Abstract
In this study we evaluate the antifibrotic properties of PG-490-88, a water-soluble derivative of triptolide. Triptolide is an oxygenated diterpene that is derived from a traditional Chinese herb that has potent immunosuppressive and antitumor activity. We used the intratracheal bleomycin mouse model and found that PG490-88 inhibits fibrosis in the bleomycin group when given the same day or 5 days after bleomycin. PG490-88 also markedly reduced the number of myofibroblasts in the bleomycin treatment group. An enzyme-linked immunosorbent assay of transforming growth factor (TGF)-β in the bronchoalveolar lavage fluid showed a significant decrease in TGF-β in the PG490-88-treated groups compared to the bleomycin-treated group. Additionally, triptolide blocked bleomycin-induced increase in TGF-β mRNA in cultured normal human lung fibroblasts. The efficacy of PG490-88 when administered late after bleomycin installation suggests a potential role in the treatment of idiopathic pulmonary fibrosis.
Idiopathic pulmonary fibrosis is a progressive interstitial lung disease of unknown etiology. The disease most commonly affects middle-aged adults although infants and children are also affected. It is characterized by the excessive deposition of extracellular matrix in the lung interstitium. The pathological features of inflammation and fibrosis are well appreciated but little is known about its etiology and pathogenesis. 1,2 To date there is no satisfactory treatment for idiopathic pulmonary fibrosis. Various anti-inflammatory agents such as corticosteroids, 3 colchicine, 4 and cytotoxic agents such as azathioprine 5 and cyclophospamide 6 have been used alone or in combination to treat the disease. However, less than one-third of patients respond to treatment with corticosteroids and/or cytotoxic therapy.
A newer class of agents with antifibrotic properties is currently under investigation. For example, pirfenidone, 7 interferon-β, 8 and interferon-γ 9 are in clinical trials. The exact mechanism by which these agents exert their antifibrotic property is yet to be defined. Triptolide (PG-490) is a diterpene triepoxide derived from the Chinese herb, Tripterygium Wilfordii hook. It has potent antiproliferative and immunosuppressive properties. Triptolide, for example, has been used in traditional Chinese medicine in the treatment of rheumatoid arthritis. 10 Triptolide has also shown to possess antileukemic activities and to inhibit proliferation of transformed cell lines in vitro. 11,12 An extract of Tripterygium, PG 27, has been shown to prolong heart and kidney allograft survival in rat transplantation models. 11 PG27, along with cyclosporine prolongs cardiac xenograft survival from a hamster donor to a rat recipient by inhibiting the production of serum anti-hamster IgM and IgG antibodies. 11 Wei and co-workers 12 have shown that PG27 suppresses the development of graft-versus-host disease associated with allogenic bone marrow transplantation. Another extract of Tripterygium, T2 (5% triptolide) has been shown to block mitogen-induced early cytokine gene transcription in T cells. 13 Qiu and colleagues 14 have shown that PG490 (97% pure triptolide) blocks cytokine gene expression by human peripheral blood lymphocytes and 16HBE human bronchial epithelial cells by transcriptional inhibition of nuclear factor-κB (NF-κB). We have recently shown that PG490 promotes tumor necrosis factor-α mediated apoptosis of tumor cell lines by inhibiting NF-κB. 15 Triptolide, therefore, exhibits diverse immunosuppresive and antitumor effects.
In this study, we show that PG-490-88, a water-soluble succinate salt derivative of triptolide, blocks both inflammation and fibrosis in the bleomycin model of mouse lung fibrosis. PG490-88 at a dose of 0.25 mg/kg/day for 14 days, administered intraperitoneally, decreased inflammation and fibrosis after a single intratracheal instillation of bleomycin in C57BL/6 mice. PG490-88 blocked fibrosis and decreased inflammation when treatment was initiated both on the same day as bleomycin administration or if the treatment was initiated 5 days after bleomycin instillation. The bronchoalveolar lavage fluid (BALF) level of TGF-β also showed a significant decrease in the PG490-88-treated group compared to the bleomycin group. Additionally, reverse transcriptase-polymerase chain reaction (RT-PCR) of normal human lung fibroblasts treated with bleomycin and PG490 (triptolide) showed an inhibition in TGF-β gene expression in the PG-490-treated cells compared to bleomycin alone.
