Abstract
Ultraviolet (UV) light is an epidemiological risk factor for melanoma, but its specific contribution to melanoma induction is not known. The first critical step of melanoma development, ie, the uncontrolled proliferation of melanocytes, may be induced by a combination of UV damage and an imbalance of growth factor production by cells in the immediate area of the melanocyte. Among several candidates, basic fibroblast growth factor (bFGF) is the major autocrine growth factor in melanoma and associated with tumor progression. Overexpression of bFGF via adenoviral gene transfer in human skin xenografted to severe combined immunodeficiency mice led to black-pigmented macules within 3 weeks of treatment. Immunofluorescence analysis demonstrated pathological hyperpigmentation, proliferation and hyperplasia of activated melanocytes, but no malignant transformation. Similar changes were observed in skin reconstructs. When bFGF was combined with UVB, pigmented lesions with hyperplastic melanocytic cells were detected, including a lesion with high-grade atypia resembling lentiginous forms of malignant melanoma. Donor-matched control grafts revealed no melanocytic changes. bFGF was overexpressed in dermal fibroblasts demonstrating the co-carcinogenic influence of paracrine-acting growth factors by cells of the microenvironment. This is the first report suggesting that an imbalance of physiological growth factor production in the skin may cause melanoma in combination with UVB.
Loss of growth control is a hallmark of cancer and frequently associated with aberrant growth factor production. Many tumor cell populations release mitogenic factors that sustain autonomous growth via autocrine stimulation and generate a microenvironment favoring tumor survival and invasion via paracrine effects. 1 Although many of these growth factors have been characterized and described in detail in tumor progression and metastasis, especially in breast cancer, prostate cancer, and melanoma, their roles in early stages of tumor development have been addressed only marginally.
In melanoma, several growth factors are expressed, including basic fibroblast growth factor (bFGF), melanoma growth stimulatory activity/Gro, interleukin (IL)-8, platelet-derived growth factor-A, IL-6, vascular endothelial growth factor, and granulocyte/macrophage-colony stimulating factor. 2 In contrast, normal melanocytes produce none or only low levels of these factors and during normal skin development and homeostasis, depend on the production of bFGF, endothelin-1 and -3, stem cell factor (SCF), hepatocyte growth factor (HGF), and melanocyte-stimulating hormone by keratinocytes and fibroblasts. 3 Disruption of this homeostatic balance might have an impact not only on melanocyte development and distribution, but also on nevus and melanoma development. 4 A potent environmental candidate for inducing an imbalance of growth factor production in skin is ultraviolet (UV) light, whose association with nevus and melanoma development has been documented by epidemiological studies. 5,6 Limited experimental data have demonstrated UV induction of melanoma in animal models (Xiphophorus hybrid fish and opossum) as well as in a human skin graft/immunodeficient mouse model when combined with 7,12-dimethyl(a)benzanthracene. 7-9 In addition to its direct DNA-damaging effects, UVB has been shown to stimulate expression of IL-1, IL-3, IL-6, tumor necrosis factor-α, granulocyte/macrophage-colony stimulating factor, endothelin-1, IL-8, IL-12, and vascular endothelial growth factor in keratinocytes and IL-1α and bFGF expression in HeLa cells. 10-15 UVA could induce IL-6 and tumor necrosis factor-α expression in keratinocytes and dermal fibroblasts. 16 In UVB-irradiated murine skin in vivo, the epidermal expression of bFGF increased, whereas interferon-β decreased, alterations that were associated with enhanced cutaneous angiogenesis. 17
bFGF, also called FGF-2, is one of 21 members of the FGF gene family known to modulate cell growth, differentiation, motility, and angiogenesis. 18 bFGF binds to low-affinity receptors on the cell surface and in the extracellular matrix. These low-affinity receptors that are heparan sulfate proteoglycans are required for binding of FGF to the four different types of high-affinity receptors. 19 In melanoma, bFGF is the most important autocrine growth factor. Inhibition of bFGF production by antisense oligodeoxynucleotides led to inhibition of melanoma proliferation in vitro and in vivo. 20,21 Through its mitogenic effects on endothelial cells and fibroblasts, bFGF production by melanoma can also promote angiogenesis and fibrous stroma formation via a paracrine mode. 22 Although expression of bFGF is absent in normal melanocytes, it is moderate to high in compound and dysplastic nevi and always present in melanomas. 23-26 This change in bFGF expression early in melanoma development suggests an alteration in the growth control mechanisms during melanocyte transformation. However, bFGF alone cannot induce complete melanocyte transformation, as demonstrated by different groups. Infection of murine melanocytes with a retrovirus carrying the cDNA for bFGF caused autonomous growth and suppressed differentiation properties in vitro, but was insufficient to form malignant tumors in vivo. 27,28 Transfection of human melanocytes with bFGF via retroviral gene transfer still required exogenous bFGF for growth, whereas adenoviral gene transfer of the bFGF gene in human melanocytes reduced dependence on growth factors in vitro, induced anchorage-independent growth in vitro, and increased survival and proliferation in vivo. 29,30 These observations led to the hypothesis that melanocytes may be activated by bFGF, but require additional stimulation by a cooperating factor for complete transformation.
In this study, the effects of bFGF on melanocytes in vivo with and without exposure to UVB were analyzed in human skin grafted to immunodeficient mice. To achieve high and sustained levels of bFGF in the skin, adenoviral gene transfer for bFGF was used.
A highly mitogenic effect with hyperpigmentation and melanocytic hyperplasia was found by bFGF overexpression alone. When combined with UVB irradiation a lentiginous melanoma-like lesion developed within 2 months of treatment. This is the first report suggesting that human melanoma in vivo can be experimentally induced by a growth factor and UVB.
Materials and Methods
Cell Culture
Normal human keratinocytes and melanocytes were isolated from the epidermis, and fibroblasts from the dermis of neonatal human foreskins. Keratinocytes were cultured in serum-free medium (Life Technologies, Inc., Gaithersburg, MD) supplemented with human recombinant epidermal growth factor and bovine pituitary extract. Melanocytes were cultured in MCDB153 (Sigma, St. Louis, MO) supplemented with 2% fetal bovine serum (FBS), 10% chelated FBS, 2 mmol/L glutamine, 20 pmol/L cholera toxin (Sigma), 150 pmol/L recombinant human bFGF, 100 nmol/L recombinant human endothelin-3 (Peninsula, Belmont, CA), and 10 ng/ml recombinant human SCF (R&D Systems, Minneapolis, MN). Fibroblasts were cultured in Dulbecco’s modified Eagle’s medium with glutamine (Life Technologies, Inc.), 8 mmol/L Hepes (Sigma), and 10% FBS (Hyclone, Logan, UT).
Adenoviral Vectors
The adenoviral vector bFGF/Ad5 carrying the gene for the 18-kd form of the bFGF protein has been described. 30 The control adenoviral vector LacZ/Ad5 (Vector Core, University of Pennsylvania, Philadelphia, PA) induces expression of the reporter gene β-galactosidase from Escherichia coli. The adenoviral vector for HGF was kindly provided by Dr. J. M. Wilson (Institute for Human Gene Therapy, The Wistar Institute, Philadelphia, PA). 31 The adenoviral vectors for platelet-derived growth factor-A and insulin-like growth factor-1 were generated from d17001 and AdEasy-1 viruses, respectively, with deleted E1 and E3 regions and the transgenes driven by the CMV promoter (Satyamoorthy K, Li G, Vaidya B, Patel D, Herlyn M, unpublished). The vectors were prepared, purified, and titered to 1 to 5 × 10 10 plaque-forming units (p.f.u.)/ml.
