Abstract
It has recently been proposed that gastrointestinal stromal tumors (GISTs) originate from stem cells that differentiate toward a phenotype of interstitial cells of Cajal (ICCs). Nestin is a newly identified intermediate filament protein, and is predominantly expressed in immature cells, such as neuroectodermal stem cells and skeletal muscle progenitor cells, and tumors originating from these cells. In this study, we examined, using immunohistochemistry, the nestin expression in GISTs and ICCs to clarify the origin of GISTs. Strong immunoreactivity for nestin was observed in all 18 GISTs, and its expression was confirmed by Western blot and Northern blot analyses. In contrast, three leiomyomas and a schwannoma that developed in the gastrointestinal tract showed no apparent immunoreactivity for nestin. Among 17 mesenchymal tumors (seven leiomyosarcomas, five malignant peripheral nerve sheath tumors, and five fibrosarcomas) that occurred in sites other than the gastrointestinal tract, only two malignant peripheral nerve sheath tumors were moderately immunoreactive for nestin. Furthermore, with fluorescence double immunostaining of the normal small intestine, nestin expression was demonstrated in ICCs. These results show that nestin may be a useful marker for diagnosis of GISTs, and support the current hypothesis that GISTs are tumors of stem cells that differentiate toward an ICC phenotype.
Mesenchymal tumors develop in the gastrointestinal tract, and have been believed to be of smooth muscle or neuronal cell origin. However, subsequent immunohistochemical and ultrastructural studies have shown that a large proportion of the tumors do not have typical features of smooth muscle or neuronal cells. As a result, a major subset of mesenchymal tumors, that differ from typical leiomyomas and schwannomas, are presently designated as gastrointestinal stromal tumors (GISTs). 1-3 Recently, most GISTs have been shown to express c-kit receptor tyrosine kinase (KIT) and CD34, both of which are expressed in hematopoietic stem cells. 4-7 In the normal gastrointestinal tract, interstitial cells of Cajal (ICCs), that are predominantly located between the circular and longitudinal muscle layers, express KIT and CD34. These cells are functionally important for gastrointestinal motility and show pacemaker activity. 8-10 ICCs are assumed to originate from mesenchymal progenitor cells that can also differentiate into smooth muscle cells. 11-13 Because of the shared expression of KIT and CD34 in GISTs and ICCs, a hypothesis that GISTs may originate from stem cells that differentiate toward an ICC phenotype has been recently proposed. 2,3,14-17
Intermediate filaments are a major component of the cellular cytoskeleton, and their cell-specific expression in normal tissues and differential expression in tumors has been of enormous value in tumor diagnosis. 18,19 Nestin is a newly identified intermediate filament protein that belongs to the sixth class of intermediate filaments. 20 Previous studies have demonstrated that nestin is abundantly expressed in neuroectodermal stem cells and is extinguished from differentiated cells of the central nervous system. 21,22 Nestin is also expressed in skeletal muscle progenitor cells, but is down-regulated on cellular differentiation. 22-24 In addition, nestin is shown to be predominantly expressed in tumors that are thought to arise from immature cells, such as primitive neuroectodermal tumors, medulloblastomas, and pediatric rhabdomyosarcomas. 25-30 These findings suggest that nestin may be a useful molecular tool with which to characterize tumors originating from immature cells, such as stem or progenitor cells. In this study, we examined the nestin expression in GISTs and ICCs to clarify the hypothesis that GISTs originate from stem cells that differentiate toward an ICC phenotype.
Materials and Methods
Tissue Preparation and Immunohistochemistry
The polyclonal antibody against nestin (no. 130) was produced in a rabbit immunized with a bacterially produced fusion protein containing the 4,000 carboxy-terminal base pair of the rat nestin gene. The specificity of this antibody has been described previously; the rabbit polyclonal antibody (no. 130) is specific for nestin and reactive with human nestin, as well as rat nestin. 25 In this study, we used this antibody (no. 130) to examine the nestin expression in human tissues.
