Abstract
Fractalkine (CX3CL1) is synthesized as a type I transmembrane protein. Its unique CX3C chemokine domain is attached to a 241-amino acid mucin stalk, a 19-amino acid transmembrane domain, and a 37-amino acid intracellular domain of unknown function. A soluble form of fractalkine can be generated by proteolytic cleavage at the base of the mucin stalk. Novel monoclonal and polyclonal antibodies that specifically recognize only the amino- or carboxyl-terminal ends of the human fractalkine molecule have revealed that epithelial cells are the predominant cell type expressing transmembrane forms of fractalkine in human skin, the tonsil, and the large intestine. Using these specific anti-fractalkine reagents we do not detect high-level expression of fractalkine on endothelial cells in normal or inflamed colon samples obtained from patients with Crohn’s disease or ulcerative colitis. In contrast to previous reports we do not detect fractalkine expression by Langerhans cells or immature dendritic cells in mucosal-associated lymphoid tissues in vivo. We show that the reagent used in previous studies, an anti-fractalkine N-terminal peptide antisera, cross-reacts with human CD84. Finally we discuss potential roles for fractalkine in constitutive leukocyte trafficking based on its observed pattern of expression in epithelia.
Fractalkine (CX3CL1) has a number of unique properties that mediate a range of distinct biological activities. Structurally, the chemokine is characterized by the 3-amino acid spacing between the first two conserved cysteines within its chemokine domain. It is synthesized as a type-I transmembrane molecule with the chemokine domain tethered by a 241-amino acid glycosylated stalk, a 19-amino acid transmembrane region, and 37-amino acid intracellular tail. 1,2 A soluble form of the molecule comprised of the chemokine domain and most of the stalk region, is generated by cleavage at a conserved dibasic motif, proximal to the cell membrane. 1
Fractalkine is the only known chemokine shown to mediate strong cell adhesion mediated via in vitro binding to its receptor, a member of the TM7 family of receptors. 1,3,4 Monocytes, natural killer cells, T cells, 3 and microglia 5 express the CX3CR1 receptor, migrate in response to fractalkine, and adhere to immobilized fractalkine in vitro. 1,4 Cell adhesion to immobilized forms of fractalkine or transfected cells expressing full-length fractalkine can occur under flow conditions in vitro. 6,7 Binding to immobilized forms of fractalkine does not require G-protein signaling, does not require the mucin stalk, and can occur via an integrin-independent pathway. 4,7-9 Fractalkine mRNA and protein expression have been shown to be up-regulated in human umbilical cord endothelial cells (HUVECs) treated with a number of inflammatory cytokines 1 in vitro and it has been proposed that binding to fractalkine offers an alternative pathway for leukocyte adhesion under conditions of physiological flow. 4 Immunocytochemical studies using reagents reactive to peptide sequences taken from the chemokine domain of fractalkine, have shown labeling of neurons in the brain, 10 of endothelium, and dendritic cells (DCs) within the tonsil and skin. 11 Reagents reactive to a different set of peptides were reported to detect endothelium and epithelial cells in the human gut. 12
To identify the distribution of full-length transmembrane fractalkine in vivo, we have developed antibody reagents that specifically detect the intracellular tail of human fractalkine. Using novel polyclonal and monoclonal reagents that specifically recognize the chemokine domain of fractalkine in human tissue samples, we show that epithelial cells are the predominant cell type that expresses transmembrane forms of fractalkine in normal and inflamed tissues. We propose that constitutive epithelial cell production of fractalkine within the periphery may play a role in the trafficking of CX3CR1 receptor-positive DC precursors to these sites.
Materials and Methods
Production of Full-Length Transmembrane Fractalkine-Specific Reagents
Rabbit and chicken polyclonal antiserum were raised against the synthetic peptide MAEGLRYIPRSCGSNSYVL, which represents amino acids 352 to 370 located in the intracellular tail region of fractalkine, coupled at the C terminus to KLH. Rabbit antisera was raised against the synthetic peptide CADPKEQWVKDA MQHLDRQAAAL that represents amino acids 74 to 96 located within the chemokine domain of fractalkine and previously used to generate an anti-fractalkine N-terminus antisera. 1 Peptide-specific IgG was affinity-purified using NH2-activated Sepharose (Pharmacia, Uppsala, Sweden). Peptide was coupled overnight on a wheel at 4°C by using 10 mg immunizing peptide/ml of beads, in coupling buffer containing 0.1 mol/L NaHCO3 and 0.5 mol/L NaCl, pH 8.5, as per the manufacturer’s recommendations.
Human Tissue Samples
Human tonsils were obtained after routine tonsillectomies conducted at the Nuffield Infirmary, Oxford, UK. Noninvolved human skin samples were obtained after excision of skin tumors at the Department of Dermatology, Churchill Hospital, Oxford, UK. Human gut tissue was obtained either during routine colonoscopy or after surgical procedures. Tissue was embedded in OCT mounting medium (Miles Inc., Elkhart, IL), and stored at −80°C. Ethical approval for this study was obtained from the Central Oxford Research Ethics Committee (CORREC No. C98.127). For immunohistochemical and immunofluorescence analysis, 6- to 10-μm frozen tissue sections were mounted onto slides, allowed to dry at room temperature, and either used immediately or transferred to −20°C.
Cell Lines Used
The human colorectal adenocarcinoma cell line, DLD-1, the murine fibroblast line NIH/3T3, and the hamster epithelial line CHO-K1 were obtained from ATCC (Manassas, VA). The CD84-transfected cell line 300.19 CD-84 13 was kindly provided by Dr. P. Engel, Department of Cell Biology and Pathology, University of Barcelona, Spain. NIH/3T3 cells were grown in Dulbecco’s modified Eagle’s medium, DLD-1 and 300.19 CD-84 were grown in RPMI 1640 media, and CHO-K1 was grown in Ham’s F-12K medium, with each medium supplemented with 10% fetal calf serum, glutamine (2 mmol/L), and penicillin/streptomycin (5 IU/ml, 50 μg/ml, respectively; Lifetech, Paisley, UK) and maintained under standard culture conditions. In addition the 300.19 CD-84 cells were maintained in the presence of 1 mg/ml G418. In some experiments, DLD-1 cells were grown to confluence on 15-mm diameter glass coverslips (BDH, Poole, UK) placed within 6-well dishes (Corning Costar Corp., Cambridge, MA) containing culture media, as described above.
Cell Transfections
CHO-K1 or NIH/3T3 cells were plated into 9-cm Petri dishes and grown to 80% confluence then transfected using a ratio of 2 μg DNA/60 μg of lipofectamine (Lifetech) with either full-length fractalkine cloned in the mammalian expression vector pcDNA3 (Invitrogen, Carlsbad, CA), or β-galactosidase cloned in the same expression vector. Cells were washed three times in ice-cold phosphate-buffered saline (PBS) and incubated at room temperature for 5 to 10 minutes with PBS containing 2 mg/ml lidocaine (Sigma-Aldrich, St. Louis, MO) and 0.05 mol/L ethylenediaminetetraacetic acid (Sigma-Aldrich). Cells were harvested by vigorously pipetting the solution over the surface of the dishes. Collected cells were washed and either resuspended in PBS containing 10% fetal calf serum and used for either cytospin or fluorescence-activated cell sorting (FACS) studies, or placed in lysis buffer containing protease inhibitors (Complete mini; Roche, Lewes, UK) on ice for 20 minutes before centrifugation at 10,000 × g for 20 minutes, and stored at −20°C before use in Western blotting analysis.
Cytospin Studies
Transfected NIH/3T3 cells were suspended at a concentration of 1 × 10 6 cells/ml and then 200 μl was applied to 1% gelatin-coated glass laboratory slides (BDH) using a Cytospin 3 centrifuge (600 rpm, 6 minutes; Shandon, Pittsburgh, PA). Slides were air-dried and stored at −20°C until used.