Human skin grafts were injected intradermally with the adenoviral vectors using a 26-gauge needle at a concentration of 5 × 10 8 p.f.u. in a total volume of 100 μl sterile phosphate-buffered saline (PBS). The needle was inserted 2 mm apart from the edge of the graft and directed toward the center of the graft during injection. Generally, 100 μl were injected at one site into foreskin grafts and 50 μl were injected at two sites into trunk skin grafts, in which the fluid penetration was usually slower. Injections were performed once per week by the same person (CB).
Human Skin Grafting
Human foreskins from newborns and abdominal or breast skin from adult donors, who underwent plastic surgery (Cooperative Human Tissue Network, Philadelphia, PA), were kept in sterile transport media (RPMI-1640 or Hanks’ balanced salt solution supplemented with antibiotics) and grafted within 48 hours of excision as described with modifications. 9 Female and male C.B-17 SCID mice were bred at the Animal Facility of the Wistar Institute and housed under pathogen-free conditions in groups of up to five animals per isolator cage. At 6 to 10 weeks of age, a 1 to 3 cm 2 skin segment behind the shoulder of the animal was excised, leaving the panniculus carnosus muscle intact. The wound was immediately covered with full-thickness human skin that was held in place by the bandage alone or by 6-0 nonabsorbable polyviolene sutures. The bandage consisted of nonadhesive Vaseline dressing, sterile sponges, and surgical tape and was changed after 2 weeks. Grafts were well healed after 4 to 6 weeks and used for the experiments. The Wistar Institutional Animal Care and Use Committee approved all protocols.
Histology, Immunohistochemistry, and Immunofluorescence
At the end of each experiment, mice were sacrificed by CO2 inhalation and skin grafts were excised. Half of the grafts were fixed in 10% neutral-buffered formalin (Fisher Scientific, Pittsburgh, PA) for 6 to 12 hours at room temperature and embedded in paraffin. The other half was dehydrated by increasing concentrations of sucrose solutions (5%, 10%, and 20%) at 4°C overnight, embedded in OCT medium (Miles, Elkhart, IN), snap-frozen and stored at −70°C until cryosectioning at 6 to 8 μm. Formalin-fixed sections were stained with hematoxylin and eosin (H&E) for histopathological evaluation. The DNA-binding fluorochrome Hoechst 33258 (Sigma) was used to distinguish human from murine cells.
Immunohistochemistry was performed on serial sections using an avidin-biotin-peroxidase system kit (Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine tetrahydrochloride (Sigma) or 3-amino-9-ethylcarbazole (Vector) as chromogens. Antigens in the formalin-fixed tissues were retrieved by trypsin digestion at 37°C or microwave heat treatment in citrate buffer. Cryostat sections of 6 to 8 μm were air-dried and fixed in ice-cold acetone for 10 minutes. Before incubation with the primary antibodies in a humidified chamber at 4°C overnight or at room temperature for 1 to 2 hours, nonspecific binding was blocked with 10% normal horse or 10% normal goat serum. Primary monoclonal antibodies used in this study were: mouse anti-bFGF (bFGF-8, IgG1); 30 mouse anti-human TRP-1/gp75 (clone TA99, IgG2a; kind gift from Dr. V. Setaluri, Winston-Salem, NC); mouse anti-human Ki-67 (clone MIB-1, IgG1; Immunotech, Westbrook, ME); and mouse anti-human HMB45 (IgG1; Biogenex, San Ramon, CA). A mouse IgG1 isotype antibody (P3) was used as negative control for each staining. Between each incubation step, slides were rinsed twice in PBS for 3 to 5 minutes. A biotin-labeled anti-mouse secondary antibody was applied for 30 minutes at room temperature followed by incubation with a preformed avidin-biotinylated enzyme complex for 30 minutes. After color development by addition of the chromogen and counterstaining with Mayer’s hematoxylin (Sigma), sections were mounted and evaluated under a light microscope.
For immunofluorescence detection of the proliferation marker Ki-67 or the melanoma/activated melanocyte marker HMB-45, a biotin-labeled goat anti-mouse IgG1 secondary antibody (Jackson Immunoresearch, West Grove, PA) was used followed by incubation with streptavidin-conjugated Cy3 (Jackson Immunoresearch). For immunofluorescence detection of the melanocyte-specific antigen TRP-1, a horse anti-mouse IgG2a secondary antibody directly conjugated with fluorescein isothiocyanate (Jackson Immunoresearch) was used. Double-immunofluorescence staining was performed for Ki-67 (red) and TRP-1 (green), and cells were counterstained with Hoechst 33258 (blue).
Sections were scored in a blinded manner by counting five fields (∼1,000 epidermal basal cells) at ×200 magnification in each of three randomly selected sections using a fluorescence microscope (Leika, Wetzlar, Germany). Cells stained for TRP-1 (green), for Ki-67 (red), and for both TRP-1 and Ki-67 (yellow) were counted. All data are expressed as mean ± SD of the mean of observations. Individual groups were compared with Student’s unpaired t-test. P < 0.05 was considered significant.
UV Irradiation
UV light was provided by two Westinghouse FS72T12/UVB lamps (UV Resources International, Lakewood, OH) with a peak output at 313 nm and a range of 280 to 370 nm. The light was filtered through cellulose triacetate Kodacel TA 407 sheets (Eastman Kodak, Rochester, NY) to exclude wavelengths <295 nm. The UV dose was continuously monitored with a PMA 2100 radiometer (Solar Light, Philadelphia, PA) and ranged between 30 and 50 mJ/cm 2 for UVB and 0.1 and 0.2 J/cm 2 for UVA in the in vivo experiments. During irradiation, mice were separated from each other and allowed to move freely in the cage. Irradiation was performed three times weekly for ∼10 minutes each time throughout a period of 2 to 10 months.
Skin Reconstruction
Skin reconstructs were prepared essentially as described with modifications. 32 Human fibroblasts (FF2441) were added to neutralized bovine type I collagen (Organogenesis, Canton, MA) to a final concentration of 0.8 to 1 mg/ml of collagen in MEM (Biowhittaker, Walkersville, MA), 1.66 mmol/L l-glutamine (Life Technologies, Inc.), 10% FBS, and 0.21% sodium bicarbonate (Biowhittaker). Three milliliters of fibroblast-containing collagen (2.5 × 10 4 cells/ml) were added to each insert of a 6-well tissue-culture tray (Organogenesis) after precoating with 1 ml of acellular collagen. Mixtures were allowed to constrict in Dulbecco’s modified Eagle’s medium with 10% FBS for 5 to 7 days. The day before seeding, keratinocytes or melanocytes were infected with bFGF/Ad5, and controls with LacZ/Ad5 at 20 p.f.u./cell for 4 hours in protein-free, serum-free medium and then incubated overnight in complete serum-free medium. Keratinocytes were mixed with melanocytes at a ratio of 5:1 or 2.5:1 in low-calcium epidermal growth medium containing Dulbecco’s modified Eagle’s medium, F-12 Ham’s (Life Technologies, Inc.), 1% newborn calf serum (Hyclone), 4 mmol/L glutamine, 1.48 × 10−6 mol/L hydrocortisone, 4 pmol/L progesterone, 20 pmol/L triiodothyronine, 0.1 mmol/L O-phosphorylethanolamine, 0.18 mmol/L adenine (Sigma), 5 mg/ml insulin, 5 mg/ml transferrin, 5 mmol/L ethanolamine, 5 g/ml selenium (Biowhittaker) and 50 μg/ml gentamicin (Mediatech, Hemdon, VA). A total of 5 to 6 × 10 5 cells was seeded on each contracted collagen gel. Cultures were maintained submerged in low-calcium epidermal growth medium for 2 days and in normal calcium (1.88 mmol/L) epidermal growth medium for another 2 days, and then raised to the air-liquid interface for 10 to 12 days with feeding from below with normal calcium high-serum (20%) epidermal growth medium.
