Abstract
Cancer with high levels of microsatellite instability (MSI-H) is the hallmark of hereditary nonpolyposis colorectal cancer syndrome, and MSI-H occurs in ∼15% of sporadic colorectal carcinomas that have improved prognosis. We examined the utility of histopathology for the identification of MSI-H cancers by evaluating the features of 323 sporadic carcinomas using specified criteria and comparing the results to MSI-H status. Coded hematoxylin and eosin sections were evaluated for tumor features (signet ring cells; mucinous histology; cribriforming, poor differentiation, and medullary-type pattern; sponge-like mucinous growth; pushing invasive margin) and features of host immune response (Crohn’s-like lymphoid reaction, intratumoral lymphocytic infiltrate, and intraepithelial T cells by immunohistochemistry for CD3 with morphometry). Interobserver variation among five pathologists was determined. Subjective interpretation of histopathology as an indication for MSI testing was recorded. We found that medullary carcinoma, intraepithelial lymphocytosis, and poor differentiation were the best discriminators between MSI-H and microsatellite-stable cancers (odds ratio: 37.8, 9.8, and 4.0, respectively; P = 0.000003 to <0.000001) with high specificity (99 to 87%). The sensitivities, however, were very low (14 to 38%), and interobserver agreement was good only for evaluation of poor differentiation (kappa, 0.69). Mucinous histopathological type and presence of signet ring cells had low odds ratios of 3.3 and 2.7 (P = 0.005 and P = 0.02) with specificities of 95% but sensitivities of only 15 and 13%. Subjective interpretation of the overall histopathology as suggesting MSI-H performed better than any individual feature; the odds ratio was 7.5 (P < 0.000001) with sensitivity of 49%, specificity of 89%, and moderate interobserver agreement (kappa, 0.52). Forty intraepithelial CD3-positive lymphocytes/0.94 mm2, as established by receiver operating characteristic curve analysis, resulted in an odds ratio of 6.0 (P < 0.000001) with sensitivity of 75% and specificity of 67%. Our findings indicate that histopathological evaluation can be used to prioritize sporadic colon cancers for MSI studies, but morphological prediction of MSI-H has low sensitivity, requiring molecular analysis for therapeutic decisions.
Molecular studies have identified 10 to 15% of colorectal cancers in which a primary genetic abnormality is defective DNA nucleotide mismatch repair. This abnormality results in extensive instability in repeated nucleotide sequences called microsatellites that is termed microsatellite instability (MSI; also termed DNA replication errors, RER, or ubiquitous somatic mutation). 1-3 MSI 2,4-7 is caused by inactivation of one of a group of genes responsible for nucleotide mismatch repair, including hMSH2, hMLH1, PMS1, PMS2, hMSH6/GTBP, and hMSH3. 8-14 Hereditary nonpolyposis colorectal cancer syndrome (HNPCC) is usually the result of a germline mutation in hMSH2 or hMLH1. 9-11,13-16 High levels of MSI (MSI-H) resulting from somatic inactivation of the other wild-type allele are the molecular hallmark of cancers in patients with HNPCC. Sporadic MSI-H colon cancers 2,17-20 are much more common than colon cancers occurring in HNPCC, which accounts for only 3 to 5% of all colorectal carcinomas. Sporadic MSI-H tumors usually occur because of transcriptional silencing by methylation of the promoter of the hMLH1 gene. 21,22 Calculations based on the frequency of MSI-H among colorectal cancers and the annual incidence suggest that ∼20,000 to 26,000 MSI-H colorectal cancers occur each year in the United States.
A workshop sponsored by the National Cancer Institute developed criteria for distinction of MSI colorectal cancers from the much more common microsatellite-stable (MSS) type and for classification of MSI into high and low levels (MSI-H and MSI-L, respectively). 19 Microsatellite alterations that have accumulated in the clonal tumor cell population are identified by comparison of polymerase chain reaction amplification products from tumor DNA and non-neoplastic DNA (adjacent tissue or peripheral blood). 19,23 Alternatively, tumor DNA alone can be studied for changes in lengths of microsatellites that rarely show polymorphism, such as the 10-bp polyadenine repeat within the transforming growth factor-β1-type II receptor gene (TGF-β1-RII) or BAT-26 within an intron of hMSH2. 24-28 Mutation in the TGF-β1-RII gene is present in the vast majority of MSI-H colorectal cancers. 27,29,30
Identification of MSI-H cancers has clinical implications for recognition of HNPCC and for management and prognosis of patients with colorectal carcinoma. Clinicopathological criteria have been developed to identify those patients for whom MSI analysis should be performed to increase recognition of HNPCC probands. 31 These “Bethesda criteria” include familial, individual, and tumor characteristics. 32 Emerging issues such as segmental versus total colectomy as primary surgery for HNPCC patients with cancer require timely prediction of the molecular subtype for patient management. 33 In addition, patients with inherited or sporadic MSI-H colon cancers have inherently better stage-specific survival after surgical and adjuvant therapies. 2,7,18,34-43
Molecular analysis of tumors for MSI is currently time-consuming and expensive to perform, presenting obstacles to thorough molecular study of all colon cancers before decisions about therapy. HNPCC cohort analysis suggests a preponderance of poorly differentiated and mucinous colonic carcinomas, 15 which subsequent studies of sporadic MSI-H colon cancer have reiterated. 18,44 Pronounced features of host-tumor interaction, such as lymphoid reaction, 17,45 either Crohn’s-like with lymphoid nodules including germinal centers 46,47 or more diffuse with increased intraepithelial lymphocytes, 48 have also been described. If recognizable morphology is characteristic of MSI-H colon cancer, 20,49 histopathology could be used to identify cases. However, the clinical applicability of these morphological features has not been studied in detail. We therefore applied histopathological criteria to a cohort of sporadic colon cancer that had been subjected previously to comprehensive MSI analysis, to test the ability of histopathology to select for MSI-H colon carcinoma.