Tissues of 18 GISTs, three leiomyomas, and one schwannoma developed in the gastrointestinal tract and those of seven leiomyosarcomas, five malignant peripheral nerve sheath tumors, and five fibrosarcomas that occurred in sites other than the gastrointestinal tract were obtained from 39 cases who underwent surgical resection. The tissues were fixed with 10% formalin, embedded in paraffin, and then sections (5 μm thick) were prepared. Some sections were used for hematoxylin and eosin staining and others for immunohistochemistry. Immunohistochemical analysis was performed as follows. Sections for KIT, CD34, and nestin immunohistochemistry were heated in 10 mmol/L sodium citrate buffer (pH 6.0) for 10 minutes with a microwave oven (Matsushita Electric Inc., Osaka, Japan) at 600 W, and those for α-smooth muscle actin (α-SMA) immunohistochemistry were digested with 0.05% trypsin (Sigma Chemical Co., St. Louis, MO) in 0.1 mol/L Tris-HCl and 0.1% CaCl2, pH 7.8, for 5 minutes at 37°C. All sections for immunohistochemistry were incubated in 0.3% H2O2 in methanol for 30 minutes to inactivate internal peroxidase, washed with 10 mmol/L phosphate-buffered saline (pH 7.4) with 0.2% Tween 20 (PBST), and incubated with normal goat serum or normal horse serum (Vector Laboratories, Burlingame, CA) to block nonspecific binding of antibodies. The sections were then incubated with a rabbit polyclonal antibody against KIT (C-19; Santa Cruz Biotechnology, Santa Cruz, CA), a mouse monoclonal antibody against CD34 (QBEnd 10; DAKO, Glostrup, Denmark), a mouse monoclonal antibody against α-SMA (Nichirei, Tokyo, Japan), a rabbit polyclonal antibody against S-100 (Nichirei), or a rabbit polyclonal antibody against nestin (no. 130) at 4°C overnight. After washing with PBST, the sections were incubated with biotin-conjugated goat anti-rabbit or horse anti-mouse IgG for 60 minutes, and immunoreacted cells were then visualized using the streptavidin-biotin peroxidase complex method with a Vectastain ABC Elite kit (Vector Laboratories) and diaminobenzidine tetrahydrochloride (Sigma Chemical Co.). The sections were lightly counterstained with hematoxylin.
Western Blot Analysis
Tissue samples were homogenized in RIPA buffer (10 mmol/L Tris-HCl, pH 7.2, 150 mmol/L NaCl, 1 mmol/L O,O′-bis(2-ami-noethyl) ethylene glycol-N,N,N′,N′-tetraacetic acid, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecylsulfate, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) using a handy microhomogenizer, Physcotron (NITI-ON, Tokyo, Japan). After incubation in ice for 30 minutes, the homogenate was centrifuged at 15,000 × g for 30 minutes and the supernatant was collected. Aliquots of the supernatant were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 10% polyacrylamide. After electrophoresis, proteins in the gel were electrophoretically transferred onto a nitrocellulose transfer membrane (Nitrobind; Micron Separations, Inc., Westborough, MA). Immunoblotting was then performed with either a rabbit polyclonal antibody against KIT or a rabbit polyclonal antibody against nestin (no. 130).
Northern Blot Analysis
Total RNAs were extracted from tissue samples using TRIZOL reagent (Life Technologies, Inc., Grand Island, NY) according to the manufacturer’s instructions. The RNAs (20 μg) were fractionated by agarose-formaldehyde gel electrophoresis, transferred to a Hybond-N+ nylon membrane (Amersham International, Buckinghamshire, UK), and hybridized with 32P-labeled human c-kit cDNA, 31 nestin cDNA, 32 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA. 33 The blot was hybridized in Church’s buffer (1 mmol/L ethylenediaminetetraacetic acid, 7% sodium dodecyl sulfate, and 0.5 mol/L Na2HPO4, pH 7.2) at 65°C overnight. 34 The blot was washed at 65°C in 2× standard saline citrate (300 mmol/L NaCl and 30 mmol/L trisodium citrate, pH 7.4) and subjected to autoradiography.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNAs (5 μg) were reverse-transcribed in 20 μl of the reaction mixture containing Superscript II reverse transcriptase (Life Technologies, Inc.) and random hexamer. One μl of each reaction product was amplified in 50 μl of PCR mixture containing Takara Ex Taq (Takara Shuzou, Kyoto, Japan) and each set of sense and antisense primers with 30 cycles of 30 seconds of denaturation at 94°C, 30 seconds of annealing at 58°C, and 1 minute of synthesis at 72°C. The following oligonucleotides were used for PCR: sense (5′-TTTGGTCTAGCCAGAGACATCAAG-3′, 2431 to 2454) and antisense (5′-AGGGCTGAGCATCCGGAAGCCTTC-3′, 2653 to 2676) for the human c-kit gene, 31 sense (5′-ATCCAGGACTCCCAGGTTCCTTTG-3′, 1597 to 1620) and antisense (5′-ATGTCTCTAGATTACCTTCAAGAG-3′, 1910 to 1933) for the human nestin gene, 32 and sense (5′-CGGAGTCAACGGATTTGGTCGTAT-3′, 78 to 101) and antisense (5′-AGCCTTCTCCATGGTGGTGAAGAC-3′, 361 to 384) for the human GAPDH gene. 33 Ten μl of the PCR products were electrophoresed in a 2% agarose gel containing ethidium bromide.