Results
bFGF Induces Pigmented Macules in Human Skin
Six human skin grafts were injected intradermally with bFGF/Ad5 once weekly receiving up to seven treatments (Table 1) ▶ . In the third week, one abdominal skin graft developed a brown macule (Figure 1A) ▶ , one foreskin graft showed a small black spot centrally (not shown), and one foreskin graft turned from pink to an almost complete black pigmentation (Figure 1B) ▶ . Other bFGF/Ad5-injected skin grafts showed no pigmentation changes, although thickening of the skin was observed (Figure 1C) ▶ . Eight skin grafts injected with bFGF/Ad5 once only and evaluated 3 days later revealed no change in pigmentation (not shown).
Table 1.
Clinical and Histopathological Characteristics of Human Skin Grafted to SCID Mice and Injected with bFGF/Ad5*
| Treatment group | Skin type and age of donor(years) | Clinical appearance* in weeks 1 to 3† | Clinical appearance* in weeks 4 to 6† | Total bFGF/Ad5 injections‡ | Total UVB irradiations§ | Biopsy† | Histological melanocytic changes¶ |
|---|---|---|---|---|---|---|---|
| bFGF | Breast (unknown) | Normal pink | Normal pink | 7 | 0 | Month 7 | Increase |
| Foreskin, neonatal | Normal pink | 1 Brown macule | 6 | 0 | Month 8 | Hyperplasia | |
| Foreskin, neonatal | 70% black | 70% black | 4 | 0 | Month 7 | Hyperplasia | |
| Abdomen (54) | 1 Brown macule | 1 Brown macule | 2 | 0 | Month 3 | Hyperplasia | |
| Foreskin, neonatal | 1 Black spot | — | 2 | 0 | Week 2 | Increase | |
| Abdomen (39) | Normal pink | — | 1 | 0 | Day 7 | None | |
| Foreskins, neonatal (n = 8) | Normal pink | — | 1 | 0 | Day 3 | None | |
| bFGF+ UVB§ | Foreskin, neonatal | Black spots and streaks | 6 Black macules | 9 | 41 | Month 3 | Increase |
| Foreskin, neonatal | Dark brown | 70% dark brown/black | 8 | 117 | Month 10.5 | Increase | |
| Abdomen (52) | 1 Black macule | 5 Black macules | 7 | 26 | Month 2 | Lentiginous form of melanoma | |
| Abdomen (52) | 1 Dark brown macule | 4 Black spots | 7 | 90 | Months 5 and 7 | Hyperplasia | |
| Foreskin, neonatal | Entirely black | Entirely black | 6 | 18 | Months 1 and 2 | Hyperplasia | |
| Abdomen (39) | 3 Black macules | 4 Black macules | 3 | 36 | Months 3 and 6 | Increase |
*At the start of treatment all skin xenografts were pale to pink and showed no pigmented lesions.
†After beginning of treatment.
‡Intradermally at 5 × 108 p.f.u. in 100 μl of sterile PBS once weekly.
§UVB irradiations were done three times weekly at a dose of 30 to 50 mJ/cm2.
¶Other histological changes, such as increase in vessels, extracellular matrix, or stromal cells are not listed.
Figure 1.
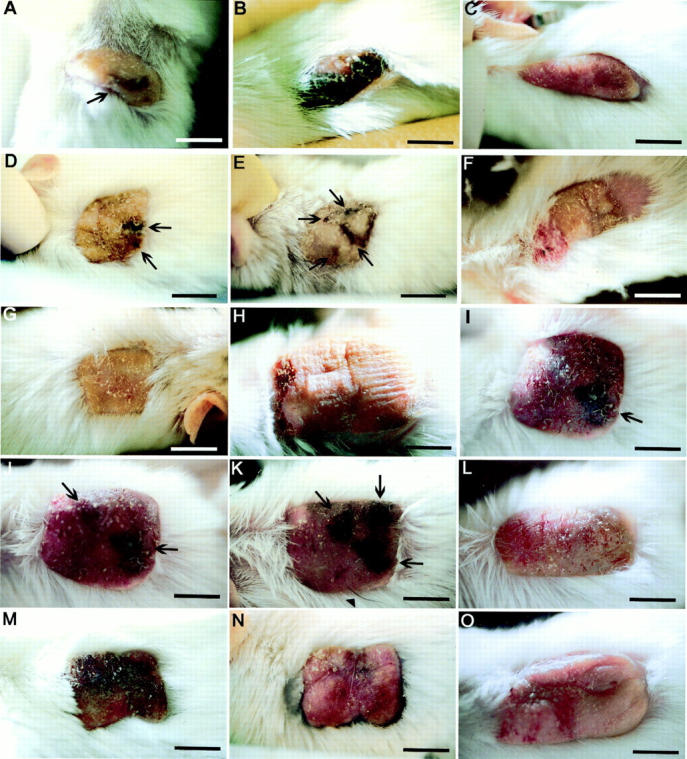
A-O: Photographs of human skins grafted to SCID mice and injected intradermally with 100 μl of bFGF/Ad5 or LacZ/Ad5 in PBS once per week. Where stated, grafts were additionally irradiated with 30 to 50 mJ/cm 2 UVB three times weekly. The total number of irradiations is given for each presented example. A: Adult abdominal skin graft 3 weeks after the last of two injections of bFGF/Ad5 (no UVB irradiation), showing a brown macule (arrow). B: Neonatal foreskin graft after three injections of bFGF/Ad5 (no UVB irradiation; week 4). Note the black hyperpigmentation. C: Neonatal foreskin graft after six injections of bFGF/Ad5 (no UVB irradiation; week 6). Note the graft thickness and lack of pigmented lesions. D–F: Adult abdominal skin graft after two injections of bFGF/Ad5 and seven UVB irradiations (week 3) (D), after seven injections of bFGF/Ad5 and 40 UVB irradiations (month 5) (E), and after seven injections of bFGF/Ad5 and 90 UVB irradiations (month 7) (F). Note the development of black macules (arrows) during the treatment and their disappearance after the discontinuation of bFGF/Ad5 injections. G: Control adult abdominal skin graft from the same donor as in A–C after 90 UVB irradiations (month 7). H–K: Adult abdominal skin graft before treatment (H), after two injections of bFGF/Ad5 and six UVB irradiations (week 3) (I), after three injections of bFGF/Ad5 and eight UVB irradiations (week 4) (J), and after four injections of bFGF/Ad5 and 10 UVB irradiations (week 5) (K). Note the development of black macules (arrows) during the treatment. L: Control adult abdominal skin graft from the same donor as in H–K after three injections of LacZ/Ad5 and eight UVB irradiations (week 4). M and N: Neonatal foreskin graft after two injections of bFGF/Ad5 and seven UVB irradiations (week 2) (M), and 3 months after the last of eight injections of bFGF/Ad5 and after 65 UVB irradiations (month 5) (N). Note the prominent thickness of the graft and black hyperpigmentation of the skin during the injection treatment. O: Control neonatal foreskin graft after three injections of LacZ/Ad5 and eight UVB irradiations (week 4). Scale bars, 1 cm.