Materials and Methods
Murine Model of Bleomycin-Induced Pulmonary Fibrosis
Eight- to 10-week-old male C57BL/6 mice weighing between 22 to 26 g were obtained from the Stanford University (Palo Alto, CA) animal facility. The animals were caged in groups of five and housed in the Stanford animal care facility. They had free access to rodent chow and water. All care was in accordance with the National Institutes of Health Guide for Animal Welfare Act. Mice in each set of experiment were randomly assigned into five groups of five each. Mice in groups 1 and 2 were treated intratracheally with 0.1 ml of sterile saline alone or saline plus intraperitoneal PG490-88 (a gift from Pharmagenesis, Palo Alto, CA). To induce pulmonary fibrosis, mice in groups 3, 4, and 5 were treated on day 1 with 0.1 ml of bleomycin (Blenoxane, Novaplus, 15 U/vial; VHA Inc., Nippon Kayaku Co., Tokyo, Japan) at a dosage of 0.1 U/mouse by intratracheal injection under anesthesia. The anesthetic solution used is a mixture of 1 ml of ketamine (100 μg/ml) and 1 ml of xylazine (100 μg/ml) and 4.6 ml of sterile bacteriostatic saline (Stanford comparative medicine pharmacy) at a dose of 0.1 ml/30 g body weight. Mice in groups 4 and 5 were treated daily with PG490-88 (0.25 mg/kg) starting the same day as bleomycin treatment or 5 days after bleomycin installation. The animals were weighed every other day. All of the animals that reached 17 g or below were euthanized and the lungs were not used for analysis. Animals that died before day 15 were also not included in the analysis. All of the animals were sacrificed on day 15 after bleomycin or saline and PG490-88 treatment. The lungs were removed and appropriately processed for light microscopy or were frozen in liquid nitrogen for analysis of hydroxyproline content. Separate experiments were conducted for each of the analysis. The experiment was conducted four times (five animals per group) for hydroxyproline analysis, two times (five animals per group) for light microscopy with one lung for hematoxylin and eosin (H&E) and one lung for trichrome staining. A single experiment was used for the analysis of α-smooth muscle actin expression by immunohistochemistry (five animals per group) and a different experiment was used for the enzyme-linked immunosorbent assay (ELISA) of TGF-β from bronchoalveolar lavage (three animals per group). The actual number of animals used for analysis is given in Results and in the figures.
Histological Scoring of Fibrosis
The lung samples were fixed by inflation with buffered 10% formalin solution for 24 hours. After embedding in paraffin, the sections were prepared and stained by H&E or by trichrome stain. The severity of fibrosis was semiquantitatively assessed according to the method proposed by Ashcroft and co-workers. 16 Briefly, the grade of lung fibrosis was scored on a scale from 0 to 8 by examining 44 randomly chosen fields per sample at a magnification of ×100. Criteria for grading lung fibrosis were as follows: grade 0, normal lung; grade 1, minimal fibrous thickening of alveolar or bronchiolar walls; grade 3, moderate thickening of walls without obvious damage to lung architecture; grade 5, increased fibrosis with definite damage to lung structure and formation of fibrous bands or small fibrous masses; grade 7, severe distortion of structure and large fibrous areas; grade 8, total fibrous obliteration of fields. If there was difficulty in deciding between two odd-numbered categories, the field would be given the intervening even-numbered grade. The observations were then entered into a repeated measures analysis of variance. Treatment (three levels) was considered fixed and field (44 levels) was repeated with a compound symmetric structure. Scoring of the untreated and PG490-alone groups both gave values of zero so the data were not included in the analysis of variance because they violated the normality assumptions underlying the analysis.
Immunohistochemistry
Paraffin-embedded tissue from control and bleomycin-treated lungs were processed for immunohistochemical localization of α-smooth muscle actin to identify myofibroblasts. Briefly, tissue sections were dewaxed in xylene and rehydrated through graded concentrations of ethanol. Slides were blocked with protein block serum-free (DAKO Corp., Carpinteria, CA) and overlaid with a 1:400 dilution of monoclonal mouse anti-α-smooth muscle actin antibody overnight (Sigma-Aldrich Inc., St. Louis, MO) or phosphate-buffered saline (PBS) as negative control at 4°C. Slides were then rinsed and overlaid with biotinylated anti-mouse link immunoglobulins (Universal Dako LSAB Kit, Peroxidase; DAKO) for 60 minutes. After washing with PBS, slides were then overlaid with horseradish peroxidase conjugated to streptavidin (DAKO) and incubated for 60 minutes in 3,3-diaminobenzidine (DAB) chromogen for chromogenic localization of actin (liquid DAB substrate kit; ZYMED Laboratories, Inc., San Francisco, CA). After optimal color development, sections were immersed in sterile water, counterstained with Harris alum hematoxylin, and coverslipped using an aqueous mounting solution.
Hydroxproline Assay
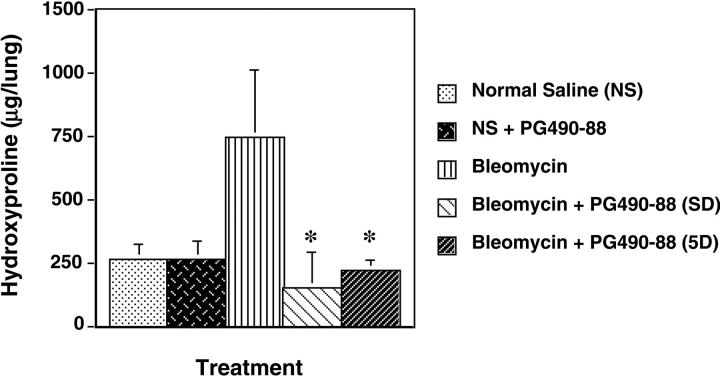
To estimate the total amount of collagen deposited as an indicator of pulmonary fibrosis, the hydroxyproline content of whole lung was measured in each group according to the procedure described by Woessner. 17 A spectrophotometric assay was used to quantify the lung hydroxyproline. Briefly, lungs were harvested and homogenized in 5 ml of saline with a Polytron homogenizer (IKA-Labortechnik, Staufen, Germany). Each sample (0.5 ml) was then digested in 2 ml of 6 N HCl for 16 hours at 110°C. The samples were neutralized with NaOH until pH 7. One ml of 0.5 mol/L Chloramine T reagent was then added and the samples were left at room temperature for 20 minutes. Next, 1 ml of 3.15 N perchloric acid and P-dimethylaminobenzaldehyde were added to each sample, and the samples were incubated for 20 minutes at 65°C. Samples were cooled for 10 minutes and read at 557 nm on a DU-64 spectrophotometer (Beckman Coulter, Inc., Hialeah, FL). trans−4-hydroxy-l-proline (Sigma) concentrations from 0 to 5 μg/ml were used to construct a standard curve. The data were first log-transformed to remove the observed correlation between the means and variances. The observations were then entered into a mixed model analysis of variance, with experiment (four levels) considered random and treatment (five levels) considered fixed. The geometric least squares means were obtained by exponentiation from the least squares means (see Figure 3 ▶ ).