Fluorescence Double Immunolabeling
Fluorescence double immunostaining was performed using a combination of a mouse monoclonal antibody against KIT (Novocastra Laboratories, Ltd., Newcastle, UK) and a rabbit polyclonal antibody against nestin (no. 130). Freshly dissected tissues were fixed with 4% paraformaldehyde in 0.1 mol/L phosphate buffer, pH 7.2, for 6 hours, then placed overnight in 20% sucrose in 0.1 mol/L phosphate buffer at 4°C. The tissues were covered with Tissue-Tek OCT compound (Miles Inc., Elkhart, IN), quickly frozen in liquid nitrogen, and cut with a cryostat. Frozen sections (5 μm thick) were sequentially incubated with normal goat serum to block nonspecific binding of antibodies, a mouse monoclonal antibody against KIT, and R-phycoerythrin-conjugated anti-mouse IgG (DAKO). After rigorous washing with PBST, the sections were incubated sequentially with a rabbit polyclonal antibody against nestin, biotin-conjugated anti-rabbit IgG, and fluorescein isothiocyanate-conjugated streptavidin (DAKO). The sections were examined with a confocal laser-scanning microscope (LSM310; Carl Zeiss Jena GmbH, Jena, Germany) with an excitation wavelength of 568 nm for R-phycoerythrin and 488 nm for fluorescein isothiocyanate.
Results
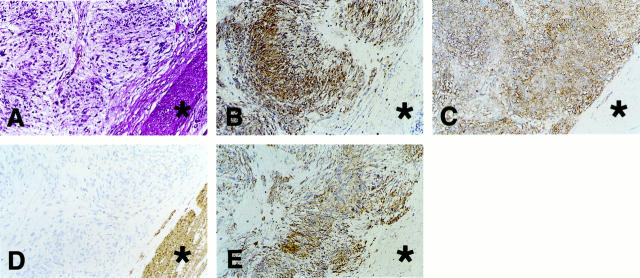
We collected 22 mesenchymal tumors that developed in the gastrointestinal tract, comprising 18 GISTs, three leiomyomas, and one schwannoma. The results of the immunohistochemical studies on these tumors are summarized in Table 1 ▶ . As previously reported, 1-7,14-17 the neoplastic cells of all GISTs expressed both KIT and CD34 (Figure 1; A, B, and C ▶ ). In contrast the neoplastic cells of three leiomyomas and a schwannoma were completely negative for KIT and CD34, although a few CD34-positive fibroblast-like cells and endothelial cells were observed within the leiomyoma nodules. α-SMA immunoreactivity was observed in mature smooth muscle cells (Figure 1D) ▶ , five of 18 GISTs, and all three leiomyomas. The α-SMA immunoreactivity in five of 18 GISTs was weak compared with that in mature smooth muscle cells and leiomyomas, and was confined to a focal area. The remaining 13 GISTs were completely negative for α-SMA (Figure 1D) ▶ . S-100 immunoreactivity was observed in the schwannoma, but not in any of the 18 GISTs. As for nestin immunoreactivity, all GISTs were strongly positive (Figure 1E) ▶ . On the other hand, nestin immunoreactivity was not detected in the surrounding nontumor smooth muscle cells and neoplastic cells of the three leiomyomas, whereas a few nestin-positive spindle cells were scattered within the leiomyoma nodules. Nestin immunoreactivity was also hardly detected in neoplastic cells of the schwannoma.
Table 1.
Summary of Mesenchymal Tumors that Developed in the Gastrointestinal Tract
| Case no. | Age (years) | Sex | Histology | Primary site | Immunohistochemistry | ||||
|---|---|---|---|---|---|---|---|---|---|
| KIT | CD34 | α-SMA | S-100 | Nestin | |||||
| 1 | 63 | F | GIST | Stomach | ++ | +++ | − | − | +++ |
| 2 | 74 | M | GIST | Stomach | +++ | +++ | − | − | +++ |
| 3 | 68 | F | GIST | Stomach | +++ | +++ | − | − | +++ |
| 4 | 51 | F | GIST | Stomach | +++ | +++ | − | − | +++ |
| 5 | 69 | M | GIST | Stomach | +++ | +++ | − | − | ++ |
| 6 | 69 | F | GIST | Stomach | +++ | +++ | − | − | +++ |
| 7 | 59 | F | GIST | Stomach | ++ | ++ | − | − | ++ |
| 8 | 53 | F | GIST | Stomach | +++ | +++ | − | − | +++ |
| 9 | 73 | F | GIST | Stomach | ++ | +++ | +* | − | +++ |
| 10 | 71 | F | GIST | Stomach | +++ | +++ | − | − | +++ |
| 11 | 62 | M | GIST | Stomach | ++ | +++ | − | − | +++ |
| 12 | 53 | M | GIST | Stomach | ++ | +++ | − | − | ++ |
| 13 | 75 | F | GIST | Stomach | +++ | +++ | +* | − | +++ |
| 14 | 62 | F | GIST | Stomach | ++ | +++ | +* | − | +++ |
| 15 | 74 | F | GIST | Stomach | ++ | ++ | − | − | ++ |
| 16 | 64 | F | GIST | Stomach | +++ | +++ | − | − | +++ |
| 17 | 42 | M | GIST | Jejunum | +++ | +++ | +* | − | +++ |
| 18 | 72 | M | GIST | Rectum | +++ | +++ | +* | − | +++ |
| 19 | 62 | F | Leiomyoma | Stomach | − | −† | +++ | − | −‡ |
| 20 | 35 | M | Leiomyoma | Stomach | − | −† | ++ | − | −‡ |
| 21 | 39 | F | Leiomyoma | Colon | − | − | +++ | − | − |
| 22 | 80 | F | Schwannoma | Stomach | − | − | − | +++ | ±§ |
KIT, c-kit receptor tyrosine kinase; α-SMA, α-smooth muscle actin; M, male; F, female; GIST, gastrointestinal stromal tumor.