Another six human skin grafts were injected weekly with bFGF/Ad5 and irradiated with UVB (Table 1) ▶ . Pigmented lesions were detected in all grafts in the third week of treatment. Adult abdominal skin developed black or brown macules, which increased in size and number with further injections (Figure 1; D, E, I, J, and K ▶ ), whereas foreskins tended to become entirely black (Figure 1M) ▶ . All pigment changes gradually disappeared 1 to 2 months after discontinuation of bFGF/Ad5 injections and despite continuation of UVB irradiations (Figure 1, F and N) ▶ . Control skin grafts injected with adenoviral vectors for LacZ and irradiated with UVB did not develop pigmented lesions during the observation period of 3 weeks to 8 months (Figure 1, L and O ▶ , and Table 2 ▶ ). Control skin grafts of the same donors UVB-irradiated only (Figure 1G) ▶ also showed no changes except for some tanning as expected and previously reported. 9 Overexpression of other growth factors such as HGF, insulin-like growth factor-1, and platelet-derived growth factor-A had no detectable effect on the pigment cell system of the skin grafts (Table 2) ▶ .
Table 2.
Clinical and Histopathological Characteristics of Human Skin Grafted to SCID Mice and Injected with Different Adenoviral Vectors*
| Treatment group and number of skin grafts (n) | Skin type of donors† (number of grafts) | Total injections* | Total UVB irradiations‡ | Clinical appearance until biopsy | Biopsy§ | Histological melanocytic changes¶ |
|---|---|---|---|---|---|---|
| LacZ (20) | Foreskin (15) | 1–2 | 0 | Normal pink | Day 3–Month 5 | N/D |
| Foreskin (2) | 6, 7 | 45, 16 | Tan | Months 4.5, 2 | N/D, increase | |
| Abdomen (2) | 6, 7 | 108, 16 | Tan | Months 12, 2 | N/D | |
| Breast (1) | 1 | 0 | Normal pink | Week 3 | N/D | |
| PDGF-1 (8) | Foreskin (3) | 1 | 0 | Pink | Day 3 | N/D |
| Breast (3) | 9, 10, 10 | 27, 89, 89 | Tan | Months 3, 13, 15 | N/D | |
| Face (1) | 9 | 58 | Tan | Month 12 | N/D | |
| HGF (10) | Foreskin (6) | 1 | 0 | Normal pink | Day 3 | N/D |
| Foreskin (2) | 5, 10 | 27, 119 | Tan | Months 2, 10.5 | N/D | |
| Abdomen (1) | 11 | 109 | Tan | Month 9 | N/D | |
| Breast (1) | 4 | 91 | Tan | Month 13 | N/D | |
| IGF-1 (8) | Foreskin (4) | 1 | 0 | Normal pink | Day 3 | N/D |
| Foreskin (4) | 11, 12, 12, 12 | 75, 82, 83, 103 | Tan | Months 9, 8, 14, 14 | N/D |
*Intradermally at 5 × 108 p.f.u. in 100 μl of sterile PBS once weekly.
†Foreskins were from newborns; all other skin specimens were from adult donors.
‡UVB irradiations were done three times weekly at a dose of 30 to 50 mJ/cm2.
§After beginning of treatment.
¶Other histological changes, such as acanthosis of the epidermis, increase in vessels, extracellular matrix, or stromal cells are not listed.
N/D = Not detectable.
Overall, bFGF alone induced pigmented lesions in 50% of the grafts, whereas the combination of bFGF and UVB led to pigmented lesions in 100% of the cases.
bFGF Induces Dermal Thickening and Angiogenesis
Thickening of the skin after two or more bFGF/Ad5 injections was observed in the foreskin grafts and remained even after discontinuation of injections (Figure 1, C and N) ▶ . Three of six foreskin grafts became hypervascular after 1 to 2 weeks of treatment, which was noticed by easy bleeding during subsequent injections.
Intradermal Injection of bFGF/Ad5 Induces bFGF Expression in Fibroblasts
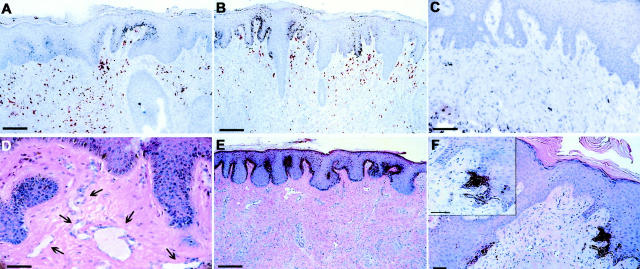
Immunohistochemical detection of bFGF protein to assess the transduction efficiency of the intradermal bFGF/Ad5 injections indicated abundant expression of bFGF in fibroblasts in the upper dermis (Figure 2, A and B) ▶ . Generally, no bFGF expression was detected in the epidermis except for one confined area in one section where single keratinocytes showed strong bFGF expression. This area was hyperplastic and parakeratotic and appeared to be the entrance site of the injection needle through the epidermis (not shown). Controls showed no or only weak bFGF expression (Figure 2C) ▶ .
Figure 2.
Histological sections of human skin xenografts injected intradermally with bFGF/Ad5 or LacZ/Ad5 once per week and irradiated or not with 30 to 50 mJ/cm 2 UVB three times weekly. A–C: Immunohistochemical detection of bFGF protein (in red) in a foreskin graft after six injections of bFGF/Ad5 and 18 UVB irradiations (week 7), showing positively stained fibroblasts in the upper dermis (A and B, scale bars, 100 μm), and in a control abdominal skin graft after six injections of LacZ/Ad5 and 15 UVB irradiations (week 6) (C, scale bar, 100 μm). D–F: H&E staining of a foreskin graft 6 months after the last of four injections of bFGF/Ad5, showing high number of vessels in the dermis (arrows) (D, scale bar, 50 μm), of a foreskin graft after six injections of bFGF/Ad5 and 18 UVB irradiations (week 7), showing pathological hyperpigmentation of the entire epidermis and hypercellularity of the thickened dermis (E, scale bar, 200 μm), and of an abdominal skin graft after seven injections of bFGF/Ad5 and 26 UVB irradiations (week 9), showing localized black hyperpigmentation in the epidermis (F, scale bar, 50 μm).
bFGF Induces Proliferation of Endothelial Cells and Fibroblasts
Capillaries and small vessels were markedly increased in the dermis of some of the bFGF/Ad5-injected foreskin grafts (Figure 2D) ▶ . The clinically observed thickening of the skin during the treatment was reflected by an increase in extracellular matrix and fibroblasts in the dermis (Figure 2E) ▶ .
bFGF Induces Pathological Hyperpigmentation and Proliferation of Activated Melanocytes
A strong increase in pigment in the epidermis was noted in the bFGF/Ad5-injected skin grafts (Figure 2, E and F) ▶ . Accumulation of black pigment in confined areas of the epidermis (Figure 2F) ▶ correlated macroscopically with black macules. In other sections, hyperpigmentation was present throughout the human epidermis (Figure 2E) ▶ . Pigment-laden melanophages in the subepidermis were commonly seen and remained present after the epidermal hyperpigmentation had disappeared weeks after discontinuation of the bFGF/Ad5 injections (data not shown).