FACS Studies
DLD-1 cells were washed and fixed in 2% paraformaldehyde in PBS for 30 minutes at 4°C. Cells were then washed and permeabilized in 0.5% saponin/0.5% bovine serum albumin/PBS (Sigma-Aldrich) containing 5% normal human serum (National Blood Service, Bristol, UK), a solution used for all subsequent staining steps. Primary antibodies were applied for 20 minutes at 4°C, cells were washed, and fluorescein isothiocyanate-conjugated secondary antibodies applied for 20 minutes at 4°C in the dark. Cells were subsequently washed, fixed in 2% paraformaldehyde in PBS, and analyzed by FACS, using a FACScan and CellQuest software (Becton Dickinson, Franklin Lakes, NJ).
Isolation of Total RNA and Semi-Quantitative Reverse Transcriptase-Polymerase Chain Reaction (PCR)
DLD-1 cell pellets were resuspended in total RNAzol B isolation reagent (Biogenesis, Poole, UK) and total RNA isolated according to the manufacturer’s instructions. Dried RNA pellets were resuspended in nuclease-free water and stored at −80°C before analysis. HUVEC cDNA, was a kind gift from Dr. Dicken Koo, Nuffield Department of Surgery, University of Oxford, Oxford, UK. Total RNA was reverse-transcribed using oligo dT 12-18 and Superscript reverse transcriptase (Lifetech). Reactions were incubated at 42°C for 40 minutes and enzyme-inactivated at 95°C for 5 minutes. Triplicate PCR reactions were assembled containing cDNA from 25 ng of total RNA and Taq DNA polymerase (Bioline, London, UK). PCR for the housekeeping gene hypoxanthine phosphoribosyltransferase (HPRT) was performed using the primers 5′-AATTATGGACAG GACTGAACGTC-3′ (forward) and 5′-CGTGGGGTCCTTTTCACCAGCAAG-3′ (reverse), generating a 386-bp PCR product. PCR for fractalkine was performed using the primers 5′-CACGTGCAGCAAGATGACATC-3′ (forward) and 5′-CACTCGGAAAA GCTCCGTGC-3′(reverse), generating a 462-bp PCR product. Reactions were subjected to touchdown PCR using a PTC-200 thermal cycler (MJ Research, Watertown, MA) with the following parameters: after an initial denaturing step of 96°C for 1 minute, five cycles of 96°C for 25 seconds, 70°C for 45 seconds, and 72°C for 45 seconds; followed by 31 cycles of 96°C for 25 seconds, 60°C for 50 seconds, and 72°C for 45 seconds; and finally four cycles of 96°C for 25 seconds, 55°C for 1 minute, and 72°C for 2 minutes. After agarose gel electrophoresis PCR products were analyzed under a UV lamp and product intensities measured by AlphaEase image analysis software (Alpha Innotech Corporation, San Leonardo, CA). Fractalkine PCR product intensities were divided by those of the HPRT PCR product intensities to give a fractalkine:HPRT ratio to generate comparative fractalkine mRNA data. The specificity of fractalkine PCR products was confirmed by digestion with BsmA1 restriction endonuclease (New England Biolabs, Hitchin, UK; data not shown).
Antibodies Used
Tissue and cytospin samples were labeled using a range of chemokine-specific and lineage-related reagents (Table 1) ▶ . The immunostaining conditions were optimized for each antibody reagent used in this study. The following reagents and fixation conditions were used: murine anti-human monoclonal IgG1 antibodies reactive to fractalkine chemokine domain (1 to 10 μg/ml, clone 51636.11; 1 μg/ml, clone 51637.11; R&D Systems, Minneapolis, MN), anti-human CD1a (1 to 10 μg/ml, clone NA1/34, isotype IgG2a; Serotec, Kidlington, UK) anti-cytokeratin AE1/AE3 (1 μg/ml, clone AE1/AE3 isotype IgG1; DAKO AS, Glostrup, Denmark); goat anti-fractalkine chemokine domain polyclonal (5 μg/ml; R&D Systems); rabbit anti-fractalkine N-terminal peptide 1 (Chemocentryx, San Carlos, CA) used at 0.1 to 2 μg/ml; rabbit anti-fractalkine C-peptide reagent, chicken anti-fractalkine C-peptide reagent, used at 1 to 5 μg/ml. Murine anti-human CD84 mAb (IgG1) was obtained from the Seventh International Workshop on Human Differentiation Antigens, Harrogate, UK, 2000. These reagents were used on sections fixed using either 4% paraformaldehyde in PBS containing 1 mmol/L HEPES at room temperature or ice-cold acetone for 10 minutes. Specificity controls used for the polyclonal antibodies were their specific absorption with 10× molar excess of recombinant human fractalkine chemokine domain (362-CX-025, R&D Systems) or immunizing peptide for 30 minutes at room temperature and subsequent application to serial tissue sections. For the monoclonal reagents, isotype control antibodies of immunoglobulin classes IgG1 and IgG2a (Serotec) were applied to tissue sections at the same concentration as the specific reagents and assessed for reactivity. In addition to the two anti-fractalkine chemokine domain mAb clones described above, two further clones (clones 51621.11, 51643.11; R&D Systems) also showed the same patterns of reactivity as clone 51636.11 on human tonsil tissue.
Table 1.
Antihuman Fractalkine Reagents Used in this Study
| Reagent | Control reagent | Used at | Source |
|---|---|---|---|
| Mouse anti-human fractalkine chemokine domain clone 51636.11 mAb | Mouse IgG1; preabsorbtion with 10X molar excess Rh Fractalkine | 1–10 μg/ml | R&D Systems, Minneapolis, MN |
| Mouse anti-human fractalkine chemokine domain clone 51637.11 mAb | Mouse IgG1 | 1 μg/ml | R&D Systems |
| Goat anti-human fractalkine chemokine domain polyclonal | Goat IgG; preabsorbtion with 10X molar excess Rh Fractalkine | 5 μg/ml | R&D Systems |
| Rabbit anti-human fractalkine N-terminal peptide polyclonal | Rabbit IgG; preabsorbtion with 10X molar excess immunizing peptide | 0.1–2 μg/ml | Chemocentryx, San Carlos, CA & this paper |
| Rabbit anti-human fractalkine C-terminal peptide polyclonal | Rabbit IgG; preabsorbtion with 10X molar excess immunizing peptide | 1–5 μg/ml | This paper |
| Chicken anti-human fractalkine C-terminal peptide polyclonal | Preabsorbtion with 10X molar excess immunizing peptide | 1–5 μg/ml | This paper |
Immunofluorescent Labeling
All antibodies were diluted in PBS containing 1% bovine serum albumin and 0.2% Triton X-100. Single labeling involved incubation of primary antibodies for 2 hours at 4°C. Concentrations of the secondary and tertiary reagents was typically 0.1 mg/ml. In cases in which a red label was required for visualization, samples were incubated with anti-species-specific IgG conjugated to the fluorochrome Texas Red (Jackson Laboratories, Bar Harbor, ME) for 1 hour at 4°C. In cases in which a green label was required for visualization, samples were incubated with anti-species-specific IgG conjugated to biotin (DAKO), for 1 hour at 4°C. Finally a streptavidin-fluorescein isothiocyanate reagent (Amersham, Amersham, UK) was applied for 30 minutes at 4°C. Double-immunofluorescent labeling involved mixing the mouse and rabbit primary antibodies, during the first incubation period and the biotinylated and Texas Red-conjugated anti-species-specific IgG during the second. Finally a streptavidin-fluorescein-isothiocyanate reagent (Amersham) was applied for 30 minutes at 4°C.
Immunohistochemistical Labeling
Primary antibodies were incubated from times ranging from 2 to 12 hours at 4°C. Neutralization of endogenous peroxidases was achieved by incubating tissue sections with 3% H2O2 in PBS containing 0.1 g/ml NaN3 (Sigma-Aldrich) for 10 minutes at room temperature. Concentrations of the secondary and tertiary reagents were typically 0.1 g/ml. Double labeling involved mixing the mouse and rabbit primary antibodies during the first incubation period, and the biotinylated and horseradish peroxidase-conjugated anti-species-specific IgG (DAKO) during the second. Finally a streptavidin-alkaline-phosphatase reagent (DAKO) was applied for 30 minutes at 4°C. The sections were then developed using diaminobenzidine tetrahydrochloride (PolySciences, Warrenton, PA), washed in PBS and then incubated with streptavidin-alkaline-phosphatase for 30 minutes at room temperature and developed using BCIP/VBT substrate (Vector Laboratories).