Signal strength is shown as an increasing scale (−, ±, +, ++, and +++).
*The α-SMA immunoreactivity was weak compared with that of the nontumor smooth muscle cells and leiomyomas, and was confined to a focal area.
†A few CD34-positive fibroblast-like cells and endothelial cells were observed within the tumor nodule, but neoplastic cells were negative for CD34.
‡A few nestin-positive spindle cells were scattered, but neoplastic cells were negative for nestin.
§Immunoreactivity was hardly detected in the tumor.
Figure 1.
Expression of nestin in GISTs determined by immunohistochemistry. A–E: Sections of case 1 (GIST) are shown. A: Staining with H&E. B: Immunostaining with anti-KIT. C: Immunostaining with anti-CD34. D: Immunostaining with anti-α-SMA. E: Immunostaining with anti-nestin. Marked immunoreactivity for nestin is observed in GIST. Asterisks in A to E indicate nontumor mature smooth muscle cells that are positive for α-SMA. Original magnification, ×73.
To clarify whether nestin has any specificity toward GIST, we examined, using immunohistochemistry, the nestin expression in 17 mesenchymal tumors, which comprised seven leiomyosarcomas, five malignant peripheral nerve sheath tumors, and five fibrosarcomas that occurred in sites other than the gastrointestinal tract. Nestin immunoreactivity was not observed in neoplastic cells of all seven leiomyosarcomas and of all five fibrosarcomas. Moreover, three of five malignant peripheral nerve sheath tumors showed no apparent immunoreactivity, although the remaining two tumors were moderately immunoreactive for nestin.
We next examined the KIT and nestin expression in GISTs and nontumor tissues by Western blot analysis. The nontumor tissues, comprising the circular and longitudinal muscle layers of the intestinal tract, were prepared from the surrounding tissues of GISTs. As shown in Figure 2A ▶ , KIT and nestin were markedly expressed in GISTs, but hardly detected in nontumor tissues. Northern blot analysis also showed marked expression of c-kit and nestin mRNAs in GISTs, even when autoradiography was performed for only 5 hours (Figure 2B) ▶ . In nontumor tissues, the expression of c-kit and nestin mRNAs was slightly detected if autoradiography persisted for more than 72 hours (data not shown). In accord with the findings on Northern blot analysis, RT-PCR showed faint expression of c-kit and nestin mRNAs in nontumor tissues (Figure 2C) ▶ .
Figure 2.
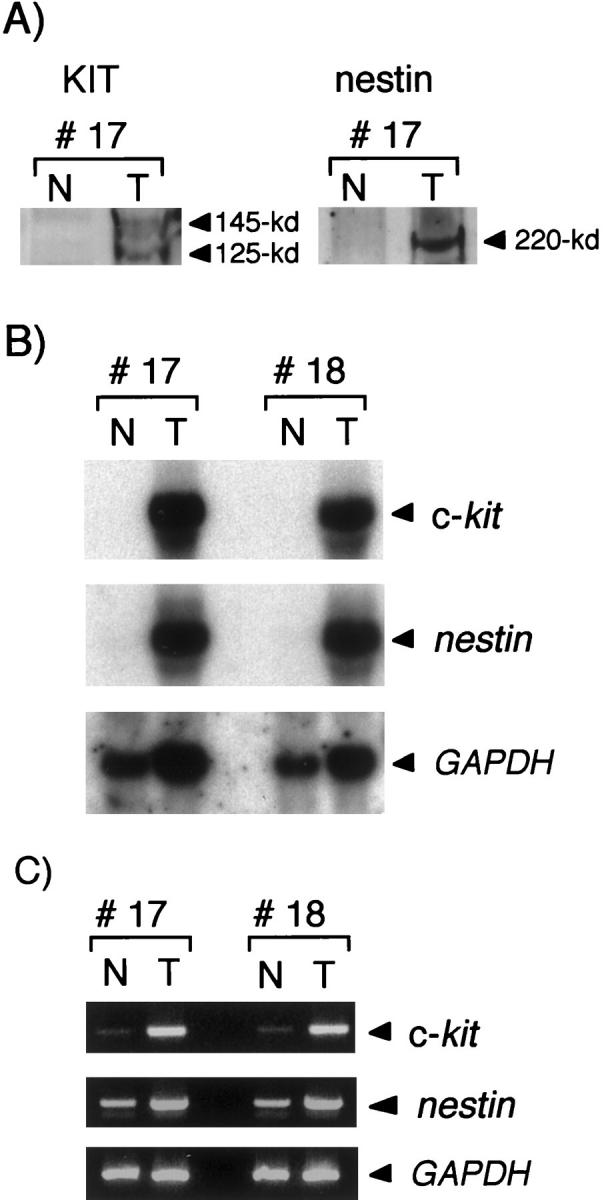
Expression of nestin in GISTs. A: Western blot analysis after sodium dodecyl sulfate-polyacrylamide gel electrophoresis with nontumor and GIST tissues obtained from case 17. KIT is expressed in GIST as 145- and 125-kd proteins, which are mature and immature forms of KIT, respectively. Nestin is expressed in GIST as an ∼220-kd protein. B: Northern blot analysis using total RNAs extracted from nontumor and GIST tissues of cases 17 and 18. Subsequent hybridization with the GAPDH cDNA probe served as a quantitative internal control. Each transcript of c-kit, nestin, and GAPDH gave a single band of ∼5.5 kb, 6.0 kb, and 1.4 kb, respectively. C: RT-PCR using total RNA extracted from nontumor and GIST tissues of cases 17 and 18. The GAPDH gene was used as an endogenous internal control. The fragments of the c-kit, nestin, and GAPDH genes were amplified, corresponding to transcripts of 246 bp, 337 bp, and 307 bp, respectively. N, nontumor tissues; T, GIST tissues.