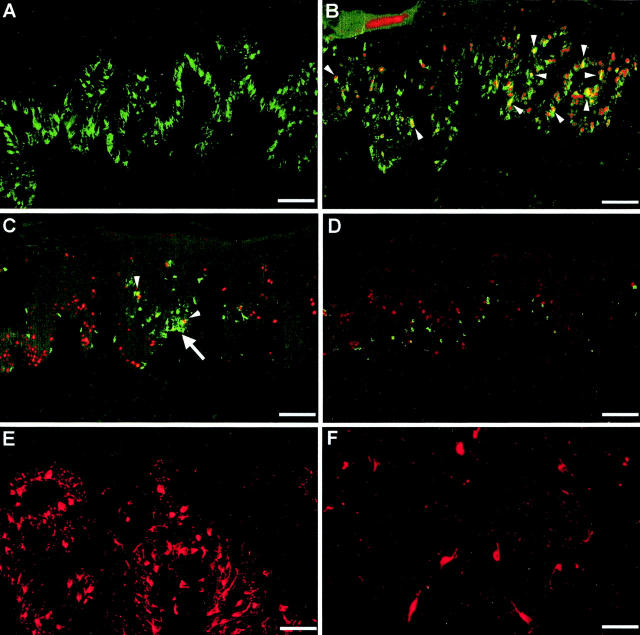
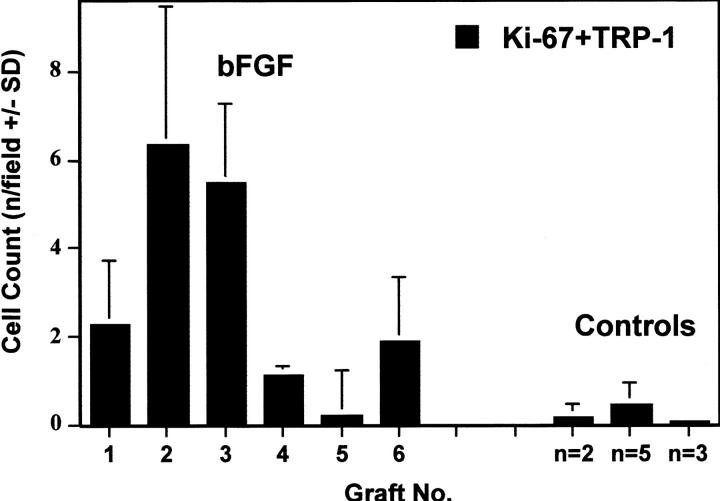
In the bFGF/Ad5-treated skin grafts, there was a striking increase in the number of melanocytes in the epidermis, as confirmed by immunofluorescence staining for TRP-1. The melanocytes showed prominent dendricity and were located close to each other along the basement membrane of the epidermis (Figure 3, A and B) ▶ ; in some areas, incipient cluster formation was noted (Figure 3C) ▶ . Proliferating melanocytes were identified by dual staining for TRP-1 and Ki-67 (Figure 3, B and C) ▶ . The increased number of melanocytes and proliferating melanocytes compared to controls was also demonstrated by counting the TRP-1 and/or Ki-67 positively stained cells in randomly chosen fields of different histological sections. There were significantly more proliferating melanocytes in the bFGF/Ad5-treated skin grafts compared to the controls (P < 0.006) with up to 10 cells per counting field in contrast to 0 to 1 cell in the controls (Figure 4) ▶ . The number of melanocytes varied considerably among the different skin grafts ranging from 20 per counting field in both groups to 90 in the bFGF group only.
Figure 3.
Immunofluorescence staining of sections of human abdominal skin xenografts injected intradermally with bFGF/Ad5 in PBS once per week and irradiated with 30 to 50 mJ/cm 2 UVB three times weekly. Control xenografts were injected with LacZ/Ad5 and irradiated with UVB following the same protocol. A: Melanocyte-specific marker TRP-1 (green). Note the high number of dendritic melanocytes along the basement membrane of the epidermis. Scale bar, 100 μm. B and C: TRP-1 (green) and the nuclear proliferation marker Ki-67 (red). Scale bars, 50 and 100 μm, respectively. Cells stained for both markers appear yellow and represent proliferating melanocytes (arrowheads). Note the incipient nest formation by melanocytes (arrow). Scale bar, 100 μm. D: Control skin graft stained for TRP-1 (green) and Ki-67 (red). Scale bar, 100 μm. E: HMB-45 (red). Note the high number of positively stained melanocytes. Scale bar, 70 μm. F: Control graft stained for HMB-45 (red). A few single melanocytes in the LacZ/Ad5-injected control graft also stain red-positive. Scale bar, 50 μm.
Figure 4.
Quantitation of proliferating melanocytes (TRP-1+Ki-67) in the epidermis of human skin grafts. Melanocytes were immunostained for TRP-1 and detected with fluorescent green (fluorescein isothiocyanate). Proliferating cells were immunostained for Ki-67 and detected with fluorescent red (Cy3). The numbers (mean + SD) are the positively stained cells for TRP-1 and Ki-67 per 1-mm length of epidermis, respectively, in five randomly chosen fields at ×200 magnification (∼1000 epidermal basal cells). Grafts were analyzed at different treatment time points ranging from 2 weeks to 6.5 months. bFGF, treated with bFGF/Ad5 injections once weekly. Grafts 1 to 6: week 1, week 2, month 3, month 7, month 7, and month 8 after one, two, two, seven, four, and six injections, respectively, once weekly. Controls: n = 2, average of two grafts treated with LacZ/Ad5 injections; n = 5, average of five grafts treated with UVB irradiations; and n = 3, average of three grafts treated with LacZ/Ad5 injections and UVB irradiations.
Activation of the melanocytes in the bFGF/Ad5-injected skin grafts was documented by a strong positive staining for HMB-45 (Figure 3E) ▶ . Skin grafts injected with control adenoviral vectors and/or irradiated with UVB were negative for HMB-45 (not shown) or showed only single positive cells when injected with LacZ/Ad5 and irradiated with UVB (Figure 3F) ▶ .
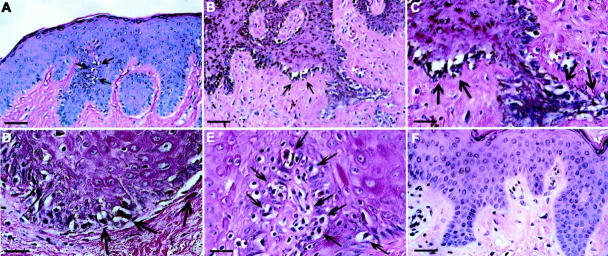
bFGF Induces Melanocyte Hyperplasia and, in Combination with UVB, Lentiginous Melanoma
In sections of the bFGF/Ad5-treated skin grafts, melanocytes displayed a hyperplastic morphology that was still present months after the last treatment (Figure 5A) ▶ . Hyperplasia with additional atypia was detected in one bFGF/Ad5-treated abdominal skin graft that concomitantly had been irradiated with UVB for 2 months. In this pathologically pigmented lesion, the hyperplastic, high-grade atypical melanocytic cells were distributed in a lentiginous growth pattern resembling lentiginous forms of malignant melanoma in humans (Figure 5; B, C, and E ▶ ). Control grafts from the same donor, either not treated or UVB-irradiated only showed no melanocytic abnormalities (Figure 5F) ▶ .
Figure 5.