Figure 3.
Total hydroxyproline content ± SE in μg/lung. In the bleomycin plus PG490-88 (5 days) group, PG490-88 was administered 5 days after bleomycin. Four independent experiments were used for the statistical analysis that is outlined in Materials and Methods. *, P < 0.01 compared to bleomycin alone.
BALF and Measurement of TGF-β Level in BALF by ELISA
The BALF was obtained 14 days after bleomycin treatment from three mice in each group. TGF-β levels in the BALF were assayed using a commercially available TGF-β ELISA kit (TGF-β E max ImmunoAssay System; Promega Corp., Madison, WI). The kit contains a TGF-β coat monoclonal antibody for a 96-well microtiter plate coating and immunomobilized mouse polyclonal antibody to TGF-β with a reported sensitivity of 15.6 pg/ml. BALF was obtained from the mice under anesthesia using 1 ml of sterile isotonic saline. Lavage was performed four times in each mouse and the total volume collected separately. The volume of fluid collected in each mouse ranged from 3.0 to 3.5 ml. The lavage fluid was centrifuged at 1,500 rpm at 4°C for 15 minutes. To assay for total TGF-β, the supernatant was first acidified to process TGF-β from a latent form to the bioactive form. The active form is immunoreactive and detected by the anti-TGB-β antibody. The representative standard curve was generated using the TGF-β standard provided with the kit. The observations were entered into a mixed model analysis of variance, with experiment (two levels) considered random and treatment (four levels) considered fixed.
Culture of Normal Human Lung Fibroblasts (NHLFs)
The NHLF cell line was obtained from Clonetics-BioWhittaker (San Diego, CA). NHLFs were cultured in fibroblast growth medium (Clonetics) at 37°C in a 5% CO2 atmosphere. The cultures were incubated at various concentrations of bleomycin (0, 0.1, and 10 μg/ml; Blenoxane, Novaplus, 15 U/Vial) for 16 hours and the cells were then harvested for RNA extraction.
Analysis of TGF-β mRNA Expression
RT-PCR for TGF-β mRNA was done on NHLFs treated with varying doses of bleomycin (0, 0.1, and 10 U). TGF-β mRNA was measured in the presence or absence of PG490 (20 ng/ml) that was added the same time as TGF-β and samples were harvested after 24 hours. Total RNA from NHLFs was extracted using Total RNA/mRNA isolation reagent (RNA STAT-60; Tei-Test“B”, Inc.). Four mg of total RNA was used for reverse-transcription into cDNA with a cDNA synthesis kit (Life Technologies, Inc., Rockville, MD) and then amplified for 35 cycles in MiniCycle PCR system (MJ Research, Inc.) with denaturation at 95°C for 30 seconds, primer annealing at 55°C for 30 seconds, and primer extension at 72°C for 2 minutes. TGF-β and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense and antisense oligonucleotides were: TGF-β sense, 5*-CGC ATA CAG TTA TCT CGG ACG A C-3*; antisense, 5*-TTT GTT GGC TGC TCT CAC GG-3*; GAPDH sense, 5*-GGA GCC AAA AGG GTC ATC TC-3*, antisense, 5*-AGT GGG TGT CGC TGT TGA AGT C-3*. PCR products were separated by electrophoresis on 2% agarose gel with ethidium bromide (EB) and were visualized with a electronic UV Transilluminator (Ultra-Lum, Inc.).
Analysis of Cell Viability
Cell viability was measured by an of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Briefly, untreated cells or cells treated with PG490 in a 96-well plate at the dosages shown were harvested at 24 hours followed by the addition 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to the cells. Cells were then solubilized with 0.1 N acidified CH3Cl-HCl. The 96-well plate was read at a wavelength of 590 nm on an iEMS Labsystems plate reader.