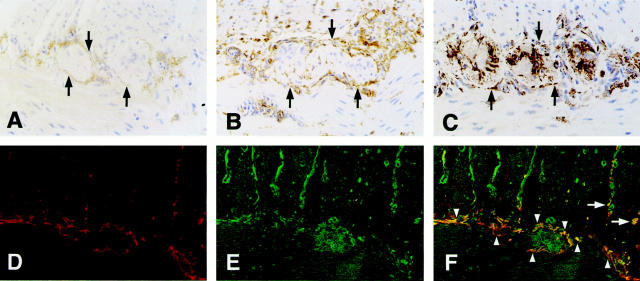
We further investigated whether the nestin expression of nontumor tissues was caused by ICCs. Because ICCs between the circular and longitudinal muscle layers of the small intestine are easily identified by their specific localization, they were immunohistochemically examined. As reported by other investigators, 2-4,9 the cells enclosing the myenteric ganglia, assumed to be ICCs, showed immunoreactivity for both KIT and CD34 (Figure 3, A and B) ▶ . The cells, of which the distribution was similar to that of KIT- and CD34-positive cells, also showed nestin expression, suggesting that ICCs may express nestin (Figure 3C) ▶ . On the other hand, immunoreactivity for nestin was not observed in mature smooth muscle cells of the circular and longitudinal muscle layers (Figure 3C) ▶ . When fluorescence double staining for KIT and nestin on the same small intestinal section was performed to further clarify the nestin expression in ICCs, KIT-positive ICCs between the circular and longitudinal muscle layers showed definitive nestin expression. In addition, some KIT-positive ICCs, that were present along the fiber bundles of the circular muscle layer, simultaneously expressed nestin (Figure 3; D, E, and F ▶ ). However, nestin-positive, but KIT-negative, cells were also observed in the intercellular space of myenteric ganglion cells (Figure 3C) ▶ and along the fiber bundles of the circular muscle layer (Figure 3E) ▶ .
Figure 3.
Expression of nestin in ICCs of the normal human small intestine. A, B, and C: Immunohistochemistry of the normal human small intestine. A: Immunostaining with anti-KIT. B: Immunostaining with anti-CD34. C: Immunostaining with anti-nestin. D, E, and F: Demonstration by confocal laser-scanning microscopy. D: Binding of mouse anti-KIT (red). E: Binding of rabbit anti-nestin (green). F: Merged confocal image of D and E. The co-localization of KIT and nestin is shown as yellow in F. Arrows in A, B, and C show KIT-, CD34-, or nestin-positive cells enclosing the myenteric ganglion. Arrowheads and arrows in F show KIT and nestin double-positive ICCs between the circular and longitudinal muscle layers and those lying along the fiber bundles of the circular muscle layer, respectively. Original magnifications, ×146 (A–C) and ×88 (D–F).
Discussion
In this study, we examined immunohistochemically the nestin expression in GISTs, and found that all 18 GISTs were strongly immunoreactive for nestin, as well as for KIT and CD34. The striking nestin expression in GISTs was confirmed by Western blot and Northern blot analyses. On the other hand, apparent immunoreactivity for nestin was not observed in three leiomyomas and a schwannoma that developed in the gastrointestinal tract. It therefore seems that nestin is exclusively expressed in GISTs among tumors developing in the gastrointestinal tract. These findings further support the point that GISTs are distinct from genuine leiomyomas and schwannomas, and show that nestin, as well as KIT and CD34, may be a useful marker for diagnosis of GISTs.
