Abstract
Fas-L molecules expressed by in vitro stimulated T cells may be critically involved in suicidal activation-induced cell death (AICD) of such cells through engagement of their Fas receptors. A similar suicide of T cells was postulated to occur even in vivo, to eliminate dangerous activated lymphocytes; however, the demonstration of suicidal AICD of T cells in healthy humans in vivo is still lacking. We therefore investigated the possible occurrence of Fas-L-linked suicidal apoptosis of T cells in normal human peripheral blood. For this purpose, we took advantage of immunoelectron microscopy, which allows simultaneous visualization of the morphological apoptotic cellular changes together with surface expression of Fas-L molecules. Very few T lymphocytes were observed showing the ultrastructural features of apoptotic lymphocytes; these occasional apoptotic T cells, together with the majority of the normal T cell population, expressed the Fas molecule on the plasma membrane, as expected. Interestingly, the apoptotic cells were also Fas-L-positive, whereas normal T cells were Fas-L-negative. Such Fas-L-associated T cell suicide operating in vivo in healthy individuals is presumably able to suppress immune responses and prevent autoreactivity, thus maintaining the homeostasis of human blood.
Evidence supports the proposition that there is a suicide program inherent in vertebrate cells that can be activated when the cell’s death is desirable for the good of the rest of the community. 1 In fact, in order to maintain cellular homeostasis and protect the body from the continued secretion of potentially harmful amounts of cytokines, activated T cells, on re-encountering the specific antigen, are removed through the induction of apoptosis, 2 a program termed activation-induced cell death (AICD). 3 Such an apoptotic process can occur through triggering an internal suicide program that involves the stimulation, on the membrane of the activated T cell, of suicide signals from death-inducing molecules, such as CD95 (Fas), on interaction with their ligands, such as CD95-ligand (Fas-L). 4,5 In fact, whereas peripheral resting T cells virtually do not express Fas-L and express moderate amounts of Fas, 6-10 activated peripheral T cells express abundant quantities of both Fas and Fas-L. 8,11-14 In this way, it can be assumed, a T lymphocyte undergoing suicidal AICD is recognizable because it simultaneously shows both Fas-L expression and apoptotic signs. 2,15,16
Although AICD of T cells occurring through Fas/Fas-L interaction has been demonstrated in vitro 15,17,18 and has been postulated to occur in vivo by investigations conducted in genetically manipulated mice, 19-22 as well as in diseased humans, 23-25 its spontaneous occurrence in healthy subjects has never been shown. Such an occurrence, however, should be expected, since AICD provides an essential mechanism for clonal deletion in the peripheral immune system and serves to limit immune responses, being therefore necessary to preserve tissue homeostasis. 3 The aim of this study, therefore, was to recognize suicidal AICD of T cells in peripheral blood of normal human volunteers: for such a purpose, although immuno-electron microscopy (IEM) had not previously been used to show AICD, we took advantage of pre-embedding IEM, which allows the simultaneous detection of both ultrastructural morphology, ie, apoptotic T cellular changes, 1,26,27 and cell membrane antigenic immunophenotype, ie, Fas-L expression. In this way, we succeeded in demonstrating the occurrence of occasional Fas-L-positive T cells undergoing apoptosis, presumably representing suicidal T lymphocytes, in normal human peripheral blood.
Materials and Methods
T Cell Preparation
Fresh normal human peripheral blood was provided from six randomly selected healthy volunteers. Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) gradient centrifugation and separated from adherent cells by incubation on Petri dishes for 2 hours at 37°C. T cells (E-rosetting) were isolated by a single-step rosetting method 28 and subsequently separated by a second Ficoll-Hypaque gradient centrifugation. This cell fraction contained more than 95% E-rosetting cells. T cells were finally resuspended in RPMI 1640, containing gentamycin 50 μg/ml, at 5 × 10 6 cells/ml.