Materials and Methods
Tumor Specimens
The tumors were derived from a previous study of prognostic/predictive markers, reported in detail elsewhere, in an Eastern Cooperative Oncology Group cohort. 50 The 520 coded specimens were stage II or stage III sporadic colon carcinomas from patients initially accrued to two clinical trials of postoperative adjuvant chemotherapy. E2284 (INT 0035) compared postoperative 5-fluorouracil (5-FU)/levamisole with postoperative levamisole or surgery alone in resectable Dukes’ B or C adenocarcinoma of the colon; E2288 (INT 0089) compared low-dose leucovorin/5-FU, high-dose leucovorin/5-FU, levamisole/5-FU, and low-dose leucovorin/levamisole/5-FU after curative resection of Dukes’ B or C colon cancer. One formalin-fixed paraffin-embedded block of tumor from the pre-adjuvant resection specimen of each patient was obtained through the Eastern Cooperative Oncology Group Pathology Coordinating Office at Evanston Hospital in Evanston, IL, with the approval of the Coordinating Center of the Eastern Cooperative Oncology Group in Brookline, MA. Specimens remained coded for the entire molecular and histopathological evaluation.
Molecular Analysis for MSI Status
Laboratory analysis of molecular markers in the coded specimens was done previously in the Division of Gastrointestinal-Liver Pathology of The Johns Hopkins University School of Medicine, Baltimore, MD, as reported in detail elsewhere. 50 Eight dinucleotide repeats (D18S55, D18S58, D18S61, D18S64, D18S69, D17S1176, D17S520, and TP53) and two mononucleotide repeats in polyadenine tracts of TGF-β1-RII or BAT-26 were amplified by polymerase chain reaction. Three hundred six cases had MSI status determined by both dinucleotide and mononucleotide markers, of which 22% (67 of 306) were MSI-H, based on shifts of allele size in at least two markers representing 30% or more of evaluable markers. 19,50 The number of shifted markers ranged from 2 to 10 with an average of 5.5 shifted markers. Cases with a shift in only one dinucleotide marker representing <30% of evaluable markers were classified as MSI-L and were included with MSS tumors for analysis, because of the reported similarity between cases with low levels of MSI and MSS 17,18,51 and the small number of MSI-L cases in our series that precluded meaningful subset analysis. Our molecular data showed that all tumors with TGF-β1-RII or BAT-26 mutations also had shifts in dinucleotide repeats. Therefore, the remaining 214 cases for which control genomic DNA was not retrievable by microdissection were amplified for TGF-β1-RII and BAT-26 mutations, revealing an additional 31 cases classifiable as MSI-H. 27-30 The remainder of the cases (n = 183) were classified as indeterminate for MSI status and excluded. Of the 98 MSI-H cancers, 92 were available for histopathological evaluation, and 231 of the 239 MSS tumors were available as morphological controls, producing a study group total of 323 cases with prevalence of MSI-H of 28% (92 of 323). The mean age of the MSI-H group was 61 years with a median of 63 years; 45% were women. The MSS cases had a mean age of 64 years and median of 65 years; 40% were women. The differences in age and gender were not statistically significant. No attempt was made to distinguish HNPCC and sporadic MSI-H cases.
Histopathological Features
Coded hematoxylin and eosin (H&E) slides (n = 323), one representative of each case, were examined individually by five pathologists without access to clinical data. Each section was cut from the same block used for DNA retrieval in the previous molecular study. 50 Histopathological evaluation of tumor features and host response was performed using the following criteria.
Tumor Features
Signet Ring Cells: The presence of tumor cells with an intracytoplasmic mucin-filled vacuole causing lateral compression of the nucleus, whether within extracellular mucin lakes or infiltrating directly in stroma.
Mucinous (Colloid) Histology: Extracellular mucin accumulation bounded either by neoplastic epithelium or by host stroma. Semiquantitative assessment characterized two subgroups, >50% of tumor area involved and 10 to 50% of tumor area involved. 52
Cribriforming Growth Pattern: Invasive tumor epithelial islands showing punched-out, gland-like spaces with sharp margins, rounded or ovoid in shape. Tumors were semiquantitatively subgrouped into >50% involvement of the tumor area or 10 to 50% involvement.
Poor Differentiation: Solid or sheet-like epithelial growth in >70% of the tumor area, with <30% of tumor showing gland formation. 2 This feature was assessed away from the invasive edge.
Medullary Pattern (Figure 1) ▶ : Rare subtype of poor differentiation consisting of nests, trabecula, and sheets of small- to medium-sized cells with scant to abundant eosinophilic cytoplasm, 53,54 frequent mitotic figures and a distinct stromal population of small lymphocytes. Two subgroups were defined, >70% of area 55 and 10 to 70% of area.
Figure 1.

Histopathology of medullary carcinoma of the colorectum, a tumor-type characteristic of MSI-H. The malignant cells are large with abundant pink cytoplasm and vesicular nuclei with prominent nucleoli. Numerous lymphocytes are evident in the malignant epithelium.
Mixed Growth Patterns: Distinct and different growth patterns as described above, identified adjacent to each other in the same histological section. For example, mucinous and medullary pattern with poor differentiation produced a mosaic appearance in some carcinomas, with no dominant growth pattern.
Sponge-Like Architecture: Finger-like extensions of mucin extending radially from the tumor, in association with strips of neoplastic epithelium, producing a net-like or sponge-like low-power architecture.
Pushing Margin: The presence of an expansile appearance to the advancing tumor margin when viewed at low power. This characteristic was only assessable if the slide included the infiltrating edge of the tumor and adjacent stroma.
Extensive Necrosis: More than 50% of the tumor area occupied by necrotic material. Although the presence of ‘dirty’ necrosis is a common finding in colorectal carcinomas, widespread necrosis was the feature sought.
Host Immune Response Features
Crohn’s-Like Peritumoral Reaction: Pronounced lymphoid reaction to the tumor, composed of lymphoid follicles with germinal centers at the tumor edge, not associated with either mucosa (eg, diverticular origin) or pre-existing lymph node. Two or more large lymphoid aggregates in a section were required for the presence of this feature. 46,47
Intratumoral Lymphocytic Infiltrate (Figure 1) ▶ : The presence of small round lymphocytes within the tumor epithelium using H&E staining, often seen in association with a peritumoral, stromal lymphocytic, or inflammatory infiltrate.
