Abstract
We have previously described decreased immunostaining of nidogen-1/entactin; laminin chains α1, α5, β1,γ1; and epithelial integrin α3β1 in human diabetic retinopathy (DR) corneas. Here, using 142 human corneas, we tested whether these alterations might be caused by decreased gene expression levels or increased degradation. By semiquantitative reverse transcription-polymerase chain reaction, gene expression levels of the α1, α5, and β1 laminin chains; nidogen-1/entactin; integrin α3 and β1 chains in diabetic and DR corneal epithelium were similar to normal. Thus, the observed basement membrane and integrin changes were unlikely to occur because of a decreased synthesis. mRNA levels of matrix metalloproteinase-10 (MMP-10/stromelysin-2) were significantly elevated in DR corneal epithelium and stroma, and of MMP-3/stromelysin-1, in DR corneal stroma. No such elevation was seen in keratoconus corneas. These data were confirmed by immunostaining, zymography, and Western blotting. mRNA levels of five other proteinases and of three tissue inhibitors of MMPs were similar to normal in diabetic and DR corneal epithelium and stroma. The data suggest that alterations of laminins, nidogen-1/entactin, and epithelial integrin in DR corneas may occur because of an increased proteolytic degradation. MMP-10 overexpressed in the diabetic corneal epithelium seems to be the major contributor to the observed changes in DR corneas. Such alterations may bring about epithelial adhesive abnormalities clinically seen in diabetic corneas.
Diabetic retinopathy (DR) remains the leading cause of legal blindness in elderly people in the Western world. 1 It is a well-recognized vision-impairing complication of diabetes that mainly involves retinal vasculature. However, both insulin-dependent and noninsulin-dependent diabetes mellitus affect not only retina and lens, but also cornea, tear film, eyelids, iris, ciliary body, and cranial nerves. 2 Corneal abnormalities such as epithelial defects and fragility, recurrent epithelial erosions, decreased sensitivity, abnormal wound repair, increased susceptibility to injury, ulcers, edema, and increased autofluorescence have been clinically observed in patients with diabetes and DR. 2-7 At the cellular and molecular levels, these diabetic abnormalities may be related to increased epithelial basement membrane (BM) thickness, 2,8 decreased number of hemidesmosomes, 9-11 impaired function of corneal endothelium, 12 altered collagen, 13 and abnormal deposition of complement proteins. 14 These alterations have been discussed in detail in a recent review. 6
Because many of the diabetic corneal abnormalities are apparently related to changes in cell adhesion and tissue repair, they might be due to alterations of adhesive molecules of the extracellular matrix (ECM) and BM. We have recently conducted a systematic study of >30 various ECM and BM components and their integrin receptors in corneas from patients with diabetes and DR. 15 Our data showed that DR corneas had a significant decrease in immunostaining for major epithelial BM components, nidogen-1/entactin, laminin-1 (α1β1γ1), laminin-10 (α5β1γ1), and of their binding integrin, α3β1. These alterations, especially of α3β1 integrin, seemed to be specific for diabetic corneas because they were not pronounced in corneas from patients with a common edematous corneal disease, bullous keratopathy. 15
We tested here whether BM and integrin alterations in human diabetic and DR corneas could occur because of decreased synthesis or increased degradation. Because gene expression of the affected components did not change with diabetes and DR, decreased synthesis could not easily explain our previous data. On the other hand, gene and protein expression of two matrix metalloproteinases (MMP), MMP-3 and MMP-10 (stromelysin-1 and stromelysin-2, respectively) was increased in diabetic corneas without DR and especially, in diabetic corneas with DR. These data give reason to suggest that overexpression of MMP-10, found both in corneal epithelium and stroma (MMP-3 was expressed only in stroma), may lead to the diabetes-related alterations of corneal epithelial BM and laminin-binding integrin, α3β1.
Materials and Methods
Tissue
Normal and diabetic autopsy corneas were obtained from the National Disease Research Interchange (Philadelphia, PA) within 40 hours after death. Keratoconus corneas removed during penetrating keratoplasty were received from collaborating surgeons within 30 hours after surgery. They were used as a control group to assess specificity of changes observed in diabetic corneas. For reverse transcription-polymerase chain reaction (RT-PCR) only corneas received within 30 hours after death or surgery were used. The diabetic group consisted of 33 age-matched normal corneas (from 30 individuals; mean age, 70.2 years; 31 corneas without ocular history, and two corneas after uneventful radial keratotomy that were used only for immunostaining), 45 diabetic corneas without DR (from 34 individuals; 23 with insulin-dependent diabetes mellitus and 22 with noninsulin-dependent diabetes mellitus; mean age, 67.5 years), and 37 diabetic corneas with DR (from 26 individuals; 22 with proliferative DR, 27 with insulin-dependent diabetes mellitus, and 10 with noninsulin-dependent diabetes mellitus; mean age, 65.6 years). The keratoconus group, in which corneas came from significantly younger people than diabetics, consisted of eight age-matched normal corneas (from eight individuals; mean age, 27.9 years) and 11 keratoconus corneas (from 11 individuals; mean age, 33.7 years). Eight diseased corneas were used as positive controls to test antibodies to various proteinases in immunohistochemistry. They included one neovascularized cornea with an ulcer, one neovascularized cornea with an acid burn, one cornea with herpetic scar, three failed corneal transplants, and two corneas after failed laser in situ keratomileusis. To confirm staining specificity, antibodies to proteinases were additionally used to stain sections of one normal brain, one meningioma, three glioblastomas multiforme, and one astrocytoma.
In diabetic corneas, all ECM and integrin abnormalities were previously documented in the central part only where Bowman’s layer lies underneath the epithelium. 15 Also, in keratoconus corneas, only a central part removed during transplantation was available. Therefore, for gene expression analysis by RT-PCR, normal and diabetic corneas were trephined before freezing and central parts only analyzed to ensure adequate comparison between groups. For some RT-PCR experiments, epithelium, stroma, and endothelium of trephined corneas were mechanically separated and then frozen. The vast majority of corneas were analyzed individually, without being pooled. Only in six diabetic and DR cases, trephined corneas were cut in half and then one half was embedded in OCT for immunofluorescence and the other one used for RT-PCR or Western blotting with pooling halves from fellow eyes. Because of this fact and the small amount of tissue available from a trephined cornea, a different set of normal and diabetic or keratoconus corneas was analyzed by semiquantitative RT-PCR, Western blotting/zymography, and immunofluorescence.
Semiquantitative RT-PCR
Immediately on arrival, corneas were trephined, frozen in liquid nitrogen, and stored at −80°C. The RT-PCR procedure has been described in detail previously. 16 Briefly, total RNA was isolated from whole corneas or from mechanically separated epithelium, stroma, and endothelium with TRIZOL reagent (Life Technologies Inc., Gaithersburg, MD) as per the manufacturer’s instructions. cDNA was synthesized from 2.0 μg of total RNA using SuperScript II reverse transcriptase (Life Technologies, Inc.). cDNA samples were subjected to PCR using specific primers for different ECM proteins, proteinases, and for β2-microglobulin (β2-MG) that served as an internal standard for sample normalization. Most primers were designed using the Primer3 Internet software program (The Whitehead Institute, Boston, MA) and their specificity was confirmed by BLAST (National Library of Medicine, Bethesda, MD) Internet software-assisted search of nonredundant nucleotide sequence database. 16-18 The sequences of primers for RT-PCR are shown in Table 1 ▶ .
Table 1.