Western Blot Analysis
To test the specificity of the anti-peptide antiserum raised against the carboxyl terminus of human fractalkine, Western blotting was performed on protein lysates of CHO-K1 cells transfected with full-length fractalkine cloned in the mammalian expression vector pcDNA3 1 (Invitrogen, Carlsbad, CA), or untransfected CHO-K1 cells. Samples, which included supernatant taken from the fractalkine-transfected cells, were run under reducing conditions on 7.5% polyacrylamide gels (Pharmacia) and transferred to nitrocellulose membranes (Hybond C; Amersham) overnight at 4°C. Membranes were probed using goat anti-chemokine domain polyclonal and chicken and rabbit anti-C peptide reagents at 0.5 μg/ml. Specific signal was amplified by addition of horse anti-species-specific IgG conjugated to horseradish peroxidase and visualized using an enhanced chemiluminescence system (ECL, Amersham).
Results
Generation of a Transmembrane Fractalkine-Specific Reagent
To differentiate between transmembrane-expressed fractalkine and cleaved forms of the molecule, we developed a number of polyclonal anti-peptide reagents (rabbit α-C-pep and chicken α-C-pep), using a peptide sequence from the intracellular tail of the molecule.
We tested the specificity of these anti-C-peptide reagents in the following ways. First, cell lysates prepared from CHO-K1 WT or CHO-K1 transfected with a construct 1 containing the full-length transcript of fractalkine and samples of supernatant taken from the transfected cells, were separated via electrophoresis under reducing conditions and analyzed by Western blotting. Figure 1A ▶ shows a representative experiment comparing the reactivity of goat anti-fractalkine chemokine domain polyclonal (R&D Systems) and the chicken anti-C-peptide polyclonal. Both reagents detected two bands of similar size, ∼95 kd, within the transfected cell lysates (Figure 1 ▶ , lanes 2 and 5). These two bands may represent different glycosylated forms of the molecule. No nonspecific bands were detected by either reagent within WT CHO-K1 samples (Figure 1 ▶ , lanes 1 and 4). The anti-chemokine domain reactive polyclonal detected a broad band of 85 to 90 kd (Figure 1 ▶ , lane 3) within the transfected cell supernatant whereas the anti-C-peptide reagent showed no reactivity against proteins within the supernatant (Figure 1 ▶ , lane 6). In other experiments neither anti-C-peptide reagent detected various recombinant forms of fractalkine- or macrophage-derived chemokine (R&D Systems, data not shown). Thus it is possible to use these reagents to discriminate between full-length and cleaved forms of fractalkine. Second, we compared the anti-C-peptide reagents ability to detect nondenatured fractalkine expressed on transiently transfected NIH/3T3 cells with other anti-fractalkine reagents. Each of the anti-fractalkine reagents used: mouse anti-chemokine domain mAb clone 51636.11 (Figure 1C ▶ , R&D Systems), goat anti-chemokine domain reagent (Figure 1E) ▶ , chicken anti-C-peptide reagent (Figure 1F) ▶ , rabbit anti-C-peptide (Figure 1H) ▶ , and anti-N-peptide reagent 1 (Figure 1I) ▶ strongly stained the surface of ∼80% of the cells. In contrast the murine IgG1 isotype control mAb (Figure 1B) ▶ , no primary antibody control (1D), and rabbit IgG control, showed no specific staining of the cell surface.
Figure 1.

Distinguishing between cleaved and membrane-tethered fractalkine, generation of specific reagents. A: Samples of Western lysates from WT CHO-K1 and CHO-K1 cells transfected with a human fractalkine expression vector 1 along with samples of supernatant taken from fractalkine-transfected CHO-K1 cells, were run on 7.5% acrylamide gels under standard reducing conditions. Samples were transferred to nitrocellulose membranes and identical membranes probed using goat anti-fractalkine polyclonal reagent (goat α-Fkn, R&D Systems) (lanes 1–3) or chicken anti-C-peptide polyclonal reagent (chicken α-C-pep, lanes 4–6). The goat α-Fkn reagent is reactive against the chemokine domain of the molecule and specifically detects twobands at the predicted size of 95 kd (lane 2, asterisk). These two bands are also detected by the chicken α-C-pep reagent (lane 5, asterisk). In addition, these reagents discriminate between cleaved and intact forms of the molecule as the goat α-Fkn detects the cleaved form of fractalkine within transfected cell supernatant (lane 3, 85 to 90 kd), whereas the chicken α-C-pep does not (lane 6). Furthermore, the goat α-Fkn detects one larger (lane 2, 100 kd) and two smaller bands (lane 2, 75 and 66 kd) within transfected CHO-K1 samples that are not detected by the chicken α-C-pep (lane 5). The larger band may be nonspecific because it has no counterpart detected by the chicken α-C-pep. The two smaller bands may indicate partially degraded forms of fractalkine, still containing the N-terminus chemokine domain. B–I: The specificity of a range of anti-fractalkine antibodies was evaluated by immunohistochemistry. Cytospins were prepared from NIH/3T3 cells transiently transfected as above, with fractalkine (3T3-Fkn) and were stained as follows. B: 3T3-Fkn stained with mouse IgG1 control as a control for C. C: 3T3-Fkn stained with mouse anti-fractalkine chemokine domain (mouse α-Fkn, clone 51636.11; R&D Systems) mAb. D: 3T3-Fkn stained with no primary antibody as a control for E and F. E: 3T3-Fkn stained with goat α-Fkn. F: 3T3-Fkn stained with chicken α-C-pep. G: 3T3-Fkn stained with rabbit IgG as a control for H and I. H: 3T3-Fkn stained with rabbit α-C-peptide. I: 3T3-Fkn stained with rabbit α-N-pep polyclonal reagent. 1 Note that although there is light nonspecific staining of the nucleus within the control sections (B, D, and F) this is in marked contrast to the strong cell surface staining in sections stained with the specific reagents. Similar results were obtained using transfected CHO-K1 cells and via immunofluorescence. Original magnification, ×400.
Fractalkine Is Constitutively Expressed on a Human Colorectal Adenocarcinoma Cell Line, DLD-1
Initial analysis of human tonsil sections with both the anti-C peptide reagents revealed a very different pattern of staining to published patterns, which were obtained using an anti-fractalkine N-terminal peptide reagent. 12 The predominant cell type detected with our anti-C-terminal peptide reagents was epithelial cells. To confirm that epithelial cells express full-length transmembrane fractalkine we used the adenocarcinoma cell line DLD-1. We stained confluent monolayers of unstimulated DLD-1 cells for the presence of transmembrane fractalkine by immunofluorescent double labeling (Figure 2A) ▶ using mouse anti-chemokine domain (clone 51636.11; green) mAb and the rabbit anti-C-peptide reagents (red). This revealed a subset of DLD-1 cells strongly positive for both epitopes, whereas most of the rest of the cells were positive for the chemokine domain of fractalkine. The specificity of the anti-fractalkine chemokine domain mAb was verified (Figure 2B) ▶ by double labeling using a mouse IgG control mAb (green) and rabbit anti-C-peptide reagents (red). Furthermore, the reactivity of the mAb could be competed away by the addition of a 10× molar excess of recombinant human fractalkine (Figure 2C) ▶ . Interestingly, when these cells were double-labeled for the presence of cytokeratin AE1/AE3 (green) and the intracellular epitope of fractalkine (red), the transmembrane fractalkine-positive cells correspond to those cells with the highest levels of cytokeratin expression (Figure 2D) ▶ .
Figure 2.