Quantitation of Intratumoral T cell Infiltrate (Figure 2) ▶ : An available section from 169 cases (52 MSI-H, 117 MSS) was stained immunohistochemically using anti-CD3 pan-T cell antibody (DAKO, Carpinteria, CA), with diaminobenzidine as the chromogen in the TechMate 1000 automated system (Ventana, BioTek Solutions, Tucson, AZ). The number of T cells within the tumor epithelium was assessed by counting within the area of five high-power fields (Olympus D-Plan ×40 objective, total area of five fields 0.94 mm2).
Figure 2.
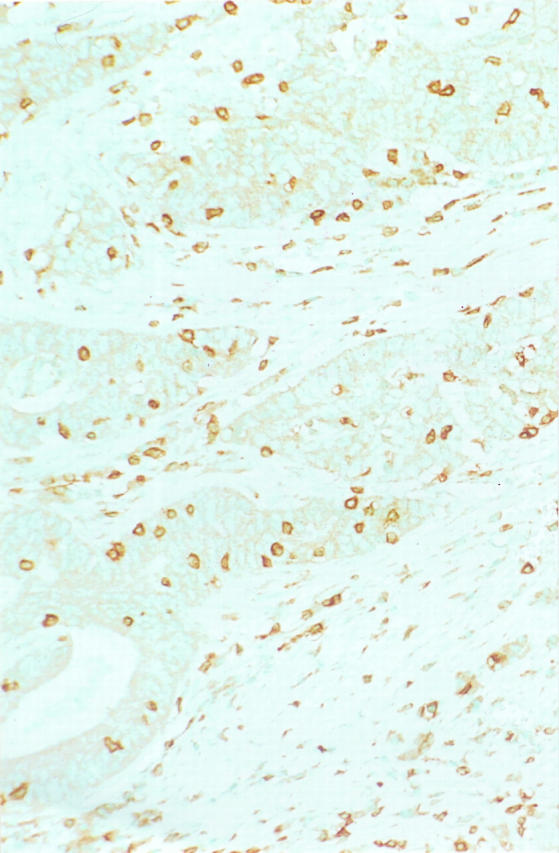
Immunohistochemistry for CD3 pan-T cell marker with methyl green counterstain. Numerous T lymphocytes are present in the neoplastic epithelium of this MSI-H colon carcinoma as well as in the surrounding nonneoplastic stroma. (Same specimen as Figure 1 ▶ .)
Study of Interobserver Variation
One set of slides was circulated among the five pathologists. Checklist case-sheets with definitions for each feature were provided. Each observer was also asked to indicate whether, based on the histopathological features identified, each case should be tested for MSI (MSI test request).
In light of interobserver variation, final classification of the histopathological features present in each slide was based on a weighted consensus score, with two points assigned for each of two pathologists experienced with MSI-H tumors and one point for the other three pathologists. A feature was classified as present if the score was ≥4. All data were entered on to True Epistat statistical software (Richardson, TX) and analyzed for agreement using the kappa statistic. Fisher’s exact test for a two-tailed P value for differences in histologies, odds ratio with 95% confidence limits, sensitivity, specificity, and Mann-Whitney U test for skewed distribution with reference to the molecular subgroups were used. A receiver operating characteristic curve 56 was derived for the continuous variable of intraepithelial T lymphocyte counts as compared with dichotomous MSI-H/MSS molecular status.
Results
The results are tabulated in Table 1 ▶ .
Table 1.
Histopathological Associations with MSI Status
| Histopathology (n=323) | MSI-H (n = 92), n | Prevalence & sensitivity for MSI-H, % | MSS (n = 231), n | Prevalence in MSS, % | Specificity for MSI-H, % | Exact P value | Odds ratio for MSI-H | 95% Confidence limits | Kappa statistic |
|---|---|---|---|---|---|---|---|---|---|
| Signet ring cells | 12 | 13 | 12 | 5 | 95 | 0.02 | 2.7 | 1.1, 6.8 | 0.66 |
| Mucinous carcinoma | 14 | 15 | 12 | 5 | 95 | 0.005 | 3.3 | 1.4, 7.9 | — |
| Mucinous type >10% | 20 | 22 | 16 | 7 | 93 | 0.0003 | 3.7 | 1.7, 8.0 | 0.54 |
| Cribriforming pattern | 12 | 13 | 65 | 28 | 72 | 0.004 | 0.4 | 0.2, 0.8 | 0.36 |
| Poor differentiation | 35 | 38 | 31 | 13 | 87 | 0.000003 | 4.0 | 2.2, 7.3 | 0.69 |
| Medullary-type carcinoma | 13 | 14 | 1 | 0.4 | 99 | <.000001 | 37.8 | 5.0, 139.9 | — |
| Medullary type >10% | 23 | 25 | 6 | 3 | 97 | <.000001 | 12.5 | 4.6, 35.9 | 0.32 |
| Mixed patterns | 6 | 6 | 9 | 4 | 96 | 0.38 | 1.7 | 0.5, 5.5 | 0.20 |
| Sponge-like pattern | 6 | 6 | 7 | 3 | 97 | 0.11 | 2.6 | 0.7, 9.5 | 0.29 |
| Lymphocytosis | 19 | 21 | 7 | 3 | 97 | <.000001 | 9.8 | 3.5, 28.5 | 0.27 |
| No. of slides showing invasive margin | 68 | 168 | |||||||
| Pushing margin | 10 | 15 | 13 | 8 | 92 | 0.14 | 2.1 | 0.8, 5.3 | — |
| No. of slides with peripheral stroma | 71 | 133 | |||||||
| Crohn’s-like reaction in stroma | 35 | 49 | 48 | 36 | 64 | 0.07 | 1.7 | 0.9, 3.2 | 0.45 |
| MSI test request by a majority of observers | 45 | 49 | 26 | 11 | 89 | <.000001 | 7.5 | 4.1, 14.0 | 0.52 |
Tumor Features
Signet ring cells were reproducibly identified by the five observers (kappa value, 0.66), but were found in only 13% of MSI-H (12 of 92) and 5% of MSS (12 of 231) tumors (P = 0.02; odds ratio, 2.7). Furthermore, only three cases contained signet ring cells in the absence of mucinous differentiation, and all three were MSS cancers.