RT-PCR Primers and Hybridization Oligonucleotides Used in this Study
| Gene | Forward primer | Reverse primer | Product size, bp | Hybridization oligonucleotide |
|---|---|---|---|---|
| α1 LN | AAGTGTGAAGAATGTGAGGATGGG | CACTGAGGACCAAAGACATTTTCCT | 441 | CCTGCGTACAGTGTTCCTTGGGTTACAGAG |
| α5 LN | CTGAGGACTGAAGTGAAAACTCAAG | AACTGGTAGGAGTCTCGGGTG | 473 | GTCTGGCACCATTTACAACTTCAGTGG |
| β1 LN | CCAAGATCCTTATCATGAGACCCTG | GATCTAAAGCACGAAATATCACCTC | 342 | GTATAGATACTTCGCCTATGACTGTGAG |
| N-1/EN | CTGGGGAAGGTTTATTATCGAG | TGAGAATGTCGTATGGAACTGC | 276 | GGAGATCTCTTTCCAGCCTAGTAGCGCGGTGGT |
| α3 INT | TACTACTTCGAGAGGGAAAGAGGAAG | GAAGGTCTGGGTAGAAGTTCTCATC | 358 | GCAAAGTGTACATCTATCACAGTAGCTCTA |
| β1 INT | TTATCCTTCTATTGCTCACCTTGTC | ATAACCTCTACTTCCTCCGTAAAGC | 426 | CCTAAGTCAGCAGTAGGAACATTATCTG |
| MMP-1* | CGACTCTAGAAACACAAGAGCAAGA | AAGGTTAGCTTACTGTCACACGCTT | 786 | ND |
| MMP-2 | GGGACAAGAACCAGATCACATAC | CTTCTCAAAGTTGTAGGTGGTGG | 446 | AATGGCAAGGAGTACAACAGCTGCACTGAT |
| MMP-3 | GAACAATGGACAAAGGATACAAC | AAATGAAAACGAGGTCCTTGCTAG | 460 | CGGAACCTGTCCCTCCAGAACCTGGGAC |
| MMP-7* | GGTCACCTACAGGATCGTATCATAT | CATCACTGCATTAGGATCAGAGGAA | 373 | ND |
| MMP-9* | GTATTTGTTCAAGGATGGGAAGTAC | GCAGGATGTCATAGGTCACGTAG | 515 | ND |
| MMP-10 | ACCTGGGCTTTATGGAGATATTC | TCATATGCAGCATCCAAATATGATG | 452 | CAAAATCTGTTCCTTCGGGATCTGAGATG |
| MMP-14 | CGCTACGCCATCCAGGGTCTCAAA | CGGTCATCATCGGGCAGCACAAAA | 497 | CATAATGAAATCACTTTCTGCATCCAGAATTACACC |
| TIMP-1 | CTGTTGTTGCTGTGGCTGATAG | CTTTTCAGAGCCTTGGAGGAC | 507 | ACCTTATACCAGCGTTATGAGATCAAGATGACC |
| TIMP-2 | TCTGGAAACGACATTTATGG | GTTGGAGGCCTGCTTATGGG | 501 | TGGGGTCTCGCTGGACGTTGGAGGAAAGAAGGAA |
| TIMP-3 | CATCAAGCAGATGAAGATGTACC | GGTAGTAGCAGGACTTGATCTTGC | 269 | CTCACCCTCTCCCAGCGCAAGGGGCTGAACTATC |
| CV/L2† | TACGGCTTTGAAGGAGCAAAT | AGAATTAAGCAATGAGTCCTTTGA | 378 | GGACAGCATGTCTGGGGAAATTTTATCTTGAAACTG |
| U-PA | CACACACTGCTTCATTGATTACC | TGGTGACTTCAGAGCCGTAGTAG | 404 | TCGTCTACCTGGGTCGCTCAAGGCTTAACTCCAACA |
| T-PA | TGTGTGGAGCAGTCTTCGTTTC | CCTGGTATCTATTTCACAGCAC | 334 | GAGCCAGATCTTACCAAGTGATCTGCAGAGATGAA |
| β2-MG | CTCGCGCTACTCTCTCTTTCTG | GCTTACATGTCTCGATCCCACTT | 335 | GTCTTTCAGCAAGGACTGGTCTTTCTATCTCTTGTA |
LN, laminin; N-1/EN, nidogen-1/entactin; INT, integrin; CV/L2, cathepsin V/L2; U-PA, urokinase-type plasminogen activator; T-PA, tissue plasminogen activator; β2-MG, β2-microglobulin; bp, base pairs; ND, not designed. *, from Giambernardi et al.; †, from Adachi et al. All primers are from 5′ to 3′.
PCR was performed with 5 to 25 ng of reverse-transcribed RNA, Taq polymerase buffer (Promega Biotech, Madison, WI) containing 200 μmol/L dNTPs, 1.25 U Taq polymerase, and 250 nmol/L of sense and anti-sense primers, in a total volume of 50 μl. Each cycle consisted of 30 seconds of denaturation at 94°C, 30 seconds of annealing, and 45 seconds elongation at 72°C. β2-MG amplification was used to normalize the samples. Normalized samples were amplified in a linear range established using serial cDNA dilutions and varying the number of cycles. Routine RT-PCR controls without RT or with normal human genomic DNA as a template were negative. Amplified products were separated by electrophoresis in 3% agarose gels and visualized under UV light after staining with ethidium bromide.
For Southern blot analysis, PCR products were transferred to positively charged Hybond N+ (Amersham Inc., Arlington Heights, IL) nylon membranes using an alkaline blotting procedure. Filters were hybridized with oligonucleotide probes (Table 1) ▶ 5′ end-labeled with [γ-32P]ATP (222 TBq/mmol; Dupont-New England Nuclear, Boston, MA), washed at high stringency, and exposed to Kodak X-OMAT AR (Eastman Kodak, Rochester, NY) X-ray film. To confirm sequence identity, PCR fragments were excised from the gels, purified using Wizard PCR Prep (Promega Biotech), reamplified, cloned into Plasmid PCR II using TA cloning kit (Invitrogen, Carlsbad, CA), and sequenced in an automated sequencer at the Cedars-Sinai DNA Sequencing Core Facility.
Indirect Immunofluorescence
Corneas were embedded in OCT and OCT blocks and sections were stored at −80°C. General procedure, secondary antibodies, and routine controls were as described previously. 15,16 Primary antibodies are listed in Table 2 ▶ . Because there are controversial data in the literature about tissue localization of various proteinases and little work has been done on cryostat sections, we have compared many antibodies using various pathological cases as positive controls and several fixation protocols. The necessity for this strategy has been recently emphasized. 19 The recommendations for immunohistochemical use of particular antibodies are also described in Table 2 ▶ .
Table 2.
Antibodies to Proteinases Used for Immunostaining in this Study
| Proteinase | Antibody and name | Use for immunostaining | Source |
|---|---|---|---|
| MMP-1/collagenase | mAb VI3 | Good | NeoMarkers |
| mAb COMY 4A2 | Weak staining | NeoMarkers | |
| mAb 41-1E5 | Unusual staining | Chemicon | |
| MMP-2/gelatinase A | mAb 42-5D11 | Nonspecific staining | Chemicon |
| mAb CA-4001* | Nonspecific staining | NeoMarkers | |
| mAb VB3 | Weak staining | NeoMarkers | |
| mAb VID2 | Good | NeoMarkers | |
| mAb A-Gel VC2* | Unusual staining | NeoMarkers | |
| mAb Gel-B IIA5 | Weak staining | NeoMarkers | |
| mAb KO VIIB1 | Some nonspecific staining | NeoMarkers | |
| Sheep pAb 0211 | Weak staining | Biogenesis | |
| MMP-3/stromelysin-1 | mAb SL-1 IIIC4 | Weak staining | Chemicon |
| mAb 55-2A4 | Good | Calbiochem | |
| mAb 10D6 | Good | R&D Systems | |
| mAb SL-1 IID4 | No staining detected | L.J. Windsor | |
| Rabbit pAb AB812 | Also detects MMP-10 | Chemicon | |
| MMP-7/matrilysin | mAb ID2 | Good | NeoMarkers |
| MMP-9/gelatinase B | mAb GE-213 | Good, stronger than pAb | NeoMarkers |
| mAb IA4 | Weak staining | NeoMarkers | |
| mAb IA5 | No staining detected | NeoMarkers | |
| mAb IIA5 | Weak staining | NeoMarkers | |
| mAb IVA2 | Some nonspecific staining | NeoMarkers | |
| mAb VIIC2 | Weak staining | NeoMarkers | |
| mAb 56-2A4 | Weak staining | Chemicon | |
| Sheep pAb 0911 | Good | Biogenesis | |
| MMP-10/stromelysin-2 | mAb IVC5 | Good, somewhat weak | L.J. Windsor† |
| mAb IB1 | No staining detected | NeoMarkers | |
| mAb VC3 | Weak staining | NeoMarkers | |
| mAb IIA1 | No staining detected | NeoMarkers | |
| mAb IIIB3 | Mostly stains epithelium | L.J. Windsor | |
| mAb IID6 | No staining detected | L.J. Windsor | |
| mAb IIID1 | Weak staining | L.J. Windsor | |
| mAb 142-9B11 | Unusual staining | Chemicon | |
| Sheep pAb D248-6 | Good | G. Murphy | |
| MMP-14/MT1-MMP | mAb 114-6G6 | Good | Chemicon |
| Urokinase | Goat pAb #398 | Weak staining | American Diagnostica |
| mAb #394 | Some nonspecific staining | American Diagnostica | |
| mAb 2B2.10.6 | Good | Serotec | |
| Rabbit pAb 8701 | Good | L.J. Windsor | |
| Rabbit pAb 8703 | Good, stronger than 8701 | L.J. Windsor | |
| Goat pAb AB776 | High background | Chemicon | |
| Tissue plasminogen activator | mAb PAM-3 | Good | American Diagnostica |
| Procathepsin V/L2 | Rabbit pAb AS-923 | Good | W. Adachi/S. Kinoshita |
Unusual staining, the pattern did not match those of any other antibody to the same MMP.
