Abstract
Nuclear factor-interleukin-6 (NF-IL-6) is one of several nuclear transcription factors (NF-IL-6, NF-κB, PU.1, interferon-regulatory factor 1, Egr-1, and Stat-1). NF-IL-6 and NF-κB are expressed in macrophages and is induced by bacterial lipopolysaccharides. To evaluate whether NF-IL-6 is required for the inflammatory immune response to mycobacterial infection, in which epithelioid macrophages comprise the leading cell population, we generated NF-IL-6 knockout (KO) mutant mice. Airborne infection of these mice with Mycobacterium tuberculosis strains induced disseminated tuberculosis lacking granuloma formation, although interferon-γ, tumor necrosis factor-α, and interleukin-12 mRNA expression levels were within the normal range compared with those of wild-type mice. Generation of O2− and mycobacterial killing by neutrophils from these mice were impaired severely compared with wild-type mice. We conclude that NF-IL-6 is a critical transcription factor in mycobacterial control as well as in granulocyte-colony stimulating factor induction resulting in neutrophil activation.
Nuclear factor-interleukin-6 (NF-IL-6) is one of several nuclear transcription factors (NF-IL-6, NF-κB, PU.1, interferon-regulatory factor 1, Egr-1, and Stat-1). Especially, NF-IL-6 and NF-κB are expressed in macrophages and induced by bacterial lipopolysaccharides. 1,2 NF-IL-6 was originally identified as a nuclear factor that binds to the IL-1 response element of the human IL-6 gene. 3 It is reported that NF-IL-6 expression level is dramatically increased during macrophage differentiation. 4,5 Thus, NF-IL-6 may be closely related to macrophage functions.
NF-IL-6-deficient mice displayed a high susceptibility to Salmonella, Listeria and Brucella abortus infections, indicating that NF-IL-6 plays a role in controlling intracellualr parasite proliferation by an as yet unknown mechanism. Macrophages acquire enhanced bactericidal capacity when fully activated after their interaction with Mycobacterium tuberculosis. Tuberculosis is a chronic disease induced by M. tuberculosis, one of typical intracellular pathogens, and characterized by clusters of epithelioid macrophages with central caseous necrosis. 6 Thus, it is of great interest to examine the relationship between NF-IL-6 expression and tuberculosis, because the direct role of NF-IL-6 in mycobacterial infection has not been established fully. To evaluate whether NF-IL-6 is required for the inflammatory immune response to mycobacterial infection, in which epithelioid macrophages comprise the leading cell population, we generated NF-IL-6 knockout (KO) mutant mice 7 and demonstrated that NF-IL-6 is an essential transcription factor in defense against mycobacterial infection.
Materials and Methods
Generation of NF-IL-6 KO Mice with C57BL/6 Background
An 11-kb genomic fragment spanning from 8.5 kb 5′ of the NK-IL6 transcription initiation site to 2.3 kb 3′ of the end of the exon was subcloned into pUC18 plasmid vector. The MC1-herpes simplex virus thymidine kinase was inserted into the unique HindIII site in the 5′ end of the homologous region. E14–1 ES cells were electroporated in 800 μl of phosphate-buffered saline (PBS) with 32 μg of SalI-linearized targeting vector DNA in a Bio-Rad Gene Pulser. G418- and Gancyclovir-resistant colonies were picked up 10 to 12 days later. Homologous recombination was screened by polymerase chain reaction (PCR) and subsequently confirmed by genomic Southern blot hybridization. 7 These NF-IL-6 mice with C57BL/6 background were back-crossed over six generations. After birth, the NF-IL-6 KO mice developed normally and they have normal number of lymphocytes, leukocytes, and leukocyte subsets. They were kept under specific pathogen-free conditions in an environmentally controlled clean room at the laboratory animal facility at the Research Institute of Tuberculosis.