Mucinous carcinoma with >50% of the tumor having mucinous histology was identified in 15% of the MSI-H tumors (14 of 92) and 5% of MSS tumors (12 of 231; P = 0.005; odds ratio, 3.3). Mucinous histology involving >10% of the tumor was moderately reproducible (kappa value, 0.54). There was a highly significant difference in the frequency of mucinous component between the MSI-H group and the MSS group (P = 0.0003; odd ratio, 3.7), but sensitivity for identification of MSI-H cases was low (22%).
Focal cribriforming growth pattern was common (24%, 77 of 323), although it was rarely dominant (1%, five of 323). Identification was poorly reproducible (kappa value, 0.36), and this feature was more frequent in MSS than MSI-H cases (P = 0.004).
The reproducibility for identification of poor differentiation was good (kappa value, 0.69) and was the highest among the histopathological characteristics studied. Poorly differentiated cancers in the cohort (20%, 66 of 323) included the medullary-type cancers as a subgroup and were associated strongly with MSI-H (38% of MSI-H cases compared to 13% of MSS cases; P = 0.000003; odds ratio, 4.0). Medullary-type carcinoma showed high specificity for MSI-H status (99%; P < 0.000001; odds ratio, 37.8), but the low frequency of this tumor type (prevalence of only 4%, 14 of 323) resulted in low sensitivity of only 14%. Areas of medullary-type, poorly differentiated growth were seen in 29 cases, including those categorized as medullary type (25% of MSI-H tumors and 3% of MSS tumors; P < 0.000001; odds ratio, 12.5). Reproducibility for this feature was low (kappa value, 0.32).
Mixed or variegated histopathological patterns were recognized in only 15 tumors, without a significant difference between molecular subtypes. Sponge-like architecture was also uncommon, identified in only six tumors from each molecular group. Pushing margin was assessable in 236 tumors; 15% (10 of 68) of MSI-H tumors showed a pushing margin as compared to 8% (13 of 168) of MSS tumors, but the difference was not statistically significant (P = 0.14). Each of these features was poorly reproducible and infrequently applied by observers.
Host Response Features
A conspicuous Crohn’s-like reaction was common, with most cases including some lymphoid aggregates but many lacking germinal centers. Peripheral stroma was present for evaluation in 204 sections. Among assessable MSI-H cases with peripheral stroma, 49% (35 of 71) showed a Crohn’s-like reaction, versus 36% (48 of 133) of assessable MSS cases (P = 0.07; odds ratio, 1.7). Interobserver agreement was moderate (kappa value, 0.45).
Intraepithelial lymphocytosis by H&E stain was variable, being marked in 21% (19 of 92) of MSI-H tumors but in only 3% (six of 231) of MSS tumors (P < 0.000001; odds ratio, 9.8). However, this feature showed one of the lowest reproducibility statistics among the five observers (kappa value, 0.27).
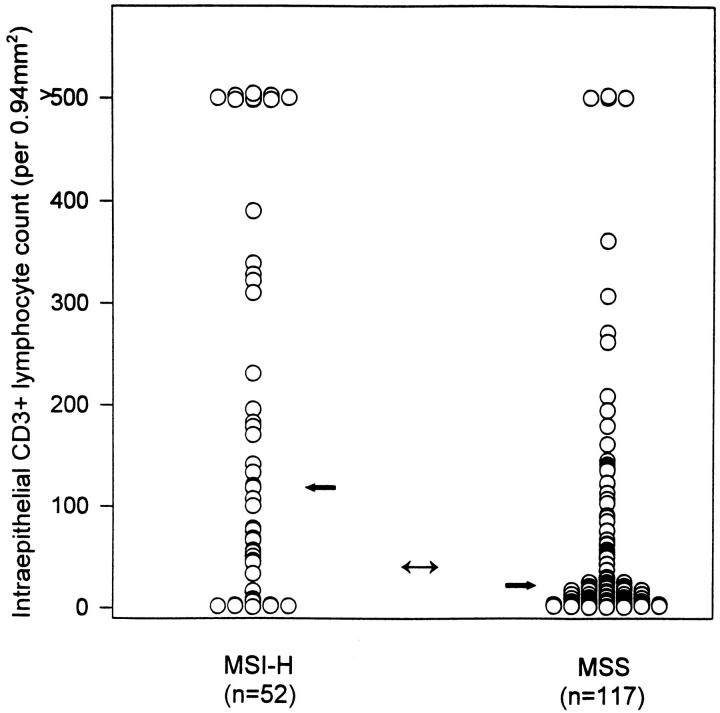
With intraepithelial lymphocyte morphometry using the CD3 immunohistochemical marker for T cells applied to 166 cases, the median count of T cells/0.94 mm 2 for MSI-H cases was 120 and for MSS cases 18 T cells/0.94 mm2, with overall median of 26 T cells/0.94 mm 2 (Figure 3) ▶ . The distributions of lymphocyte counts for the MSI-H and MSS tumors were skewed and significantly different (Mann-Whitney U test, one-tailed, P = 0.000001). A receiver operating characteristic curve (Figure 4) ▶ was used to examine the ability of the CD3+ lymphocyte count to predict the molecular classification of cancer as MSI-H or MSS. A cut-point of 40 T cells/0.94 mm 2 was found and was between the medians of the distributions for the molecular subtypes. Seventy-five percent of MSI-H tumors had counts >40 T cells/0.94 mm 2 versus 33% of MSS tumors (P < 0.000001; odds ratio, 6.0). The sensitivity of >40 CD3+ cells per 0.94 mm 2 to select MSI-H tumors was 75% with specificity of 67%.
Figure 3.
Scattergram of tumor-infiltrating T cell counts for MSI-H and microsatellite-stable colon carcinomas. The median (single-headed arrow) for the MSI-H cancers was 120 T cells/0.94 mm2, and the median for the microsatellite-stable cancers was 18 T cells/0.94 mm2. An intermediate cut-point (double-headed arrow) of 40 T cells/0.94 mm 2 was selected using a receiver operating characteristic curve (see Figure 4 ▶ ).
Figure 4.