Results
The histological appearance of the mouse lungs in the different treatment groups is shown in Figure 1A ▶ after sacrifice on day 15. The saline control group and the saline plus PG490-88 (0.25 mg/kg/day) group showed normal lung architecture. The bleomycin-alone group shows extensive inflammation and fibrosis. The PG490-88 treatment groups showed a marked decrease in inflammation and fibrosis when treatment is initiated on the same day as the bleomycin insult or 5 days later. The animals treated with triptolide 5 days after bleomycin were also sacrificed on day 15 so that they received treatment with PG490-88 for 9 days. The extent of fibrosis was scored by the method proposed by Ashcroft and colleagues. 16 Forty-four randomly chosen fields from each animal (5 animals per group) were used for analysis. Each sample was scored by a single observer and numerical scores were assigned based on the degree of fibrosis (see Materials and Methods for details). The mean scores are as follows: saline control, 0; saline plus PG490, 0; bleomycin alone, 3.36; bleomycin plus PG490 treatment starting the same day, 1.07; bleomycin plus PG490 treatment started 5 days later, 1.67 (Table 1) ▶ . The degree of fibrosis was significantly less (P < 0.001) in the treatment group both when the treatment was started on the same day as bleomycin or 5 days later. PG-490-88 was well tolerated with no change in activity, skin color, and respiration. The PG490-88-treated mice also showed an increase in body weight compared to the bleomycin group (Table 1) ▶ . There are no apparent adverse effects on long-term treatment with PG490-88 at this dosage based on studies with up to 3 months of dosing in a mouse xenograft tumor model (J. H. Fidler and G. D. Rosen, unpublished results). However, dosages >0.75 to 1.0 mg/kg/day are associated with gastrointestinal toxicity manifested by intestinal hemorrhage (J. H. Fidler and G. D. Rosen, unpublished results).
Figure 1.
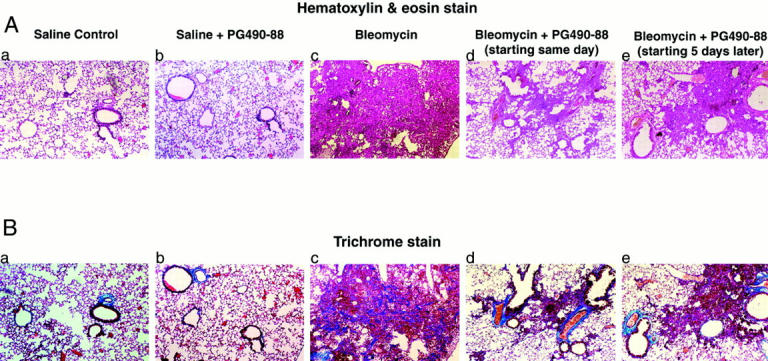
A: H&E stain: ×100. a: Saline control, normal lung architecture. b: Saline plus PG490-88, normal lung architecture. c: Bleomycin alone, extensive inflammation and fibrosis. d: Bleomycin plus PG490-88 (same day), patchy areas of inflammation with minimal fibrosis. e: Bleomycin plus PG490-88 (5 days later), patchy areas of inflammation with minimal fibrosis. B: Comparable sections of mouse lung to Figure 1 ▶ stained with trichrome: saline control: normal lung architecture (a); saline plus PG490-88, normal lung architecture (b); bleomycin alone, extensive areas of collagen (stained blue) (c); bleomycin plus PG490-88 (same day), minimal collagen (d); bleomycin plus PG490-88 (5 days later), minimal collagen (e).
Table 1.
Mean Body Weight and Fibrosis Scores
| Groups | Mean body weight (g) Day 0 | Mean body weight (g) Day 15 | Mean fibrosis scores |
|---|---|---|---|
| Saline | 25.2 (27.7–22.6) | 27.3 (29.7–25.4) | 0 (0–0) |
| Saline+ PG 490-88 | 24.9 (26.5–23.4) | 26.6 (28.7–24.2) | 0 (0–0) |
| Bleomycin alone | 26.1 (27.8–25.2) | 20.2 (22.3–18.0) | 3.36 (3.05–3.65) |
| Bleomycin+ PG 490-88 (same day) | 26.0 (27.1–25.2) | 22.6 (24.5–20.1) | 1.10* (0.77–1.37) |
| Bleomycin+ PG 490-88 (5 days later) | 25.3 (26.7–23.2) | 22.9 (24.1–20.6) | 1.67* (1.37–1.96) |
The mean fibrosis scores reveal a significant effect of treatment compared to bleomycin alone (*P < 0.01). The numbers in parentheses from the mean fibrosis scores correspond to upper and lower 95% confidence intervals.
We then stained the tissue sections with a trichrome stain that detects type I collagen. Type I collagen is the dominant form of collagen that is expressed in patients with pulmonary fibrosis. The bleomycin-alone group shows excessive accumulation of collagen in the lung tissue compared to the treatment groups (Figure 1B) ▶ . The accumulation of collagen is inhibited even when PG490-88 treatment is initiated 5 days after bleomycin instillation.
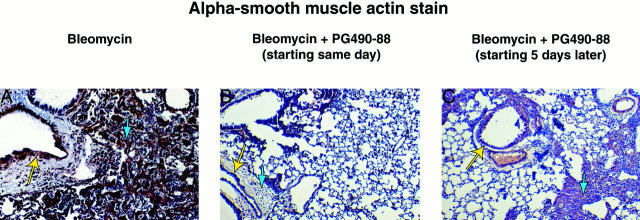
We also immunostained the mouse lung tissue with α-smooth muscle actin that identifies myofibroblasts. Myofibroblast proliferation is thought to be a hallmark of lung fibrosis and myofibroblasts in lung fibrosis express α-smooth muscle actin. There was a decrease in the presence of myofibroblasts in the PG490-88-treated groups compared to the bleomycin-alone group, again when given the same day or 5 days after bleomycin (Figure 2) ▶ .