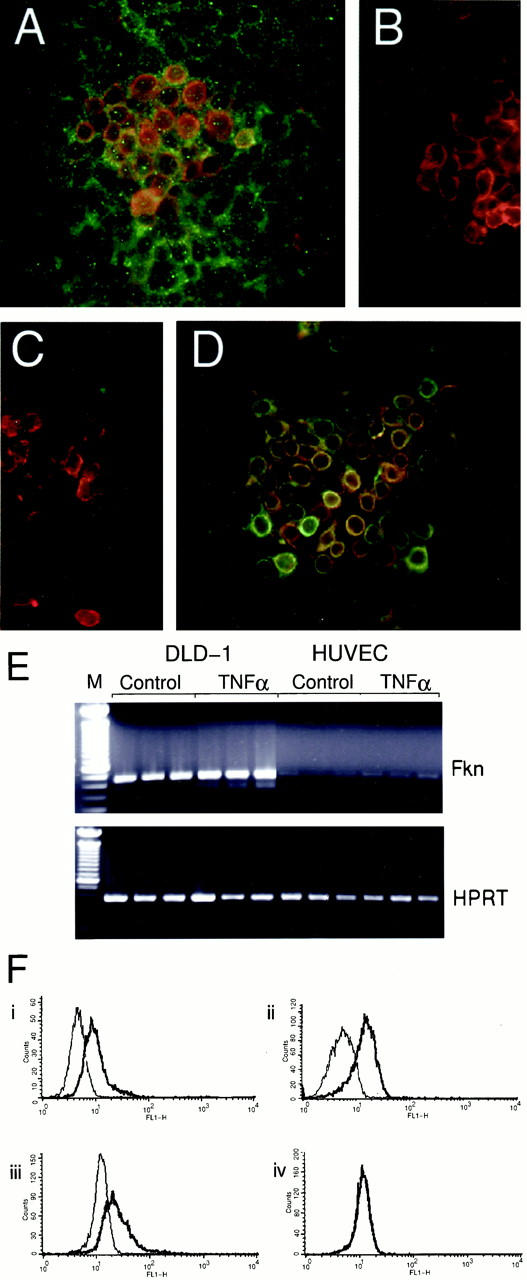
The transmembrane form of fractalkine is expressed by the human colorectal adenocarcinoma cell line, DLD-1. A: DLD-1, cells were grown to confluence on glass coverslips and stained using indirect immunofluorescence for transmembrane-expressed fractalkine using the anti-fractalkine chemokine domain (mouse α-Fkn, clone 51636.11; green) mAb and rabbit anti-C-peptide reagent (α-C-pep; red). Strong double labeling (orange) occurred on a subset of cells where the intracellular epitope was most strongly expressed. Lower levels of anti-chemokine domain staining could be detected on most cells. B: Anti-chemokine domain reagent specificity was demonstrated by double labeling using an isotype control antibody for the anti-chemokine mAb (green) and α-C-pep (red). α-C-pep staining was also competed out by addition of 10× molar excess of the immunizing peptide (data not shown). C: The α-Fkn (green) but not α-C-pep staining (red) couldbe competed totally by pre-incubation with a 10× molar excess of recombinant human fractalkine chemokine domain (rhFkn; 362-CX-025; R&D Systems). D: Cells were double-labeled with α-cytokeratin (clone AE1/AE3, DAKO; green). Original magnifications, ×400 (A–D). E: Total RNA was prepared from DLD-1 and HUVECs cultured with or without 10 U/ml TNF-α. RNA was reverse-transcribed and triplicate 25 ng cDNA samples subjected to PCR reactions using primers specific for fractalkine (Fkn) or HPRT. There was no fractalkine or HPRT signal amplified in reverse transcriptase samples (data not shown). F: DLD-1 cells were permeabilized and stained using i) mouse α-Fkn (clone 51636.11) mAb or control mouse IgG1 mAb (Serotech), ii) goat α-Fkn polyclonal or 10% goat serum, iii) α-C-pep or rabbit IgG, iv) α-N-pep polyclonal 1 or rabbit IgG, and fractalkine expression analyzed by FACS. The bold trace shows the fluorescence of cells stained with the specific antibody, whereas the normal trace shows the background fluorescence of cells stained with the control reagent.
We analyzed in a qualitative manner, the relative levels of fractalkine mRNA expression in DLD-1 cells and in HUVECs, which have been shown to express fractalkine after activation with inflammatory cytokine. 1 Reverse transcriptase-PCR analysis was performed on triplicate samples of 25 ng of cDNA derived after reverse transcription of RNA harvested from DLD-1 and HUVECs incubated with or without 10 U/ml tumor necrosis factor (TNF)-α for 24 hours. A representative experiment is presented in Figure 2E ▶ where DLD-1 samples show abundant expression of fractalkine message transcripts with and without TNF-α treatment. In contrast HUVECs show a weak level of constitutive expression that is slightly increased with TNF-α treatment.
Finally we compared the anti-N-peptide reagents reactivity to fractalkine expressed by permeabilized DLD-1 cells with a range of anti-fractalkine chemokine domain and anti-C-peptide reagents via FACS (Figure 2F) ▶ . The bold traces show cells labeled with either 1) mouse anti-fractalkine chemokine domain (clone 51636.11) mAb, 2) goat anti-fractalkine chemokine domain polyclonal reagent, 3) rabbit anti-C-peptide polyclonal reagent, or 4) rabbit anti-N-peptide polyclonal reagent. The thin traces show labeling of the cells with 1) mouse IgG1 isotype control, 2) 10% goat serum in PBS control, 3) and 4) rabbit IgG control. Both anti-fractalkine chemokine domain reagents (1 and 2) and the anti-C-peptide reagent (3) detect clear shifts in fluorescence relative to their controls, indicating the detection of fractalkine on these cells, however the anti-N-peptide reagent shows no shift in fluorescence relative to the rabbit IgG control antibodies. This failure of the anti-N-peptide reagent to detect fractalkine on the DLD-1 line was also observed when staining confluent DLD-1 via immunofluorescence (data not shown).
Polyclonal Antibodies Derived from a Fractalkine N-Terminal Peptide Cross-React with Human CD84
During a screening of human tonsil with a panel of antibodies from the seventh Human Leukocyte Differentiation Antigens workshop we noticed that the staining pattern of the anti-fractalkine N-terminal peptide reagent (α-N-pep) (Figure 3A) ▶ was very similar to that for human CD84 (admixture of α-CD84 mAb from HLDA human CD84 panel) (Figure 3B) ▶ . CD84 is a cell surface antigen of the Ig super family, with no ascribed function. 13 To test whether α-N-Fkn cross-reacted to CD84 protein we used 20 ng of recombinant human fractalkine (Rh fractalkine) containing the N-terminus and mucin stalk (R&D Systems) and 100 ng of cell lysate from the human CD84-transfected cell line 300.19 CD-84. 13 Duplicate Western blot membranes were incubated with α-N-pep, α-CD84 (clone 2G7), goat α-Fkn, and chicken α-C-pep antisera (Figure 3C) ▶ . The α-N-pep reagent clearly detects Rh fractalkine (lane 1) at the predicted size of 90 kd and also a degradation product (∼70 kd), it also strongly detects a band ∼70 kd in size in the lysate from the CD84-transfected cell line (asterisk, lane 2). The α-CD84 reagent does not detect Rh fractalkine (lane 3) but clearly detects an ∼70-kd band (asterisk, lane 4) in CD84-transfected cells, consistent with the reported size of human CD84. 13 The goat α-Fkn reagent also detects the major 90-kd band and degradation band within the Rh fractalkine sample (lane 5) but does not detect any proteins within the CD84-transfected cell line sample (lane 6). In contrast the α-C-pep reagent does not detect Rh fractalkine (lane 7) or any proteins within the CD84-transfected cell line sample (lane 8).
Figure 3.