Immunogold Labeling
The immunogold labeling was performed according to the protocol previously described by us, 29 with slight modifications. Briefly, T cells were prefixed in 3% paraformaldehyde in 0.1 mol/L phosphate buffer, pH 7.2, for 15 minutes at room temperature, washed twice in glycine 50 mmol/L buffer, and subjected to a blocking preincubation for 30 minutes at 4°C to avoid nonspecific antibody binding to Fc receptors by using PBS containing 20% normal goat serum (BioCell Research, Cardiff, UK) plus 1% bovine serum albumin (BSA). T cells, after washings with 0.1% BSA in PBS, were incubated with the following anti-human mouse monoclonal antibodies (mAb): anti-Fas mAb (DX-2, Chemicon International Inc., Temecula, CA), anti-Fas-L mAb (G247–4, PharMingen, San Diego, CA), and, as a control, anti-CD3 mAb (Leu 4, Becton Dickinson, Mountain View, CA); mAb were diluted 1:1000 in PBS containing 0.1% BSA, and the incubation was 1 hour at room temperature. After washings in PBS containing BSA 0.1% and then in 0.1% BSA buffer (0.02 mol/L Tris-HCL buffer, pH 8.2, containing 0.1% BSA and 2% sodium azide), T cells were incubated with goat anti-mouse IgG coupled to 15-nm colloidal gold particles (Aurion, Wageningen, The Netherlands), diluted in the incubation buffer system, pH 7.6, as suggested by the manufacturer, for 1 hour at room temperature.
Ultrastructural Studies
Labeled T cells were subjected to an electron microscopy protocol according to a previously described procedure. 30 Briefly, cells were fixed in 1% glutaraldehyde in 0.1 mol/L cacodylate buffer, pH 7.4, 3 hours at room temperature, washed in 0.15 mol/L cacodylate buffer at pH 7.4, packaged in 2% Bacto Agar (Difco Laboratories, Detroit, MI) at 45°C, postfixed in potassium-ferricyanate-reduced osmium tetroxide, 31 dehydrated in graded acetone, embedded in Durcupan ACM (Fluka, Buchs, Switzerland), and cut at a LKB III ultramicrotome to be observed with a transmission electron microscope.
Controls for Method Specificity
Four types of negative controls were carried out: (i) omitting the incubation with primary antibody, (ii) substituting for the primary antibody with unreactive mouse IgG of the same isotype, (iii) pre-incubating by a unlabeled goat anti-mouse antibody before the secondary antibody, and (iv) substituting for the secondary (labeled) antibody with noncoupled colloidal gold particles of the same size.
Results
Freshly Isolated, E-Rosetting PBMC Are T Lymphocytes
Virtually the entire population of PBMC observed at the electron microscope, ie, E-rosetting cells, showed the ultrastructural characters of normal peripheral blood lymphocytes 32 (Figure 1A) ▶ . They were T lymphocytes, as they were isolated by E-rosetting. 28 In fact, the great majority of the control cells observed in IEM were CD3-positive; that is, when incubated by the control Leu 4 mAb, they showed gold particles on the cell membrane.
Figure 1.