Figure 2.
Alpha smooth-muscle actin stain of mouse lung sections (original magnification, ×100) that detects myofibroblasts (blue arrow) and airway smooth muscle cells that are a positive control (yellow arrow). Left: Bleomycin alone, extensive accumulation of α-smooth muscle actin-positive myofibroblasts. Center: Bleomycin plus PG490-88 (same day), small number of myofibroblasts. Right: Bleomycin plus PG490-88 (5 days later), scattered areas of myofibroblasts. Consecutive sections of mouse lung tissue stained with H&E are shown for comparison.
Total lung hydroxyproline has been shown to be a sensitive measure of collagen turnover in the lungs, ie, synthesis, deposition, and degradation. We, therefore, measured total hydroxyproline in the different groups according to the protocol described in Materials and Methods. Total lung hydroxyproline content was significantly decreased in the PG490-88-treated groups (P < 0.01) compared with the bleomycin group (1.47 μg/lung; n = 12) both when the treatment was initiated on the same day (0.292 μg/lung; n = 11) as bleomycin installation or 5 days later (0.43 μg/lung; n = 11) (Figure 3) ▶ . The saline plus PG490 group had hydroxyproline levels (0.519 μg/lung; n = 19) that are almost identical to the control group (0.521 μg/lung; n = 18) (Figure 3) ▶ .
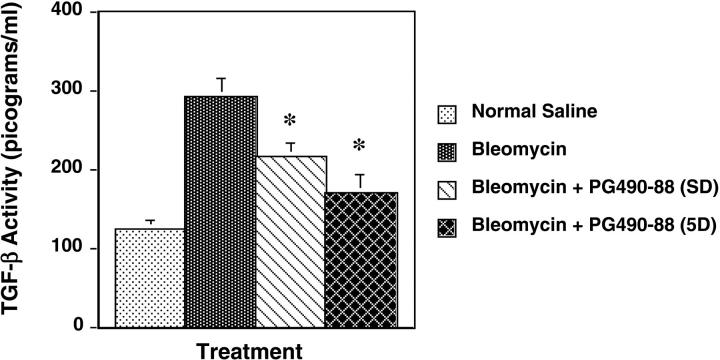
TGF-β is known to play a central role in the pathogenesis of pulmonary fibrosis. 18-24 To examine the effect of PG490-88 on TGF-β, we assayed TGF-β activity in BALF level by ELISA: group 1: intratracheal saline plus PG490-88 (0.25 mg/kg); group 2: intratracheal bleomycin; group 3: bleomycin plus intraperitoneal PG490-88 starting same day; and group 4: bleomycin plus intraperitoneal PG490-88 (0.25 mg/kg) starting 5 days later. Bronchoalveolar lavage was performed on day 15 in all of the groups under anesthesia and the animals were then sacrificed. TGF-β activity was significantly lower in the treatment groups (P < 0.01): PG490-88 plus bleomycin (SD) (215.3 pg/ml) and PG490-88 plus bleomycin (5 days) (169.0 pg/ml) versus bleomycin alone (290.5 pg/ml) (Figure 4) ▶ .
Figure 4.
BALF levels of active TGF-β (pg/ml) ± SE measured by ELISA. *, P < 0.01 compared to bleomycin alone.