*The antibody recognizes only the proform of MMP-2.
†Also available from NeoMarkers.
mAb, mouse monoclonal antibody; pAb, polyclonal antibody.
The evaluated fixation protocols included: 1) 100% acetone at −20°C for 15 minutes, 2) 100% methanol at −20°C for 15 minutes, 3) 1% formalin in phosphate-buffered saline (PBS) (0.37% formaldehyde) at room temperature for 5 minutes, 4) 2% formalin in PBS at room temperature for 5 minutes, 5) 70% ethanol plus 1% formalin at room temperature for 10 minutes, and 6) no fixation. Acetone, ethanol-formalin, and methanol fixations dramatically reduced fluorescence intensity when compared with 1 or 2% formalin. Also, when no fixative was used, staining intensity was somewhat lower than after formalin fixation. For most experiments, 2% formalin fixation was used. However, 1% formalin was used for MMP-10 analysis because 2% formalin yielded strong, apparently nonspecific, staining of cell borders in the corneal epithelium with antibody IVC5 (not shown here), which was not seen with any other fixation method, nor with any other MMP-10 antibody.
Routine controls without primary antibodies were included with each experiment and were negative (see Figure 8 ▶ ). Each antibody was analyzed at least twice on most cases, with identical results. Since this work was being done over a considerable amount of time, different number of cases was analyzed for different proteinases. Purified monoclonal antibodies were used at 20 to 30 μg/ml, and polyclonal antibodies were diluted according to suppliers’ recommendations.
Figure 8.
Immunofluorescent analysis of MMP-3 and MMP-10 protein localization in normal, diabetic, and keratoconus corneas. No specific staining is seen in normal and keratoconus (KC) corneas for both MMPs, as well as on sections of normal corneas incubated only with a secondary antibody (second AB, negative controls). Epithelial and keratocyte staining for MMP-10 (polyclonal antibody D248-6) is seen in non-DR diabetic corneas and it is significantly increased in DR corneas, especially in the epithelium. MMP-3 (monoclonal antibody 55-2A4) is only seen in stromal keratocytes in non-DR diabetic and especially, DR corneas. e, epithelium; s, stroma.
Zymography
Gelatin zymography was done as described. 20 Briefly, frozen whole corneas were pulverized under liquid nitrogen, and extracted with 50 mmol/L Tris-HCl, pH 7.4, with 10 mmol/L CaCl2 and 0.25% Triton X-100, for 30 minutes at 4°C. The cleared solution is referred to as S1 fraction. The pellet was heat-extracted again in 50 mmol/L Tris-HCl, pH 7.4, with 10 mmol/L CaCl2 and 0.15 mol/L NaCl at 60°C for 6 minutes. The extract was cleared again and the supernate is referred to as S2 fraction. Samples were normalized for protein using the bicinchoninic acid protein assay as per the manufacturer’s recommendations (Pierce Chemical Co., Rockford, IL). Equal amounts of protein per sample were electrophoresed in 10% nonreducing Laemmli sodium dodecyl sulfate gels with 1% gelatin. Gels were soaked in 1% Triton X-100 for 30 minutes, rinsed and incubated overnight in the assay buffer containing 50 mmol/L Tris-HCl, pH 7.4, with 5 mmol/L CaCl2 and 0.02% NaN3. The gels were then stained with Coomassie Brilliant Blue and destained in acetic acid/methanol (10%/10% v/v). Gelatinase bands appeared clear against the blue background. Casein zymography was done in the same way as gelatin zymography, using precast 12.5% casein-containing gels (Bio-Rad, Hercules, CA). Separate control gels were incubated in the assay buffer supplemented with 2 mmol/L phenylmethyl sulfonyl fluoride to block serine proteinases or with 10 mmol/L ethylenediaminetetraacetic acid to inhibit MMPs. Addition of phenylmethyl sulfonyl fluoride did not alter the band pattern, and ethylenediaminetetraacetic acid eliminated gelatinolytic and caseinolytic bands.
Western Blot Analysis
Frozen and powdered whole corneas were extracted as above and the protein was determined by the bicinchoninic acid method (Pierce Chemical Co.). The extracts were heated at 95°C for 10 minutes in 4× Laemmli’s sample buffer with proteinase inhibitors and 2-mercaptoethanol (Boehringer Mannheim, Indianapolis, IN). Equal amounts of protein per sample were electrophoresed in 4 to 20% gradient sodium dodecyl sulfate gels (Bio-Rad). Western blotting with goat anti-mouse alkaline phosphatase conjugate as a secondary antibody was as described. 16 Primary mouse monoclonal antibodies 10D6 to MMP-3 (R&D Systems, Minneapolis, MN) and IVC5 to MMP-10 (NeoMarkers, Fremont, CA) were used at 3 to 5 μg/ml.
Statistical Analysis
Immunostaining results were analyzed using a double-sided Fisher’s exact test, and RT-PCR results, by nonparametric Mann-Whitney test (InStat software program; GraphPad Software, San Diego, CA).
Results
BM and Integrin Gene Expression Is Not Decreased in Diabetic Corneas
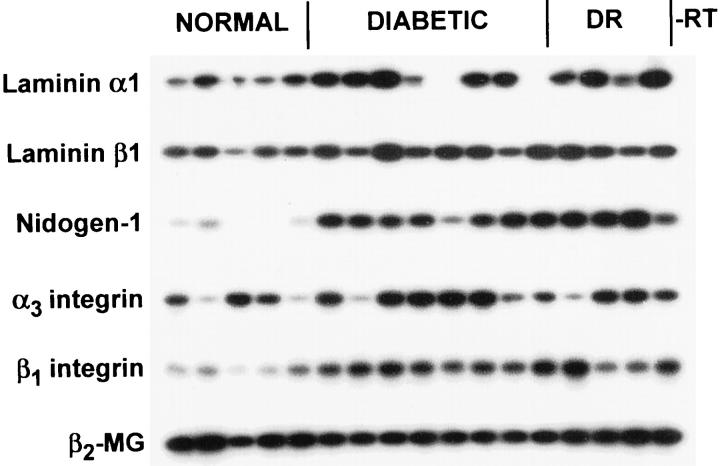
Our previous finding of decreased immunostaining of non-DR diabetic and especially DR corneas for laminins, nidogen-1/entactin, and epithelial integrin α3β1 could be due either to decreased synthesis or increased degradation. To assess changes in synthesis, gene expression levels were analyzed by semiquantitative RT-PCR. When whole corneal RNA was used, gene expression of α1 and β1 laminin chains, nidogen-1/entactin, and integrin subunits α3 and β1 was increased approximately twofold in non-DR diabetic or DR corneas (Figure 1 ▶ and Table 3 ▶ ). Because the diabetes-related BM and integrin alterations 15 likely concerned epithelial cell products, gene expression of the affected components was evaluated in separated corneal epithelium. Gene expression of α1, α5, β1 laminin chains; nidogen-1/entactin; and integrin subunits α3 and β1 did not significantly change in non-DR diabetic or DR corneal epithelium (Figure 2 ▶ and Table 3 ▶ ).
Figure 1.
RT-PCR analysis of BM and integrin gene expression in whole human corneas. Lanes 1 to 5: Normal corneas. Lanes 7 to 13: Non-DR diabetic corneas. Lanes 14 to 17: DR corneas. −RT, negative control without RT. Note increased expression of mRNAs for BM components and integrin subunits in non-DR diabetic and DR corneas. Autoradiograms of Southern blots are shown. Samples were normalized with respect to β2-MG cDNA amplification.