Receiver operating characteristic curve for tumor-infiltrating T cell counts. The curve was constructed to evaluate the continuous variable in separating MSI-H from MSS colonic carcinomas. As the area under the curve is 0.73 (>0.5), the T cell count shows sensitivity in discriminating MSI-H colon cancer, and with a cut-point count of 40 T cells/0.94 mm2, this sensitivity was 75% with specificity of 67%.
Combinations of Features
Histopathological features that discriminated between MSI-H tumors and MSS tumors were used in combination to attempt to increase the sensitivity of identification of MSI-H while maintaining specificity. Use of marked intraepithelial lymphocytosis in the H&E-stained section, followed by use of areas of mucinous histology in the remaining cases, was both sensitive (74%) and specific (83%) at discriminating MSI-H tumors from MSS tumors. Analysis of the possible combinations suggested that the major determinant for identifying the MSI-H group from the cohort of sporadic colon carcinomas was tumor intraepithelial lymphocytosis, but this feature would consistently fail to permit recognition of the 10% of MSI-H tumors in which a marked lymphocytic infiltrate was absent on H&E staining.
Subjective interpretation of the overall histopathological appearance as indicating the need for molecular analysis (MSI test result request in Table 1 ▶ ) performed better than any single H&E feature at discrimination of MSI-H cancers when used between observers (P < 0.000001; odds ratio, 7.5). However, this assessment selected only 49% (45 of 92) of MSI-H tumors, with moderate interobserver agreement (kappa value, 0.52). Of note, no subjective test request by any observer was entered for 33% (30 of 92) of the MSI-H tumors.
The minimum threshold for identification of each feature that discriminated MSI-H (at least one of five observers identified one feature among signet cells; mucinous, poor, medullary, or mixed differentiation; sponge-like pattern or marked intratumoral lymphocytic infiltration) and combination of the responses were also used to examine possible algorithms for detecting MSI-H cancer by morphology. Six percent (five of 92) of the MSI-H cancer group had none of the discriminating features identified by any of the five observers. Four of these cases also had a CD3 stain performed for counting intraepithelial lymphocytes, and in only one was the lymphocyte count more than the 40 T cells/0.94 mm 2 cut-point (one CD3 count of 67 T cells/0.94 mm2; three remaining cases <8 T cells/0.94 mm2).
Discussion
Histopathological recognition of phenotypic subtypes that correlate with molecular subclassification links classical surgical pathology with new techniques of molecular analysis. 57 The importance of this linkage depends on the clinical relevance of molecular analysis in patient management. Our study demonstrates that MSI-H colorectal carcinomas that are characteristic of HNPCC and of patients with more favorable prognosis and survival after adjuvant therapy 50 can be recognized on the basis of histopathology. We also provide data on observer variability in applying histopathological criteria.
We found that MSI-H in a group of apparently sporadic colon carcinomas was significantly associated with a distinct, reproducibly recognized pattern of extracellular mucin production that may be focal and with poor differentiation. Our findings also emphasize the mosaic low-power patterns in MSI-H tumors with mucinous growth abutting conventional gland-forming cancer, and the need to recognize areas of sheet-like growth. In addition, MSI-H cancers often evoke a host immune response that results in migration of activated T cells into the malignant epithelium of the tumor. 17 This feature was independent of mucinous histopathological type in our study and could be used with that feature to discriminate sensitively and specifically between most MSI-H tumors and MSS tumors. In cases without obvious lymphocytic infiltration on H&E stain, we found that CD3 immunostaining with morphometric analysis was helpful to highlight otherwise subtle intraepithelial lymphocyte populations.
Infiltrating T lymphocytes hold promise for future therapeutic strategies in elimination of circulating or seeded metastatic cells from colorectal carcinoma. The immune system recognizes neoplasia poorly, but in MSI-H colon cancer with infiltrating lymphocytes, it has been shown that mechanisms of T cell cytotoxicity are activated. 58 The cells in the epithelial compartment of the tumors are predominantly CD8+ TCR αβ+ cells, 58 and the intraepithelial lymphocytes, rather than the mixed peritumoral or stromal lymphocytic infiltrates, were the feature of value in our study. Whether intratumoral lymphocytosis represents an epiphenomenon or a genuine marker of host-tumor interaction is uncertain, but the lymphocytes do express perforin, a molecule participating in one mechanism of cell killing, 58 which would be unusual in recapitulation of the normal mucosa-associated lymphoid population. Whether the improved prognosis of MSI-H colon cancer is related to up-regulated immune response to prevent the establishment or emergence of metastatic deposits is not known. Intratumoral lymphocyte populations have been of interest for a number of years 48 and have been independently associated with improved survival after curative rectal cancer surgery. 45 Vaccines have been used in clinical trials in patients with colorectal carcinoma, and adjuvant immunotherapy to eliminate residual subclinical disease after curative resection remains a rational avenue for investigation.
We found that the presence of peritumoral Crohn’s-like lymphoid reaction was an insensitive marker for MSI-H tumors. This feature has been identified previously as an independent prognostic variable. 46,47 In a comprehensive study of 344 right-sided colonic adenocarcinomas, this feature was shown to predict improved 5-year survival in the 27% (96 of 344) of patients in which it was conspicuous. 47 The frequency of a distinct Crohn’s-like reaction among the cases in our study (40%) did not seem to be limited by examination of one slide per case, versus a mean of four slides interpreted per case in the previous study.
Similarities exist between MSI carcinomas arising in other organs and the sporadic MSI-H group of colon cancers. Medullary-type, poorly differentiated pancreatic adenocarcinoma is specifically associated with MSI-H. 59,60 Medullary-type carcinoma has been described in the stomach, 61 and a similar pattern of poorly differentiated growth in the breast has been identified. 55
Evaluation of interobserver variation in the identification of morphological features can be criticized for extrapolating good results beyond the study participants, who are often expert in the field, to the usual practice of the surgical pathologist. Our study of interobserver reproducibility, however, was conducted without previous consensus meetings or pilot studies to establish a generally applicable baseline. We found interobserver agreement to be lower than we expected, indicating that refinement of these criteria is needed before they can be used effectively for histopathological classification affecting clinical management.