To clarify whether nestin has any specificity toward GIST, we examined by immunohistochemistry the nestin expression in mesenchymal tumors occurring in sites other than the gastrointestinal tract. Among 17 mesenchymal tumors, which comprised seven leiomyosarcomas, five malignant peripheral nerve sheath tumors, and five fibrosarcomas, 15 mesenchymal tumors showed no apparent immunoreactivity. Although two malignant peripheral nerve sheath tumors were immunoreactive for nestin, their immunoreactivity was weak compared with that in GISTs. Because nestin expression has been shown in a subpopulation of Schwann cells in the peripheral nervous system, 35,36 the nestin immunoreactivity in two of five malignant peripheral nerve sheath tumors may reflect the nestin expression in a subset of Schwann cells that were considered to be the origin of these tumors. As for fibrosarcomas, consistent with our observations, Kobayashi and colleagues 30 showed no nestin immunoreactivity in the fibrosarcoma examined in their study. These results indicate that nestin is a valuable marker for GISTs among mesenchymal tumors.
Based on the current hypothesis that GISTs are tumors of stem cells that differentiate toward an ICC phenotype, 2,3,14-17 we examined whether nestin is expressed in ICCs. Although the magnitude of the nestin gene expression in nontumor tissues was very small, the presence of nestin mRNA was convincingly shown by RT-PCR, as was the case with the c-kit gene. Moreover, the immunoreactivity for nestin was observed in cells that were assumed to be ICCs by their distribution, and fluorescence double staining confirmed nestin expression in KIT-positive ICCs. In the murine gastrointestinal tract, both ICCs and smooth muscle cells have been shown to arise from common mesenchymal progenitor cells that express KIT. 11-13,37 Moreover, Miettinen and colleagues 38 recently showed nearly consistent KIT immunoreactivity in GISTs occurred as primary tumors outside of the gastrointestinal tract, including the omentum and mesentery. These observations suggest that a primitive ancestor cell present inside and outside the gastrointestinal tract, rather than ICCs, may give rise to GISTs, and that common mesenchymal progenitor cells that can differentiate into both ICCs and smooth muscle cells could be a logical candidate for the origin of GISTs. Nestin is predominantly expressed in immature cells, such as neuroectodermal stem cells and skeletal muscle progenitor cells, 21-24 and this present study has demonstrated the shared expression of nestin in GISTs and ICCs. Furthermore, we more recently found strong immunoreactivity for nestin in a tumor phenotypically identical with GISTs, but occurring in peritoneum (unpublished data). These findings further strengthen the hypothesis that GISTs may originate from stem cells that differentiate toward an ICC phenotype, although nestin expression by mesenchymal stem or progenitor cells in the gastrointestinal tract remains to be determined.
In addition to the nestin expression in KIT-positive ICCs, immunoreactivity for nestin was observed in the intercellular space of myenteric ganglion cells. Because the intercellular space of myenteric ganglion cells is likely to be consistent with the location of glial cells, the immunoreactivity for nestin in this area may reflect the nestin expression of glial cells. Supporting this, Eaker and colleagues 39 showed, by primary culture of the rat myenteric plexus, that glial cells, but not neurons of the myenteric plexus, are capable of expressing nestin. Immunoreactivity for nestin was also observed in cells lying along the fiber bundles of the circular muscle layer. Although some KIT and nestin double-positive ICCs were present, the immunoreactive cells for nestin were mostly KIT-negative. Recently, KIT-negative cells, that were close to ICCs and had a morphological resemblance to ICCs, in the muscle layer were shown to be CD34-positive fibroblasts. 40 Thus, fibroblasts may be a candidate for these KIT-negative and nestin-positive cells. However, it is possible that cells other than fibroblasts may contribute to the immunoreactivity for nestin, because Redies and colleagues 41 reported that induced immortalization of primary fibroblasts did not produce nestin-expressing cell lines, whereas that of neuroectodermal and myogenic cells did. Moreover, we failed to detect nestin immunoreactivity in all five fibrosarcomas that seemed to originate from fibroblasts, although these fibrosarcomas occurred in sites other than the gastrointestinal tract. To identify the type of cells that are KIT-negative and nestin-positive in the gastrointestinal tract, further studies will be necessary.
The biological function of the nestin expressed by ICCs is yet unknown, but it is possibly as follows. ICCs have long cytoplasmic processes and form complex networks in the lamina muscularis. In addition, ICCs have a pacemaker activity and are involved in the generation of peristaltic motor activity. 9,10 Based on these characteristics of morphology and function in ICCs, it seems likely that the nestin expressed by ICCs may play an important role in the formation of long cytoplasmic processes and the cell network. Supporting this, Matsuda and colleagues 42 reported that nestin participates in the organization or maintenance of elongated cell shapes in the developing central nervous system. Alternatively, nestin may serve as a framework for other intermediate filaments, because it is predominantly expressed in immature cells and its expression precedes expression of other types of intermediate filaments. 21-24
In summary, we found concomitant expression of nestin with KIT and CD34 in both ICCs and GISTs. These findings show that nestin, as well as KIT and CD34, may be a useful marker for diagnosis of GISTs, and support the current hypothesis that GISTs are tumors of stem cells that differentiate toward an ICC phenotype.