Table 3.
Changes in Gene Expression in Diabetic and DR Corneas Revealed by RT-PCR
| Gene | Normal | Diabetic | DR |
|---|---|---|---|
| α1 laminin | |||
| Whole cornea | 0.08 ± 0.02 | 0.20 ± 0.04* | 0.23 ± 0.06* |
| Epithelium | 0.29 ± 0.1 | 0.07 ± 0.03*‡ | 0.25 ± 0.06 |
| α5 laminin | |||
| Whole cornea | ND | ND | ND |
| Epithelium | 0.42 ± 0.08 | 0.41 ± 0.08 | 0.35 ± 0.07 |
| β1 laminin | |||
| Whole cornea | 0.10 ± 0.01 | 0.20 ± 0.02* | 0.20 ± 0.03* |
| Epithelium | 0.61 ± 0.10 | 0.80 ± 0.05 | 0.78 ± 0.08 |
| Nidogen-1/entactin | |||
| Whole cornea | 0.13 ± 0.06 | 0.45 ± 0.05* | 0.49 ± 0.11* |
| Epithelium | 0.35 ± 0.18 | 0.70 ± 0.17 | 0.70 ± 0.20 |
| α3 integrin | |||
| Whole cornea | 0.11 ± 0.02 | 0.26 ± 0.01* | 0.24 ± 0.04* |
| Epithelium | 0.41 ± 0.11 | 0.47 ± 0.07 | 0.54 ± 0.07 |
| β1 integrin | |||
| Whole cornea | 0.05 ± 0.01 | 0.14 ± 0.01* | 0.12 ± 0.02* |
| Epithelium | 0.34 ± 0.07 | 0.37 ± 0.06 | 0.43 ± 0.04 |
| MMP-2† | |||
| Stroma | 0.11 ± 0.03 | 0.56 ± 0.05* | 0.41 ± 0.08* |
| MMP-3† | |||
| Stroma | 0.09 ± 0.02 | 1.10 ± 0.45* | 1.74 ± 0.32* |
| MMP-10 | |||
| Epithelium | 0.35 ± 0.24 | 0.87 ± 0.24 | 1.28 ± 0.14* |
| Stroma | 0.07 ± 0.06 | 0.77 ± 0.15* | 0.38 ± 0.13* |
| MMP-14 | |||
| Epithelium | ND | ND | ND |
| Stroma | 1.60 ± 0.29 | 1.66 ± 0.09 | 1.99 ± 0.36 |
| TIMP-1 | |||
| Epithelium | 0.19 ± 0.07 | 0.21 ± 0.04 | 0.24 ± 0.07 |
| Stroma | 1.41 ± 0.91 | 2.03 ± 0.63 | 1.62 ± 0.10 |
| TIMP-2 | |||
| Epithelium | 1.42 ± 0.16 | 0.98 ± 0.18 | 1.27 ± 0.14 |
| Stroma | 1.73 ± 0.79 | 1.97 ± 0.71 | 1.58 ± 0.10 |
| TIMP-3 | |||
| Epithelium | 0.28 ± 0.07 | 0.32 ± 0.05 | 0.38 ± 0.03 |
| Stroma | 1.29 ± 0.26 | 0.84 ± 0.19 | 0.72 ± 0.19 |
| Cathepsin V/L2 | |||
| Epithelium | 0.66 ± 0.05 | 0.64 ± 0.06 | 0.50 ± 0.13 |
| Stroma | 0.98 ± 0.84 | 0.38 ± 0.18 | 0.12 ± 0.11 |
| u-PA | |||
| Epithelium | 0.45 ± 0.17 | 0.42 ± 0.07 | 0.52 ± 0.12 |
| Stroma | 0.40 ± 0.40 | 0.44 ± 0.14 | 0.25 ± 0.09 |
| t-PA | |||
| Epithelium | 0.66 ± 0.17 | 0.69 ± 0.08 | 0.76 ± 0.13 |
| Stroma | 0.80 ± 0.45 | 0.66 ± 0.23 | 0.60 ± 0.14 |
Each number (mean ± standard error) represents a Southern blot band intensity of a given gene’s RT-PCR product relative to the respective β2-microglobulin band intensity.
*Statistically significant when compared to normal group.
†No product was detected in the epithelium.
‡Significant decrease due to several negative samples (Fig. 1) ▶ .
u-PA, urokinase; t-PA, tissue plasminogen activator; ND, not done.
Figure 2.
RT-PCR analysis of BM and integrin gene expression in normal and diabetic corneal epithelium. Lanes 1 to 8: Normal corneas. Lanes 9 to 19: Non-DR diabetic corneas. Lanes 20 to 24: DR corneas. −RT, negative control without RT. No significant gene expression difference is observed between normal, non-DR diabetic, and DR corneas. Autoradiograms of Southern blots are shown. Samples were normalized with respect to β2-MG cDNA amplification.
Gene Expression of MMP-3 and MMP-10 Is Elevated in Diabetic But Not in Keratoconus Corneas
The results presented above suggested that the observed alterations in diabetic and DR corneas were unlikely to be caused by a decreased synthesis of affected components. Another possibility was that there was increased degradation of these components in diabetic corneas. Therefore, we have next analyzed gene and protein expression of a number of extracellular or surface proteinases in corneal epithelium and stroma including MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, and MMP-14, two plasminogen activators (urokinase and tissue plasminogen activator), and a recently described cathepsin V/L2 localized at the level of corneal epithelial BM. 21
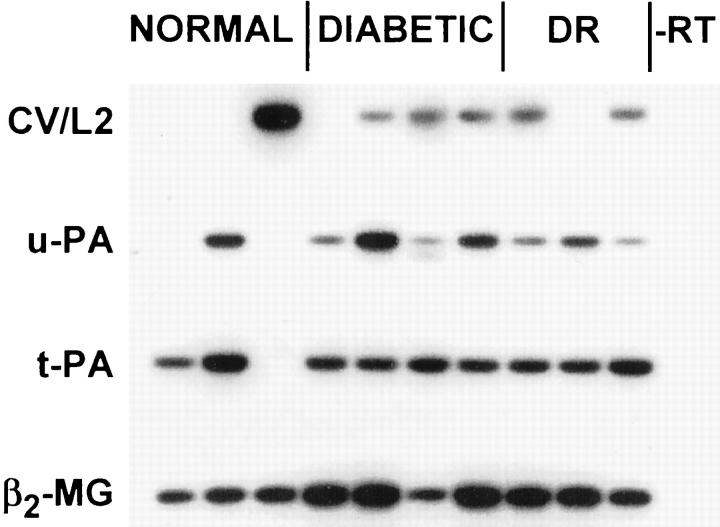
Gene expression of MMP-1, MMP-7, and MMP-9 in corneal epithelium or stroma could not be revealed by semiquantitative RT-PCR in the conditions used, with several sets of primers for each enzyme (not shown here). In the epithelium, MMP-2 and MMP-3 were not expressed and gene expression of cathepsin V/L2, urokinase, or tissue plasminogen activator did not differ in normal, non-DR diabetic, or DR corneas (Figure 3 ▶ and Table 3 ▶ ). In contrast, MMP-10 showed significantly increased gene expression in non-DR diabetic and DR corneal epithelium compared to normal (Figure 3 ▶ and Table 3 ▶ ). In the stroma, gene expression of MMP-14, cathepsin V/L2, urokinase, or tissue plasminogen activator did not differ significantly in diabetic versus normal corneas (Figures 4 and 5 ▶ ▶ and Table 3 ▶ ). However, significantly increased gene expression of MMP-2, MMP-3, and MMP-10 was noted in diabetic and DR stromal cells (Figure 4 ▶ and Table 3 ▶ ).
Figure 3.
RT-PCR analysis of proteinase gene expression in normal and diabetic corneal epithelium. Lanes 1 to 5: Normal corneas. Lanes 6 to 13: Non-DR diabetic corneas. Lanes 14 to 18: DR corneas. −RT, negative control without RT. Note increased expression of MMP-10 mRNA in non-DR diabetic and DR corneal epithelium. No change in cathepsin V/L2 (CV/L2), urokinase (u-PA), and tissue plasminogen activator (t-PA) gene expression is observed between normal, non-DR diabetic, and DR corneas. MMP-2 and MMP-3 are not expressed. Autoradiograms of Southern blots are shown. Samples were normalized with respect to β2-MG cDNA amplification.
