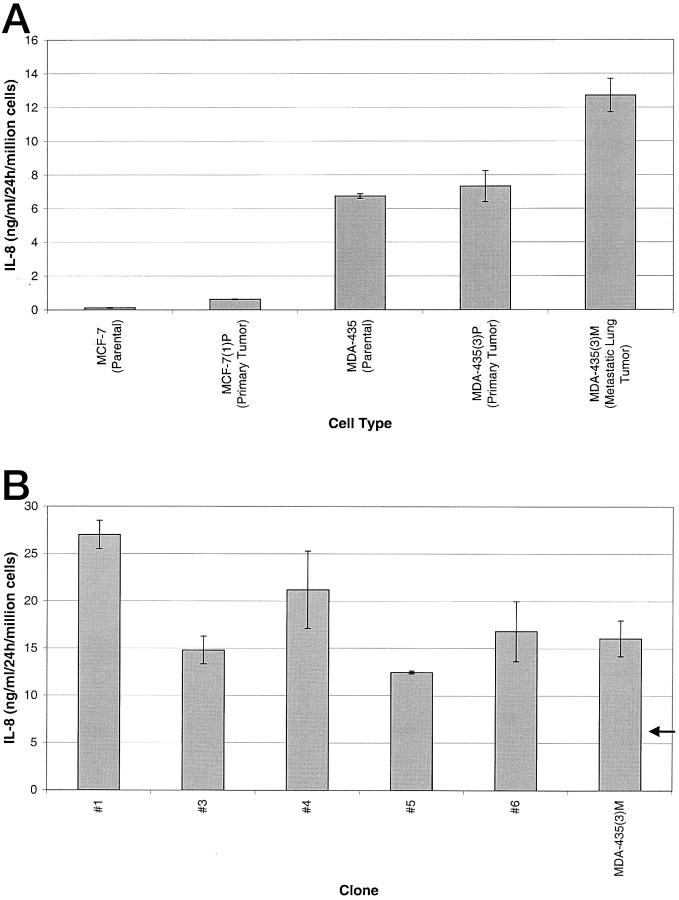
Given the correlation between IL-8 release and metastasis, it is conceivable that cells from the metastatic lesions express higher levels of IL-8 than those from the primary tumors. Cell cultures were established from primary and metastatic tumors from animals injected with MDA-435 cells and from primary tumors of animals injected with MCF-7 cells. The level of IL-8 released by these newly established cultures was compared to the IL-8 levels of the corresponding parental lines. The culture of tumor cells established from the primary MCF-7 tumor produced approximately fivefold more IL-8 than the parental line (0.621 ng/ml versus 0.123 ng/ml) (Figure 5A) ▶ . The culture of cells derived from a primary MDA-435 tumor produced approximately the same amount of IL-8 as the parental cells (7.325 ng/ml versus 6.740 ng/ml). However, the culture of metastatic cells from the lung of the same mouse produced 1.9-fold the level of IL-8 as the parental MDA-435 cells on a per cell basis (Figure 5A) ▶ . Because the lungs from which this culture was derived had >100 tumors, clones were isolated from this culture to determine whether a clonal variation exists in the level of IL-8 being expressed. The level of IL-8 production was determined for five clones. All five clones released higher levels of IL-8 than the culture developed from the primary tumor (arrow versus bars in Figure 5B ▶ ). When the levels of IL-8 expressed by the clones are compared to the level of expression of the mixed culture of metastatic lung cells, some were lower than the mixed population and some were higher. However, all five clones release substantially more IL-8 (1.8- to 3.8-fold) than that released by cells derived from the primary tumor. Together, the findings indicate cells derived from the primary tumor release more IL-8 than the parental cells, and the cells derived from the metastatic lesion release more IL-8 than the cells derived from the primary tumor.
Figure 5.
IL-8 expression by cells cultured from primary tumor and lung tissue sections. Cells were seeded in six-well plates in DMEM containing 10% FBS or RPMI 1640 containing 10% FBS. At 24 hours, 1.5 ml of media was collected from each well, clarified of cells and organelles, and an IL-8 ELISA was performed. The values are calculated for 1 million cells. Arrow in B indicates level of IL-8 released by MDA-435 primary tumor cell line.
The metastatic potential of MDA-435(3)M clone 1, the highest IL-8-producing clone from a metastatic lung tumor (Figure 5B) ▶ , was compared in vivo to the metastatic potential of the parental cells that gave rise to clone 1. Lung tumors were found in four of four animals inoculated with clone 1. Only one of the four animals inoculated with cells of the parental culture developed lung metastases. This further supports the correlation between higher levels of IL-8 release and an increased metastatic potential.
