Abstract
We have previously demonstrated that intrathecal pretreatment with dextro-morphine or morphine attenuates the morphine-produced antinociception. The phenomenon has been defined as antianalgesia, which is mediated by a non-opioid receptor (Wu et al. 2005). To determine if p38 mitogen-activated protein kinase (MAPK) is involved in the antianalgesia, the effects of p38 MAPK inhibitor 4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole (SB203580) on the attenuation of the morphine-produced tail-flick inhibition induced by dextro-morphine or morphine were studied in male CD-1 mice. Intrathecal pretreatment with SB203580 (24.2 nmol) reversed the attenuation of the morphine-produced tail-flick inhibition induced by dextromorphine (33 fmol) or morphine (0.3 nmol) pretreatment. The finding indicates that the antianalgesia induced by dextro-morphine or morphine is mediated by the activation of p38 MAPK in the mouse spinal cord.
Keywords: mitogen-activated protein kinase, antianalgesia, analgesia, opioid, spinal cord
1. Introduction
The naturally occurring morphine alkaloid, which is isolated from the juice of the opium poppy, papaver somniferum, is stereochemically identified as a levorotatory form. The synthetic dextro-isomer of morphine, dextro-morphine, virtually is inert to produce any analgesic and other μ-opioid receptor-mediated pharmacological actions, because it does not have any affinity for μ-opioid receptors (Jacquet et al., 1977). We have previously demonstrated that pretreatment with dextro-morphine at a femtomolar dose or morphine at a picomolar dose given intrathecally attenuates the tail-flick inhibition produced by morphine in the mouse. The phenomenon has been defined as antianalgesia (Wu et al., 2004, 2005). The antianalgesia induced by dextro-morphine or morphine is mediated by a non-opioid receptor. This view is evidenced by the findings that dextro-morphine does not have any affinity for μ-opioid receptors (Jacquet et al., 1977) and that the antianalgesia induced by dextro-morphine or morphine is blocked by the non-opioid dextro-naloxone (Iijima et al., 1978; Wu et al., 2005). In addition, pretreatment with dextro-morphine or morphine also attenuates the antinociception produced by δ-opioid receptor agonist deltorphin II and κ-opioid receptor agonist trans-(1S,2S)-3,4-Dichloro-N-methyl-N-[2-(1-pyrolidinyl)cyclohexyl]benzeneacetamide hydrochloride in μ-opioid receptor knockout mice, indicating that μ-opioid receptors are not involved in dextro-morphine- and morphine-induced antianalgesia (Wu et al., 2006).
The p38 mitogen-activated protein kinase (MAPK) is involved in regulating numerous cellular responses (Nebreda and Porras, 2000). The p38 MAPK responds to environmental stress and its pathway is crucial to inflammatory cytokine production and signaling (Kumar et al., 2003). Activation of p38 MAPK in spinal microglia contributes to hyperalgesia and allodynia following peripheral nerve injury (Jin et al., 2003; Schafer et al., 2003; Tsuda et al., 2004) and the inflammation-induced spinal pain processing (Svensson et al., 2003, 2005). The activation of p38 MAPK is required for μ-opioid receptor endocytosis (Mace et al., 2005) and chronic morphine treatment increases p38 MAPK phosphorylation, which is associated with the development of antinociceptive tolerance to morphine (Ma et al., 2001; Cui et al., 2006).
To determine if the activation of p38 MAPK is involved in the antianalgesia induced by dextro-morphine or morphine, the effects of p38 MAPK inhibitor 4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole (SB203580) on the attenuation of the morphine-produced tail-flick inhibition induced by dextro-morphine or morphine were studied in male CD-1 mice. We now report that pretreatment with SB203580 reversed the attenuation of levo-morphine-produced antinociception induced by dextro-morphine or morphine. The finding provides the evidence that activation of p38 MAPK is involved in the antianalgesia induced by dextro-morphine and morphine.
2. Materials and Methods
2.1. Animals
Male CD-1 mice weighing 25-30 g (Charles River Breeding Laboratory, Wilmington, MA) were used. Animals were housed five per cage in a room maintained at 22 ± 0.5°C with an alternating 12-h light-dark cycle. Food and water were available ad libitum. Each animal was used only once. All experiments were approved by and conformed to the guidelines of the Animal Care Committee of the Medical College of Wisconsin.