Figure 4.
RT-PCR analysis of MMP gene expression in normal and diabetic corneal stroma. Lanes 1 to 5: Normal corneas. Lanes 6 to 10: Non-DR diabetic corneas. Lanes 11 to 15: DR corneas. −RT, negative control without RT. Note increased expression of MMP-2, MMP-3, and MMP-10 mRNA in non-DR diabetic and DR corneas with no change in MMP-14 mRNA levels. Autoradiograms of Southern blots are shown. Samples were normalized with respect to β2-MG cDNA amplification.
Figure 5.
RT-PCR analysis of proteinase gene expression in normal and diabetic corneal stroma. Lanes 1 to 3: Normal corneas. Lanes 4 to 7: Non-DR diabetic corneas. Lanes 8 to 10: DR corneas. −RT, negative control without RT. No change in cathepsin V/L2 (CV/L2), urokinase (u-PA), and tissue plasminogen activator (t-PA) gene expression is observed between normal, non-DR diabetic, and DR corneas. Autoradiograms of Southern blots are shown. Samples were normalized with respect to β2-MG cDNA amplification.
To verify that changes of MMP-3 and MMP-10 gene expression were specific for diabetic corneas, semiquantitative RT-PCR was conducted on corneas from an unrelated common corneal thinning disorder, keratoconus. In keratoconus corneas, gene expression of MMP-10 was not changed compared to age-matched normal corneas, and that of MMP-3 was significantly decreased (Figure 6) ▶ . Therefore, MMP-3 and MMP-10 gene expression seemed to be selectively elevated in diabetic corneas.
Figure 6.
RT-PCR analysis of MMP-3 and MMP-10 gene expression in normal and keratoconus human corneas. Lanes 1 to 8: Normal corneas. Lanes 9 to 19: Keratoconus corneas. −RT, negative control without RT. Note a decreased MMP-3 gene expression in keratoconus corneas and no change in MMP-10 gene expression. Autoradiograms of Southern blots are shown. Samples were normalized with respect to β2-MG cDNA amplification.
Corneas contain three natural tissue inhibitors of metalloproteinases (TIMPs) that modulate MMP activity. 22-24 Therefore, it was important to find out whether TIMP gene expression was also elevated in diabetic corneas. When gene expression of TIMP-1, TIMP-2, and TIMP-3 was analyzed in the epithelial or stromal cells, no significant changes were noted in non-DR diabetic and DR groups (Figure 7 ▶ and Table 3 ▶ ).
Figure 7.
RT-PCR analysis of TIMP gene expression in normal and diabetic corneal epithelium (left) and stroma (right). Left: Normal corneas (lanes 1 to 5); non-DR diabetic corneas (lanes 6 to 13); and DR corneas (lanes 14 to 18). Right: Normal corneas (lanes 1 to 3); non-DR diabetic corneas (lanes 4 to 7); and DR corneas (lanes 8 to 10). −RT, negative control without RT. No change in epithelial expression of all genes is seen. A slight overexpression of TIMP-2 and TIMP-3 genes in diabetic corneal stroma was not statistically significant. Autoradiograms of Southern blots are shown. Samples were normalized with respect to β2-MG cDNA amplification.
MMP-3 and MMP-10 Show Increased Immunostaining in Diabetic Corneas
Carefully pretested antibodies to various proteinases (Table 2) ▶ have been used in immunofluorescence studies of normal, diabetic, and keratoconus corneas. In agreement with our inability to detect MMP-1, MMP-7, and MMP-9 gene expression by RT-PCR, these enzymes were only seen in occasional keratocytes of 1 to 2 cases per each group of corneas (not shown here). The same was true for MMP-2 and MMP-14 despite the presence of their mRNA in the corneas. Possibly, their amounts were too low to be detected by the immunostaining method used.
Procathepsin V/L2 was revealed in the epithelial BM and limbal blood vessels. The staining intensity was similar among different groups of corneas (Table 4) ▶ . Urokinase antibodies stained the epithelium and some keratocytes. Tissue plasminogen activator was revealed in some keratocytes (often also positive for urokinase) and weakly in the epithelium. The epithelial staining for urokinase and tissue plasminogen activator remained similar in normal and diabetic corneas, but some diabetic and DR cases had more positive keratocytes than normal (Table 4) ▶ .
Table 4.
Results of Corneal Immunostaining for Various Proteinases
| Proteinase* | Increase in diabetic corneas | Increase in DR corneas |
|---|---|---|
| MMP-1, stroma | 0 /10 | 2 /10 |
| MMP-2, stroma | 1 /10 | 0 /10 |
| MMP-3, stroma | 8 /20†‡ | 13 /15†‡> |
| MMP-7, stroma | 0 /10 | 2 /10 |
| MMP-9, stroma, epithelium | 1 /10 | 2 /10 |
| MMP-10 | ||
| Stroma | 5 /10† | 8 /10† |
| Epithelium | 2 /10‡ | 8 /10†‡ |
| MMP-14, stroma | 0 /10 | 2 /10 |
| Cathepsin V/L2, EBM | 0 /10 | 0 /10 |
| u-PA | ||
| Epithelium | 1 /10 | 2 /10 |
| Stroma | 3 /10 | 4 /10 |
| t-PA | ||
| Epithelium (weak) | 1 /10 | 2 /10 |
| Stroma | 3 /10 | 5 /10 |
Number of abnormal cases to total number of cases is shown in each cell.
*Staining localization is included.
†P < 0.05 between diabetic or DR group versus normal.
‡P < 0.05 DR versus diabetic.
EBM, epithelial basement membrane; u-PA, urokinase; t-PA, tissue plasminogen activator.
MMP-3 and MMP-10 displayed little if any staining in normal and keratoconus corneas (Figure 8) ▶ . However, in non-DR diabetic corneas, specific punctate staining for MMP-3 was revealed in some keratocytes and the number of positive cells significantly increased in DR corneas (Figure 8 ▶ and Table 4 ▶ ). The epithelium was negative in accordance with RT-PCR data. The intensity of diffuse epithelial staining for MMP-10 was at the background level in normal corneas. The staining significantly increased in a minority of non-DR diabetic and in most of DR corneas (Figure 8 ▶ and Table 4 ▶ ). In normal corneas, keratocytes were mostly negative. The number of cases with positive keratocyte staining increased in non-DR diabetic and especially in DR corneas (Figure 8 ▶ and Table 4 ▶ ). These findings were in complete accordance with RT-PCR data.
Stromelysin Activity Is Increased and MMP-2 Activity Is Not Changed in Diabetic Corneas
There was a discrepancy between our RT-PCR data showing increased MMP-2 expression in diabetic corneas and our inability to detect it by immunostaining in most cases. To resolve this controversy, gelatinase activity (mostly due to MMP-2 and/or MMP-9) was assayed by zymography in detergent corneal extracts. We have used our previous extraction protocol and obtained a more soluble (detergent extract, S1) and a more insoluble (heat extract, S2) fractions. Gelatin zymography revealed one major band of lysis at ∼65 kd, consistent with the proform of MMP-2 (migration is increased in nonreducing gels 20 ) that is the major corneal MMP (Figure 9) ▶ . This band was present in all corneas and its intensity did not differ significantly between groups in either S1 or S2 fraction. In normal corneas, very weak bands in the MMP-9 size region were seen, and they were absent from diabetic corneas.
Figure 9.
Gelatin zymography of Triton X-100-soluble (S1) and heat-extractable (S2) material from normal, diabetic, and DR corneas. Lanes have been normalized for total protein. One major band with mobility corresponding to pro-MMP-2 (arrow) is seen. No significant differences can be observed in gelatinase activity between corneal groups.