Discussion
In this study, we examined four breast carcinoma cell lines, two metastatic cell lines and two nonmetastatic cell lines. We demonstrate that a strong correlation exists between the level of IL-8 released in cell culture and the metastatic potential of these cell lines, as observed in spontaneous metastasis assays. More specifically, the basal level of IL-8 expression is substantially higher in the metastatic cell lines when compared to the nonmetastatic lines. Furthermore, when the metastatic cells are treated with IL-1β or TNF-α, the levels of IL-8 released are greatly enhanced. The nonmetastatic cells, however, release little or no IL-8 constitutively, and the levels of IL-8 induced by IL-1β or TNF-α are significantly lower than the constitutive levels released by the metastatic cells. Other reports have also provided data supporting a role for IL-8 in tumor metastasis. What is obvious from these reports is that IL-8 could be exerting its effects in a multiplicity of ways, working to the benefit of the tumor. It has been demonstrated, using in vivo models of metastasis, that tumor cells expressing ectopic IL-8 have increased growth potential and enhanced vascularization and metastatic potential, 7-9,16 further strengthening the correlation between metastatic potential and ectopic IL-8 production.
On examination of two breast carcinoma lines in an orthotopic model of spontaneous metastasis, we find their tumorigenic and metastatic potential as reported. Both cell lines were highly tumorigenic in this model and had comparable growth rates. The MCF-7 tumors did not metastasize, whereas the MDA-435 cells metastasized in 50% of the cases. In vitro cultures of metastatic cells that had colonized the lungs produce more IL-8 than cultures derived from either the parental line or the line derived from the primary tumor. The results are consistent with the hypothesis that cells producing high levels of IL-8 are able to metastasize more readily than cells in primary tumors, which produce little if any IL-8. This correlation is also supported by the fact that no lung metastases were seen in the mice injected with MCF-7 cells. The cells from the primary MDA-435 tumor release >10 times the amount of IL-8 on a per cell basis than do cells from the primary MCF-7 tumor and perhaps this is what enables MDA-435 cells to establish metastatic lung tumors.
Further examination of these cells revealed the presence of other characteristics that seem to correlate with their metastatic potential, the presence or absence of estrogen dependence and expressed morphological phenotype. The nonmetastatic cell lines are estrogen-dependent, whereas, the metastatic cell lines are estrogen-independent. The morphological features of these cell lines also parallel their reported metastatic potential. The MDA-435 and MDA-231 cell lines, which constitutively produce IL-8 at elevated levels, exhibit a more mesenchymal cellular morphology in vitro with less cell-cell interaction. The MCF-7 and T47D cell lines, which produce little IL-8, exhibit a more epithelioid morphology, forming tightly adhesive colonies of nonmigratory cells. The in vivo morphology of the MDA-435 and MCF-7 also follows this trend. The MDA-435 forms a less well-differentiated carcinoma with a more solid appearance, whereas the MCF-7 forms a well-differentiated carcinoma that grows in glandular structures.
The data indicate IL-8 expression, constitutively and under induction by IL-1β or TNF-α, is lower in epithelioid carcinoma cell lines and higher in fusiform carcinoma cell lines. In vitro cultures of untransformed mesenchymal cells are reported to express IL-8 levels that are highly inducible by either IL-1β or TNF-α. 17 We also found this to hold true for HDF cells. Their level of constitutively released IL-8 was between those released by the two metastatic breast carcinoma cell lines. On induction by inflammatory mediators, their level of IL-8 release increased between ∼320- and 1730-fold, depending on treatment. This raises the possibility that morphological appearance or state of phenotypic differentiation may be a contributing factor in the regulation of IL-8 expression. For example, normal mesenchymal cells, such as the HDF cell line, which function in inflammation and wound healing, would use the increases in IL-8 release as a protective mechanism. The increase in IL-8 released by cells at the site of a wound or inflammation would draw in neutrophils to fight infection and eventually, endothelial cells for the formation of new blood vessels to supply the remodeled tissues. In the case of breast carcinoma cells, these physiological functions may enhance the metastatic potential of tumor cells ectopically expressing IL-8. Where IL-8 release is attenuated in the epithelial, nonmetastatic breast carcinoma cell lines, minimizing the IL-8 contribution to angiogenic and metastatic potential, it is enhanced in the mesenchymal, metastatic cell lines, aiding in angiogenesis and metastasis.