Bacterial Strains and Mouse Infections
Virulent M. tuberculosis H37Rv (ATCC358121) and more virulent Kurono (ATCC25618) strains were passed through mice and grown in 7H9 liquid medium once before storing in aliquots at −85°C and titered. The cultured strains were filtered with a membrane filter of 4 μm pore size (Millipore, Bedford, MA) before use so that they were dispersed evenly. The NF-IL-6 KO and wild-type (WT) mice were infected by an airborne route by placing them in the exposure chamber of an airborne infection apparatus (Model 099CA4241; Glas-Col, Inc., Terre Haute, IN). The nebulizer compartment was filled with 5 ml of a suspension of 10 6 CFU of H37Rv or Kurono strain. 8 The concentration was calculated to result in uptake of ∼200 viable bacilli by the lungs just after inhalation exposure for 90 minutes under the experimental conditions for this study. 19 The survival of groups of mice for 50 days after infection with Kurono strain was recorded, and survival curves were plotted. The lungs and spleens from NF-IL-6 KO and WT mice infected with H37Rv were retrieved 1, 2, 3, 4, 5, 6, and 7 weeks after infection, homogenized, diluted with physiological saline, plated on Ogawa slant agar, and incubated at 37°C for 21 days. The resulting CFU were counted. Permission (no. 991) to experiments on animals was given by the Animal Experiment Committee of The Research Institute of Tuberculosis.
Histology
Some mice were sacrificed just before death after infection. Tissue sections from paraffin blocks containing lung, liver, and spleen tissue were stained with hematoxylin and eosin or the Ziehl-Neelsen method for acid-fast bacilli.
Reverse Transcriptase (RT)-PCR
Pulmonary tissue samples were taken from infected NF-IL-6 KO and WT mice just before death after infection (usually 35 days), frozen in liquid nitrogen, and stored at −80°C until required for use, when RNA was extracted as described previously. 8 RT-PCR was carried out with gene-specific primer sets for interferon-γ (IFN-γ), IL-12 p40, tumor necrosis factor-α (TNF-α), and IL-1β (CLP Inc., San Diego, CA). The granulocyte-colony stimulating factor (G-CSF) cDNA was amplified by primer sets specific for G-CSF. 7 The size of β-actin used as a positive control was 514 bp. The same amounts of β-actin RNA from the lung tissues used as an internal control were used in RT-PCR analysis.
Macrophage Nitric Oxide (NO) Assay
Alveolar macrophages were obtained by bronchoalveolar lavage. Briefly, the murine trachea was cannulated and 1 ml of physiological saline was poured in. The saline was recovered using a 1-ml disposable syringe. The cells thus obtained contained more than 99% macrophages as assessed by phagocytosis of BCG and anti-Mac-1 immunostaining. The alveolar macrophages were cultured with H37Rv overnight at the multiplicity of infection of 5:1. Nitrite concentrations were determined by the Griess assay as described previously. 9
Enzyme-Linked Immunosorbent Assay (ELISA)
The spleens were collected from the NF-IL-6 KO and WT mice and pooled, and a single-cell suspension was prepared. The cells were treated for 5 minutes with a 0.155 mol/L ammonium chloride/0.01 mol/L potassium bicarbonate solution for hemolysis of RBC, washed, and resuspended in RPMI 1640 medium with heat-inactivated 10% fetal calf serum. The cell suspensions were plated at 5 × 10 5 cells/well in 96-well flat-bottomed culture plates and incubated for 3 days at 37°C in 5% CO2 in air. The cells were stimulated with either medium alone or live H37Rv (10 5 CFU). The concentrations of IFN-γ, TNF-α, IL-1β, and IL-12 in cell culture supernatants from the cells incubated in the presence of H37Rv were measured by sandwich ELISA (Biosource International, Camarillo, CA). The values were expressed as the mean and SE (in picograms per milliliter) of triplicate determinations for pooled cells from five animals.
O2− and Neutrophil CFU Assays
The 10% peptone broth-elicited peritoneal exudate cells were plated and cultured on Petri dishes for 6 hours. Non-adherent cells were collected after gentle shaking. The neutrophils (105; more than 98% pure as evaluated by morphology) from NF-IL-6 KO and WT mice were stimulated with H37Rv strain (5 × 10 6 CFU) in vitro overnight. Their O2− production was examined using a nitroblue tetrazolium (NBT) reagent. 10 Briefly, 0.1 ml of 0.1% HEPES-saline-glucose buffer containing NBT was added to 0.1 ml neutrophil cell suspension (1 × 106/ml PBS) and the cell suspension was incubated at 37°C for 7 minutes. Then, 10 μl of 0.2 mol/L EDTA solution was added. The cell suspension was placed on a hemocytometer. The 200 cells were counted, and cells possessing NBT formazan in cytoplasm (blue-stained neutrophils) were judged as positive. At the same time, the supernatants containing neutrophils subjected to lysis with 0.05% sodium dodecyl sulfate were cultured in Lowenstein-Jensen media for 4 weeks before counting mycobacterial colonies.