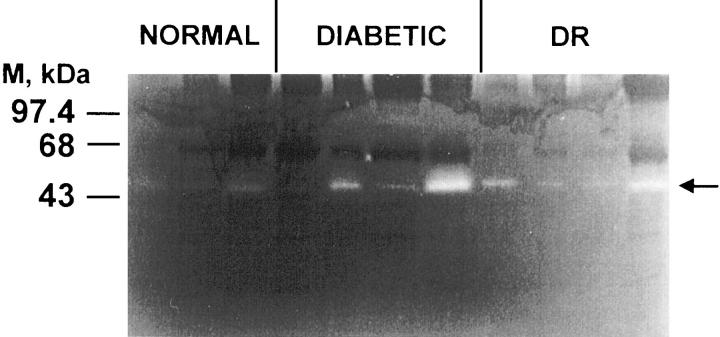
Casein zymography was used to determine stromelysin activity. Because MMP-3 and MMP-10 have similar sizes, this approach does not allow to separately assess their activity. S1 fraction did not show any caseinolytic bands (not shown here). In the minor S2 fraction, one band at ∼50 kd was observed, corresponding in size to the slower moving band of the doublet of the MMP-3 standard. Some activity was seen in normal corneas and it appeared to be increased in diabetic and DR corneas (Figure 10) ▶ . As with gelatinolytic activity, heterogeneity in the band intensity from cornea to cornea within the same group was evident.
Figure 10.
Casein zymography of heat-extractable (S2) material from normal, diabetic, and DR corneas. Lanes have been normalized for total protein. One band of activity is seen around 50 kd (arrow), with mobility corresponding to the upper band of the doublet of the MMP-3 standard. It is more pronounced in diabetic corneas.
In the presence of ethylenediaminetetraacetic acid but not phenylmethyl sulfonyl fluoride, no gelatinolytic or caseinolytic bands were revealed strongly suggesting that they represented only MMP but not serine proteinase activity (not shown here).
Increased expression of MMP-10 and MMP-3 mostly in DR corneal S2 fractions was confirmed by Western blotting although the bands were rather weak (not shown here).
Discussion
In human diabetic and especially DR corneas, we previously found a significantly decreased immunostaining of several major BM components and of an epithelial integrin α3β1. 15 These results agreed well with clinical observations of corneal epithelial abnormalities in diabetic corneas. 6 In this study, the mechanism of BM and epithelial integrin alterations in diabetic human corneas was explored. Two major possibilities were tested: 1) decreased synthesis of BM components and α3β1 integrin, and 2) increased degradation of these components by extracellular proteinases.
Laminin gene expression was previously found to be increased in diabetic mouse kidneys. 25 We also found increased gene expression of laminin chains α1, α5, β1; nidogen-1/entactin; and integrin chains in diabetic and DR human corneas. This was probably due to stromal and/or endothelial cell activity because the increase was not seen in the epithelium (Figures 1 and 2) ▶ ▶ . Although posttranscriptional changes could not be ruled out by these experiments, the data presented here suggested that the observed alterations in diabetic corneas were unlikely to be occuring because of decreased synthesis of the affected components.
To explore a possible role of proteolytic degradation in the observed BM abnormalities, gene and protein expression of various proteinases was studied. Seven MMPs known to cleave select or many BM components 24,26-29 were chosen including MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, and MMP-14 as a membrane-type MMP. We also looked at the expression of the plasminogen activator system including urokinase and tissue plasminogen activator, which is important for both BM degradation and activation of pro-MMPs. 30,31 Finally, a newly discovered cathepsin V/L2 was studied because it can degrade BM components and is localized at the corneal epithelial BM. 18,21,32
Gene expression of MMP-1, MMP-7, and MMP-9 was not revealed in any corneal group. Gene expression of MMP-14, urokinase, tissue plasminogen activator, and cathepsin V/L2 did not change in diabetic corneal epithelium and stroma. These data were essentially corroborated at the protein level. Despite increased gene expression of MMP-2 in diabetic and DR corneal stroma, immunostaining and gelatin zymography did not reveal any diabetes-associated changes in its localization or activity.
In the epithelium of diabetic and DR corneas, an increased gene expression of MMP-10 was observed. In the stroma of diabetic and DR corneas, an increased gene expression of MMP-10 and especially MMP-3, was found. Elevated gene expression of MMP-3 or MMP-10 in diabetic corneas seemed specific because it was not observed in keratoconus corneas.
Differences in gene expression of stromelysins in diabetic corneas paralleled the increase in respective protein expression in diabetic and especially DR corneas. Casein zymography of corneal extracts also revealed a band similar in size to stromelysins that appeared to be more expressed in diabetic and DR corneas. Similar results were obtained for both MMP-3 and MMP-10 by Western blotting (not shown here). Noteworthy, elevated levels of MMP-3 and MMP-10 were not accompanied by significantly increased TIMP levels, which would potentially result in net increase of proteolytic activity.
These data support the idea that BM and integrin alterations in diabetic and DR corneas may result from degradation by select proteinases activated in diabetic conditions. MMP-10 might well be one such proteinase because it was the only one that was overexpressed in diabetic corneal epithelium. The presence of collagenous Bowman’s layer in human corneas between the epithelium and the stroma makes it less likely that a stromal proteinase may be involved in epithelial BM alterations. However, it cannot be excluded completely.
MMP-3 and MMP-10 are closely related proteins and belong to a group of broad spectrum MMPs capable of degrading various ECM and BM components and activating other pro-MMPs. 33-35 Their expression is important for tissue development and remodeling, immune response, as well as for wound healing. 36-42 MMP-3 and/or MMP-10 expression is also induced or increased in various pathological conditions including cancer, osteoarthritis, rheumatoid arthritis, and chronic gastrointestinal and leg ulcers. 24,43-50 MMP-3 may play a direct role in mammary tumor development and may be considered as a natural mammary tumor promoter. 50,51
MMP-3 seems to be involved in the regulation of glomerular ECM turnover. Its expression is decreased in diabetic nephropathy and this may contribute to mesangial ECM expansion. 52 To the best of our knowledge, no studies have been conducted on MMP-10 in diabetes. This report thus provides the first demonstration of MMP-10 mRNA and protein in the cornea. It also documents MMP-10 increase in diabetic and DR corneas, which may contribute to BM alterations and subsequent epithelial abnormalities typical for these corneas.
What may be the mechanisms of increased expression of MMP-3 and MMP-10 in diabetic corneas? The first possibility concerns an abnormal activity of certain corneal growth factors and cytokines. 53 Our preliminary data show that in diabetic and DR corneas, gene expression of one such growth factor, insulin-like growth factor-I (IGF-I), is significantly increased (M. Saghizadeh and A. V. Ljubimov, unpublished data). IGF-I has been implicated in DR development both directly 54 and indirectly, by up-regulating a potent angiogenic factor, vascular endothelial growth factor. 55,56 It was recently shown that IGF-I can increase MMP-3 expression in the trabecular meshwork cells. 57 Moreover, at least MMP-3 can degrade IGF-binding proteins and in turn, modulate IGF-I activity. 58,59 However, other growth factors and cytokines known to modulate the expression of stromelysins 33,37,39 might also influence their activity in diabetic corneas.
The second possibility concerns nonenzymatic glycosylation (glycation) of ECM and BM proteins as a result of long-term diabetic hyperglycemia. Advanced glycation end products have been found in the diabetic corneas primarily in the epithelial BM. 60 Interestingly, cultured corneal epithelium showed reduced adhesion and spreading on glycated laminin-1 (α1β1γ1) that could lead to abnormal adhesion of diabetic corneal epithelium. Glycated BM type IV collagen may be turned over more slowly than normal. 61 This may result in turn, in an increase of MMP expression, which would lead to enhanced proteolysis of other BM components, eg, laminins and nidogen-1/entactin, and exacerbate diabetic epitheliopathy.
In summary, alterations of BM and epithelial integrin in diabetic human corneas could occur because of proteolytic degradation by specific proteinases, notably by MMP-10. Increased proteolysis in the corneal epithelial layer may be the molecular mechanism leading to abnormalities of epithelial cell adhesion and wound healing typical for diabetic corneas. It should be noted, however, that more direct experiments using, for example, treatment of normal, diabetic, and DR organ-cultured corneas with purified MMPs and their inhibitors, are needed to demonstrate the actual involvement of select MMPs in diabetic corneal alterations. Alternative mechanisms, such as abnormal posttranscriptional and/or posttranslational modifications of BM proteins and integrins leading to their decreased expression in diabetic corneas, should also be explored.
Note Added in Proof
When this paper was in press, the upregulation of MMP-10/stromelysin-2 mRNA and protein was described in a variety of skin wounds and ulcers, especially in chronic inflamed diabetic and venous ulcers. 62 The authors also show that MMP-10 expression in keratinocytes can be increased by several cytokines but not by cell-ECM interactions and conclude that MMP-10 is important for the normal wound healing process.