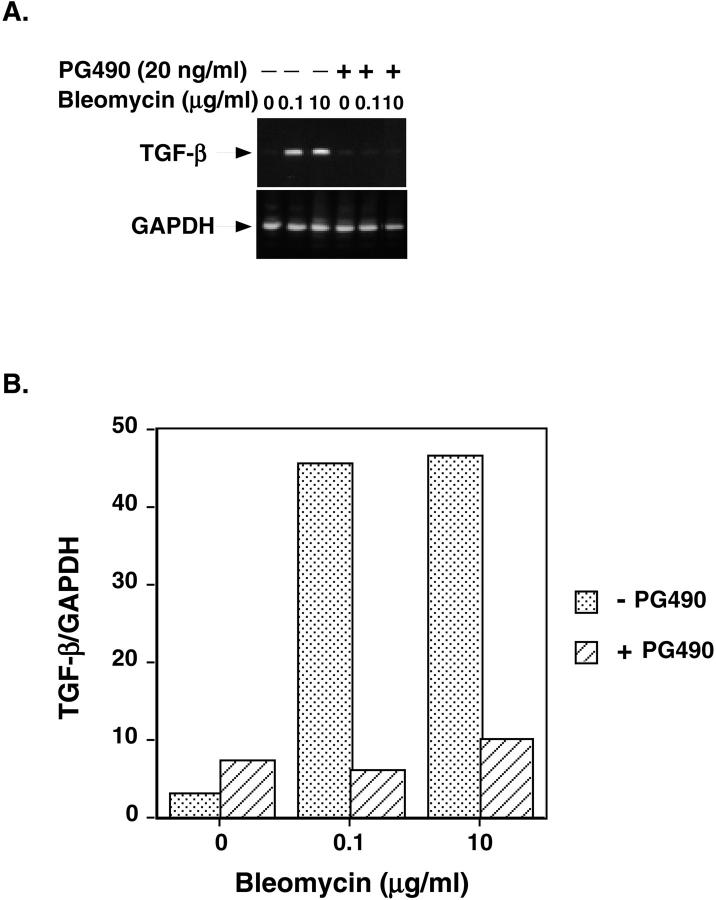
In view of recent studies that show triptolide inhibits activation-induced cytokine gene transcription, we examined the effect of PG490 (purified triptolide) on TGF-β mRNA expression in normal human lung fibroblasts. Normal human lung fibroblasts (Colonetics Inc., Walkersville, MD) were stimulated with bleomycin at various dose ranges (0, 0.1, 1, and 10 μg/ml) and RT-PCR for TGF-β mRNA was done in the presence or absence of PG490 (20 ng/ml). We observed that PG490 caused a significant reduction in TGF-β mRNA expression (eightfold for 0.1 μg/ml and fivefold for 10 μg/ml) in response to bleomycin (Figure 5) ▶ . It was possible that the PG490-mediated suppression in TGF-β transcription is a result of antiproliferative or cytotoxic effects of PG490 on NHLFs. PG490 (20 ng/ml), however, only reduced NHLF viability by 15% after 24 hours of treatment (Table 2) ▶ . These data suggest, therefore, that PG490 inhibits the bleomycin-mediated increase in TGF-β activity, at least in part, through inhibition of TGF-β gene expression.
Figure 5.
A: RT-PCR of TGF-β mRNA in NHLFs stimulated for 24 hours with 0.1 μg/ml or 10 μg/ml of bleomycin in the presence or absence of PG490 (20 ng/ml). GAPDH is used as a loading control. B: The ratio of TGF-β/GAPDH mRNA is shown.
Table 2.
Effect of Triptolide on Viability of NHLF
| Groups | Viable cells (%) ± SE |
|---|---|
| Control | 100 |
| PG-490 (2 ng/ml) | 108 ± 1.8 |
| PG-490 (5 ng/ml) | 95.6 ± 1.95 |
| PG-490 (20 ng/ml) | 84.8 ± 3.2 |
Normal human lung fibroblasts (NHLFs) were treated for 24 hours with triptolide (2 to 20 ng/ml) and then harvested for analysis of viability by an MTT assay. The data shown is the average of 10 samples for each condition from two independent experiments ± SE of the mean of the two experiments. The control (unstimulated) sample is assigned a viability of 100%.
Discussion
Pulmonary fibrosis is characterized by excessive accumulation of extracellular matrix and collagen in the lung parenchyma leading to progressive hypoxemia and death. Current treatment modalities are minimally effective. The current focus on treatment for this relentlessly progressive disease is to suppress or decrease the fibrosis in the lung with antifibrotic agents. 2,25 Lung fibrosis is a result of a complex interplay between various cell types, immunological, genetic factors, and cytokines that regulate collagen synthesis and degradation that ultimately results in excessive extracellular matrix deposition.
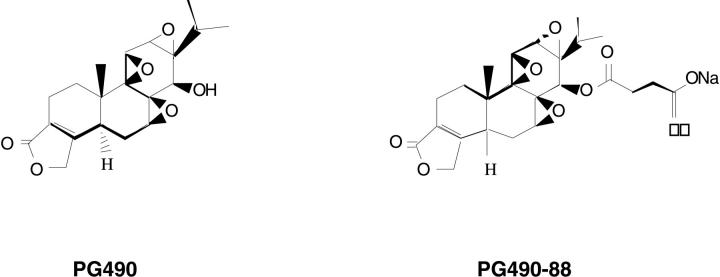
This study was undertaken to demonstrate the antifibrotic properties of PG490-88, a derivative of triptolide. Triptolide is an oxygenated diterpine triepoxide isolated from Tripterygium wilfordii hook.f (Celastraceae). 26 The molecular structure of this compound is shown in Figure 6 ▶ . Various extracts from this plant have been used in traditional Chinese medicine as a treatment for inflammatory conditions such as rheumatoid arthritis. 10 Triptolide has also been shown to have antiproliferative, antileukemic, and immunosuppressive properties in animal models and in transplant rejection models. 15 For example, we have recently shown that triptolide sensitizes tumor cells to tumor necrosis factor-α-induced apoptosis by blocking tumor necrosis factor-α-mediated activation of NF-κB. 15 Also, Qiu and colleagues showed that triptolide inhibits expression of the proinflammatory cytokines interleukin-2 and interleukin-8 by blocking activation of NF-κB. 14 Here we show that PG490-88, a water-soluble derivative of triptolide inhibits bleomycin-mediated inflammation and fibrosis. Triptolide may therefore exert its action, in part, by blocking NF-κB-mediated cytokine production by immune cells in this model. Most agents that have been studied using the bleomycin animal model show efficacy only when the experimental drug is given simultaneously or before bleomycin. We show here that PG490-88 inhibits pulmonary fibrosis even when administered 5 days after bleomycin.