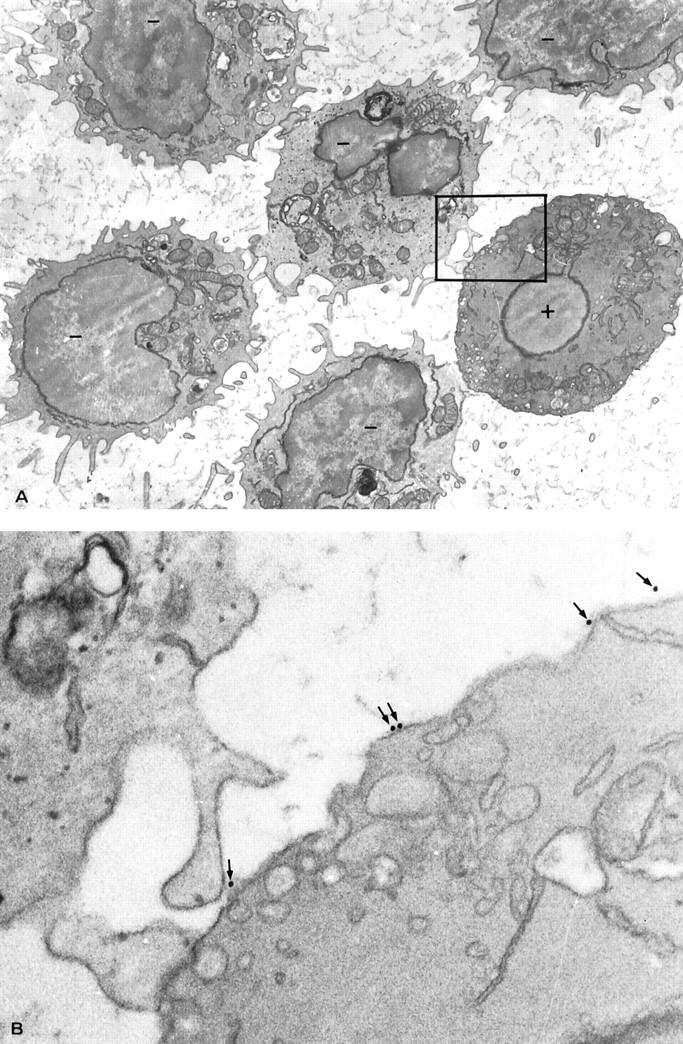
A peripheral blood T lymphocyte from a healthy donor shows both morphological characters of apoptosis and immunogold Fas-L labeling on the plasma membrane. A: Several T cells suspended from peripheral blood, enriched by E-rosetting, subjected to an immunogold Fas-L labeling double-layer procedure in IEM, are visible. The lymphocyte visible on the right side shows, unlike the others, some ultrastructural signs of apoptosis, including loss of plasma membrane microvilli, collapsed nucleus, and peripherally tightly packed cytoplasmic organelles. Note that the plasma membrane is intact and that no spilling of intracellular contents is visible. Such an apoptotic lymphocyte is, moreover, immunogold labeled (+), although gold particles present along the plasma membrane are not clearly observed at this magnification; by contrast, the other, viable lymphocytes are unlabeled (−). The boxed area is shown at higher magnification in B. Immunogold electron microscopy; magnification, ×5200. B: The area boxed in A is herein shown enlarged. The facing portions of two lymphocytes are observed: one, on the right side, belongs to the apoptotic lymphocyte, and shows some gold particles (arrows) along the plasma membrane, thus revealing the expression of the Fas-L molecule; the other, on the left side, belongs to a normal, viable lymphocyte, and does not show any gold granule on the surface, thus yielding a Fas-L-negative result. Immunogold electron microscopy; magnification, ×26,000.
Normal Peripheral T Cells Are Fas-L-Negative
When the anti Fas-L mAb was used, lymphocytes at the ultrastructural observation resulted negative. These cells, in fact, did not show virtually any gold particle along the plasma membrane (Figure 1A) ▶ . This Fas-L-negativity of resting T cells observed in IEM is consistent with similar results obtained by other immunostaining methods, including cytofluorimetry and Western blot analysis. 6-10
Apoptotic Peripheral T Cells Are Fas-L-Positive
By contrast, occasional (ie, <1%) lymphocytes were visible that exhibited at least some of the morphological characteristics that are typical of lymphocytes undergoing apoptosis, 1,26,27 namely, loss of plasma membrane microvilli (Figure 1A) ▶ , often blebbed plasma membrane, condensed cytoplasm, tightly packed cytoplasmic organelles (Figure 1A) ▶ , swelling of the cisternae of endoplasmic reticulum, condensed nuclear chromatin, and, especially, uniform and collapsed nucleus (Figure 1A) ▶ , which was recently outlined as the defining morphological feature of an apoptotic cell. 27 Despite these remarkable changes, however, the plasma membrane was normal, allowing no spilling of intracellular contents outside the cell (Figure 1, A and B) ▶ . In addition, although the intensity of immunostaining did not correlate directly with ultrastructural apoptotic features, the occasional apoptotic lymphocytes showed a number (about 10–30) of colloidal gold particles per section surface area scattered along the plasma membrane (Figure 1B) ▶ , therefore resulting Fas-L-positive: in fact, more than three gold granules decorating the plasma membrane is a prerequisite for classifying a cell as gold-immunolabeled. 33 It was possible to consider such cells, therefore, T lymphocytes undergoing suicidal apoptosis, 4 because they simultaneously showed in IEM the hallmarks of such a special cell death: both apoptotic morphological changes 1,27 (Figure 1A) ▶ and Fas-L expression on the cell membrane 2,16 (Figure 1B) ▶ .