Reconstitution of NF-IL-6 KO Mice with Exogenous G-CSF
The mice were injected subcutaneously with 2 μg of recombinant murine G-CSF (PeproTech, Rocky Hill, NJ) in PBS or PBS alone three times at weekly intervals to see whether or not recombinant G-CSF affects development of mycobacterial infection. The lungs, spleens, and livers from NF-IL-6 KO mice treated subcutaneously with recombinant G-CSF were retrieved from the infected mice 7 weeks after infection, and their histopathology was evaluated.
Statistical Method
The values were compared by Student’s t-test. For all statistical analyses, a P value <0.01 was considered significant.
Results
Course of M. tuberculosis Infection in NF-IL-6 Mice
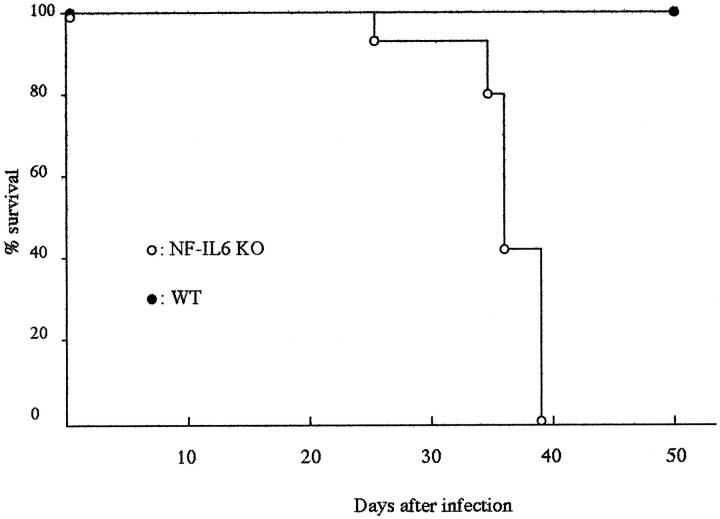
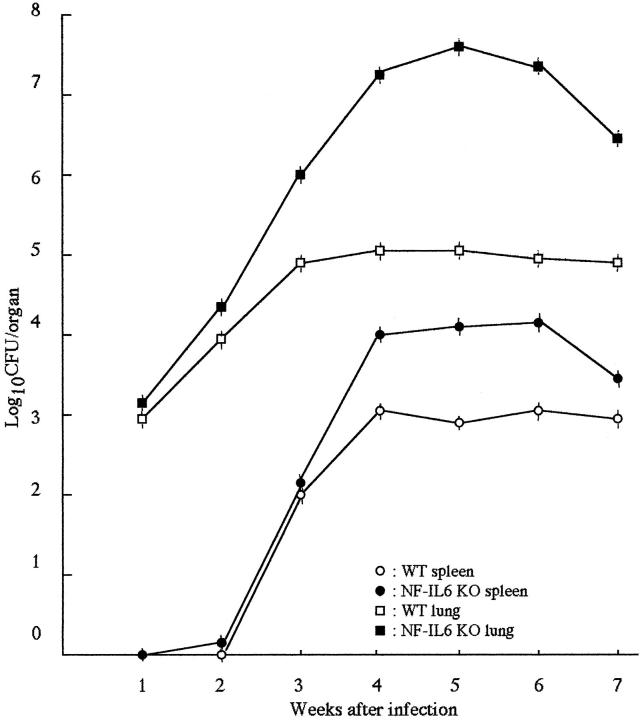
We infected WT and NF-IL-6 KO mice with either H37Rv (ATCC358121) or Kurono (ATCC25618) M. tuberculosis strains (10 6 each). All of the mice infected with the Kurono strain (each group of 10) died of mycobacteremia within 40 days, whereas WT mice survived until the date of sacrifice at 50 days (Figure 1) ▶ . When NF-IL-6 KO mice were infected with H37Rv, the CFU number was much higher than that from WT mice at weeks 2 through 7 after infection (Figure 2) ▶ . Figure 3 ▶ shows that there were extensive necroses in the lungs, spleen, and liver of NF-IL-6 KO mice infected with Kurono strain. On the contrary, pulmonary granulomas were observed in WT mice. Histopathologically, multiple foci of abscesses consisting of neutrophils and macrophages were noted in the lungs, spleen, and liver, whereas WT mice developed granulomatous lesions lacking neutrophil infiltration (Figure 4) ▶ . When infected with H37Rv by an airborne route, NF-IL-6 KO mice survived but developed severe lobar pneumonia with pulmonary edema. Necrotic lesions induced in NF-IL-6 KO mice were inhibited significantly by treatment with exogenous recombinant murine G-CSF, as shown in Figure 4c ▶ . No neutrophil infiltration was recognized in the lung treated with recombinant G-CSF. The NF-IL-6 KO mice treated with recombinant murine G-CSF lived longer than the WT mice.