H&E-stained sections of human skin xenografts injected intradermally with bFGF/Ad5 once per week and/or irradiated with 30 to 50 mJ/cm 2 UVB three times weekly. A: Foreskin graft 7 months after six injections of bFGF/Ad5 (no UVB irradiation). Note the hyperplastic melanocytes along the rete ridges (arrows). Scale bar, 50 μm. B–E: Lentiginous form of malignant melanoma in an abdominal skin graft after seven injections of bFGF/Ad5 and 26 UVB irradiations (week 9). Note the hyperplastic atypical melanocytic cells (arrows) in the epidermis in a dense lentiginous growth pattern, and the pathological pigmentation (C and E). Scale bars, 50 and 25 μm. F: Abdominal skin graft from the same donor as in B–E without bFGF/Ad5 injections, but with 114 UVB irradiations throughout 9 months. Scale bar, 40 μm.
bFGF Overexpression in Skin Reconstructs Induces Hyperpigmentation and Fibroblast Proliferation
Like the pigmentation changes observed in the human skin in vivo, increased pigmentation was observed in human skin reconstructs in vitro after overexpression of bFGF in keratinocytes (Figure 6 ▶ , top). Compared to LacZ controls, pigmentation of the epidermis was stronger and, as expected, darkest in reconstructs with the highest number of initially seeded melanocytes (ratio 1:2.5). The same pigmentation difference was seen when melanocytes were transduced with bFGF/Ad5 (Figure 6 ▶ , bottom). Clusters of black pigment were observed histologically in the epidermis and on top of the keratin layer. The number of dermal fibroblasts was strikingly increased, demonstrating a paracrine mitogenic effect by the bFGF overexpression.
Figure 6.
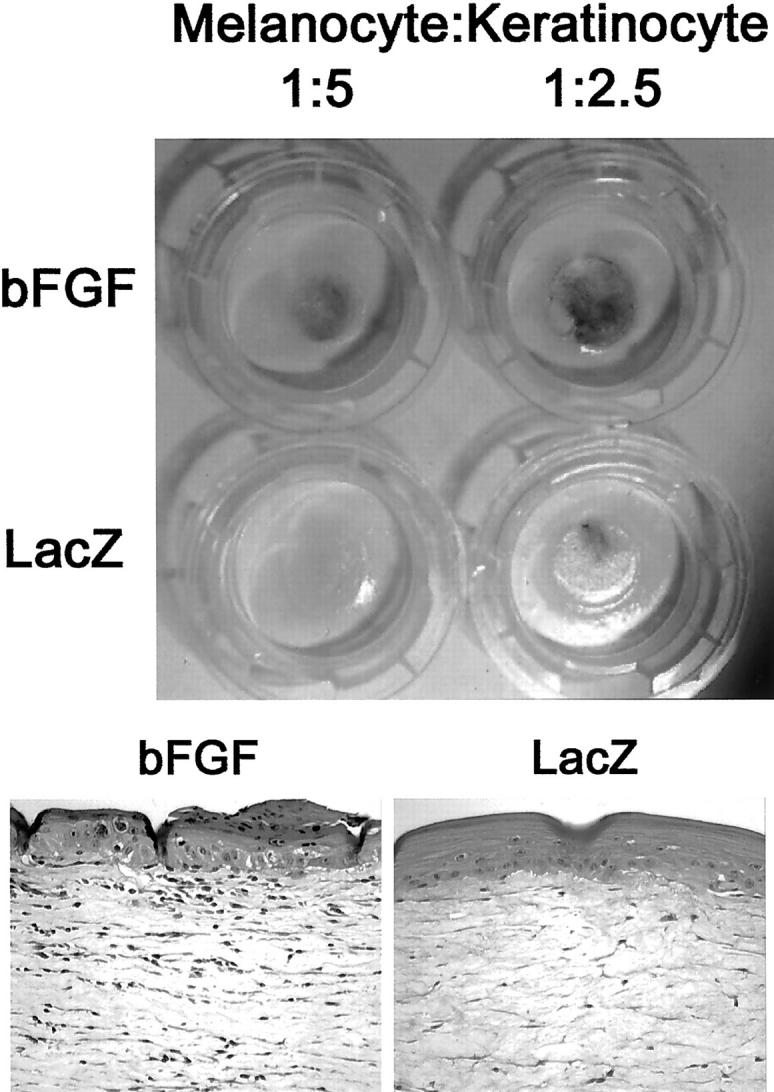
Top: Human skin reconstructs with bFGF/Ad5- or LacZ/Ad5-transduced keratinocytes mixed with normal melanocytes at a ratio of 5:1 and 2.5:1. Pigmentation is stronger in the bFGF reconstructs. The histopathological findings were the same as presented at the bottom. Bottom: H&E-stained sections of formalin-fixed, paraffin-embedded human skin reconstructs with bFGF/Ad5-transduced melanocytes versus controls mixed with normal keratinocytes at a ratio of 1:2. Note the black pigment clusters in and on top of the epidermis as well as the increased number of fibroblasts in the dermis. Original magnification, ×200.
Discussion
The present study demonstrates clinically and histologically the mitogenic effects of bFGF on melanocytes, fibroblasts, and endothelial cells in human skin in vivo and in human skin reconstructs in vitro. The observed hyperpigmentation in the skin grafts supports in vitro observations that bFGF can induce melanogenesis, although it is not known as a major melanogenic factor such as α-melanocyte-stimulating hormone. 33,34 The more widespread hyperpigmentation in the foreskin grafts as opposed to the more confined black macule formation in the trunk skin grafts might have been because of a more widespread penetration of the injected fluid in foreskin tissue than in the more compact trunk skin tissue. The hyperplasia of melanocytes after bFGF treatment suggests an activated stage of melanocytes superior to mere proliferation, but inferior to malignant transformation, which parallels the in vitro bFGF transduction studies of murine and human cells where melanocytes displayed a transformed phenotype but no malignancy. 27,30 The requirement for additional cooperating factors that confer a malignant phenotype to melanocytes has been suggested. 35 We focused on UV light as an additional melanocyte-stimulating factor, because it has been associated with melanoma development, although its carcinogenic effects on melanocytes in humans have remained only speculative. 36 Histopathological analysis suggested that the combination of bFGF overexpression in the skin and UVB irradiation in our model led to melanocyte transformation in vivo and a consequent lentiginous form of malignant melanoma. Experimental induction of human melanoma by UVB has already once been reported before, however, only in combination with topical treatment of the chemical carcinogen 7,12-dimethyl(a)benzanthracene, which is not found in the natural environment. 9 The present study suggests that 1) melanoma can be induced by naturally occurring factors in the immediate micro- and macroenvironment of epidermal melanocytes, specifically by bFGF and UVB light; 2) although each factor alone can activate melanocytes, both are needed to induce malignant transformation; and 3) factors beneficial or necessary for melanocyte proliferation and survival can become carcinogenic when released in overdose. An acute overdose of UV light is clinically reflected by sunburn that is a clearly documented risk factor for melanoma development. 37,38 However, the conditions in vivo that can lead to an imbalance and/or overdose of growth factor production with an activating potential on melanocytes remain to be elucidated. Numerous stress-inducing factors in addition to UV light, such as external heat, shock, local trauma, or inflammatory diseases can induce local overproduction of cytokines and growth factors. Recently, it has been demonstrated that bFGF expression is significantly increased in rat skin during wound healing after burning. 39 On the other hand, there are no epidemiological data showing that certain inflammatory diseases or trauma are risk factors for melanoma development. It is not known how genetic factors predispose to exogenous influences by growth factors and UV light, apart from the known increased risk in DNA damage repair enzyme-deficient patients.