2.2. Assessment of antinociception
Nociceptive responses were measured with the tail-flick test (D’Amour and Smith, 1941). To measure the latency of the tail-flick response, mice were gently held with the tail put on the apparatus (Model TF6, EMDIE Instrument Co., Maidens, VA). The tail-flick response was elicited by applying radiant heat to the dorsal surface of the tail. The heat stimulus was set to provide a pre-drug tail-flick response time of 3 to 4 s and the cutoff time was set at 10 s to avoid tissue damage.
2.3. Drugs and drug-administration
Morphine sulfate and dextro-morphine base were obtained from National Institute of Drug Abuse (Baltimore, MD). 4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole (SB203580) was purchased from Sigma (St. Louis, MO). Morphine and SB203580 were dissolved in 0.9% saline. The dextro-morphine was dissolved in 10 N hydrochloric acid and then titrated with 1 N sodium hydroxide to pH 7, which was then diluted to intended dose in 0.9% saline. Drugs were injected intrathecally in an injection volume of 5 μl using a 25-μl Hamilton syringe with a 30-gauge needle according to the procedure described by Hylden and Wilcox (1980).
2.4. Statistical analysis
The nociceptive responses (tail-flick latencies) were presented as the mean ± S.E.M. One-way analysis of variance (ANOVA) followed by Dunnett’s post-test and upaired Student t-test were used to test the differences between groups. The GraphPad Prism software was used to perform the statistics (version 4.1; GraphPad Software, Inc., San Diego, CA).
3. Results
3.1. Effect of the pretreatment with SB203580 on the attenuation of morphine-produced tail-flick inhibition induced by dextro-morphine or morphine
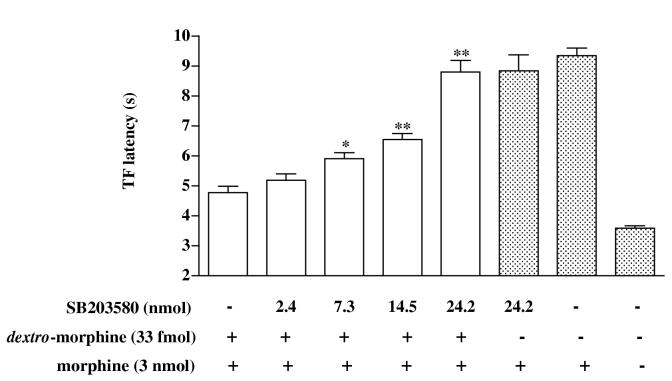
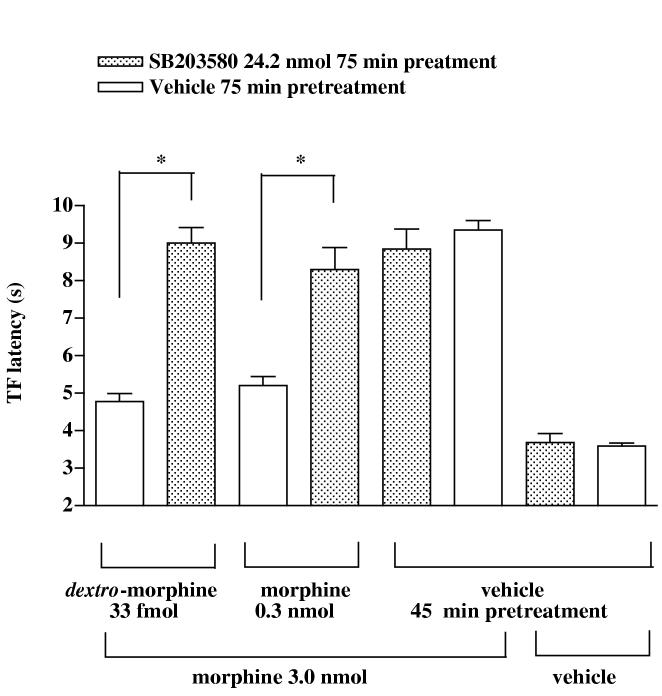
The p38 MAPK inhibitor SB203580 was used to determine if the activation of p38 MAPK is involved in mediating the antianalgesia induced by dextro-morphine or morphine. Groups of mice were pretreated intrathecally with various doses (2.4-24.2 nmol) of SB203580 30 min (Cui et al., 2006) before intrathecal injection of dextro-morphine (33 fmol). Morphine (3.0 nmol) was injected intrathecally 45 min after dextro-morphine injection and the tail-flick response was measured 15 min after morphine (3.0 nmol) injection. Intrathecal pretreatment with SB203580 (2.4-24.2 nmol) dose-dependently reversed the attenuation of the tail-flick inhibition produced by morphine (3.0 nmol). SB 203580 at a dose of 24.2 nmol completely reversed the attenuation of the morphine-produced tail-flick inhibition induced by dextro-morphine (Fig 1). Similarly, intrathecal pretreatment with SB203580 (24.2 nmol) completely reversed the attenuation of the morphine-produced tail-flick inhibition induced by morphine (0.3 nmol) (Fig. 2). Intrathecal pretreatment with SB203580 (24.2 nmol) given alone did not affect the morphine-produced tail-flick inhibition, nor did it affect the tail-flick latency in mice pretreated with vehicle (Fig. 2).