The Great Majority of Peripheral T Cells Are Fas-Positive
When the anti-Fas mAb was used, the vast majority of lymphocytes at the ultrastructural level were positive, ie, showed gold particles along the plasma membrane. This was the case in both normal-appearing and apoptotic T lymphocytes. Similarly, Fas expression was also noticed on T cells when immunodetection methods other than IEM were used. 6-10
Control Experiments
The four controls for method specificity gave negative results.
Discussion
This is the first paper demonstrating the occurrence of T cells, freshly isolated from normal human peripheral blood, simultaneously showing both apoptotic changes and the expression of the Fas-L molecule on their plasma membrane. In fact, freshly isolated PBMC were depleted of adherent cells and subjected to an E-rosetting method; thus, T cells (namely, E-rosetting lymphocytes) were separated. 28 On the other hand, such an engagement of CD2 in this method presumably did not contribute to the occurrence of AICD, because CD2 engagement was shown not to influence, or even to decrease, lymphoid cell apoptosis, since it inhibits up-regulation of Fas and Fas-L. 34 Fas-L-expressing T cells were, moreover, recognized by a double-step gold-immunolabeling protocol, and were therefore revealed by the detection of colloidal gold particles along the plasma membrane. 35 Apoptotic ultrastructural changes characteristic of lymphocyte apoptosis, including collapse of the nucleus and other well known morphological apoptotic features, 1,27 were finally detected at the electron microscope. It was possible, therefore, to realize that all of the occasional T cells showing apoptotic features coexpressed Fas-L on the cell membrane, whereas the great majority of freshly isolated, unstimulated, normal-appearing T cells were Fas-L-negative.
Since in vitro studies on stimulated T cells demonstrated that the phenotype characterized by the simultaneous presence of Fas-L expression on the plasma membrane and of apoptotic changes is a prerequisite of T cells undergoing suicidal AICD, 15,16 the present results demonstrate that such an AICD also occurs spontaneously in vivo. As with the in vitro findings, it can be envisaged that, even in vivo, resting, Fas-L-negative T lymphocytes can be activated by antigenic stimulation, which induces Fas-L (mRNA and surface) expression and up-regulates Fas expression. 11-14 On restimulation, membrane-bound Fas-L molecules may bind to Fas receptors, eg, by membrane folding, to trigger the death signaling cascade that causes the apoptotic suicide of the T cell. 19,20 Alternatively, Fas-L may be cleaved from the cell membrane and engage, in a soluble form, Fas expressed by the same cell (autocrine AICD), 15 although the precise role played by the cleaved Fas-L molecule is still matter of debate. 36 It remains to be determined whether preformed Fas-L and newly synthesized Fas-L are preferentially involved in distinct cellular functions, such as paracrine killing of Fas-positive targets and autocrine suicidal AICD of T cells, respectively. 37,38
The present study shows that the AICD, which was postulated to occur in healthy individuals because it is missing in pathological Fas/Fas-L-lacking conditions in both mouse 19-22 and man, 23-25 can be effectively observed in normal conditions, ie, it can occur in healthy subjects. In fact, AICD of mature T cells through Fas-L/Fas interactions has been shown to be lacking in mice defective in Fas or Fas-L, 19,20 and therefore display enhanced lymphoproliferation and impairment of peripheral deletion. 21,22 Similarly, patients with a syndrome characterized by homologous lymphoproliferation and with autoimmune disease have mutations in their Fas or Fas-L genes. 23-25 By contrast, the current results demonstrate T cells from healthy human volunteers directly showing spontaneous features resembling AICD (namely, Fas-L expression together with apoptotic changes).