Acknowledgments
We thank Drs. Gregory Goldberg and Barry Marmor (Washington University School of Medicine, St. Louis, MO) for MMP-3 plasmid, Mina Bissell (Lawrence Berkeley National Laboratory, Berkeley, CA) for helpful suggestions, and Atul Tandon (NeoMarkers, Fremont, CA) for advice concerning the antibodies to MMPs.
Footnotes
Address reprint requests to Alexander V. Ljubimov, Ph.D., Burns and Allen Research Institute, Cedars-Sinai Medical Center, Davis-5069, 8700 Beverly Blvd., Los Angeles, CA 90048. E-mail: ljubimov@cshs.org.
Supported by Cedars-Sinai Medical Center Young Investigator Award (to A. V. L.); the Iris and B. Gerald Cantor Foundation (to M. C. K. and A. V. L.); the National Institutes of Health grant no. EY06807 (to M. C. K.), and The Discovery Fund for Eye Research.
References
- 1.Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, III, Klein R: Diabetic retinopathy. Diabetes Care 1998, 21:143-156 [DOI] [PubMed] [Google Scholar]
- 2.Herse PR: A review of manifestations of diabetes mellitus in the anterior eye and cornea. Am J Optom Physiol Opt 1988, 65:224-230 [DOI] [PubMed] [Google Scholar]
- 3.Rao GN: Dr. P. Siva Reddy oration. Diabetic keratopathy. Indian J Ophthalmol 1987, 35:16–36 [PubMed]
- 4.Saini JS, Khandalavla B: Corneal epithelial fragility in diabetes mellitus. Can J Ophthalmol 1995, 30:142-146 [PubMed] [Google Scholar]
- 5.Ohashi Y: Diabetic keratopathy (Japanese). Nippon Ganka Gakkai Zasshi 1997, 101:105-110 [PubMed] [Google Scholar]
- 6.Sánchez-Thorin JC: The cornea in diabetes mellitus. Int Ophthalmol Clin 1998, 38:19-36 [PubMed] [Google Scholar]
- 7.Van Schaik HJ, Benitez del Castillo JM, Caubergh MJ, Gobert A, Leite E, Moldow B, Rosas V, Van Best JA: Evaluation of diabetic retinopathy by fluorophotometry. European concerted action on ocular fluorometry. Int Ophthalmol 1998–1999, 22:97–104 [DOI] [PubMed]
- 8.Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK: Decreased penetration of anchoring fibrils into the diabetic stroma. A morphometric analysis. Arch Ophthalmol 1989, 107:1520-1523 [DOI] [PubMed] [Google Scholar]
- 9.Tabatabay CA, Bumbacher M, Baumgartner B, Leuenberger PM: Reduced number of hemidesmosomes in the corneal epithelium of diabetics with proliferative vitreoretinopathy. Graefe’s Arch Clin Exp Ophthalmol 1988, 226:389–392 [DOI] [PubMed]
- 10.Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK: Altered epithelial-basement membrane interactions in diabetic corneas. Arch Ophthalmol 1992, 110:537-540 [DOI] [PubMed] [Google Scholar]
- 11.Meller D, Augustin AJ, Koch FH: A modified technique of impression cytology to study the fine structure of corneal epithelium. Ophthalmic Res 1996, 28:71-79 [DOI] [PubMed] [Google Scholar]
- 12.Saini JS, Mittal S: In vivo assessment of corneal endothelial function in diabetes mellitus. Arch Ophthalmol 1996, 114:649-653 [DOI] [PubMed] [Google Scholar]
- 13.Sady C, Khosrof S, Nagaraj R: Advanced Maillard reaction and crosslinking of corneal collagen in diabetes. Biochem Biophys Res Commun 1995, 214:793-797 [DOI] [PubMed] [Google Scholar]
- 14.Weiss JS, Sang DN, Albert DM: Immunofluorescent characteristics of the diabetic cornea. Cornea 1990, 9:131-138 [PubMed] [Google Scholar]
- 15.Ljubimov AV, Huang Z, Huang GH, Burgeson RE, Gullberg D, Miner JH, Ninomiya Y, Sado Y, Kenney MC: Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J Histochem Cytochem 1998, 46:1033-1041 [DOI] [PubMed] [Google Scholar]
- 16.Ljubimov AV, Saghizadeh M, Spirin KS, Khin HL, Lewin SL, Zardi L, Bourdon MA, Kenney MC: Expression of tenascin-C splice variants in normal and bullous keratopathy human corneas. Invest Ophthalmol Vis Sci 1998, 39:1135-1142 [PubMed] [Google Scholar]
- 17.Giambernardi TA, Grant GM, Taylor GP, Hay RJ, Maher VM, McCormick JJ, Klebe RJ: Overview of matrix metalloproteinase expression in cultured human cells. Matrix Biol 1998, 16:483-496 [DOI] [PubMed] [Google Scholar]
- 18.Adachi W, Kawamoto S, Ohno I, Nishida K, Kinoshita S, Matsubara K, Okubo K: Isolation and characterization of human cathepsin V: a major proteinase in corneal epithelium. Invest Ophthalmol Vis Sci 1998, 39:1789-1796 [PubMed] [Google Scholar]
- 19.Lebeau A, Nerlich AG, Sauer U, Lichtinghagen R, Lohrs U: Tissue distribution of major matrix metalloproteinases and their transcripts in human breast carcinomas. Anticancer Res 1999, 19:4257-4264 [PubMed] [Google Scholar]
- 20.Brown D, Chwa MM, Opbroek A, Kenney MC: Keratoconus corneas: increased gelatinolytic activity appears after modification of inhibitors. Curr Eye Res 1993, 12:571-581 [DOI] [PubMed] [Google Scholar]
- 21.Adachi W, Kawasaki S, Kinoshita S, Okubo K: Localization of cathepsin V protein in normal cornea. ARVO Abstracts. Invest Ophthalmol Vis Sci 1999, 40:S392 [Google Scholar]
- 22.Fini ME, Cook JR, Mohan R, Brinckerhoff CE: Regulation of MMP gene expression. Parks WC Mecham RP eds. Matrix Metalloproteinases. 1998, :pp 299-356 Academic Press, New York [Google Scholar]
- 23.Kenney MC, Chwa M, Alba A, Saghizadeh M, Huang ZS, Brown DJ: Localization of TIMP-1, TIMP-2, TIMP-3, gelatinase A and gelatinase B in pathological human corneas. Curr Eye Res 1998, 17:238-246 [DOI] [PubMed] [Google Scholar]
- 24.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM: Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol 2000, 18:1135-1149 [DOI] [PubMed] [Google Scholar]
- 25.Yang CW, Hattori M, Vlassara H, He CJ, Carome MA, Yamato E, Elliot S, Striker GE, Striker LJ: Overexpression of transforming growth factor-β 1 mRNA is associated with up-regulation of glomerular tenascin and laminin gene expression in nonobese diabetic mice. J Am Soc Nephrol 1995, 5:1610-1617 [DOI] [PubMed] [Google Scholar]
- 26.Durko M, Navab R, Shibata HR, Brodt P: Suppression of basement membrane type IV collagen degradation and cell invasion in human melanoma cells expressing an antisense RNA for MMP-1. Biochim Biophys Acta 1997, 1356:271-280 [DOI] [PubMed] [Google Scholar]
- 27.Wojtowicz-Praga SM, Dickson RB, Hawkins MJ: Matrix metalloproteinase inhibitors. Invest New Drugs 1997, 15:61-75 [DOI] [PubMed] [Google Scholar]
- 28.Nagase H, Woessner F, Jr: Matrix metalloproteinases. J Biol Chem 1999, 274:21491-21494 [DOI] [PubMed] [Google Scholar]
- 29.Seiki M: Membrane-type matrix metalloproteinases. APMIS 1999, 107:137-143 [DOI] [PubMed] [Google Scholar]
- 30.Haeckel C, Krueger S, Roessner A: Antisense inhibition of urokinase: effect on malignancy in a human osteosarcoma cell line. Int J Cancer 1998, 77:153-160 [DOI] [PubMed] [Google Scholar]
- 31.Murphy G, Stanton H, Cowell S, Butler G, Knaüper V, Atkinson S, Gavrilovic J: Mechanisms for pro matrix metalloproteinase activation. APMIS 1999, 107:38-44 [DOI] [PubMed] [Google Scholar]
- 32.Guinec N, Dalet-Fumeron V, Pagano M: “In vitro” study of basement membrane degradation by the cysteine proteinases, cathepsins B, B-like and L. Digestion of collagen IV, laminin, fibronectin, and release of gelatinase activities from basement membrane fibronectin. Biol Chem Hoppe Seyler 1993, 374:1135-1146 [DOI] [PubMed] [Google Scholar]