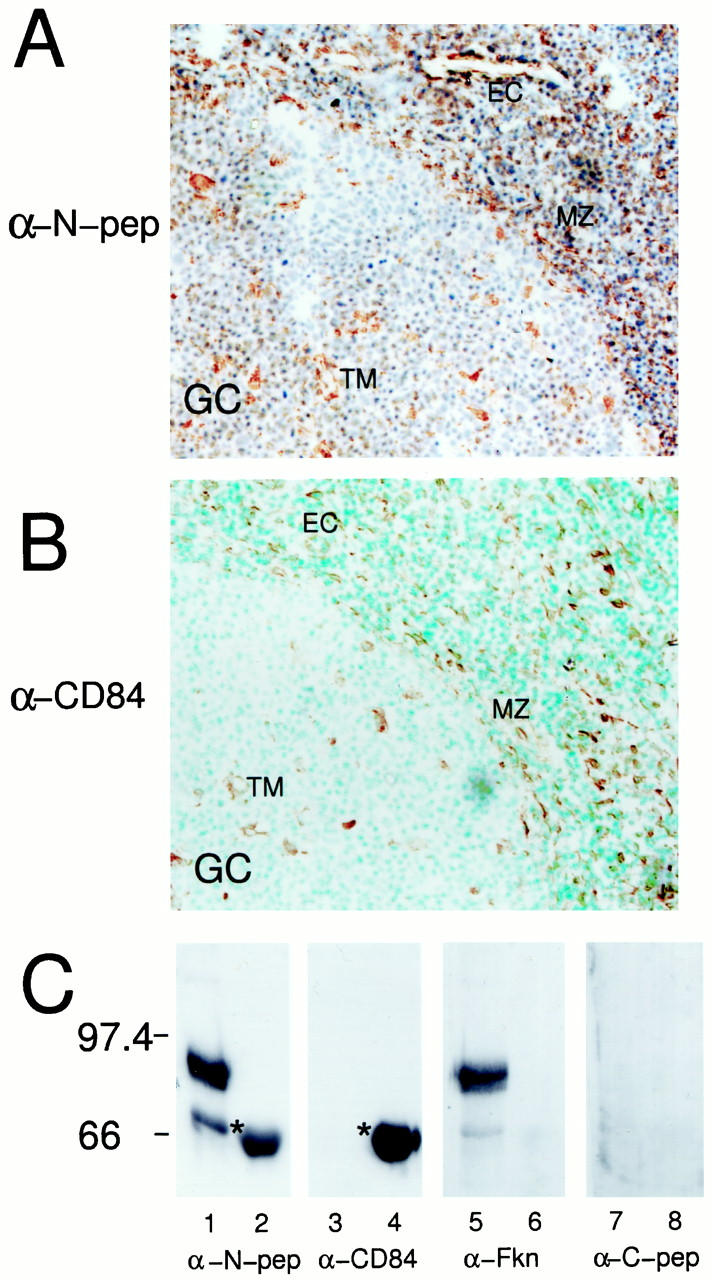
Rabbit α-N-pep polyclonal reagent cross-reacts with human CD84. Human tonsils were collected after routine tonsillectomy. Frozen sections (8 to 10 μm) were cut and examined for fractalkine expression by immunohistochemistry. Sections were stained using the rabbit α-N-pep polyclonal, counterstained with hematoxylin (A) or mouse anti-CD84 mAb, counterstained with methyl green (B) (α-CD84 13 ). Similar regions of sections from different tonsils are shown, which include part of a germinal center (GC). Similar cell types are detected by both reagents, including tingible body macrophages (TM), cells within the marginal zone (MZ), and endothelial cells (EC). An identical staining pattern was obtained using an independently produced rabbit α-N-pep polyclonal reagent. Negative control antibodies showed no background staining (data not shown). Original magnifications, ×400 (A–B). C: Western blot analysis of recombinant human fractalkine containing its N-terminus and mucin stalk (rhFKN, 365-FR-025; R&D Systems) and Western lysate taken from CD84-transfected cell line 300.19 CD-84; 13 using anti-fractalkine reagents and an α-human CD84 mAb. 13 Identical nitrocellulose membranes containing 20 ng of rhFKN (lanes 1, 3, 5, and 7) and 100 ng of CD84-transfected cell lysate (lanes 2, 4, 6, and 8), were probed using the α-N-pep (lanes 1 and 2, α-N-pep) and α-CD84 mAb (lanes 3 and 4, α-CD84). These membranes were stripped of bound antibody and then reprobed using goat α-Fkn (lanes 5 and 6) or chicken α-C-pep (lanes 7 and 8). α-N-pep detected two bands in the rhFKN sample (lane 1). The major band (90 kd) being the predicted size of this fractalkine product, the minor band possibly representing a degradation product. Furthermore, this reagent also detected a band in the CD84-transfected cell sample (lane 2, asterisk; 66 kd). The α-CD84 mAb detected this 66-kd band only within the CD84-transfected cell sample (lane 4, asterisk). The membrane probed with α-CD84 was stripped and reprobed with goat α-Fkn that detected the same two bands within the rhFKN sample as the α-N-pep (lane 5), but did not detect any bands within the CD84-transfected cell sample (lane 6). The membrane probed with α-N-pep was stripped and reprobed with chicken α-C-peptide and no bands were detected in either sample (lanes 7 and 8).
Fractalkine Is Predominantly Expressed on the Basal Layer of the Epidermis within Noninflamed Human Skin
Langerhans cells, melanocytes, endothelial cells, and dermal dendrocytes have been reported to express fractalkine on the basis of staining using the α-N-pep polyclonal antisera. 11,14 We were interested in comparing the reactivity of our α-C-pep reagent and goat α-Fkn reagents, within similar skin samples. Serial frozen sections taken from noninflamed human skin were stained with goat α-Fkn (Figure 4A) ▶ or α-C-pep (Figure 4B) ▶ . In both cases staining was restricted to the basal keratinocytes of the epidermis, with no significant staining of structures or cells within the dermis. In contrast, the α-N-pep reagent (Figure 4C) ▶ reproduced the staining reported previously, 11,14 with clear labeling of intraepidermal Langerhans cells and melanocytes along the basement membrane of the epidermis, along with blood vessels and cells with a dendritic morphology, within the dermis (Figure 4C) ▶ . Our independently produced α-N-pep antisera obtained an identical staining pattern to Figure 4C ▶ .
Figure 4.
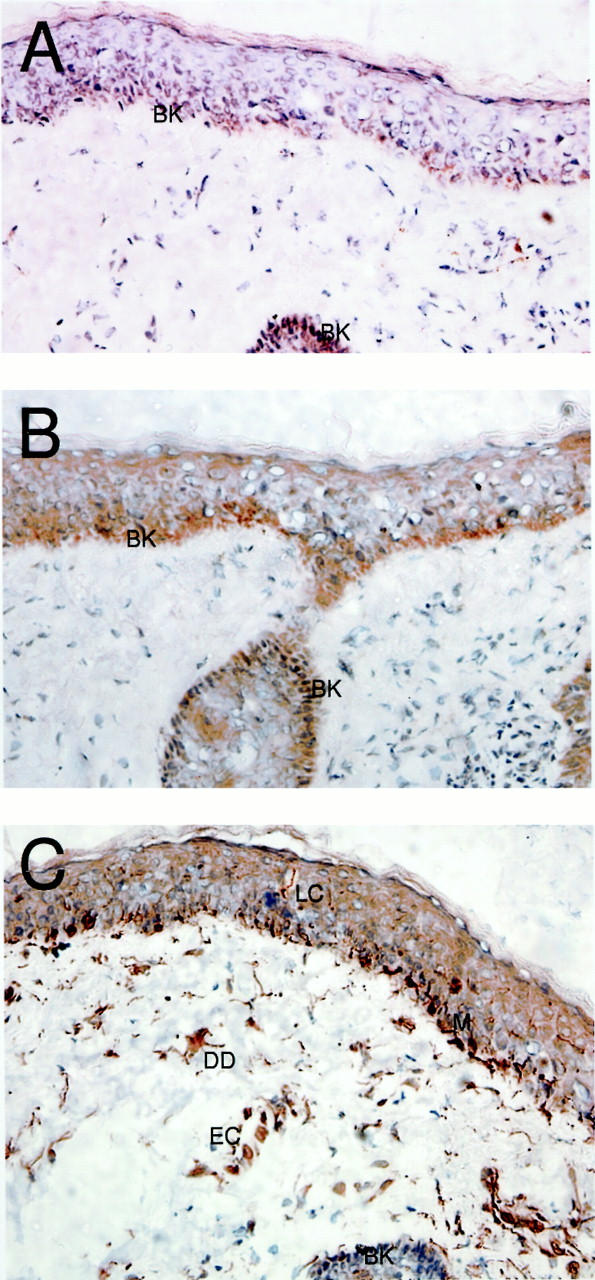
Transmembrane fractalkine is predominantly expressed in the epidermis of noninflamed human skin. Noninvolved skin samples were taken after resection of human facial skin tumors. Frozen sections (8 to 10 μm) were cut for analysis by immunohistochemistry. Immunohistochemical labeling was performed using anti-fractalkine and anti-peptide polyclonal reagents. A: The staining using the goat α-Fkn revealed positive cells restricted to the basal keratinocytes of the human epidermis, with no obvious positive cells within the dermis. B: Staining using rabbit α-C pep, which reacts to an intracellular epitope of fractalkine, revealed a similarly restricted staining pattern, again with no obvious positive cells within the dermis. C: Staining using the rabbit α-N-pep reagent showed discrete positive cell staining within the epidermis with morphology characteristic of Langerhans cells (a single cell is emphasized against the background), along the basement membrane of the epithelium with characteristics of melanocytes (M), there was also discrete staining of cells within the dermis, including structures with the appearance of blood vessels (EC) and dermal dendrocytes (DD). Negative control reagents showed no background staining (data not shown). Original magnifications, ×400.