The functional significance of a restricted subpopulation of Fas-L-positive apoptotic T cells circulating in normal peripheral blood of healthy subjects is still unknown. It is tempting to speculate that, even in such apparently normal conditions, some T cells must die, to avoid chronic inflammation 3,25,39,40 and/or autoreactivity. 38,41-44 In fact, it is now well established that the Fas/Fas-L system is involved in both the elimination of activated T cells after they have responded to foreign antigens 15-18 and the clonal deletion of autoreactive T cells in peripheral lymphoid organs. 37,45-48 As a consequence, the occurrence of preactivated and/or self-reactive Fas-L-positive T cells undergoing apoptosis in peripheral blood should be expected in normal subjects, because such apoptosis avoids accumulation of activated/autoreactive T lymphocytes. Indeed, the current demonstration of a small subset of Fas-L-expressing apoptotic T cells in normal peripheral blood fulfills such a prediction.
Acknowledgments
We thank Dr. Antonio Lavazza, head of the Electron Microscopy Laboratory at the Zooprophylactic Institute, Brescia, Italy, and his technicians, Gianni Bozzoni, Giuseppe Bonatti, and Giuseppe Bertocchi, for their excellent technical assistance.
Footnotes
Address reprint requests to Giuseppe De Panfilis, Divisione Dermatologia, Spedali Civili, I-25125 Brescia, Italy.
References
- 1.Cohen JJ: Apoptosis. Immunol Today 1993, 14:126-130 [DOI] [PubMed] [Google Scholar]
- 2.Osborne BA: Apoptosis and the maintenance of homoeostasis in the immune system. Curr Opin Immunol 1996, 8:245-254 [DOI] [PubMed] [Google Scholar]
- 3.Kabelitz D, Pohl T, Pechold K: Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol Today 1993, 14:338-339 [DOI] [PubMed] [Google Scholar]
- 4.Nagata S, Golstein P: The Fas death factor. Science 1995, 267:1449-1456 [DOI] [PubMed] [Google Scholar]
- 5.Nagata S: Apoptosis by death factor. Cell 1997, 88:355-365 [DOI] [PubMed] [Google Scholar]
- 6.Owen-Schaub LB, Yonehara S, Crump WL, III, Grimm EA: DNA fragmentation and cell death is selectively triggered in activated human lymphocytes by Fas antigen engagement. Cell Immunol 1992, 140:197-205 [DOI] [PubMed] [Google Scholar]
- 7.Klaus C, Debatin K-M, Jonker RR, Krammer PH: Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol 1993, 5:625-630 [DOI] [PubMed] [Google Scholar]
- 8.Kawakami A, Eguchi K, Matsuoka N, Tsuboi M, Koji T, Urayama S, Nakashima T, Kawabe Y, Nagataki S: Expression and function of Fas and Fas ligand on peripheral blood lymphocytes in normal subjects. J Lab Clin Med 1998, 132:404-413 [DOI] [PubMed] [Google Scholar]
- 9.Mincheff M, Loukinov D, Zoubak S, Hammett M, Meryman H: Fas and Fas ligand expression on human peripheral blood leukocytes. Vox Sang 1998, 74:113-121 [PubMed] [Google Scholar]
- 10.Caricchio R, Reap EA, Cohen PL: Fas/Fas ligand interactions are involved in ultraviolet B-induced human lymphocyte apoptosis. J Immunol 1998, 161:241-251 [PubMed] [Google Scholar]
- 11.Anel A, Buferne M, Boyer C, Schmitt-Verhulst A-M, Golstein P: T cell receptor-induced Fas ligand expression in cytotoxic T lymphocyte clones is blocked by protein tyrosine kinase inhibitors and cyclosporin A. Eur J Immunol 1994, 24:2469-2476 [DOI] [PubMed] [Google Scholar]
- 12.Vignaux F, Golstein P: Fas-based lymphocyte-mediated cytotoxicity against syngeneic activated lymphocytes: a regulatory pathway? Eur J Immunol 1994, 24:923-927 [DOI] [PubMed] [Google Scholar]