The observed effects on the melanocytes in the human epidermis in this study are all consistent with the known effects of bFGF on melanocytes in vitro and may have been mediated via paracrine secretion of bFGF by the adenoviral vector-transduced fibroblasts in the dermis. However, the bFGF protein lacks a hydrophobic secretory signal sequence and only a few cell types including melanoma cells have been shown to secrete it, presumably via a pathway independent of the endoplasmic reticulum-Golgi complex. 30,40,41 bFGF can be concentrated intracellularly and can be biologically active without secretion. Hence, it is also possible, that the strong activating effects on the melanocytes in the epidermis were not directly mediated by bFGF but by other secondarily induced factors, for example, HGF, SCF, α-melanocyte-stimulating hormone, endothelin-1, or endothelin-3. Human dermal fibroblasts are known to produce HGF, SCF, and bFGF and have been suggested to play a role in regulating cutaneous pigmentation during inflammation and aging. 42 This suggestion is supported by the observation that two primary mediators of inflammation and injury, IL-1α and tumor necrosis factor-α, can stimulate HGF and SCF secretion by fibroblasts. HGF production can also be induced by epidermal growth factor, platelet-derived growth factor, and bFGF, illustrating the complex network of growth factors mediating cell-cell and cell-stroma interactions. 43 The importance of epidermal-dermal interactions mediated by diffusible factors for tissue regeneration and maintenance of homeostasis of rapidly renewing epithelia was recently suggested by Maas-Szabowski and colleagues, 44 who demonstrated a double-paracrine pathway between fibroblasts and keratinocytes, ie, the induction of keratinocyte growth factor in the fibroblasts by IL-1 secretion by the keratinocytes. We show here that an imbalance of these paracrine-mediated mesenchymal-epithelial interactions may be also important in early steps of melanoma development. However, we cannot exclude that other major factors for melanoma development were missing in this model, because melanocyte transformation was only observed in one of four skin grafts treated with a similar regimen, ie, at least 7 weeks of bFGF overexpression and 2 months of UVB irradiation. Two of these skin grafts were even from the same donor indicating the relative rarity of melanocyte transformation in vivo independent from the individual genetic predisposition.
In light of this functional paracrine communication between cells of the epidermis and dermis, our human skin in vivo model demonstrates also that adenoviral gene transduction of cells in the dermis can efficiently and transiently target cells in the epidermis and vice versa, as shown in the reconstruct experiments. This strategy can be considered in the field of cutaneous gene therapy, eg, for wound healing. Although adenoviral gene transduction of cells in the dermis can direct local, highly efficient expression of the protein of interest, injections of recombinant proteins immediately into the tissues are often ineffective because of rapid degradation and short half-lives.
In conclusion, the experimental induction of a melanoma lesion in human skin in vivo by overexpression of bFGF in the dermis and concomitant UVB irradiation throughout 2 months strongly suggests the importance of local growth factor production and UVB light in the pathogenesis of malignant melanoma.
Acknowledgments
We thank Dr. Mark Nesbit for providing the bFGF adenovirus, Drs. Don Forbes and Frank Gasparro for helpful support for the UV irradiation equipment, Christina Peklak and Adrien Jarvis for technical assistance with the animal experiments, Dr. Jonathan Garlick for invaluable support for the skin reconstruction model, Emma DeJesus and Rena Finko for outstanding technical assistance with the skin reconstruction, Elsa Aglow for excellent histological processing of the samples, Dr. Vijay Setaluri for providing the TA-99 antibody, and Dr. John Lininger for helpful pathological discussions.
Footnotes
Address reprint requests to Meenhard Herlyn, D.V.M., The Wistar Institute, 3601 Spruce St., Philadelphia, PA 19104. E-mail: herlynm@wistar. upenn.edu.
Supported by National Institutes of Health grants CA80999, CA25874, and CA10815 (to M. H.) and a postdoctoral research fellowship BE2189/1-1 from the Deutsche Forschungsgemeinschaft (to C. B.)
References
- 1.Favoni RE, de Cupis A: The role of polypeptide growth factors in human carcinomas: new targets for a novel pharmacological approach. Pharmacol Rev 2000, 52:179-206 [PubMed] [Google Scholar]
- 2.Lazar-Molnar E, Hegyesi H, Toth S, Falus A: Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine 2000, 12:547-554 [DOI] [PubMed] [Google Scholar]
- 3.Halaban R: The regulation of normal melanocyte proliferation. Pigment Cell Res 2000, 13:4-14 [DOI] [PubMed] [Google Scholar]
- 4.Herlyn M, Berking C, Li G, Satyamoorthy K: Lessons from melanocyte development for understanding the biological events in naevus and melanoma formation. Melanoma Res 2000, 10:1-10 [DOI] [PubMed] [Google Scholar]
- 5.Elwood JM, Jopson J: Melanoma and sun exposure: an overview of published studies. Int J Cancer 1997, 73:198-203 [DOI] [PubMed] [Google Scholar]
- 6.Green A, Whiteman D, Frost C, Battistutta D: Sun exposure, skin cancers and related skin conditions. J Epidemiol 1999, 9:S7-S13 [DOI] [PubMed] [Google Scholar]
- 7.Setlow RB, Woodhead AD, Grist E: Animal model for ultraviolet radiation-induced melanoma: platyfish-swordtail hybrid. Proc Natl Acad Sci USA 1989, 86:8922-8926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson ES, VandeBerg JL, Hubbard GB, Dooley TP: Malignant melanoma in ultraviolet irradiated laboratory opossums: initiation in suckling young, metastasis in adults, and xenograft behavior in nude mice. Cancer Res 1994, 54:5986-5991 [PubMed] [Google Scholar]
- 9.Atillasoy ES, Seykora JT, Soballe PW, Elenitsas R, Nesbit M, Elder DE, Montone KT, Sauter E, Herlyn M: UVB induces atypical melanocytic lesions and melanoma in human skin. Am J Pathol 1998, 152:1179-1186 [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz T, Luger TA: Effect of UV irradiation on epidermal cell cytokine production. J Photochem Photobiol B 1989, 4:1-13 [DOI] [PubMed] [Google Scholar]
- 11.Imokawa G, Yada Y, Miyagishi M: Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem 1992, 267:24675-24680 [PubMed] [Google Scholar]
- 12.Kondo S, Kono T, Sauder DN, McKenzie RC: IL-8 gene expression and production in human keratinocytes and their modulation by UVB. J Invest Dermatol 1993, 101:690-694 [DOI] [PubMed] [Google Scholar]
- 13.Enk CD, Mahanty S, Blauvelt A, Katz SI: UVB induces IL-12 transcription in human keratinocytes in vivo and in vitro. Photochem Photobiol 1996, 63:854-859 [DOI] [PubMed] [Google Scholar]
- 14.Brauchle M, Funk JO, Kind P, Werner S: Ultraviolet B and H2O2 are potent inducers of vascular endothelial growth factor expression in cultured keratinocytes. J Biol Chem 1996, 271:21793-21797 [DOI] [PubMed] [Google Scholar]