The current theory explaining the observed increased rate of metastases in tumor cells ectopically releasing IL-8, attributes the increase to these angiogenic properties of IL-8. The angiogenic properties of IL-8 can enhance the metastatic potential of tumor cells by enhancing the vascular supply at the tumor site. Newly acquired vessels will enhance the growth rate of the tumor and possibly present a proximal means for tumor cell dissemination. The attraction of neutrophils by IL-8 may also contribute to angiogenic and metastatic potential of the tumor. The neutrophils, during their journey from the vessels from which they extravasate, to the tumor to which they are migrating, remodel the extracellular matrix by releasing proteases, a heparinase, and various other enzymes. 18 In this process of remodeling, the neutrophils are likely to release many factors previously sequestered in the extracellular matrix, such as basic fibroblast growth factor. These released factors can act as growth factors and chemoattractants for both endothelial cells and tumor cells. The liberation of these sequestered factors may increase the metastatic potential of a tumor in any number of ways, for example, by increasing angiogenesis, stimulating tumor progression, and/or enhancing tumor cell migration. 19,20 Thus, it seems nature has created a many-factored alliance surrounding cancer and IL-8. Neutrophils attracted to the tumor site may play a role in freeing or dislodging metastatic cells via the release of proteases and other enzymes.
In addition, the inflammatory milieu of the tumor often has increased levels of TNF-α and/or IL-1β, which would also disproportionately increase the production of IL-8 in both the tumor cells expressing a more mesenchymal phenotype, as well as the tumor-associated mesenchymal cells. A possible mode of IL-8 regulation by these factors is through the activation of transcription factors that recognize and bind to consensus sequences in the IL-8 promoter.
The robust response of the metastatic- or mesenchymal-appearing breast carcinoma cells to either IL-1β or TNF-α may be because of elevated expression of transcription factors needed for transcription of the IL-8 gene. Nuclear factor κB (NF-κB), a transcription factor, which can be activated by either IL-1β or TNF-α, is an example of such a transactivator. Activated NF-κB recognizes and binds to a consensus sequence in the promoter region of the IL-8 gene. This binding is essential, but not sufficient for the induction of IL-8 expression. It is possible that the metastatic breast cell lines have factors working either coordinately or synergistically with activated NF-κB to enhance IL-8 expression. 21
Epithelioid or nonmetastatic cells may either lack a component in the signal transduction pathway or may express a factor(s) that attenuates the expression of IL-8. Although several consensus sequences for the transcriptional activation of the IL-8 gene have been identified within the promoter region of IL-8, little is known about the mechanisms regulating the repression of IL-8 expression. The binding of the POU-homeodomain transcription factor (Oct-1) to the IL-8 promoter represses IL-8 expression by binding to an element that overlaps one of the transcriptional activators of IL-8 expression. 22 The differential regulation of IL-8 between the metastatic and nonmetastatic breast cells may be because of any combination of the above possibilities, ie, positive elements inducing IL-8 expression in the metastatic cells and/or the presence of a repressor(s) in the nonmetastatic tumor cells. Further work is needed to identify the key components responsible for this differential regulation.
The increase in the release of IL-8 by breast carcinoma cells may also contribute to the metastatic phenotype rather than simply being a consequence of the phenotype. As the cells progress from the epithelial, carcinoma in situ to the mesenchymal, metastatic tumor cell, the IL-8 levels, as well as, the angiogenic and metastatic potentials increase dramatically. Once the tumor cells have taken on a mesenchymal phenotype, the ectopic release of IL-8 seen by cells of this phenotype may further enhance their metastatic potential.
However, IL-8 is less likely to be responsible for the progression itself, because short-term exposure (6 weeks) of the nonmetastatic cell lines to IL-8 (at 0.1, 1, and 10 ng/ml) did not induce such morphological change (data not shown). Nonetheless, long-term exposure to IL-8 might still induce a phenotypic alteration of the morphology.
Understanding the diverse and critically important ways IL-8 can work in the body, coupled with a better understanding of the possible factors controlling IL-8 expression will likely provide important insights into the key factors contributing to the process of tumor progression. Knowledge of these mechanisms will increase our understanding of not only tumor progression and metastasis, but perhaps also the developmental process involving epithelial to mesenchymal transition. Ideally, this information will provide a means to design new therapeutic approaches to attenuate neutrophil-mediated inflammation and the ectopic expression of IL-8 by tumor cells expressing premetastatic or metastatic phenotypes.
Acknowledgments
We thank Dr. Michael Johnson of the Lombardi Cancer Center’s Tissue Culture Shared Resource facility for discussions, comments, and instruction in orthotopic transplantation; and Fiona Chan for her technical help during these experiments.