Figure 6.
Molecular structure of PG-490-88 and triptolide. PG490-88 is a succinate salt-water soluble derivative of triptolide that is converted to triptolide in the serum.
The accumulation of extracellular collagen is the hallmark of pulmonary fibrosis. There is increasing evidence that an important stimulus for the accumulation of collagen is increased production of proinflammatory and profibrotic cytokines. 27 TGF-β is the most potent profibrotic cytokine that induces collagen accumulation by several different mechanisms. It is found in relatively high levels in the normal lower respiratory tract and it is expressed by alveolar macrophages and platelets at sites of injury. 19 TGF-β increases the production of collagen and fibronectin by embryonic lung fibroblasts 20 and increases the production of types I, III, and V collagen by adult human fibroblasts. 21 It also increases α-1 collagen (I) mRNA gene transcription and increases the RNA stability of collagen and fibronectin. 22 In addition, in combination with platelet-derived growth factor and epidermal growth factor, it is mitogenic for fibroblasts, increases the production of fibronectin by macrophages, and stimulates fibroblast chemotaxis. Moreover, TGF-β acts in concert with other cytokines to inhibit fibroblast degradation by inhibiting the expression of tissue inhibitors of metalloproteinases and plasminogen activator/plasminogen activator inhibitors. 23 In animal models of bleomycin-induced fibrosis, TGF-β mRNA expression has been shown to precede increases in collagen production that correlates with areas of active fibrosis. 24,28
We show that PG490-88 inhibits the bleomycin-mediated increase in TGF-β activity in vivo and that PG490 (triptolide) inhibits the bleomycin-mediated increase in TGF-β transcription in normal human lung fibroblasts (Figures 4 and 5) ▶ ▶ . TGF-β activity in BAL was significantly lower in PG490-88 treatment groups even when administered 5 days after bleomycin. The mechanism of this suppression may result, in part, from triptolide-mediated inactivation of other transcription factors such as nuclear factor of activated T cells and Sp1 (M. Gao and G. D. Rosen, unpublished results). 14 We have also observed that triptolide is a potent antitumor agent in vivo (J. J. Fidler and G. D. Rosen, unpublished results). We are presently looking for intracellular targets of triptolide in an effort to decipher its potent antifibrotic, immunosuppresive, and antitumor effects. Our finding that PG490-88 inhibits bleomycin-mediated fibrosis even when administered 5 days after bleomycin suggests a potential role in the treatment of patients with pulmonary fibrosis.
Acknowledgments
We thank Mitchell J. Rosen for the statistical analysis and John A. Fidler from Pharmagenesis for providing PG490-88 and PG490.
Footnotes
Address reprint requests to Glenn D. Rosen, M.D., Associate Professor, Stanford University Medical Center, Division of Pulmonary and Critical Care Medicine, 300, Pasteur Dr., Stanford, CA 94305-5236. E-mail: grosen@leland.stanford.edu.
Supported by the Suha and Khalid Shoman Family fund.
G. K. and K. L. both contributed equally to preparation of the manuscript.
References
- 1.Katzenstein AA, Myers JL: Idiopathic pulmonary fibrosis. Clinical relevance of pathologic classification. AJRCCM 1998, 157:1301-1315 [DOI] [PubMed] [Google Scholar]
- 2.Idiopathic pulmonary fibrosis: diagnosis and treatment: international consensus statement. AJRCCM 2000, 161:646–664 [DOI] [PubMed]
- 3.Mapel DW, Samet JM, Coultas DB: Corticosteroids and the treatment of idiopathic pulmonary fibrosis: past, present and future. Chest 1996, 110:1058-1067 [DOI] [PubMed] [Google Scholar]
- 4.Douglas WW, Ryu JH, Bjoraker JA, Schroder DR, Myers JL, Tazelaar HD, Swensen SJ, Peters SG, deRemee RA: Colchicine versus prednisone as treatment of usual interstitial pneumonia. Mayo Clin Proc 1997, 72:201-209 [DOI] [PubMed] [Google Scholar]
- 5.Raghu G, Depaso WJ, Cain K, Hammar SP, Wetzel CE, Dreis DF, Hutchinson J, Pardee NE, Winterbauer RH: Azathioprine combined with prednisone in the treatment of idiopathic pulmonary fibrosis: a prospective, double blind, randomized, placebo controlled trial. Am Rev Respir Dis 1991, 144:291-296 [DOI] [PubMed] [Google Scholar]
- 6.Johnson MA, Kwan S, Snell NJC, Nunn AJ, Darbyshire JH, Turner-Warwick M: Randomized controlled trial comparing prednisolone alone with cyclophospamide and low dose prednisolone in combination in cryptogenic fibrosing alveolitis. Thorax 1989, 44:280-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raghu G, Johnson WC, Lockhart D, Mageto Y: Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone. Results of a prospective, open label phase II study. AJRCCM 1999, 159:1061-1069 [DOI] [PubMed] [Google Scholar]