Thus the rabbit α-N-pep reagent that labels specifically NIH/3T3-transfected cells (Figure 1I) ▶ fails to detect transmembrane fractalkine expressed on epithelial cells in the skin but detects other cell types not labeled by anti-fractalkine chemokine domain-specific or anti-intracellular epitope-specific reagents.
Expression of Transmembrane Fractalkine within the Tonsil and Human Colon
Using our validated antibody reagents we critically examined the expression of transmembrane fractalkine expression within the human tonsil and noninvolved and chronically inflamed gut tissue. We re-examined fractalkine expression within the human tonsil using the mouse anti-fractalkine chemokine domain mAb (mouse α-Fkn, clone 51636.11) and rabbit α-C-pep reagents, looking at expression within the outer pharyngeal epithelium (Figure 5; A, B, and C ▶ ) and the cortex of the tonsil (Figure 5, D, E, and F) ▶ . Fractalkine expression within the pharyngeal epithelium was reminiscent of the pattern of fractalkine expression seen in the epidermis, with mouse α-Fkn mAb (Figure 5A ▶ , red) strongly labeling the epithelial cells but not underlying connective or lymphoid tissue. The staining was not uniform, with the basal layer of the epithelium more strongly stained than the next higher cell layers and the strongest expression on the outer layers, no obvious DC labeling was detected. Negative control antibodies showed no background staining or autofluorescence (data not shown). This staining pattern was also detected using the goat α-Fkn polyclonal reagent (data not shown). Again, similar to the epidermis, staining for the intracellular domain of fractalkine using α-C-pep reagents (Figure 5B ▶ ; red) was restricted to the basal layer of the epithelium. Double labeling using mouse α-Fkn mAb (green) and α-C-pep reagent (red) clearly showed expression of both epitopes (orange) restricted to the basal layer of the epithelium (Figure 5C) ▶ .
Figure 5.

Transmembrane fractalkine is predominantly expressed in the epithelium of human large intestine and tonsil. Colon samples were taken after resection from Crohn’s or ulcerative colitis patients, whereas human tonsils were collected after routine tonsillectomy. Frozen sections (8 to 10 μm) were cut and examined for analysis using indirect immunofluorescence. A: Staining of the pharyngeal epithelium with mouse α-Fkn mAb (clone 51636.11; red) revealed a strong labeling of the epithelial cells but not underlying connective or lymphoid tissue. The staining was not uniform, with the basal layers more strongly detected than the next higher cell layers and then the strongest expression on the outer, more squamous layers. Negative control antibodies showed no background fluorescence within the pharyngeal epithelium (data not shown). No obvious DC labeling was detected. B: Staining with rabbit α-C-pep (red) that detects an intracellular epitope of fractalkine, was restricted to the basal layer of the epithelium. C: Double labeling of the pharyngeal epithelium for the expression of the chemokine domain (green) and intracellular epitope (red) revealed that transmembrane expression of fractalkine was restricted to the basal layer of the epithelium. D: Staining for fractalkine expression within the cortex of the tonsil looking for the chemokine domain of the molecule using mouse α-Fkn mAb (clone 51636.11; green) revealed that labeling was restricted to the crypt epithelium. This staining pattern was repeated using goat α-Fkn polyclonal reagent (data not shown). There was no obvious staining of cells within T or B cell areas and no obvious staining of endothelium. E: The expression of the intracellular epitope of fractalkine (α-C-pep; red) also localized to cells with a similar morphology within the crypt epithelia. Such staining was competed completely by inclusion of 10× molar excess of immunizing peptide (data not shown). F: Double labeling for the expression of the chemokine domain (mouse α-Fkn, clone 51636.11; green) and intracellular epitope (α-C-pep; red) showed a major subset of cells were strongly double-positive (orange). There were, however, other cells that were single-labeled for the chemokine domain. Strong labeling at the external surface of the crypt represents artifactual staining present in the isotype control (data not shown). G: Double-immunofluorescent labeling for the expression of CD1a-positive immature DCs (green) and the intracellular epitope of fractalkine (α-C-pep; red) showedthat although DCs are intimately associated with transmembrane expressed fractalkine, they do not express it. H: Double-immunofluorescent labeling for the expression of CD1a-positive immature DCs and the intracellular epitope of fractalkine within the cortex of the tonsil. Similar to the pharyngeal epithelium, there is a close association of single CD1a-positive immature DCs (green) with crypt epithelial cells positive for the transmembrane form of the fractalkine (red). I: Staining of human colon taken from an ulcerative colitis patient using an anti-fractalkine chemokine domain reagent (mouse α-Fkn, clone 51636.11, green) revealed strong staining within the epithelial cells of the lamina propria (LP). No obvious endothelial staining was detectable. J: Similarly, staining for the intracellular epitope (rabbit α-C-pep, red) was also restricted to the epithelial cells within the lamina propria. K: Double labeling for the chemokine domain (green) and the intracellular epitope (red) confirmed that the transmembrane fractalkine expression on epithelial cells within the lamina propria was the predominant form. L: An identical double-labeling staining pattern was seen within noninflamed large bowel sections. Human colon samples stained with the rabbit α-Fkn-pep reagent showed extensive staining of leukocytes and endothelium within the sections (data not shown). Original magnifications, ×125 (A–I), ×225 (J–L).
Within the cortex of the tonsil, labeling with mouse α-Fkn mAb (Figure 5D ▶ , green) showed that the predominant site of fractalkine expression was cells within the crypt epithelia. This pattern was also detected using the goat α-Fkn polyclonal reagent (data not shown). There was no staining of blood vessels, or cells within T or B cell areas, relative to control antibodies. The α-C-pep reagent also showed a staining pattern restricted to the crypt epithelia (Figure 5E ▶ , red). Double labeling (Figure 5F) ▶ using the mouse α-Fkn mAb (green) and α-C-pep reagent (red) clearly showed double labeling of a subset of cells within the crypt epithelia (orange). Strong green labeling of the external surface of the crypts is a staining artifact, also restricted to this surface in the negative controls (data not shown). There is however also a minor population of chemokine-domain single positive cells, located closer to the lymphoid edge of the crypts.
The pharyngeal epithelium of the tonsil contains a resident population of immature CD1a-positive DCs. Double-immunofluorescent labeling (Figure 5G) ▶ using α-CD1a mAb detected positive immature DCs (green) and α-C-pep reagent (red) showed that although CD1a-positive DCs are intimately associated with the transmembrane-expressed fractalkine they do not express it. Similarly, immature DCs within the crypts were also shown to be negative for transmembrane fractalkine by double-immunofluorescent labeling (Figure 5H) ▶ .