Figure 1.
Survival of mice infected with M. tuberculosis Kurono strain. WT and NF-IL-6 KO mice were infected with 10 6 CFU of the Kurono strain by an airborne route. Percentages of surviving WT (solid circles) and NF-IL-6 KO mice (open squares) are shown.
Figure 2.
CFU in lung and spleen tissues of NF-IL-6 KO and WT mice exposed to 10 6 CFU of H37Rv strain by the airborne route. At the indicated weeks after infection, four mice from each group were sacrificed and homogenates of lung and spleen tissues were plated. Error bars indicate standard errors of the means.
Figure 3.
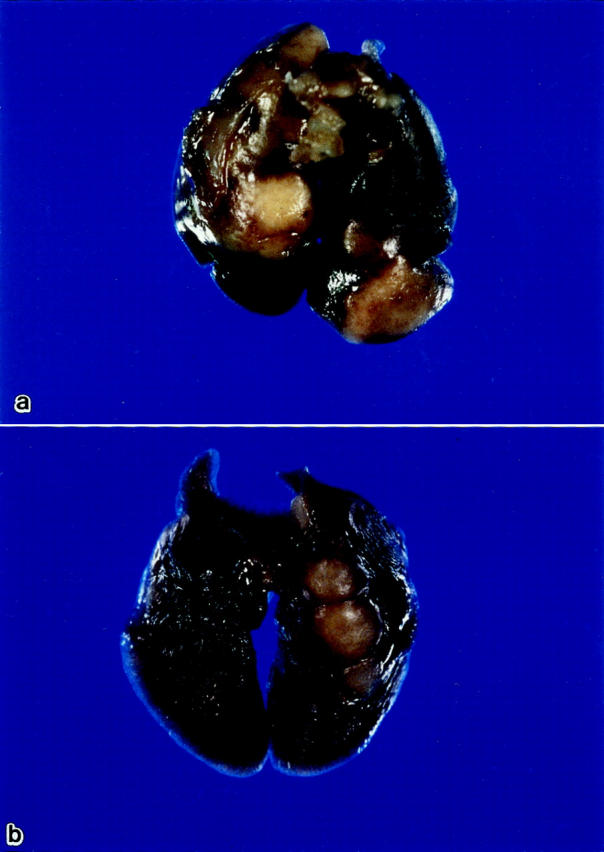
Macroscopic appearance of lungs from NF-IL-6 KO (a) and WT (b) mice infected with the Kurono strain by an airborne route. Whitish necrotic nodules are noted in the lungs of NK-IL-6 mice, but nodular lesions are noted in the lungs of WT mice.
Figure 4.
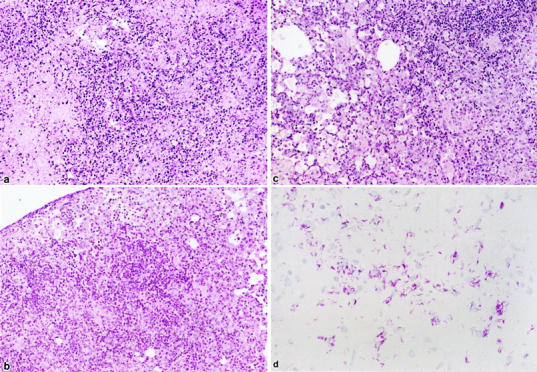
Histological analysis of lung sections after infection with M. tuberculosis (Kurono strain). Lungs were removed from NF-IL-6 KO (a) and WT mice (b) 38 days and 50 days, respectively, after infection with Kurono strain (10 6 CFU) by an airborne route. Hematoxylin and eosin stain; original magnification, ×200. c: NF-IL-6 KO mice infected with Kurono strain were injected subcutaneously three times at day 0 and at weekly intervals with recombinant murine G-CSF (2 μg/mouse). Thirty-eight days later, the mice were sacrificed and the issues were fixed with 10% buffered formalin. Hematoxylin and eosin stain; original magnification, ×200. d: Stain for acid-fast bacilli in the lung of NF-IL-6 infected with Kurono strain. Original magnification, ×600.