Fig. 1.
Effect of SB203580 on intrathecal morphine-produced tail-flick response in mice pretreated intrathecally with dextro-morphine. Groups of mice were pretreated intrathecally with SB203580 (2.4-24.2 nmol) or vehicle 30 min before intrathecal injection of dextro-morphine (33 fmol) followed by intrathecal injection of morphine (3.0 nmol) 45 min thereafter. The tail-flick latency was measured 15 min after morphine administration. Each column represents the mean and the vertical bar represents the S.E.M. with 7 to 14 mice in each group. The three columns on the right hand side of the figure represent different control data, which were not used for statistic purpose. The symbol of “+” represents “drug injection” and “-“ represent “vehicle injection”. The one-way ANOVA followed by Dunnett’s post-test was used to test the difference between groups. The F (4,47) = 34.75; *p < 0.05, ** p < 0.01 compared with the vehicle injected group (the first column from the left).
Fig. 2.
Effect of SB203580 on intrathecal morphine-produced tail-flick response in mice pretreated intrathecally with morphine and dextro-morphine. Groups of mice were pretreated intrathecally with SB203580 (24.2 nmol) or vehicle 30 min before intrathecal injection of morphine (0.3 nmol) or dextro-morphine (33 fmol) followed by intrathecal injection of morphine (3.0 nmol) 45 min thereafter. The TF latency was measured 15 min after levo-morphine administration. Each column represents the mean and the vertical bar represents the S.E.M. with 7 to 10 mice in each group. The four columns on the right hand side of the figure represent different control data, which were not used for statistic purpose. The Student t-test was used to test the difference between groups; *p < 0.01 compared with the vehicle injected group.
4. Discussion
We found in the present study that the inhibition of p38 MAPK in the spinal cord by intrathecal treatment with SB 203580 reversed the attenuation of the tail-flick inhibition induced by dextro-morphine or morphine. The finding indicates that the activation of spinal p38 MAPK is involved in inducing antianalgesia caused by dextro-morphine or morphine. The present finding also suggests that p38 MAPK may be involved in the acute antinociceptive tolerance to morphine. Cui et al. (2006) reported that repeated intrathecal pretreatment with SB203580 attenuates the antinociceptive tolerance to morphine assessed by tail-flick test, indicating that the activation of p38 MAPK in the spinal cord plays an important role in the development of tolerance to morphine analgesia.
To date, four different p38 isoforms, α, β, γ and δ, have been identified (Kumar et al., 2003). However, there are two p38 isoforms α and β found in the spinal cord. Svensson et al. (2005) recently demonstrated that the isoforms are distinctly expressed in spinal dorsal horn: p38α in neurons and p38β in microglia. Since SB 203580 non-selectively inhibits both glial p38β and neuronal p38α MAPK (Barone et al., 2001), it is not clear that dextro-morphine and morphine act on glial or neuronal p38 MAPK for inducing antianalgesia. However, we have previously demonstrated that pretreatment with a glial modulator propentofylline (Sweitzer et al., 2001) reverses the antianalgesia induced by dextro-morphine or morphine, suggesting that dextro-morphine or morphine act on glia rather than neurons for inducing antianalgesia (Wu et al., 2005). Others also reported that p38 MAP kinase is activated in the spinal microglia after a sciatic nerve ligation and inflammatory pain, which contributes to the generation of hyperalgesia and allodynia (Ji et al., 2002; Svensoon et al., 2003; Jin et al., 2003; Schafers et al., 2003; Tsuda et al., 2004).