Recently Muehlhoefer and colleagues 12 reported fractalkine protein expression on the epithelium of the lamina propria of the human gut and on the lamina propria and endothelium of chronically inflamed gut. This study was performed using a mixture of an anti-N-peptide andanti-C-peptide polyclonal reagents (Santa Cruz Biotechnology, Inc.) and thus was not able to demonstrateconclusively that the fractalkine detected was the transmembrane form on these cells. We were not able to duplicate this staining using these reagents.
We therefore stained frozen sections of human colon from Crohn’s and ulcerative colitis patients with the mouse α-Fkn mAb (Figure 5I ▶ , green) and α-C-pep reagent (Figure 5J ▶ , red), confirming the positivity of the lamina propria for extracellular and intracellular epitopes of fractalkine. Double labeling showed that the epithelial cells of the lamina propria express transmembrane fractalkine because they are clearly positive for both epitopes (Figure 5K ▶ , orange). A similar expression pattern was detected within adjacent noninvolved colon tissue (Figure 5L) ▶ . In contrast to results obtained with the α-N-pep reagent (data not shown) no obvious positive endothelial staining by either the anti-chemokine domain or intracellular epitope-reactive reagents was detected. The staining pattern revealed by the α-C-peptide reagent was consistent on 16 ulcerative colitis and 13 Crohn’s disease patient samples (for age and sex breakdown refer to Table 2 ▶ ).
Table 2.
Patient Sample Details Analyzed for Intracellular Fractalkine Epitope-Staining via Immunohistochemistry
| Disease | Sample size | Sex ratio | Mean age/age range, year |
|---|---|---|---|
| Ulcerative colitis | 16 | 7M /9F | 45.4 /(27–71) |
| Crohn’s disease | 13 | 4M /9F | 31.1 /(19–46) |
Fractalkine-Positive Cells within the Tonsil Express Cytokeratin
To confirm that fractalkine-positive cells within the epithelial crypts of the tonsil were epithelial cells, we stained adjacent serial tonsil frozen sections with α-C-pep reagent (Figure 6A ▶ , brown) and α-cytokeratin (Figure 6B ▶ , blue). Staining with either antibody was restricted to within the epithelial crypts, within the sections examined, with a characteristic labeling of small cuboid cells bordering the lymphoid tissue of the tonsil and larger cells with dendritic morphology within the epithelial crypts. Double labeling a section 30 μmol/L further into the block clearly shows these cells are double-positive (Figure 6C ▶ , purple) although the single-positive cytokeratin staining is more widespread (blue). Similar results were obtained by double labeling samples of human colon (data not shown).
Figure 6.

Transmembrane fractalkine is expressed by cytokeratin-positive cells with the human tonsil. Human tonsils were collected after routine tonsillectomy. Frozen sections (8 to 10 μm) were cut for immunohistochemical analysis. Adjacent serial sections were stained using the rabbit α-C-pep (A, brown) or mouse α-cytokeratin AE1/AE3 (B, blue). Another section ∼30 μmol/L further into the sample was double-labeled using both reagents (C). The rabbit α-C-pep reagent strongly detects a subpopulation of cells within the epithelial crypts. Staining for the cytokeratins AE1 and AE3 revealed staining restricted to the epithelial crypts with strong staining on cells with a similar distribution and morphology to A, with widespread but more diffuse staining throughout the crypts. Double labeling with rabbit α-C-pep (brown) and α-cytokeratin (blue) show clear double labeling (purple) of discrete cells within the crypts and especially along the internal border of the crypts. Original magnifications, ×400.
Discussion
Using a range of novel monoclonal and polyclonal anti-fractalkine chemokine domain and specific anti-C-terminal peptide reagents, we have demonstrated that the epithelial cells express the transmembrane form of fractalkine in a number of human peripheral tissues, namely the skin, the pharyngeal and crypt epithelia of the tonsil, and the lamina propria of the colon. Transmembrane fractalkine is constitutively expressed on the colorectal adenocarcinoma cell line DLD-1 and expression is further increased after TNF-α stimulation. Anti-fractalkine N-terminal peptide reagent 1 fails to detect fractalkine expression on this cell line. This reagent also fails to stain epithelial cells within fixed human tissues, showing a strikingly different staining pattern to the other anti-fractalkine reagents used in this study. In contrast to other studies, we have been unable to detect fractalkine expression on endothelium or immature DCs in either normal or inflamed human tissues.
Fractalkine mRNA distribution in man seems to be complex, with many large organs of diverse function, in particular the brain and the heart, showing high levels of expression. 1 Cleaved and transmembrane forms of fractalkine may potentially have quite different biological roles. In vitro descriptions of the interaction of fractalkine with its only described receptor CX3CR1 have suggested a role in arrest and extravasation of receptor-positive cells from the bloodstream. 4,7 Although the expression of fractalkine mRNA in unactivated HUVECs is low, this is increased significantly when they are stimulated with inflammatory cytokines. 1 In a similar manner, cardiac myocyte expression of fractalkine mRNA increases after treatment of rats with either lipopolysaccharide or inflammatory cytokines. 13 The reduction in inflammatory influx observed in a model of rat glomerulonephritis when CX3CR1-blocking antibodies were administered has suggested a causal connection between increased fractalkine expression, linked to local inflammation, and the enhanced trafficking of inflammatory cells. 16
In man, expression of fractalkine protein has been reported on neurons within the brain, 17 and this has been confirmed by in situ hybridization and immunohistochemistry in rodents. 5,10 Fractalkine protein expression has also been reported on DCs and endothelium within the human skin and tonsil, 11 and recently on the epithelium of the lamina propria in the small intestine. 12 Importantly, the same reagent has been used in all these human studies of brain, skin, and tonsil, namely an anti-fractalkine N-terminal peptide reagent, 1 whereas a mixture of anti-fractalkine N-terminal and anti-C-terminal antibodies was used to stain the small intestine. Using antibodies raised against the chemokine domain of fractalkine and against the intracellular tail of the molecule, we have confirmed by double labeling that the predominant site of fractalkine expression in the human colon is the lamina propria. In contrast to the previous study 12 conducted on samples of human small intestine, we were unable to detect any endothelial staining. This was also true within resection material taken from a large number of Crohn’s and ulcerative colitis resection patients (n = 13 and n = 16, respectively), in which there was a considerable inflammatory infiltrate. This observation is not consistent with a simple model of fractalkine up-regulation on endothelium in response to inflammation. The targeted replacement of CX3CR1 in mice by a GFP reporter construct fails to block monocyte extravasation from blood or migration of DCs from the skin after microbial or contact sensitizer stimulation 18 again suggesting that fractalkine’s primary role in vivo is not that of an endothelial adhesion molecule.
We could not detect either chemokine domain-reactive or transmembrane fractalkine-positive DCs within the epithelium of the tonsil and skin. Instead there was diffuse staining of fractalkine chemokine-domain single-positive cells throughout the epithelium, with a restricted expression of the transmembrane double-positive form on the basal epithelial cells. This is again in contrast to staining reported using the anti-N-terminal peptide reagent (α-N-pep) and repeated in our hands (Figure 4) ▶ . In this article we demonstrate that that α-N-pep antisera cross-react with human CD84 in CD84-transfected cells (Figure 3C) ▶ and seem to detect CD84-positive cells within the tonsil (Figure 3, A and B) ▶ . Previous reports of strong protein expression on human epidermal Langerhans cells were surprising given negligible levels of fractalkine mRNA are detected by reverse transcriptase-PCR from freshly extracted epidermal human and murine DCs, 11,19 rather message levels increase significantly as they are allowed to mature in vitro, a situation considered to be analogous to Langerhans cells that have completed their migratory journey to secondary lymphoid tissue.