RT-PCR and Cytokine Assay
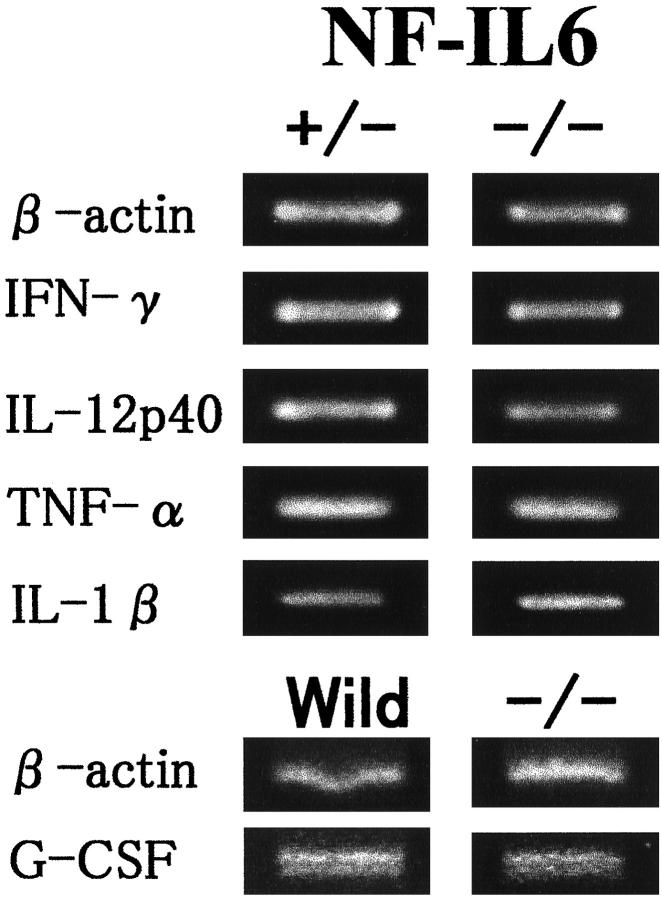
As shown in Figure 5 ▶ , RT-PCR showed that NF-IL-6 KO mice expressed IFN-γ, TNF-α, and IL-12 mRNA as strongly as did those from WT mice. G-CSF mRNA expression (704 bp) was lower in the lungs of NF-IL-6 KO mice than in the lungs of WT mice. Fibroblasts other than alveolar macrophages expressed G-CSF mRNA. To determine whether the absence of NF-IL-6 might influence the induction and control of various cytokines, the levels of several cytokines in the splenocyte cultures were determined. Table 1 ▶ shows that H37Rv-driven release of IFN-γ, TNF-α, IL-1β, and IL-12 was similar for the two types of mice.
Figure 5.
RT-PCR analysis of cytokine mRNA expression. Total RNA was isolated from lung tissues of NF-IL-6 KO and WT mice infected with M. tuberculosis and subjected to RT-PCR using cytokine-specific primers. G-CSF mRNA expression is lower in the lung of NF-IL-6 mice than that of WT mice. Lung fibroblasts from NF-IL-6 KO mice G-CSF-mRNA significantly.
Table 1.
Cytokine Release by Splenocytes from WT and NF-IL6 KO Mice
| Stimulus | Mouse type | Cytokine level | |||
|---|---|---|---|---|---|
| IL-1β | IFN-γ | TNF-α | IL-12 | ||
| H37Rv | WT | 250 ± 14 | 120 ± 10 | 750 ± 35 | 110 ± 11 |
| NF-IL6 KO | 245 ± 12 | 130 ± 10 | 800 ± 32 | 95 ± 9 | |
NO Assay
We examined the production of NO in culture supernatants of alveolar macrophages stimulated with H37Rv overnight from WT and NF-IL-6 KO mice. NO production was detectable in NF-IL-6 KO mouse macrophages to the same extent as in WT mouse macrophages (63 ± 5 vs. 60 ± 3 μmol/L).
Neutrophil Function Assay
Because induction of G-CSF production was impaired in NF-IL-6 KO mice, we next examined neutrophil functions by two methods. First, 10% peptone broth-elicited peritoneal neutrophils (105) from either NF-IL-6 KO or WT mice were cultured in vitro overnight in the presence of M. tuberculosis H37Rv strain (5 × 10 6 CFU). The supernatants containing neutrophils subject to lysis with 0.05% sodium dodecyl sulfate were cultured in Lowenstein-Jensen media for 4 weeks before counting of mycobacterial colonies. The numbers of mycobacterial colonies in these supernatants from NF-IL-6 KO mice were significantly higher than those from WT mice (54,3215 ± 2850 vs. 4356 ± 248 CFU; P < 0.01) (Figure 6) ▶ .