Acknowledgements
This work was supported by grant DA12588 from the National Institute of Health, National Institute on Drug Abuse (PI: Leon F.Tseng) and Research Affair Committee, Medical College of Wisconsin (PI: Hsiang-En Wu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, Badger AM, Legos JJ, Erhardt JA, Ohlstein EH, Hunter AJ, Harrison DC, Philpott K, Smith BR, Adams JL, Parsons AA. Inhibition of p38 mitogen-activated protein kinase provides neuroprotection in cerebral focal ischemia. Med. Res. Rev. 2001;21:129–145. doi: 10.1002/1098-1128(200103)21:2<129::aid-med1003>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Cui Y, Chen Y, Zhi J-L, Guo R-X, Feng J-Q, Chen P-X. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069:235–243. doi: 10.1016/j.brainres.2005.11.066. [DOI] [PubMed] [Google Scholar]
- D’Amour FE, Smith DL. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941;72:74–79. [Google Scholar]
- Hylden JLK, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol. 1980;167:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Iijima I, Minamikawa J-I, Jacobsen AE, Brossi A, Rice KE. Studies in the (+)-morphine series-5. Synthesis and biological properties of (+)-naloxone. J. Med. Chem. 1978;21:398–400. doi: 10.1021/jm00202a018. [DOI] [PubMed] [Google Scholar]
- Jacquet YF, Klee WA, Rice KC, Iijima I, Minamikawa J. Stereospecfic and nonstereospecific effects of (+)- and (-)-morphine: evidence for a new class of receptors? Science. 1977;198:842–845. doi: 10.1126/science.199942. [DOI] [PubMed] [Google Scholar]
- Ji RR, Baba K, Brenner GJ, Woold CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J. Neurosci. 2002;22:478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S-X, Zhuang A-Y, Woolf CJ, Ji R-R. p38 Mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J. Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signaling molecules as therapeutic targets for inflammatory diseases. Nature Rev. Drug Disc. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- Ma W, Zheng W-H, Powell K, Jhamandas K, Quirion R. Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons: an in vitro and in vivo study. Eur. J. Neurosci. 2001;14:1091–1104. doi: 10.1046/j.0953-816x.2001.01731.x. [DOI] [PubMed] [Google Scholar]
- Mace G, Miaczynska M, Zerial M, Nebreda AR. Phosphorylation of EEA1 by p38 MAP kinase regulate μ opioid receptor endocytosis. EMBO J. 2005;24:3235–3246. doi: 10.1038/sj.emboj.7600799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda A, Porras A. p38 MAPK kinases: beyond the stress response. Trends Biochem. Sci. 2000;25:257–260. doi: 10.1016/s0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- Schafer M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-α induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J. Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activtion of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J. Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Fitzsimmons B, Azizi S, Powell HC, Hua X-Y, Yaksh TL. Spinal p38β isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. J. Neurochem. 2005;92:108–1520. doi: 10.1111/j.1471-4159.2004.02996.x. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J. Pharmacol. Exp. Ther. 2001;297:1210–1217. [PubMed] [Google Scholar]
- Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- Wu H, Thompson J, Sun H, Leitermann RJ, Fujimoto JM, Tseng LF. Nonopioidergic mechanism mediating morphine-induced antianalgesia in the mouse spinal cord. J. Pharmacol. Exp. Ther. 2004;310:240–246. doi: 10.1124/jpet.104.065334. [DOI] [PubMed] [Google Scholar]
- Wu H, Thompson J, Sun H, Terashvili M, Tseng LF. Antianalgesia: stereo-selecitve action of dextro-morphine over levo-morphine on glia in the mouse spinal cord. J. Pharmacol. Exp. Ther. 2005;314:1101–1108. doi: 10.1124/jpet.105.087130. [DOI] [PubMed] [Google Scholar]
- Wu H, Sun H, Terashvili M, Schwasinger E, Sora I, Hall FS, Uhl GR, Tseng LF. dextro- and levo-morphine attenuate opioid δ and κ receptor agonist produced analgesia in μ-opioid receptor knockout mice. Eur. J. Pharmacol. 2006;531:103–107. doi: 10.1016/j.ejphar.2005.12.012. [DOI] [PubMed] [Google Scholar]