Although our study does not address the role of fractalkine expression within the brain, we have shown an interesting and apparently widespread, constitutive expression of transmembrane fractalkine by epithelial cells in the periphery. If there is active cleavage of fractalkine from these cells, which is suggested by the more widespread detection of cells single labeled for the chemokine domain of fractalkine in the skin and tonsil, a constitutive fractalkine gradient might be produced. Such a constitutive fractalkine gradient might serve to attract CX3CR1-positive cells to peripheral tissues. Indeed this is the model that has been suggested for T cell trafficking into the lamina propria of the human gut. 12
A recent report of the transient expression of CCR6 by monocyte-derived DCs produced in the presence of TGF-β, 20 suggested that cells gain or lose responsiveness to chemokines through the regulation of chemokine receptor expression. Chemokines are known to mediate constitutive and inducible leukocyte recruitment and our analysis of fractalkine expression in noninflamed tissues is consistent with fractalkine providing one of these constitutive signals. Cells potentially recruited by fractalkine include monocytes, which respond chemotactically to fractalkine. 1,15 The demonstration that monocytes have the capacity to rapidly differentiate into DCs after a series of transmigrations through an endothelial monolayer, 21 is consistent with the possibility that constitutive fractalkine expression, within the periphery, may be important in the attraction and differentiation of monocytes into dermal and/or epidermal DCs. Although the targeted replacement of CX3CR1 has not shown obvious defects of DC localization 18 the demonstration that cutaneous DCs were strongly positive for the reporter gene, is consistent with the potential importance of precursor/Langerhans cell interactions with epithelial fractalkine. Furthermore, there remains the possibility that fractalkine might act as a ligand for other as yet unidentified chemokine receptors, expressed by responding cells. Further analysis of CX3CR1 regulation on cells of the hemopoietic lineage, which presently is restricted because of a paucity of good reagents, should aid in the clarifying of which cell types are interacting with fractalkine in these distinct anatomical compartments.
Acknowledgments
We thank the surgical personal from the Nuffield Infirmary and Dr. F. Wojnarowska, at the Churchill Hospital, Oxford, UK, for their assistance in obtaining clinical samples of tonsil and skin; Dr. C. M. Quinn for CHO-K1 cell lysates; Dr. P. Engel and Dr. M. Brown for providing the human CD84-transfected cell line 300.12 CD84; Dr. M. Hadam for providing α-CD84 antibodies; and Dr. T. Schall for providing anti-fractalkine antisera.
Footnotes
Address reprint requests to David R. Greaves, Sir William Dunn School of Pathology, University of Oxford, Oxford OX1 3RE, UK. E-mail: david.greaves@path.ox.ac.uk.
Supported by the Arthritis Research Campaign (grant G0553), the National Association of Colitis and Crohn’s, and the Wellcome Trust.
A. D. L. and N. C. contributed equally to this study.
References
- 1.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ: A new class of membrane-bound chemokine with a CX3C motif. Nature 1997, 385:640-644 [DOI] [PubMed] [Google Scholar]
- 2.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos JC, Gearing D: Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature 1997, 387:611-617 [DOI] [PubMed] [Google Scholar]
- 3.Combadiere C, Salzwedel K, Smith ED, Tiffany HL, Berger EA, Murphy PM: Identification of CX3CR1. A chemotactic receptor for the human CX3C chemokine fractalkine and a fusion coreceptor for HIV-1. J Biol Chem 1998, 273:23799-23804 [DOI] [PubMed] [Google Scholar]
- 4.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O: Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 1997, 91:521-530 [DOI] [PubMed] [Google Scholar]
- 5.Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L: Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA 1998, 95:10896-10901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, Imai T, Patel DD: Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med 1998, 188:1413-1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haskell CA, Cleary MD, Charo IF: Molecular uncoupling of fractalkine-mediated cell adhesion and signal transduction. Rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activation. J Biol Chem 1999, 274:10053-10058 [DOI] [PubMed] [Google Scholar]
- 8.Fong AM, Erickson HP, Zachariah JP, Poon S, Schamberg NJ, Imai T, Patel DD: Ultrastructure and function of the fractalkine mucin domain in CX(3)C chemokine domain presentation. J Biol Chem 2000, 275:3781-3786 [DOI] [PubMed] [Google Scholar]
- 9.Goda S, Imai T, Yoshie O, Yoneda O, Inoue H, Nagano Y, Okazaki T, Imai H, Bloom ET, Domae N, Umehara H: CX3C-chemokine, fractalkine-enhanced adhesion of THP-1 cells to endothelial cells through integrin-dependent and -independent mechanisms. J Immunol 2000, 164:4313-4320 [DOI] [PubMed] [Google Scholar]
- 10.Schwaeble WJ, Stover CM, Schall TJ, Dairaghi DJ, Trinder PK, Linington C, Iglesias A, Schubart A, Lynch NJ, Weihe E, Schafer MK: Neuronal expression of fractalkine in the presence and absence of inflammation. FEBS Lett 1998, 439:203-207 [DOI] [PubMed] [Google Scholar]
- 11.Papadopoulos EJ, Sassetti C, Saeki H, Yamada N, Kawamura T, Fitzhugh DJ, Saraf MA, Schall T, Blauvelt A, Rosen SD, Hwang ST: Fractalkine, a CX3C chemokine, is expressed by dendritic cells and is up-regulated upon dendritic cell maturation. Eur J Immunol 1999, 29:2551-2559 [DOI] [PubMed] [Google Scholar]
- 12.Muehlhoefer A, Saubermann LJ, Gu X, Luedtke-Heckenkamp K, Xavier R, Blumberg RS, Podolsky DK, MacDermott RP, Reinecker HC: Fractalkine is an epithelial and endothelial cell-derived chemoattractant for intraepithelial lymphocytes in the small intestinal mucosa. J Immunol 2000, 164:3368-3376 [DOI] [PubMed] [Google Scholar]
- 13.de la Fuente MA, Pizcueta P, Nadal M, Bosch J, Engel P: CD84 leukocyte antigen is a new member of the Ig superfamily. Blood 1997, 90:2398-2405 [PubMed] [Google Scholar]
- 14.Papadopoulos EJ, Fitzhugh DJ, Tkaczyk C, Gilfillan AM, Sassetti C, Metcalfe DD, Hwang ST: Mast cells migrate, but do not degranulate, in response to fractalkine, a membrane-bound chemokine expressed constitutively in diverse cells of the skin. Eur J Immunol 2000, 30:2355-2361 [DOI] [PubMed] [Google Scholar]
- 15.Harrison JK, Jiang Y, Wees EA, Salafranca MN, Liang HX, Feng L, Belardinelli L: Inflammatory agents regulate in vivo expression of fractalkine in endothelial cells of the rat heart. J Leukoc Biol 1999, 66:937-944 [DOI] [PubMed] [Google Scholar]
- 16.Feng L, Chen S, Garcia GE, Xia Y, Siani MA, Botti P, Wilson CB, Harrison JK, Bacon KB: Prevention of crescentic glomerulonephritis by immunoneutralization of the fractalkine receptor CX3CR1 rapid communication. Kidney Int 1999, 56:612-620 [DOI] [PubMed] [Google Scholar]
- 17.Tong N, Perry SW, Zhang Q, James HJ, Guo H, Brooks A, Bal H, Kinnear SA, Fine S, Epstein LG, Dairaghi D, Schall TJ, Gendelman HE, Dewhurst S, Sharer LR, Gelbard HA: Neuronal fractalkine expression in HIV-1 encephalitis: roles for macrophage recruitment and neuroprotection in the central nervous system. J Immunol 2000, 164:1333-1339 [DOI] [PubMed] [Google Scholar]
- 18.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR: Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 2000, 20:4106-4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanazawa N, Nakamura T, Tashiro K, Muramatsu M, Morita K, Yoneda K, Inaba K, Imamura S, Honjo T: Fractalkine and macrophage-derived chemokine: T cell-attracting chemokines expressed in T cell area dendritic cells. Eur J Immunol 1999, 29:1925-1932 [DOI] [PubMed] [Google Scholar]
- 20.Yang D, Howard OM, Chen Q, Oppenheim JJ: Cutting edge: immature dendritic cells generated from monocytes in the presence of TGF-beta 1 express functional C-C chemokine receptor 6. J Immunol 1999, 163:1737-1741 [PubMed] [Google Scholar]
- 21.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA: Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 1999, 11:753-761 [DOI] [PubMed] [Google Scholar]