- 15.Kramer M, Sachsenmaier C, Herrlich P, Rahmsdorf HJ: UV irradiation-induced interleukin-1 and basic fibroblast growth factor synthesis and release mediate part of the UV response. J Biol Chem 1993, 268:6734-6741 [PubMed] [Google Scholar]
- 16.Avalos-Diaz E, Alvarado-Flores E, Herrera-Esparza R: UV-A irradiation induces transcription of IL-6 and TNF-alpha genes in human keratinocytes and dermal fibroblasts. Rev Rheum Engl Ed 1999, 66:13-19 [PubMed] [Google Scholar]
- 17.Bielenberg DR, Bucana CD, Sanchez R, Donawho CK, Kripke ML, Fidler IJ: Molecular regulation of UVB-induced cutaneous angiogenesis. J Invest Dermatol 1998, 111:864-872 [DOI] [PubMed] [Google Scholar]
- 18.Bikfalvi A, Klein S, Pintucci G, Rifkin DB: Biological roles of fibroblast growth factor-2. Endocrinol Rev 1997, 18:26-45 [DOI] [PubMed] [Google Scholar]
- 19.Klagsbrun M, Baird A: A dual receptor system is required for basic fibroblast growth factor activity. Cell 1991, 67:229-231 [DOI] [PubMed] [Google Scholar]
- 20.Becker D, Meier CB, Herlyn M: Proliferation of human malignant melanomas is inhibited by antisense oligodeoxynucleotides targeted against basic fibroblast growth factor. EMBO J 1989, 8:3685-3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Becker D: Antisense targeting of basic fibroblast growth factor and fibroblast growth factor receptor-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nat Med 1997, 3:887-893 [DOI] [PubMed] [Google Scholar]
- 22.Shih IM, Herlyn M: Autocrine and paracrine roles for growth factors in melanoma. In Vivo 1994, 8:113-123 [PubMed] [Google Scholar]
- 23.Scott G, Stoler M, Sarkar S, Halaban R: Localization of basic fibroblast growth factor mRNA in melanocytic lesions by in situ hybridization. Invest Dermatol 1991, 96:318-322 [DOI] [PubMed] [Google Scholar]
- 24.al-Alousi S, Barnhill R, Blessing K, Barksdale S: The prognostic significance of basic fibroblast growth factor in cutaneous malignant melanoma. J Cutan Pathol 1996, 23:506-510 [DOI] [PubMed] [Google Scholar]
- 25.al-Alousi S, Carlson JA, Blessing K, Cook M, Karaoli T, Barnhill RL: Expression of basic fibroblast growth factor in desmoplastic melanoma. J Cutan Pathol 1996, 23:118-125 [DOI] [PubMed] [Google Scholar]
- 26.Albino AP, Davis BM, Nanus DM: Induction of growth factor RNA expression in human malignant melanoma: markers of transformation. Cancer Res 1991, 51:4815-4820 [PubMed] [Google Scholar]
- 27.Dotto GP, Moellmann G, Ghosh S, Edwards M, Halaban R: Transformation of murine melanocytes by basic fibroblast growth factor cDNA and oncogenes and selective suppression of the transformed phenotype in a reconstituted cutaneous environment. J Cell Biol 1989, 109:3115-3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramon y Cajal S, Suster S, Halaban R, Filvaroff E, Dotto GP: Induction of different morphologic features of malignant melanoma and pigmented lesions after transformation of murine melanocytes with bFGF-cDNA and H-ras, myc, neu, and E1a oncogenes. Am J Pathol 1991, 138:349-358 [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman AB, Lugo TG: Normal human melanocytes that express a bFGF transgene still require exogenous bFGF for growth in vitro. J Invest Dermatol 1998, 110:793-799 [DOI] [PubMed] [Google Scholar]
- 30.Nesbit M, Nesbit HK, Bennett J, Andl T, Hsu MY, Dejesus E, McBrian M, Gupta AR, Eck SL, Herlyn M: Basic fibroblast growth factor induces a transformed phenotype in normal human melanocytes. Oncogene 1999, 18:6469-6476 [DOI] [PubMed] [Google Scholar]
- 31.Phaneuf D, Chen SJ, Wilson JM: Intravenous injection of an adenovirus encoding hepatocyte growth factor results in liver growth and has a protective effect against apoptosis. Mol Med 2000, 6:96-103 [PMC free article] [PubMed] [Google Scholar]
- 32.Meier F, Nesbit M, Hsu MY, Martin B, Van Belle P, Elder DE, Schaumburg-Lever G, Garbe C, Walz TM, Donatien P, Crombleholme TM, Herlyn M: Human melanoma progression in skin reconstructs: biological significance of bFGF. Am J Pathol 2000, 156:193-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stocker KM, Sherman L, Rees S, Ciment G: Basic FGF and TGF-beta 1 influence commitment to melanogenesis in neural crest-derived cells of avian embryos. Development 1991, 111:635-645 [DOI] [PubMed] [Google Scholar]
- 34.Puri N, van der Weel MB, de Wit FS, Asghar SS, Das PK, Ramaiah A, Westerhof W: Basic fibroblast growth factor promotes melanin synthesis by melanocytes. Arch Dermatol Res 1996, 288:633-635 [DOI] [PubMed] [Google Scholar]
- 35.Halaban R, Fan B, Ahn J, Funasaka Y, Gitay-Goren H, Neufeld G: Growth factors, receptor kinases, and protein tyrosine phosphatases in normal and malignant melanocytes. Immunotherapy 1992, 12:154-161 [DOI] [PubMed] [Google Scholar]
- 36.Gilchrest BA, Eller MS, Geller AC, Yaar M: The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med 1999, 340:1341-1348 [DOI] [PubMed] [Google Scholar]
- 37.Osterlind A, Tucker MA, Stone BJ, Jensen OM: The Danish case-control study of cutaneous malignant melanoma. II. Importance of UV-light exposure. Int J Cancer 1988, 42:319-324 [DOI] [PubMed] [Google Scholar]
- 38.Naldi L, Lorenzo Imberti G, Parazzini F, Gallus S, La Vecchia C: Pigmentary traits, modalities of sun reaction, history of sunburns, and melanocytic nevi as risk factors for cutaneous malignant melanoma in the Italian population: results of a collaborative case-control study. Cancer 2000, 88:2703-2710 [DOI] [PubMed] [Google Scholar]
- 39.Kibe Y, Takenaka H, Kishimoto S: Spatial and temporal expression of basic fibroblast growth factor protein during wound healing of rat skin. Br J Dermatol 2000, 143:720-727 [DOI] [PubMed] [Google Scholar]
- 40.Abraham JA, Mergia A, Whang JL, Tumolo A, Friedman J, Hjerrild KA, Gospodarowicz D, Fiddes JC: Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science 1986, 233:545-548 [DOI] [PubMed] [Google Scholar]
- 41.Mignatti P, Morimoto T, Rifkin DB: Basic fibroblast growth factor, a protein devoid of secretory signal sequence, is released by cells via a pathway independent of the endoplasmic reticulum-Golgi complex. J Cell Physiol 1992, 151:81-93 [DOI] [PubMed] [Google Scholar]
- 42.Imokawa G, Yada Y, Morisaki N, Kimura M: Biological characterization of human fibroblast-derived mitogenic factors for human melanocytes. Biochem J 1998, 330:1235-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gohda E, Matsunaga T, Kataoka H, Takebe T, Yamamoto I: Induction of hepatocyte growth factor in human skin fibroblasts by epidermal growth factor, platelet-derived growth factor and fibroblast growth factor. Cytokine 1994, 6:633-640 [DOI] [PubMed] [Google Scholar]
- 44.Maas-Szabowski N, Stark HJ, Fusenig NE: Keratinocyte growth regulation in defined organotypic cultures through IL-1-induced keratinocyte growth factor expression in resting fibroblasts. J Invest Dermatol 2000, 114:1075-1084 [DOI] [PubMed] [Google Scholar]