Acknowledgments
We thank Dr. Yoichi Tani of DAKO Japan Co., Ltd. (Kyoto, Japan), for his valuable advice; and Ms. Michiko Kakihana and Ms. Ayako Kuhara for their technical assistance.
Footnotes
Address reprint requests to Tohru Tsujimura, M.D., Ph.D., the First Department of Pathology, Hyogo College of Medicine, 1-1, Mukogawa-cho, Nishinomiya, Hyogo 663-8501, Japan. E-mail: tohru@hyo-med.ac.jp.
Supported in part by grants from the Japanese Ministry of Education, Science, and Culture.
References
- 1.Ma CK, Amin MB, Kintanar E, Linden MD, Zarbo RJ: Immunohistologic characterization of gastrointestinal stromal tumors: a study of 82 cases compared with 11 cases of leiomyomas. Mod Pathol 1993, 6:139-144 [PubMed] [Google Scholar]
- 2.Miettinen M, Sarlomo-Rikala M, Lasota J: Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol 1999, 30:1213-1220 [DOI] [PubMed] [Google Scholar]
- 3.Chan JKC: Mesenchymal tumors of the gastrointestinal tract: a paradise for acronyms (STUMP, GIST, GANT, and now GIPACT), implication of c-kit in genesis, and yet another of the many emerging roles of the interstitial cell of Cajal in the pathogenesis of gastrointestinal diseases? Adv Anat Pathol 1999, 6:19-40 [DOI] [PubMed] [Google Scholar]
- 4.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Tunio GM, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y: Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998, 279:577-580 [DOI] [PubMed] [Google Scholar]
- 5.Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M: CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol 1998, 11:728-734 [PubMed] [Google Scholar]
- 6.van de Rijn M, Hendrickson MR, Rouse RV: CD34 expression by gastrointestinal tract stromal tumors. Hum Pathol 1994, 25:766-771 [DOI] [PubMed] [Google Scholar]
- 7.Miettinen M, Virolainen M, Sarlomo-Rikala M: Gastrointestinal stromal tumor—value of CD34 antigen in their identification and separation from true leiomyomas and schwannomas. Am J Surg Pathol 1995, 19:207-216 [DOI] [PubMed] [Google Scholar]
- 8.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A: W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 1995, 373:347-349 [DOI] [PubMed] [Google Scholar]
- 9.Sander KM: A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 1996, 111:492-515 [DOI] [PubMed] [Google Scholar]
- 10.Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD: Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med 1998, 4:848-851 [DOI] [PubMed] [Google Scholar]
- 11.Young HM, Ciampoli D, Southwell BR, Newgreen DF: Origin of interstitial cells of Cajal in the mouse intestine. Dev Biol 1996, 180:97-107 [DOI] [PubMed] [Google Scholar]
- 12.Klüppel M, Huizinga JD, Malysz J, Bernstein A: Developmental origin and kit-dependent development of the interstitial cells of Cajal in the mammalian small intestine. Dev Dyn 1998, 211:60-71 [DOI] [PubMed] [Google Scholar]
- 13.Torihashi S, Nishi K, Tokutomi Y, Nishi T, Ward S, Sanders KM: Blockade of kit signaling induces transdifferentiation of interstitial cells of Cajal to a smooth muscle phenotype. Gastroenterology 1999, 117:140-148 [DOI] [PubMed] [Google Scholar]
- 14.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM: Gastrointestinal pacemaker cell tumor (GlPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998, 152:1259-1269 [PMC free article] [PubMed] [Google Scholar]
- 15.Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH: Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol 1999, 23:377-389 [DOI] [PubMed] [Google Scholar]
- 16.Sakurai S, Fukasawa T, Chong J, Tanaka A, Fukayama M: Embryonic form of smooth muscle myosin heavy chain (SMemb/MHC-B) in gastrointestinal stromal tumor and interstitial cells of Cajal. Am J Pathol 1999, 154:23-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson TL, Sircar K, Hewlett BR, Chorneyko K, Riddell RH, Huizinga JD: Gastrointestinal stromal tumors may originate from a subset of CD34-positive interstitial cells of Cajal. Am J Pathol 2000, 156:1157-1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho CL, Liem RKH: Intermediate filaments in the nervous system: implications in cancer. Cancer Metastasis Rev 1996, 15:483-497 [DOI] [PubMed] [Google Scholar]
- 19.Prasad S, Soldatenkov VA, Srinivasarao G, Dritschilo A: Intermediate filament proteins during carcinogenesis and apoptosis (Review). Int J Oncol 1999, 14:563-570 [DOI] [PubMed] [Google Scholar]