- 8.Raghu G, Bozic CR, Brown K, Lynch D, Center D, Aguayo SM, Lloyd K, Lull J, Kevitsky D, Schwartz DA, King TE: Trial of interferon beta-1a in idiopathic pulmonary fibrosis: characteristics of patients with and without surgical lung biopsy. ATS Abstract. 2000, Canada, Toronto
- 9.Ziesche R, Hofbauer E, Wittman K, Petkov V, Block L: A preliminary study of long term treatment with interferon gamma 1b and low dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med 1999, 341:1264-1269 [DOI] [PubMed] [Google Scholar]
- 10.Tao XL, Sun Y, Dong Y, Xiao YL, Hu DW, Shi YP, Zhu QL, Dai H, Zhang NZ: A prospective, controlled, double-blind, cross-over study of Tripterygium wilfordii hook F in treatment of rheumatoid arthritis. Chin Med J 1989, 102:327-332 [PubMed] [Google Scholar]
- 11.Kupchan SM, Court WA, Dailey R, Jr, Gilmore CJ, Bryan RF: Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J Am Chem Soc 1972, 94:7194-7195 [DOI] [PubMed] [Google Scholar]
- 12.Wei YS, Adachi I: Inhibitory effect of triptolide on colony formation of breast and stomach cancer cell lines. Chung Kuo Yao Li Hsueh Pao 1991, 12:406-410 [PubMed] [Google Scholar]
- 13.Tao X, Davis LS, Hashimoto K, Lipsky PE: The Chinese herbal remedy, T2, inhibits mitogen-induced cytokine gene transcription by T cells, but not initial signal transduction. J Pharmacol Exp Ther 1996, 276:316-325 [PubMed] [Google Scholar]
- 14.Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, Ng JCH, Kao PN: Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-κB transcriptional activation. J Biol Chem 1999, 274:13443-13450 [DOI] [PubMed] [Google Scholar]
- 15.Lee KY, Chang W, Qiu D, Kao PN, Rosen GD: PG490 (triptolide) cooperates with tumor necrosis factor-α to induce apoptosis in tumor cells. J Biol Chem 1999, 274:13451-13455 [DOI] [PubMed] [Google Scholar]
- 16.Ashcroft T, Simpson JM, Timbrell V: Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 1988, 41:467-470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woessner JF, Jr: The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys 1961, 93:440-444 [DOI] [PubMed] [Google Scholar]
- 18.Coker RK, Laurent GJ: Pulmonary fibrosis: cytokines in the balance. Eur Respir J 1998, 11:1218-1221 [DOI] [PubMed] [Google Scholar]
- 19.Assoian RK, Fleurdelys BE, Stevenson HC, Miller PJ, Madtes DK, Raines EW, Ross R, Sporn MB: Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA 1987, 84:6020-6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ignotz RA, Massague J: Transforming growth factor beta stimulates the expression of fibronectin and collagen and their incorporation into extra cellular matrix. J Biol Chem 1986, 261:4337-4345 [PubMed] [Google Scholar]
- 21.Raghu G, Masta S, Meyers D, Narayanan AS: Collagen synthesis by normal and fibrotic human lung fibroblasts and the effect of transforming growth factor beta. Am Rev Respir Dis 1989, 140:95-100 [DOI] [PubMed] [Google Scholar]
- 22.Raghow R, Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH: Transforming growth factor beta increases steady state level of type 1 pro collagen and fibronectin messenger RNA posttranscriptionally in cultured human dermal fibroblasts. J Clin Invest 1987, 79:1285-1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crouch E: Pathobiology of pulmonary fibrosis. Am J Physiol 1990, 259:L159-L184 [DOI] [PubMed] [Google Scholar]
- 24.Breen E, Shull S, Burne S, Absher M, Kelley J, Phan S, Cutroneo KR: Bleomycin regulation of transforming growth factor-β and fibroblast collagen synthesis in chronic pulmonary inflammation. Am J Respir Cell Mol Biol 1992, 6:146-152 [DOI] [PubMed] [Google Scholar]
- 25.Mason RJ, Schwarz MI, Hunninghake GW, Musson RA: NHLBI workshop summary: pharmacological therapy for idiopathic pulmonary fibrosis—past, present and future. AJRCCM 1999, 160:1771-1777 [DOI] [PubMed] [Google Scholar]
- 26.Shamon LA, Pezzuto JM, Graves JM, Mehta RR, Wangcharoentrakul S, Sangsuwan R, Chaichans S, Tuchinda P, Cleason P, Reutrakul V: Evaluation of the mutagenic, cytotoxic and antitumor potential of triptolide, a highly oxygenated diterpine isolated from Triptrygium wilfordii. Cancer Lett 1997, 112:113-117 [DOI] [PubMed] [Google Scholar]
- 27.McAnulty RJ, Laurent GJ: Collagen and it’s regulation. Phan SH Thrall RS eds. Pulmonary Fibrosis. 1995, :pp 135-171 Marcel Dekker, New York [Google Scholar]
- 28.King SL, Litcher AC, Rowe DW, Xie R, Long GL, Absher MP, Cutroneo KR: Bleomycin stimulates pro α-1 collagen promotor through intracellular and extra cellular signalling. J Biol Chem 1994, 269:13156-13161 [PubMed] [Google Scholar]