Footnotes
Address reprint requests to Joseph E. De Larco, Department of Laboratory Medicine and Pathology, University of Minnesota, 420 Delaware St. SE, Minneapolis, MN 55455. E-mail: delar001@tc.umn.edu.
Supported in part by Breast Cancer Program award DAMD17-97-1-7080 from the Department of Defense and National Institutes of Health grant R01-CA 29995.
References
- 1.Fidler IJ, Gersten DM, Hart IR: The biology of cancer invasion and metastasis. Adv Cancer Res 1978, 28:149-250 [DOI] [PubMed] [Google Scholar]
- 2.Folkman J, Watson K, Ingber D, Hanahan D: Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 1989, 339:58-61 [DOI] [PubMed] [Google Scholar]
- 3.Leek RD, Landers R, Fox SB, Ng F, Harris AL, Lewis CE: Association of tumour necrosis factor alpha and its receptors with thymidine phosphorylase expression in invasive breast carcinoma. Br J Cancer 1998, 77:2246-2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, Leonard EJ: Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA 1987, 84:9233-9237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green AR, Green VL, White MC, Speirs V: Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. Int J Cancer 1997, 72:937-941 [DOI] [PubMed] [Google Scholar]
- 6.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM: IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor [published erratum appears in J Immunol 1994 Oct 1;153(7): 3360]. J Immunol 1993, 151:2667-2675 [PubMed] [Google Scholar]
- 7.Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ: Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res 1994, 54:3242-3247 [PubMed] [Google Scholar]
- 8.Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM: Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J Clin Invest 1996, 97:2792-2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar-Eli M: Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol 1997, 151:1105-1113 [PMC free article] [PubMed] [Google Scholar]
- 10.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM: Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258:1798-1801 [DOI] [PubMed] [Google Scholar]
- 11.Miller LJ, Kurtzman SH, Wang Y, Anderson KH, Lindquist RR, Kreutzer DL: Expression of interleukin-8 receptors on tumor cells and vascular endothelial cells in human breast cancer tissue. Anticancer Res 1998, 18:77-81 [PubMed] [Google Scholar]
- 12.Strieter RM, Kunkel SL, Elner VM, Martonyi CL, Koch AE, Polverini PJ, Elner SG: Interleukin-8. A corneal factor that induces neovascularization. Am J Pathol 1992, 141:1279-1284 [PMC free article] [PubMed] [Google Scholar]
- 13.Mukaida N, Mahe Y, Matsushima K: Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem 1990, 265:21128-21133 [PubMed] [Google Scholar]
- 14.Hiraguri S, Godfrey T, Nakamura H, Graff J, Collins C, Shayesteh L, Doggett N, Johnson K, Wheelock M, Herman J, Baylin S, Pinkel D, Gray J: Mechanisms of inactivation of E-cadherin in breast cancer cell lines. Cancer Res 1998, 58:1972-1977 [PubMed] [Google Scholar]
- 15.Singh RK, Gutman M, Llansa N, Fidler IJ: Interferon-beta prevents the upregulation of interleukin-8 expression in human melanoma cells. J Interferon Cytokine Res 1996, 16:577-584 [DOI] [PubMed] [Google Scholar]
- 16.Singh RK, Gutman M, Reich R, Bar-Eli M: Ultraviolet B irradiation promotes tumorigenic and metastatic properties in primary cutaneous melanoma via induction of interleukin 8. Cancer Res 1995, 55:3669-3674 [PubMed] [Google Scholar]
- 17.Larsen CG, Anderson AO, Oppenheim JJ, Matsushima K: Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology 1989, 68:31-36 [PMC free article] [PubMed] [Google Scholar]
- 18.Mollinedo F, Nakajima M, Llorens A, Barbosa E, Callejo S, Gajate C, Fabra A: Major co-localization of the extracellular-matrix degradative enzymes heparanase and gelatinase in tertiary granules of human neutrophils. Biochem J 1997, 327:917-923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norrby K: Angiogenesis: new aspects relating to its initiation and control. APMIS 1997, 105:417-437 [DOI] [PubMed] [Google Scholar]
- 20.Yoshida A, Anand-Apte B, Zetter BR: Differential endothelial migration and proliferation to basic fibroblast growth factor and vascular endothelial growth factor. Growth Factors 1996, 13:57-64 [DOI] [PubMed] [Google Scholar]
- 21.Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, Appella E, Kung HF, Leonard EJ, Oppenheim JJ: Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med 1988, 167:1883-1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu GD, Lai EJ, Huang N, Wen X: Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. The role of Oct-1 as a transcriptional repressor. J Biol Chem 1997, 272:2396-2403 [PubMed] [Google Scholar]