Figure 6.
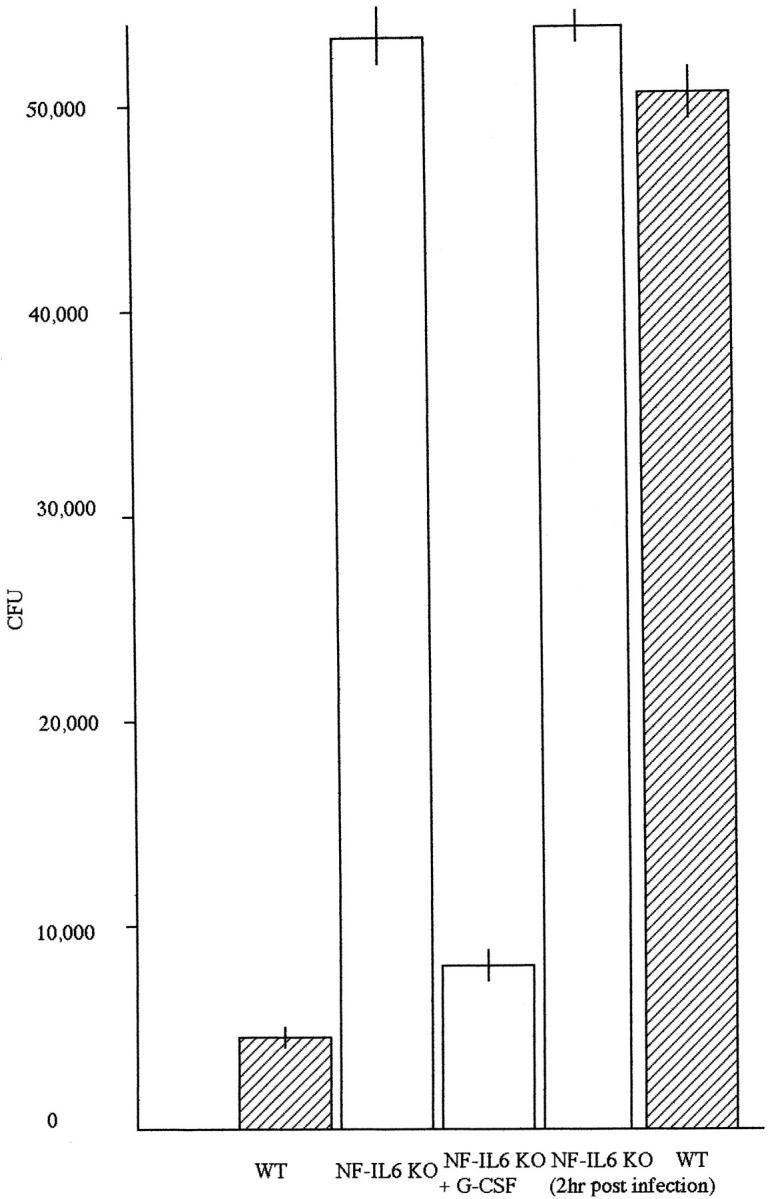
CFU assay of peritoneal neutrophils from NF-IL-6 mice. The peritoneal neutrophis (105) were cultured in the presence of H37Rv (5 × 106) in 96-well flat-bottomed microculture plates 2 hours after infection with H37Rv or overnight. The neutrophils were also cultured with H37Rv in the presence of recombinant murine G-CSF (2 × 10− 3 μg) overnight. Thereafter, the suspension containing peritoneal neutrophils were cultured for 4 weeks in Lowenstein-Jensen media and the resulting colonies were counted.
Next, 10% peptone broth-elicited peritoneal neutrophils (105) from NF-IL-6 KO and WT mice were stimulated with M. tuberculosis H37Rv strain (5 × 106) in vitro overnight. Their O2− production was examined using a NBT reagent. 10 The ratio of O2− producing neutrophils in NF-IL-6 KO mice was 15%, whereas in WT mice it was 95% (P < 0.01) (Table 2) ▶ . The O2− producing ability of neutrophils was significantly lower in NF-IL-6 KO mice.
Table 2.