- 13.Gillette-Ferguson I, Sidman CL: A specific intercellular pathway of apoptotic cell death is defective in the mature peripheral T cells of autoimmune lpr and gld mice. Eur J Immunol 1994, 24:1181-1185 [DOI] [PubMed] [Google Scholar]
- 14.Vignaux F, Vivier E, Malissen B, Depraetere V, Nagata S, Golstein P: TCR/CD3 coupling to Fas-based cytotoxicity. J Exp Med 1995, 181:781-786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhein J, Walczak H, Baumler A, Debatin K-M, Krammer PH: Autocrine T-cell suicide mediated by APO-1 (Fas/CD95). Nature 1995, 373:438-441 [DOI] [PubMed] [Google Scholar]
- 16.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH: Fas ligand mediates activation-induced cell death in human lymphocytes. J Exp Med 1995, 181:71-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF: Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T cell hybridomas. Nature 1995, 373:441-444 [DOI] [PubMed] [Google Scholar]
- 18.Ju ST, Panka DJ, Cui H, Ettinger R, El-Khatib M, Sherr D, Stanger BZ, Marshak-Rothstein A: Fas (CD95)/Fas-L interactions required for programmed cell death after T-cell activation. Nature 1995, 373:444-448 [DOI] [PubMed] [Google Scholar]
- 19.Russell JH, Rush B, Weaver C, Wang R: Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci USA 1993, 90:4409-4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell JH, Wang R: Autoimmune gold mutation uncouples suicide and cytokine/proliferative pathways in activated mature T cells. Eur J Immunol 1993, 23:2279-2282 [DOI] [PubMed] [Google Scholar]
- 21.Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, Yoshida N, Nagata S: Targeted mutation in the Fas gene causes hyperplasia in the peripheral lymphoid organs and liver. Nat Genet 1995, 11:294-300 [DOI] [PubMed] [Google Scholar]
- 22.Maldonado MA, MacDonald GC, Kakkanaiah VN, Fecho K, Dransfield M, Sekiguchi D, Cohen PL, Eisenberg RA: Differential control of autoantibodies and lymphoproliferation by Fas ligand expression on CD4+ and CD8+ T cells in vivo. J Immunol 1999, 163:3138-3142 [PubMed] [Google Scholar]
- 23.Fisher G, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM: Dominant interferring Fas gene mutations impair apoptosis in human autoimmune lymphoproliferative syndrome. Cell 1995, 81:935-946 [DOI] [PubMed] [Google Scholar]
- 24.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IAG, Debatin KM, Fisher A, De Villartay JP: Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 1995, 268:1347-1349 [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Wilson J, He J, Xiang L, Schur PH, Mountz JD: Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. J Clin Invest 1996, 98:1107-1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oishi M, Maeda K, Sugiyama S: Distribution of apoptosis-mediating Fas antigen in human skin and effects of anti-Fas monoclonal antibody on human epidermal keratinocyte and squamous cell carcinoma cell lines. Arch Dermatol Res 1994, 286:396-407 [DOI] [PubMed] [Google Scholar]
- 27.Cohen JJ: Apoptosis: mechanisms of life and death in the immune system. J All Clin Immunol 1999, 103:548-554 [DOI] [PubMed] [Google Scholar]
- 28.Saxon A, Feldhaus J, Robins RA: Single step separation of human T and B cells using AET treated SRBC rosettes. J Immunol Methods 1976, 12:285-288 [DOI] [PubMed] [Google Scholar]
- 29.Manara GC, Sansoni P, Badiali-De Giorgi L, Gallinella G, Ferrari C, Brianti V, Fagnoni FF, Ruegg CL, De Panfilis G, Pasquinelli G: New insights suggesting a possible role of a heat shock protein 70-kD family-related protein in antigen processing/presentation phenomenon in humans. Blood 1993, 82:2865-2871 [PubMed] [Google Scholar]