- 20.Lendahl U, Zimmerman LB, McKay RDG: CNS stem cells express a new class of intermediate filament protein. Cell 1990, 60:585-595 [DOI] [PubMed] [Google Scholar]
- 21.Dahlstrand J, Lardelli M, Lendahl U: Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res 1995, 84:109-129 [DOI] [PubMed] [Google Scholar]
- 22.Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay RDG, Gavin B, Mann J, Vassileva G, McMahon A: Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron 1994, 12:11-24 [DOI] [PubMed] [Google Scholar]
- 23.Kachinsky AM, Dominov JA, Miller JB: Myogenesis and the intermediate filament protein, nestin. Dev Biol 1994, 165:216-228 [DOI] [PubMed] [Google Scholar]
- 24.Sejersen T, Lendahl U: Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci 1993, 106:1291-1300 [DOI] [PubMed] [Google Scholar]
- 25.Dahlstrand J, Collins VP, Lendahl U: Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res 1992, 52:5334-5341 [PubMed] [Google Scholar]
- 26.Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RDG, Trojanowski JQ: Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest 1992, 66:303-313 [PubMed] [Google Scholar]
- 27.Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RDG, Trojanowski JQ: Monoclonal antibodies to a rat nestin fusion protein recognize a 220-kDa polypeptide in subsets of fetal and adult human central nervous system neurons and in primitive neuroectodermal tumor cells. Am J Pathol 1993, 143:258-268 [PMC free article] [PubMed] [Google Scholar]
- 28.Smits A, van Grieken D, Hartman M, Lendahl U, Funa K, Nistér M: Coexpression of platelet-derived growth factor α and β receptors on medulloblastomas and other primitive neuroectodermal tumors is consistent with an immature stem cell and neuronal derivation. Lab Invest 1996, 74:188-198 [PubMed] [Google Scholar]
- 29.Valtz NLM, Hayes TE, Norregaard T, Liu S, McKay RDG: An embryonic origin for medulloblastoma. New Biol 1991, 3:364-371 [PubMed] [Google Scholar]
- 30.Kobayashi M, Sjöberg G, Söderhäll S, Lendahl U, Sandstedt B, Sejersen T: Pediatric rhabdomyosarcomas express the intermediate filament nestin. Pediatr Res 1998, 43:386-392 [DOI] [PubMed] [Google Scholar]
- 31.Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U, Ullrich A: Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J 1987, 6:3341-3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahlstrand J, Zimmerman LB, McKay RDG, Lendahl U: Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci 1992, 103:589-597 [DOI] [PubMed] [Google Scholar]
- 33.Wong H, Anderson WD, Cheng T, Riabowol KT: Monitoring mRNA expression by polymerase chain reaction: the “primer-dropping” method. Anal Biochem 1994, 223:251-258 [DOI] [PubMed] [Google Scholar]
- 34.Church GM, Gilbert W: Genomic sequencing. Proc Natl Acad Sci USA 1984, 81:1991-1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman B, Zaremba S, Hockfield S: Monoclonal antibody rat 401 recognizes Schwann cells in mature and developing peripheral nerve. J Comp Neurol 1990, 295:43-51 [DOI] [PubMed] [Google Scholar]
- 36.Stemple DL, Anderson DJ: Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell 1992, 71:973-985 [DOI] [PubMed] [Google Scholar]
- 37.Torihashi S, Ward SM, Sanders KM: Development of c-Kit-positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology 1997, 112:144-155 [DOI] [PubMed] [Google Scholar]
- 38.Miettinen M, Monihan JM, Sarlomo-Rikala M, Kovatich AJ, Carr NJ, Emory TS, Sobin LH: Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol 1999, 23:1109-1118 [DOI] [PubMed] [Google Scholar]
- 39.Eaker EY, Sallustio JE: The distribution of novel intermediate filament proteins defines subpopulations of myenteric neurons in rat intestine. Gastroenterology 1994, 107:666-674 [DOI] [PubMed] [Google Scholar]
- 40.Vanderwinden JM, Rumessen JJ, Laet MD, Vanderhaeghen JJ, Schiffmann SN: CD34+ cells in human intestine are fibroblasts adjacent to, but distinct from, interstitial cells of Cajal. Lab Invest 1999, 79:59-65 [PubMed] [Google Scholar]
- 41.Redies C, Lendahl U, McKay RDG: Differentiation and heterogeneity in T-antigen immortalized precursor cell lines from mouse cerebellum. 1991, 30:601–615 [DOI] [PubMed]
- 42.Matsuda M, Katoh-Semba R, Kitani H, Tomooka Y: A possible role of the nestin protein in the developing central nervous system in rat embryos. Brain Res 1996, 723:177-189 [DOI] [PubMed] [Google Scholar]