Our study indicates that the identification of MSI-H by conventional histopathology can be used as a screening tool for rapid selection of sporadic colorectal cancers for molecular testing, with the potential to recover a majority of MSI-H cases. This approach can facilitate studies involving the molecular mining of archival material, to ease acquisition of uncommon cases for research. Clinical data may add to the utility of histopathology. For example, knowledge of early age of onset, multiple colorectal or other HNPCC-associated tumors, or positive family history points toward HNPCC. 15 Suspicion about sporadic MSI-H colon cancer is raised by older age of onset, right-sided location, and less advanced stage. 17 However, in our study histopathology alone failed to discriminate reliably MSI-H tumors in that minority of specimens that displayed no major difference in morphology from the usual MSS cancer. Approximately 40% of MSI-H cancers were not detected reliably in our study, and 6% were never detected by histopathology. In combination, the two features of mucinous differentiation and intraepithelial T cell infiltrate showed reasonable sensitivity and specificity for selecting MSI-H cancer from the total population of colon cancer, and the rare medullary-type pattern of poorly differentiated carcinoma is highly characteristic. Our results suggest that morphological evaluation of colorectal carcinoma for molecular subclassification is insufficient for decisions about patient management, which that require MSI testing.
Acknowledgments
We thank Drs. Al Benson, Daniel Haller, and Paul Catalano, and Eleanor McFadden of the Eastern Cooperative Oncology Group for their assistance; and Mrs. Cheryl Willis for secretarial support.
Footnotes
Address reprint requests to Stanley R. Hamilton, M.D., Head, Division of Pathology and Laboratory Medicine, MD Anderson Cancer Center, Box 85, Room G1.3754, 1515 Holcombe Blvd., Houston TX 77030. E-mail: shamilto@mdanderson.org.
Supported by the National Cancer Institute, National Institutes of Health grants CA21115 and CA78971.
References
- 1.Ionov Y, Peinado M, Malkhosyan S, Shibata D, Perucho M: Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993, 363:558-561 [DOI] [PubMed] [Google Scholar]
- 2.Kim H, Jen J, Vogelstein B, Hamilton SR: Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994, 145:148-156 [PMC free article] [PubMed] [Google Scholar]
- 3.Kolodner RD, Marsischky GT: Eukaryotic DNA mismatch repair. Curr Opin Genet Dev 1999, 9:89-96 [DOI] [PubMed] [Google Scholar]
- 4.Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, Mecklin JP, Jarvinen H, Powell SM, Jen J, Hamilton SR, et al: Clues to the pathogenesis of familial colorectal cancer. Science 1993, 260:812-816 [DOI] [PubMed] [Google Scholar]
- 5.Peltomaki P, Aaltonen LA, Sistonen P, Pylkkanen L, Mecklin JP, Jarvinen H, Green JS, Jass JR, Weber JL, Leach FS, et al: Genetic mapping of a locus predisposing to human colorectal cancer. Science 1993, 260:810-819 [DOI] [PubMed] [Google Scholar]
- 6.Peltomaki P, Lothe RA, Aaltonen LA, Pylkkanen L, Nystrom-Lahti M, Seruca R, David L, Holm R, Ryberg D, Haugen A: Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res 1993, 53:5853-5855 [PubMed] [Google Scholar]
- 7.Thibodeau SN, Bren G, Schaid D: Microsatellite instability in cancer of the proximal colon. Science 1993, 260:816-819 [DOI] [PubMed] [Google Scholar]
- 8.Boland CR: Roles of the DNA mismatch repair genes in colorectal tumorigenesis. Int J Cancer 1996, 69:47-49 [DOI] [PubMed] [Google Scholar]
- 9.Bronner CE, Baker SM, Morrison PT, Warren G, Smith LG, Lescoe MK, Kane M, Earabino C, Lipford J, Lindblom A: Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature 1994, 368:258-261 [DOI] [PubMed] [Google Scholar]
- 10.Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, Kane M, Kolodner R: The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 1993, 75:1027-1038 [DOI] [PubMed] [Google Scholar]
- 11.Leach FS, Nicolaides NC, Papadopoulos N, Liu B, Jen J, Parsons R, Peltomaki P, Sistonen P, Aaltonen LA, Nystrom-Lahti M, et al: Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 1993, 75:1215-1225 [DOI] [PubMed] [Google Scholar]
- 12.Liu B, Parsons R, Papadopoulos N, Nicolaides NC, Lynch HT, Watson P, Jass JR, Dunlop M, Wyllie A, Peltomaki P, de la Chapelle A, Hamilton SR, Vogelstein B, Kinzler KW: Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med 1996, 2:169-174 [DOI] [PubMed] [Google Scholar]
- 13.Nicolaides NC, Papadopoulos N, Liu B, Wei YF, Carter KC, Ruben SM, Rosen CA, Haseltine WA, Fleischmann RD, Fraser CM, et al: Mutations of two PMS homologues in hereditary non-polyposis colorectal cancer. Nature 1994, 371:75-80 [DOI] [PubMed] [Google Scholar]
- 14.Papadopoulos N, Nicolas NC, Wei YF, Ruben SM, Carter KC, Rosen CA, Haseltine WA, Fleischmann RD, Fraser CM, Adams MD, et al: Mutation of a mutL homolog in hereditary colon cancer. Science 1994, 263:1625-1629 [DOI] [PubMed] [Google Scholar]
- 15.Jass JR, Smyrk TC, Stewart SM, Lane MR, Lanspa SJ, Lynch HT: Pathology of hereditary non-polyposis colorectal cancer. Anticancer Res 1994, 14:1631-1634 [PubMed] [Google Scholar]
- 16.Marra G, Boland CR: Hereditary nonpolyposis colorectal cancer (HNPCC): the syndrome, the genes, and historical perspectives. J Natl Cancer Inst 1995, 87:1114-1125 [DOI] [PubMed] [Google Scholar]