Percentage of O2− Producing Neutrophils
| Neutrophils | O2− producing neutrophils (%) |
|---|---|
| WT mice | 95 |
| NF-IL6 KO mice | 15 |
The 200 peritoneal neutrophils were counted, and neutrophils containing NBT formazan were judged to be positive.
Discussion
The purpose of our study was to determine the role of NF-IL-6 as a transcription factor in the pathogenesis of murine tuberculosis. The results obtained with our NF-IL-6 KO mouse model demonstrated that NF-IL-6 was indispensable for the control and survival of the M. tuberculosis infection. As these KO mice died of mycobacteremia within 40 days, it was speculated that levels of IFN-γ , ΙL−12, and TNF-α, which are important cytokines in defense against mycobacterial infection, were lower than those of WT mice. However, the expression levels of these cytokines were within normal range as evaluated by RT-PCR and ELISA.
NO production was detectable significantly in NF-IL-6 KO mouse macrophages. There was no significant difference in NO production by alveolar macrophages between WT and NF-IL-6 mice (P < 0.01). Taken together with RT-PCR and ELISA data, these results suggest that macrophages of NF-IL-6 KO mice function normally to exert normal mycobactericidal activity.
What is the mechanism by which NF-IL-6 KO mice succumb readily to mycobacterial infection? Killing ability of mycobacteria and O2− producing ability by neutrophils was significantly lower in NF-IL-6 KO mice compared with those of WT mice. Since induction of G-CSF production by alveolar macrophages was impaired severely in NF-IL-6 KO mice, 7 we examined neutrophil functions in terms of mycobacterial killing ability by neutrophils, O2− generation, and reconstitution of NF-IL-6 KO mice with recombinant murine G-CSF. Consequently, neutrophils of NF-IL-6 KO mice did not function properly and granulomatous formation in NF-IL-6 KO mice was abrogated significantly with exogenous murine G-CSF. Since no neutrophils were recognized in the granulomatous lesions, recombinant murine G-CSF reversed neutrophil function significantly. It is also reported that G-CSF induces proliferation and differentiation of neutrophil precursors. 11-13 Electron microscopy revealed tubercle bacilli in phagosomes of macrophages from NF-IL-6 KO mice, but we saw no evidence of M. tuberculosis having escaped from phagosomes to the cytoplasm, although this has been demonstrated in infection with L. monocytogenes (data not shown). 7 Therefore, it is concluded that bactericidal activity by neutrophils may be impaired severely in these NF-IL-6 mice.
Alveolar macrophages are targets of mycobacterial infection and activated macrophages, as well as IFN-γ, TNF-α, IL-12, IL-18, and IL-1, play major roles in protective immunity against tuberculosis. 13-19 Our results demonstrate that neutrophils play an important role in the early phase of defense against murine tuberculosis when aerosol infection was used. Since G-CSF is impaired in NF-IL-6 KO mice, 4 NF-IL-6 appears to be essential for inducing G-CSF production in neutrophil proliferation and differentiation. 14-18
It has been very recently reported that addition of G-CSF, whose induction is impaired in NF-IL-6 KO mouse macrophages, restores both endocytosis and morphology of endosomes, together with bactericidal activity. 20 Taken together with our data, both bactericidal activity by neutrophils and modulation of endocytosis by macrophages may be impaired in these NF-IL-6 KO mice. We showed that neutrophils played an important role in preventing development of granulomatous lesions. Tuberculosis is of global importance. This NF-IL-6 KO mouse model may offer a strategy for developing novel anti-tuberculosis drugs that augment the NF-IL-6 expression that is closely associated with neutrophil functions.
Acknowledgments
Part of this work was presented at TB 2000 sponsored by American Society of Microbiology in New York in June, 2000.
Footnotes
Address reprint requests to Dr. I. Sugawara, Department of Molecular Pathology, The Research Institute of Tuberculosis, 3-1-24 Matsuyama, Kiyose, Tokyo 204-0022, Japan. E-mail: sugawara@jata.or.jp.
Supported in part by an International Collaborative Study Grant (to I. S.) from the Ministry of Health and Welfare, Japan.