- 30.De Panfilis G, Manara GC, Ferrari C, Torresani C, Sansoni P: Hairy cell leukemia cells express CD1a antigen. Cancer 1988, 61:52-57 [DOI] [PubMed] [Google Scholar]
- 31.Karnovski MJ: Use of ferricyanide reduced osmium tetroxide in electron microscopy. J Cell Biol 1971, 51:284-291 [Google Scholar]
- 32.Yagi H, Nakamura M, Ishii T, Kasahara S, Itoh T: Ultrastructural analysis of mouse thymocyte subpopulations. Eur J Immunol 1997, 27:2680-2687 [DOI] [PubMed] [Google Scholar]
- 33.Matutes E, Catovsky D: The fine structure of normal lymphocyte subpopulations. A study with monoclonal antibodies and the immunogold technique. Clin Exp Immunol 1982, 50:416-425 [PMC free article] [PubMed] [Google Scholar]
- 34.Ayroldi E, Migliorati G, Cannarile L, Moraca R, Delfino DV, Riccardi C: CD2 rescues T cells from T-cell receptor/CD3 apoptosis: a role for the Fas/Fas-L system. Blood 1997, 89:3717-3726 [PubMed] [Google Scholar]
- 35.Horisberger M: Evaluation of colloidal gold as a cytochemical marker for scanning electron microscopy. Biol Cell 1979, 36:253-263 [Google Scholar]
- 36.Tanaka M, Itai T, Adachi M, Nagata S: Downregulation of Fas ligand by shedding. Nat Med 1998, 4:31-36 [DOI] [PubMed] [Google Scholar]
- 37.Su X: Autocrine and paracrine apoptosis are mediated by differential regulation of Fas ligand activity in two distinct Jurkat T cell populations. J Immunol 1998, 160:5288-5293 [PubMed] [Google Scholar]
- 38.Brunner T, Mueller C: Is autoimmunity coming to a Fas(t) end? Nat Med 1999, 5:19-20 [DOI] [PubMed] [Google Scholar]
- 39.Webb S, Morris C, Sprent J: Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell 1990, 63:1249-1256 [DOI] [PubMed] [Google Scholar]
- 40.Kawabe Y, Ochi A: Programmed cell death and extrathymic reduction of Vβ8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature 1991, 349:245-248 [DOI] [PubMed] [Google Scholar]
- 41.Rocha B, Von Boehmer H: Peripheral selection of the T cell repertoire. Science 1991, 251:1225-1228 [DOI] [PubMed] [Google Scholar]
- 42.MacDonald HR, Baschieri S, Lees RK: Clonal expansion precedes anergy and death of Vβ8+ peripheral T cells responding to staphylococcal enterotoxin B in vivo. Eur J Immunol 1991, 21:1963-1966 [DOI] [PubMed] [Google Scholar]
- 43.McCormack JE, Callahan JE, Kappler J, Marrack PC: Profound deletion of mature T cells in vivo by chronic exposure to exogenous superantigen. J Immunol 1993, 150:3785-3792 [PubMed] [Google Scholar]
- 44.Webb SR, Hutchinson J, Hayden K, Sprent J: Expansion/deletion of mature T cells exposed to endogenous superantigens in vivo. J Immunol 1994, 152:586-597 [PubMed] [Google Scholar]
- 45.Musette P, Pannetier C, Gachelin G, Kourilsky P: The expansion of a CD4+ T cell population bearing a distinctive beta chain in MRL lpr/lpr mice suggests a role for the Fas protein in peripheral T cell selection. Eur J Immunol 1994, 24:2761-2766 [DOI] [PubMed] [Google Scholar]
- 46.Singer GG, Abbas AK: The Fas antigen is involved in peripheral but nonthymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity 1994, 1:365-371 [DOI] [PubMed] [Google Scholar]
- 47.Watanabe D, Suda T, Hashimoto H, Nagata S: Constitutive activation of the Fas ligand gene in mouse lymphoproliferative disorders. EMBO J 1995, 14:8-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu JL, Ramos P, Rosendorff A: Massive upregulation of Fas ligand in lpr and gld mice: implications for Fas regulation and the graft-versus-host disease-like wasting syndrome. J Exp Med 1995, 181:393-398 [DOI] [PMC free article] [PubMed] [Google Scholar]