- 17.Jass JR, Do K-A, Simms LA, Iino H, Wynter C, Pillay SP, Searle J, Radford-Smith G, Young J, Leggett B: Morphology of sporadic colorectal cancer with DNA replication errors. Gut 1998, 42:673-679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lothe RA, Peltomäki P, Meling GI, Aaltonen LA, Nystrom-Lahti M, Pylkkanen L, Heimdal K, Anderson TI, Moller P, Rognum TO: Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res 1993, 53:5849-5852 [PubMed] [Google Scholar]
- 19.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S: A National Cancer Institute Workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998, 58:5248-5257 [PubMed] [Google Scholar]
- 20.Risio M, Reato G, di Celle PF, Fizzotti M, Rossini FP, Foa R: Microsatellite instability is associated with the histological features of the tumor in nonfamilial colorectal cancer. Cancer Res 1996, 56:5470-5474 [PubMed] [Google Scholar]
- 21.Cunningham J, Christensen E, Tester DJ, Kim CY, Roche PC, Burgart LJ, Thibodeau SN: Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res 1998, 58:3455-3460 [PubMed] [Google Scholar]
- 22.Herman J, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, Markowitz S, Willson JK, Hamilton SR, Kinzler KW, Kane MF, Kolodner RD, Vogelstein B, Kunkel TA, Baylin SB: Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA 1998, 95:6870-6875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietmaier W, Wallinger S, Bocker T, Kullmann F, Fishel R, Ruschoff J: Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res 1997, 57:4749-4756 [PubMed] [Google Scholar]
- 24.Akiyama Y, Iwanaga R, Saitoh K, Shiba K, Ushio K, Ikeda E, Iwama T, Nomizu T, Yuasa Y: Transforming growth factor beta type II receptor gene mutations in adenomas from hereditary nonpolyposis colorectal cancer. Gastroenterology 1997, 112:33-39 [DOI] [PubMed] [Google Scholar]
- 25.Grady W, Myerhoff L, Swinler S, Rajput A, Thiagalingam S, Lutterbaugh JD, Neumann A, Brattain MG, Chang J, Kim SJ, Kinzler KW, Vogelstein B, Willson JK, Markowitz S: Mutational inactivation of transforming growth factor β receptor type II in microsatellite stable colon cancers. Cancer Res 1999, 59:320-324 [PubMed] [Google Scholar]
- 26.Hoang J-M, Cottu PH, Thuille B, Salmon RJ, Thomas G, Hamelin R: BAT-26, an indicator of the replication error phenotype in colorectal cancers and cell lines. Cancer Res 1997, 57:300-303 [PubMed] [Google Scholar]
- 27.Myeroff LL, Parsons R, Kim S-J, Hedrick L, Cho KR, Orth K, Mathis M, Kinzler KW, Lutterbaugh J, Park K: A transforming growth factor beta receptor type II gene mutation common in colon and gastric but rare in endometrial cancers with microsatellite instability. Cancer Res 1995, 55:5545-5547 [PubMed] [Google Scholar]
- 28.Samowitz WS, Slattery ML, Potter JD, Leppert MF: BAT-26 and BAT-40 instability in colorectal adenomas and carcinomas and germline polymorphism. Am J Pathol 1999, 154:1637-1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B: Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science 1995, 268:1336-1338 [DOI] [PubMed] [Google Scholar]
- 30.Parsons R, Myeroff LL, Liu B, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B: Microsatellite instability and mutations of the transforming growth factor β type II receptor gene in colorectal cancer. Cancer Res 1995, 55:5548-5550 [PubMed] [Google Scholar]
- 31.Jass JR, Pokos V, Arnold JL, Cottier DS, Jeevaratnam P, Van de Water NS, Browett PJ, Winship IM, Lane MR: Colorectal neoplasms detected colonoscopically in at-risk members of colorectal cancer families stratified by the demonstration of DNA microsatellite instability. J Mol Med 1996, 74:547-551 [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Bigas MA, Boland CR, Hamilton SR, Henson DE, Jass JR, Khan PM, Lynch H, Perucho M, Smyrk T, Sobin L, Srivastava S: A National Cancer Institute Workshop on hereditary nonpolyposis colorectal cancer syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst 1997, 89:1758-1762 [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Bigas MA, Vasen HF, Pakka-Mecklin J, Myrhoj T, Rozen P, Bertario L, Jarvinen HJ, Jass JR, Kunitomo K, Nomizu T, Driscoll DL: Rectal cancer risk in hereditary nonpolyposis colorectal cancer after abdominal colectomy. International Collaborative Group on HNPCC. Ann Surg 1997, 225:202-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukish JR, Muro K, DeNobile J, Katz R, Williams J, Cruess DF, Drucker W, Kirsch I, Hamilton SR: Prognostic significance of DNA replication errors in young patients with colorectal cancer. Ann Surg 1998, 227:51-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feeley KM, Fullard JF, Heneghan MA, Smith T, Maher M, Murphy RP, O’Gorman TA: Microsatellite instability in sporadic colorectal carcinoma is not an indicator of prognosis. J Pathol 1999, 188:14-17 [DOI] [PubMed] [Google Scholar]
- 36.Jernvall P, Makien MJ, Karttunen TJ, Makela J, Vihko P: Microsatellite instability: impact on cancer progression in proximal and distal colorectal cancers. Eur J Cancer 1999, 35:197-201 [DOI] [PubMed] [Google Scholar]
- 37.Liang JT, Chang KJ, Chen JC, Lee CC, Cheng YM, Hsu HC, Chien CT, Wang SM: Clinicopathologic and carcinogenetic appraisal of DNA replication error in sporadic T3NoMo stage colorectal cancer after curative resection. Hepato-Gastroenterology 1999, 46:883–890 [PubMed]
- 38.Johannsdottir JT, Bergthorsson JT, Gretarsdottir S, Kristjansson AK, Ragnarsson G, Jonasson JG, Egilsson V, Ingvarsson S: Replication error in colorectal carcinoma: association with loss of heterozygosity at mismatch repair loci and clinicopathological variables. Anticancer Res 1999, 19:1821-1826 [PubMed] [Google Scholar]
- 39.Messerini L, Ciantelli M, Baglioni S, Palomba A, Zampi G, Papi L: Prognostic significance of microsatellite instability in sporadic mucinous colorectal cancers. Hum Pathol 1999, 30:629-634 [DOI] [PubMed] [Google Scholar]