References
- 1.Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S: NF-IL-6 and NF-κB synergistically activate transcription of the inflammatory cytokines, IL-6 and IL-8. Proc Natl Acad Sci USA 1993, 90:10193-10197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein B, Cogswell PC, Baldwin AS, Jr: Functional and physical association between NF-κB and C/EBP family members: a Rel domain-bZip interaction. Mol Cell Biol 1993, 13:3964-3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isshiki H, Akira S, Tanabe O, Nakajima T, Shimamoto T, Hirano T, Kishimoto T: Constitutive and IL-1 inducible factors interact with the IL-1 responsive element in the IL-6 gene. Mol Cell Biol 1990, 10:2757-2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Natsuka S, Akira S, Nishio Y, Hashimoto S, Sugita T, Isshiki H, Kishimoto T: Macrophage differentiation specific expression of NF-IL-6, a transcription factor for IL-6. Blood 1992, 79:460-466 [PubMed] [Google Scholar]
- 5.Scott LM, Civin CI, Rorth P, Friedman AD: A novel temporal expression pattern of three C/EBP family members in differentiating myelomonocytic cells. Blood 1992, 80:1725-1735 [PubMed] [Google Scholar]
- 6.Garay SM: Pulmonary tuberculosis. Rom WN Garay SM eds. Tuberculosis. 1996, :pp 373-412 Little, Brown and Company, Boston [Google Scholar]
- 7.Tanaka T, Akira S, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T: Targeted disruption of the NF-IL-6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell 1995, 80:353-361 [DOI] [PubMed] [Google Scholar]
- 8.Sugawara I, Yamada H, Kaneko H, Mizuno S, Takeda K, Akira S: Role of interleukin-18 in mycobacterial infection in IL-18-gene-disrupted mice. Infect Immun 1999, 67:2585-2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green SJ, Crawford SRM, Hockmeyer JT, Meltzer MS, Nacy CA: Leishmania major amastigotes initiate the 1-arginine-dependent killing mechanism in IFN-gamma stimulated macrophages by induction of tumor necrosis factor-α. J Immunol 1990, 145:4290-4297 [PubMed] [Google Scholar]
- 10.Kobayashi S, Imajou-Ohmi S, Nakamura M, Kanegasaki S: Occurrence of cytochrome b558 in B-cell lineage of human lymphocytes. Blood 1990, 75:458-461 [PubMed] [Google Scholar]
- 11.Ikebuchi K, Clark SC, Ihle JN, Souza LM, Ogawa M: Granulocyte colony-stimulating factor enhances IL-3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci USA 1990, 85:3445-3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuo A, Kitagawa S, Ohsawa A, Ohta M, Miyazono K, Urabe A, Saito M, Takaku F: 19 Recombinant G-CSF as an activator of human granulocytes: potentiation of responces triggered by receptor-mediated agonists and stimulation of C3bi receptor expression and adherence. Blood 1989, 74:2144-2149 [PubMed] [Google Scholar]
- 13.Shimoda K, Okamura S, Harada N, Kondo S, Okamura T, Niho Y: Identification of a functional receptor for G-CSF on platelets. J Clin Invest 1993, 91:1310-1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme I: Disseminated tuberculosis in interferon-gamma gene-disrupted mice. J Exp Med 1993, 178:2243-2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn JL, Chan JJ, Triebold KJ, Dalton DK, Stewart TA, Bloom BR: An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J Exp Med 1993, 178:2249-2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugawara I, Yamada H, Ohtomo K, Aoki T, Doi N, Kazumi Y, Tagawa Y, Iwakura Y: Granulomas in interferon-γ gene-disrupted mice are inducible by avirulent Mycobacterium, but not by virulent Mycobacterium. J Med Microbiol 1998, 47:871-877 [DOI] [PubMed] [Google Scholar]
- 17.Cooper AM, Magram J, Ferrante J, Orme IM: Interleukin 12 is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med 1997, 188:39-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko H, Yamada H, Mizuno S, Udagawa T, Kazumi Y, Sekikawa K, Sugawara I: The role of tumor necrosis factor-alpha in Mycobacterium-induced granuloma formation in TNF-alpha deficient mice. Lab Invest 1999, 79:379-386 [PubMed] [Google Scholar]
- 19.Yamada H, Mizuno S, Horai R, Iwakura Y, Sugawara I: Protective role of interleukin-1 in mycobacterial infection in IL-1 α/β double-knockout mice. Lab Invest 2000, 80:759-767 [DOI] [PubMed] [Google Scholar]
- 20.Pizarro-Cerda J, Desjardins M, Moreno E, Akira S, Gorvel J-P: Modulation of endocytosis in nuclear factor IL-6(−/−) macrophages is responsible for a high susceptibility to intracellular bacterial infection. J Immunol 1999, 162:3519-3526 [PubMed] [Google Scholar]