- 33.Windsor LJ, Grenett H, Birkedal-Hansen B, Bodden MK, Engler JA, Birkedal-Hansen H: Cell type-specific regulation of SL-1 and SL-2 genes. Induction of the SL-2 gene but not the SL-1 gene by human keratinocytes in response to cytokines and phorbolesters. J Biol Chem 1993, 268:17341-17347 [PubMed] [Google Scholar]
- 34.Nagase H: Human stromelysins 1 and 2. Methods Enzymol 1995, 248:449-470 [DOI] [PubMed] [Google Scholar]
- 35.Murphy G, Cockett MI, Ward RV, Docherty AJ: Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J 1991, 277:277-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girard MT, Matsubara M, Kublin C, Tessier MJ, Cintron C, Fini ME: Stromal fibroblasts synthesize collagenase and stromelysin during long-term tissue remodeling. J Cell Sci 1993, 104:1001-1011 [DOI] [PubMed] [Google Scholar]
- 37.López-Barahona M, Fialka I, González-Sancho JM, Asunción M, González M, Iglesias T, Bernal J, Beug H, Muñoz A: Thyroid hormone regulates stromelysin expression, protease secretion and the morphogenetic potential of normal polarized mammary epithelial cells. EMBO J 1995, 14:1145-1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bord S, Horner A, Hembry RM, Compston JE: Stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) expression in developing human bone: potential roles in skeletal development. Bone 1998, 23:7-12 [DOI] [PubMed] [Google Scholar]
- 39.Madlener M, Mauch C, Conca W, Brauchle M, Parks WC, Werner S: Regulation of the expression of stromelysin-2 by growth factors in keratinocytes: implications for normal and impaired wound healing. Biochem J 1996, 320:659-664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madlener M: Differential expression of matrix metalloproteinases and their physiological inhibitors in acute murine skin wounds. Arch Dermatol Res Suppl 1998, 290:S24-S29 [DOI] [PubMed] [Google Scholar]
- 41.Lu PC, Ye H, Maeda M, Azar DT: Immunolocalization and gene expression of matrilysin during corneal wound healing. Invest Ophthalmol Vis Sci 1999, 40:20-27 [PubMed] [Google Scholar]
- 42.Wang M, Qin X, Mudgett JS, Ferguson TA, Senior RM, Welgus HG: Matrix metalloproteinase deficiencies affect contact hypersensitivity: stromelysin-1 deficiency prevents the response and gelatinase B deficiency prolongs the response. Proc Natl Acad Sci USA 1999, 96:6885-6889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sreenath T, Matrisian L, Stetler-Stevenson W, Gattoni-Celli S, Pozzatti RO: Expression of matrix metalloproteinase genes in transformed rat cell lines of high and low metastatic potential. Cancer Res 1992, 52:4942-4947 [PubMed] [Google Scholar]
- 44.Brinckerhoff CE, Sirum-Connolly KL, Karmilowicz MJ, Auble DT: Expression of stromelysin and stromelysin-2 in rabbit and human fibroblasts. Matrix Suppl 1992, 1:165-175 [PubMed] [Google Scholar]
- 45.Hembry RM, Bagga MR, Reynolds JJ, Hamblen DL: Immunolocalisation studies of six matrix metalloproteinases and their inhibitors, TIMP-1 and TIMP-2, in synovia from patients with osteo- and rheumatoid arthritis. Ann Rheum Dis 1995, 54:25-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saarialho-Kere UK: Patterns of matrix metalloproteinase and TIMP expression in chronic ulcers. Arch Dermatol Res Suppl 1998, 290:S47-S54 [DOI] [PubMed] [Google Scholar]
- 47.Thomasset N, Lochter A, Sympson CJ, Lund LR, Williams DR, Behrendtsen, Werb Z, Bissell MJ: Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. Am J Pathol 1998, 153:457–467 [DOI] [PMC free article] [PubMed]
- 48.Keyszer G, Lambiri I, Nagel R, Keysser C, Keysser M, Gromnica-Ihle E, Franz J, Burmester GR, Jung K: Circulating levels of matrix metalloproteinases MMP-3 and MMP-1, tissue inhibitor of metalloproteinases 1 (TIMP-1) and MMP-1/TIMP-1 complex in rheumatic disease. Correlation with clinical activity of rheumatoid arthritis versus other surrogate markers. J Rheumatol 1999, 26:251-258 [PubMed] [Google Scholar]
- 49.Leff RL: Clinical trials of a stromelysin inhibitor. Osteoarthritis, matrix metalloproteinase inhibition, cartilage loss, surrogate markers, and clinical implications. Ann NY Acad Sci 1999, 878:201-207 [DOI] [PubMed] [Google Scholar]
- 50.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier J-P, Gray JW, Pinkel D, Bissell MJ, Werb Z: The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 1999, 98:137-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sternlicht MD, Bissell MJ, Werb Z: The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene 2000, 19:1102-1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki D, Miyazaki M, Jinde K, Koji T, Yagame M, Endoh M, Nomoto Y, Sakai H: In situ hybridization studies of matrix metalloproteinase-3, tissue inhibitor of metalloproteinase-1 and type IV collagen in diabetic nephropathy. Kidney Int 1997, 52:111-119 [DOI] [PubMed] [Google Scholar]
- 53.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S: Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Ret Eye Res 2000, 19:113-129 [DOI] [PubMed] [Google Scholar]
- 54.Grant MB: IGF-I in diabetic vascular complications. Curr Opin Endocrinol Diabetes 1996, 34:335-345 [Google Scholar]
- 55.Lu M, Amano S, Miyamoto K, Garland R, Keough K, Qin W, Adamis AP: Insulin-induced vascular endothelial growth factor expression in retina. Invest Ophthalmol Vis Sci 1999, 40:3281-3286 [PubMed] [Google Scholar]
- 56.Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, Robinson G, Driver S, Bischoff J, Zhang B, Schaeffer JM, Senger DR: Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med 1999, 5:1390-1395 [DOI] [PubMed] [Google Scholar]
- 57.Alexander JP, Samples JR, Acott TS: Growth factor and cytokine modulation of trabecular meshwork matrix metalloproteinase and TIMP expression. Curr Eye Res 1998, 17:276-285 [DOI] [PubMed] [Google Scholar]
- 58.Wirtz MK, Xu H, Rust K, Alexander JP, Acott TS: Insulin-like growth factor binding protein-5 expression by human trabecular meshwork. Invest Ophthalmol Vis Sci 1998, 39:45-53 [PubMed] [Google Scholar]
- 59.Fowlkes JL, Serra DM, Nagase H, Thrailkill KM: MMPs are IGFBP-degrading proteinases: implications for cell proliferation and tissue growth. Ann NY Acad Sci 1999, 878:696-699 [DOI] [PubMed] [Google Scholar]
- 60.Kaji Y, Usui T, Oshika T, Matsubara M, Yamashita H, Araie M, Murata T, Ishibashi T, Nagai R, Horiuchi S, Amano S: Advanced glycation end products in diabetic corneas. Invest Ophthalmol Vis Sci 2000, 41:362-368 [PubMed] [Google Scholar]
- 61.Mott JD, Khalifah RG, Nagase H, Shield CF, III, Hudson JK, Hudson BG: Nonenzymatic glycation of type IV collagen and matrix metalloproteinase susceptibility. Kidney Int 1997, 52:1302-1312 [DOI] [PubMed] [Google Scholar]
- 62.Rechardt O, Elomaa O, Vaalamo M, Paakkonen K, Jahkola T, Hook-Nikanne J, Hembry RM, Hakkinen L, Kere J, Saarialho-Kere U: Stromelysin-2 is upregulated during normal would repair and is induced by cytokines. J Invest Dermatol 2000, 115:778-787 [DOI] [PubMed] [Google Scholar]