- 40.Halling KC, French AJ, McDonnell SK, Burgart LJ, Schaid DJ, Peterson BJ, Moon-Tasson L, Mahoney MR, Sargent DJ, O’Connell MJ, Witzig TE, Farr GH, Goldberg RM, Thibodeau SN: Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst 1999, 15:1295-1303 [DOI] [PubMed] [Google Scholar]
- 41.Massa MJ, Iniesta P, Gonzalez-Quevedo R, de Juan C, Caldes T, Sanchez-Pernaute A, Cerdan J, Torres AJ, Balibrea JL, Benito M: Differential prognosis of replication error phenotype and loss of heterozygosity in sporadic colorectal cancer. Eur J Cancer 1999, 35:1676-1682 [DOI] [PubMed] [Google Scholar]
- 42.Elsaleh H, Powell B, Soontrapornchai P, Joseph D, Goria F, Spry N, Iacopetta B: p53 gene mutation, microsatellite instability and adjuvant chemotherapy: impact on survival of 388 patients with Duke’s C colon carcinoma. Oncology 2000, 58:52-59 [DOI] [PubMed] [Google Scholar]
- 43.Gryfe R, Kim H, Hsieh ETK, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S: Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000, 342:69-77 [DOI] [PubMed] [Google Scholar]
- 44.Messerini L, Vitelli F, De Vitis LR, Mori S, Calzolari A, Palmirotta R, Calabro A, Papi L: Microsatellite instability in sporadic mucinous colorectal carcinomas: relationship to clinicopathologic variables. J Pathol 1997, 182:380-384 [DOI] [PubMed] [Google Scholar]
- 45.Jass JR: Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol 1986, 39:585-589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graham DM, Appelman HD: Crohn’s-like lymphoid reaction and colorectal carcinoma: a potential histologic prognosticator. Mod Pathol 1990, 3:332-335 [PubMed] [Google Scholar]
- 47.Harrison JC, Dean PJ, El-Zeky F, Vander Zwaag R: Impact of the Crohn’s-like lymphoid reaction on staging of right-sided colon cancer. Hum Pathol 1995, 26:31-38 [DOI] [PubMed] [Google Scholar]
- 48.Banner BF, Savas L, Baker S, Woda BA: Characterization of the inflammatory cell populations in normal colon and colonic carcinomas. Virchows Arch B Cell Pathol 1993, 64:213-220 [DOI] [PubMed] [Google Scholar]
- 49.Cawkwell L, Gray S, Murgatroyd H, Sutherland F, Haine L, Longfellow M, O’Loughlin S, Cross D, Kronborg O, Fenger C, Mapstone N, Dixon M, Quirke P: Choice of management strategy for colorectal cancer based on a diagnostic immunohistochemical test for defective mismatch repair. Gut 1999, 45:409-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe T, Wu T-T, Catalano PJ, Ueki T, Satriano R, Haller DG, Benson AB, Hamilton SR: Molecular predictors of survival after adjuvant chemotherapy of colon cancer. N Engl J Med 2001 (in press) [DOI] [PMC free article] [PubMed]
- 51.Jass JR, Biden KG, Cummings MC, Simms LA, Walsh M, Schoch E, Meltzer SJ, Wright C, Searle J, Young J, Leggett BA: Characterization of a subtype of colorectal cancer combining features of the suppressor and mild mutator pathways. J Clin Pathol 1999, 52:455-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiggers T, Arends J, Schutte B, Volvics L, Bosman FT: A multivariate analysis of pathologic prognostic indicators in large bowel cancer. Cancer 1988, 61:386-395 [DOI] [PubMed] [Google Scholar]
- 53.Ruschoff J, Dietmaier W, Luttges J, Seitz G, Bocker T, Zirngibl H, Schlegel J, Schackert HK, Jauch KW, Hofstaedter F: Poorly differentiated colonic adenocarcinoma, medullary type. Clinical, phenotypic and molecular characteristics. Am J Pathol 1997, 150:1815-1825 [PMC free article] [PubMed] [Google Scholar]
- 54.Jessurun J, Romero-Guadarrama M, Manivel JC: Medullary adenocarcinoma of the colon: clinicopathologic study of 11 cases. Hum Pathol 1999, 30:843-848 [DOI] [PubMed] [Google Scholar]
- 55.Gaffey MJ, Mills SE, Frierson HF, Zarbo RJ, Boyd JC, Simpson JF, Weiss LM: Medullary carcinoma of the breast: interobserver variability in histopathologic diagnosis. Mod Pathol 1995, 8:31-38 [PubMed] [Google Scholar]
- 56.Boyd JC: Mathematical tools for demonstrating the clinical usefulness of biochemical markers. Scand J Clin Lab Invest Suppl 1997, 227:46-63 [PubMed] [Google Scholar]
- 57.Jass JR: Towards a molecular classification of colorectal cancer. Int J Colorectal Dis 1999, 14:194-200 [DOI] [PubMed] [Google Scholar]
- 58.Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, Vecchiato N, Macri E, Fornasarig M, Boiocchi M: High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol 1999, 154:1805-1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goggins M, Offerhaus GJA, Hilgers W, Griffin CA, Shekher M, Tang D, Sohn TA, Yeo CJ, Kern SE, Hruban RH: Pancreatic adenocarcinomas with DNA replication errors (RER+) are associated with wild-type K-ras and characteristic histopathology: poor differentiation, a syncytial growth pattern, and pushing borders suggest RER+. Am J Pathol 1998, 152:1501-1507 [PMC free article] [PubMed] [Google Scholar]
- 60.Wilentz RE, Goggins M, Redston M, Marcus VA, Adsay NV, Sohn TA, Kadkol SS, Yeo CJ, Choti M, Zahurak M, Johnson K, Tascilar M, Offerhaus GJA, Hruban RH, Kern SE: Genetic, immunohistochemical, and clinical features of medullary carcinoma of the pancreas. A newly described and characterized entity. Am J Pathol 2000, 156:1641-1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshikawa T, Suzuki K, Kobayashi O, Sairenji M, Motohashi H, Tsuburaya A, Nakamura Y, Shimizu A, Yanoma LS, Noguchi Y: Thymidine phosphorylase/platelet-derived endothelial cell growth factor is upregulated in advanced solid types of gastric cancer. Br J Cancer 1999, 79:1145-1150 [DOI] [PMC free article] [PubMed] [Google Scholar]